Antioxidants to Defend Healthy and Youthful Skin—Current Trends and Future Directions in Cosmetology
Abstract
1. Introduction
2. Materials and Methods
3. Comprehensive Antioxidant Characteristics
3.1. Free Radicals
3.2. Oxidative Stress
3.3. Free Radicals and Aging
3.4. The Positive Impact of Free Radicals on the Human Body
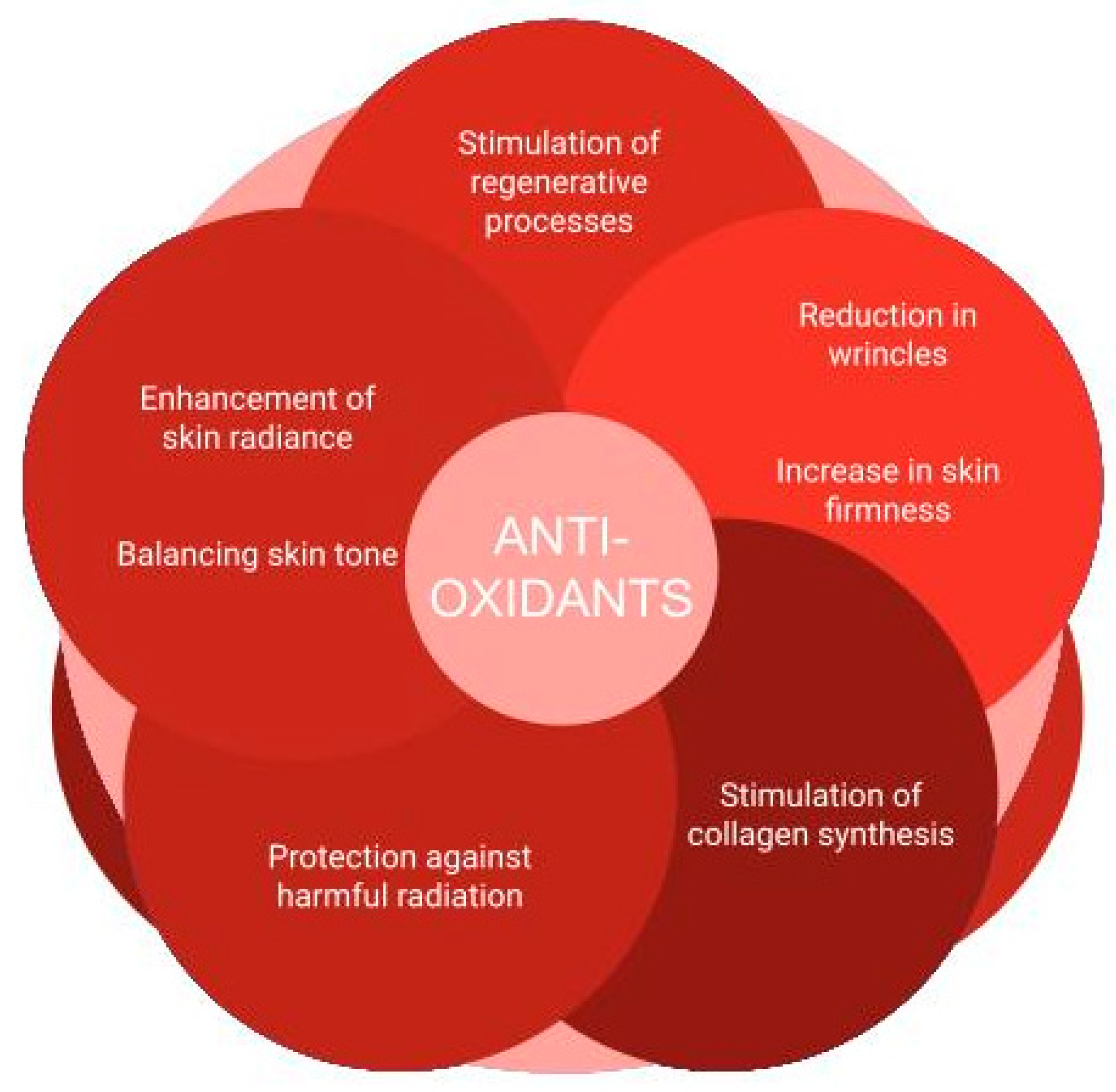
3.5. Antioxidants
4. Natural Antioxidants and Their Role in Cosmetics
4.1. Vitamins
4.1.1. Vitamin C (Ascorbic Acid)
4.1.2. Vitamin E (α-Tocopherol)
4.1.3. Vitamin A (Retinol)
4.1.4. Vitamin B5 (Pantothenic Acid)
4.1.5. Vitamin E and C Interaction
4.2. Carotenoids
4.2.1. β-Carotene
4.2.2. Lutein
4.2.3. Lycopene
4.2.4. Astaxanthin
4.2.5. Coenzyme Q10
4.3. Phenolic Compounds
4.3.1. Flavonoids
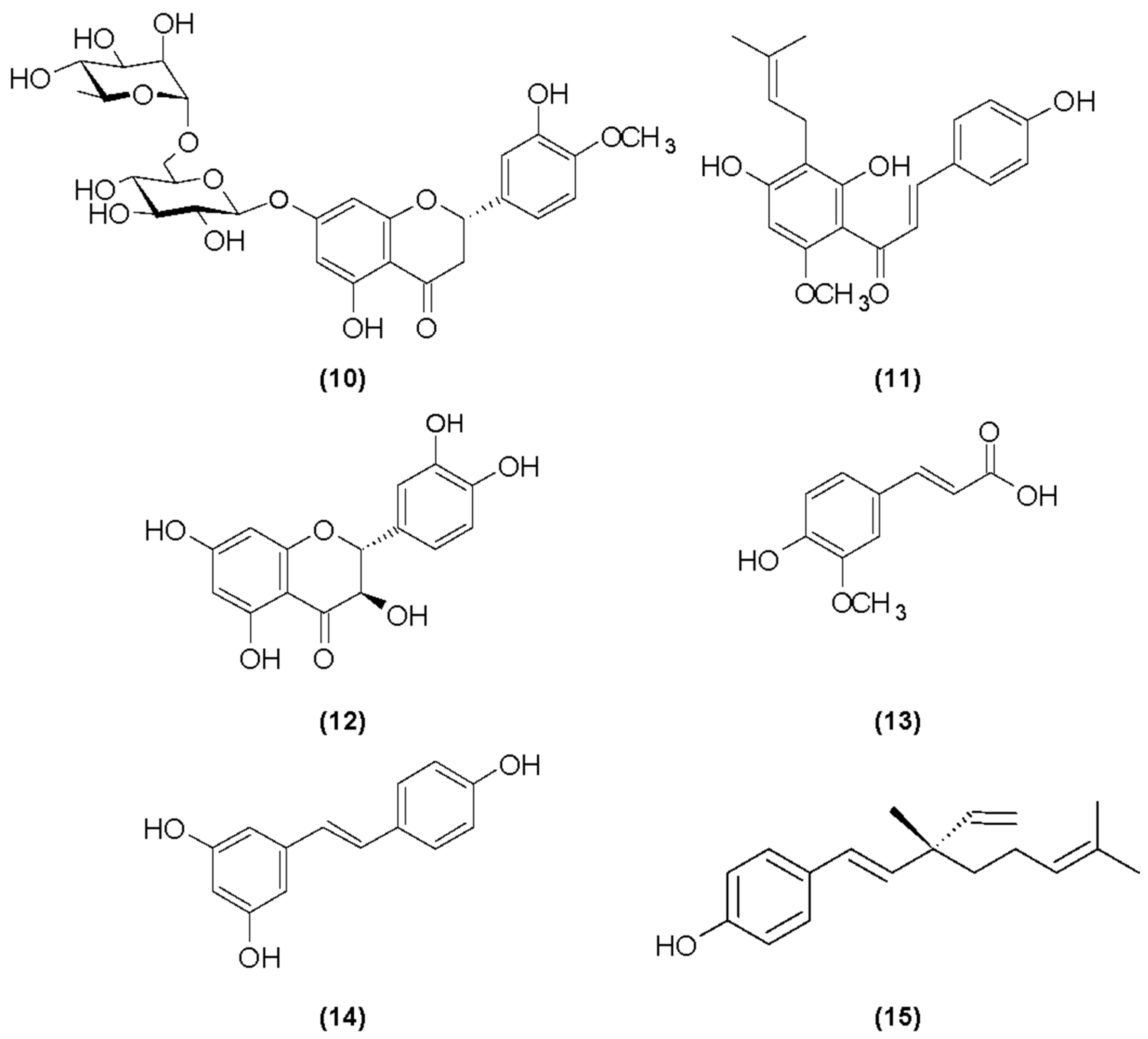
Hesperidin
Xanthohumol
Taxifolin
4.3.2. Phenolic Acids
Ferulic Acid
4.3.3. Resveratrol
4.3.4. Bakuchiol
4.4. Minerals
4.4.1. Selenium (Se)
4.4.2. Zinc (Zn)
4.5. Peptides, Amino Acids, Enzymes
4.5.1. Peptides
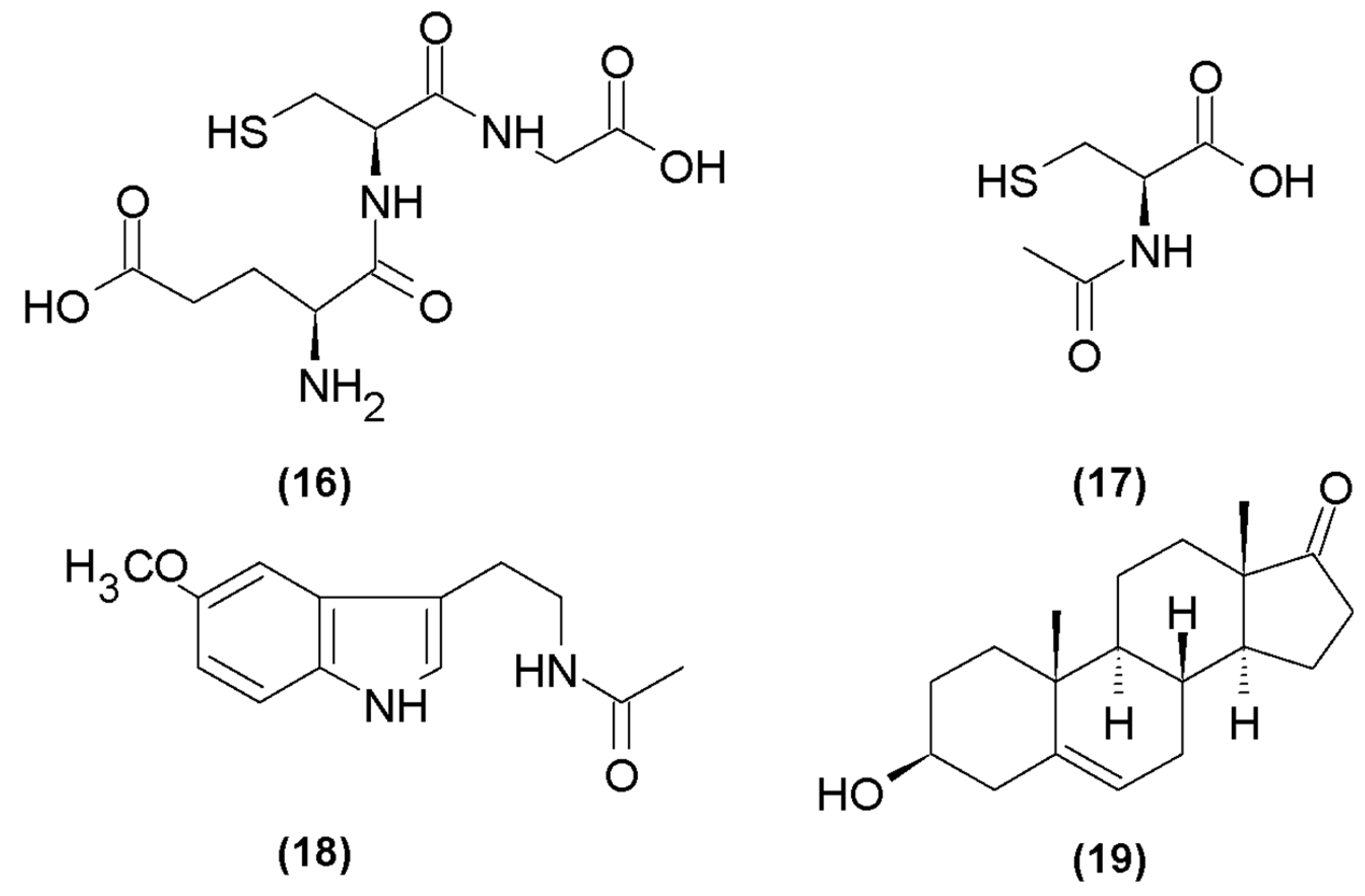
Glutathione
4.5.2. N-Acetyl-L-Cysteine
4.5.3. Superoxide Dismutases
4.6. Hormones
4.6.1. Melatonin
4.6.2. DHEA (Dehydroepiandrosterone)
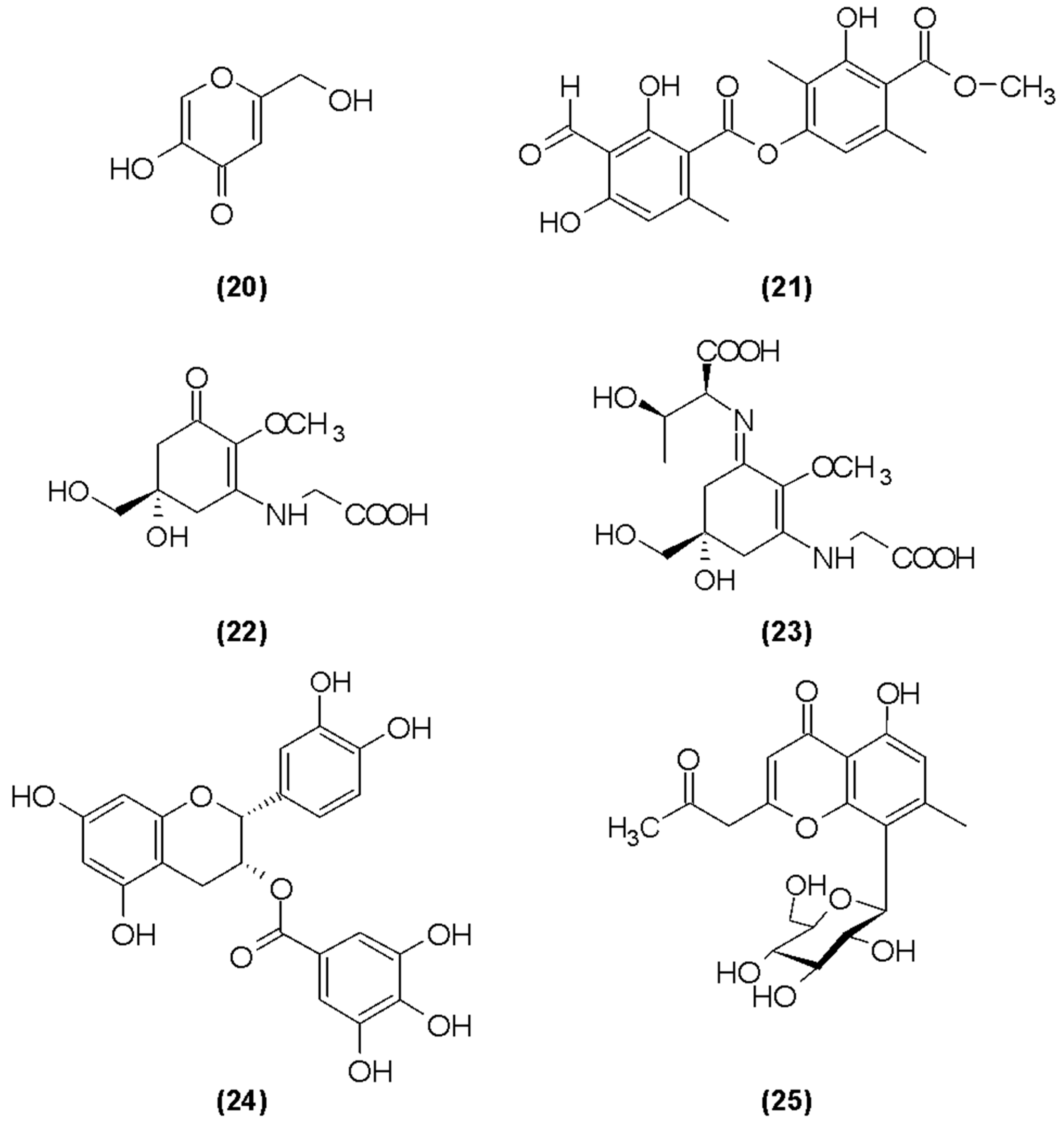
4.7. Butylated Hydroxytoluene (BHT)
4.8. Fungi-Derived Antioxidants
4.9. Lichen-Derived Antioxidants
4.10. Algae-Derived Antioxidants
4.10.1. Cyanobacteria (Blue-Green Algae)
4.10.2. Phaeophyceae (Brown Algae)
4.10.3. Chlorophyceae (Green Algae)
4.10.4. Rhodophyceae (Red Algae)
4.11. Selected Plant Species with Antioxidant Activity
4.11.1. Silybum marianum (L.) Gaertn
4.11.2. Camellia sinensis (L.) Kuntze
4.11.3. Solanum lycopersicum L.
4.11.4. Citrus Fruits
4.11.5. Vitis vinifera L.
4.11.6. Humulus lupulus L.
4.11.7. Aloe vera (L.) Webb.
4.11.8. Scutellaria baicalensis Georgi
4.11.9. Coffea arabica L.
4.11.10. Açaí Berries from Euterpe oleracea
4.12. Plant Stem Cells
5. Comparative Summary of Natural Antioxidants Used in Cosmetology
6. Delivery Systems for Transporting Cosmetic Ingredients
7. Bioavailability and Stability of Natural Antioxidant Ingredients in Cosmetics
8. Discussion
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and Aging-Related Diseases: From Molecular Mechanisms to Interventions and Treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.; Rodrigues, P.M.; Pintado, M.; Tavaria, F.K. A Systematic Review of Natural Products for Skin Applications: Targeting Inflammation, Wound Healing, and Photo-Aging. Phytomedicine 2023, 115, 154824. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Dhiman, T.; Negi, R.S.; OC, A.; Gupta, K.; Bhatti, J.S.; Thareja, S. A Comprehensive Review of the Molecular Mechanisms Driving Skin Photoaging and the Recent Advances in Therapeutic Interventions Involving Natural Polyphenols. S. Afr. J. Bot. 2024, 166, 466–482. [Google Scholar] [CrossRef]
- Debska, O.; Spiewak, R. The Meaning of the Term “Cosmetology” and the Tasks and Scope of Competences of the Cosmetologist According to Students in Cosmetology. Estetol. Med. Kosmetol. 2013, 3. [Google Scholar] [CrossRef]
- Ndhlala, A.R.; Moyo, M.; Van Staden, J. Natural Antioxidants: Fascinating or Mythical Biomolecules? Molecules 2010, 15, 6905–6930. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free Radicals, Antioxidants and Functional Foods: Impact on Human Health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Abdull Razis, A.F.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative Stress, Free Radicals and Antioxidants: Potential Crosstalk in the Pathophysiology of Human Diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef]
- Cheeseman, K.H.; Slater, T.F. An Introduction to Free Radicals Chemistry. Br. Med. Bull. 1993, 49, 481–493. [Google Scholar] [CrossRef]
- Young, I.S.; Woodside, J.V. Antioxidants in Health and Disease. J. Clin. Pathol. 2001, 54, 176–186. [Google Scholar] [CrossRef]
- Mena, S.; Ortega, A.; Estrela, J.M. Oxidative Stress in Environmental-Induced Carcinogenesis. Mutat. Res. 2009, 674, 36–44. [Google Scholar] [CrossRef]
- Dryden, G.; Deaciuc, I.; Arteel, G.; McClain, C. Clinical Implications of Oxidative Stress and Antioxidant Therapy. Curr. Gastroenterol. Rep. 2005, 7, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxid. Med. Cell. Longev. 2019, 5, 6175804. [Google Scholar] [CrossRef] [PubMed]
- Jena, A.B.; Samal, R.R.; Bhol, N.K.; Duttaroy, A.K. Cellular Red-Ox System in Health and Disease: The Latest Update. Biomed. Pharmacother. 2023, 162, 114606. [Google Scholar] [CrossRef] [PubMed]
- Afzal, S.; Abdul Manap, A.S.; Attiq, A.; Albokhadaim, I.; Kandeel, M.; Alhojaily, S.M. From Imbalance to Impairment: The Central Role of Reactive Oxygen Species in Oxidative Stress Induced Disorders and Therapeutic Exploration. Front. Pharmacol. 2023, 14, 1269581. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Lastra, J.M.P.d.l.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Chemical Insights into Oxidative and Nitrative Modifications of DNA. Int. J. Mol. Sci. 2023, 24, 15240. [Google Scholar] [CrossRef]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative Stress in Aging Human Skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef]
- Dröge, W. Free Radicals in the Physiological Control of Cell Function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Gdula-Argasińska, J.; Tyszka-Czochara, M. Role of Free Radicals in Physiological Processes: I. Med. Int. Rev. 2012, 2, 41–45. Available online: http://interrev.com/mir/index.php/mir/article/view/101/75 (accessed on 14 December 2024).
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several Lines of Antioxidant Defense against Oxidative Stress: Antioxidant Enzymes, Nanomaterials with Multiple Enzyme-Mimicking Activities, and Low-Molecular-Weight Antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Michalak, M. Plant-Derived Antioxidants: Significance in Skin Health and the Ageing Process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.-P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef] [PubMed]
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.K.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P.; et al. Vitamin C—Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef] [PubMed]
- Farbstein, D.; Kozak-Blickstein, A.; Levy, A.P. Antioxidant Vitamins and Their Use in Preventing Cardiovascular Disease. Molecules 2010, 15, 8098–8110. [Google Scholar] [CrossRef]
- Agus, D.B.; Gambhir, S.S.; Pardridge, W.M.; Spielholz, C.; Baselga, J.; Vera, J.C.; Golde, D.W. Vitamin C Crosses the Blood-Brain Barrier in the Oxidized Form Through the Glucose Transporters. J. Clin. Investig. 1997, 100, 2842–2848. [Google Scholar] [CrossRef]
- Harrison, F.E.; Bowman, G.L.; Polidori, M.C. Ascorbic Acid and the Brain: Rationale for the Use Against Cognitive Decline. Nutrients 2014, 6, 1752–1781. [Google Scholar] [CrossRef]
- Covarrubias-Pinto, A.; Acuña, A.I.; Beltrán, F.A.; Torres-Díaz, L.; Castro, M.A. Old Things New View: Ascorbic Acid Protects the Brain in Neurodegenerative Disorders. Int. J. Mol. Sci. 2015, 16, 28194–28217. [Google Scholar] [CrossRef]
- Smirnoff, N. Ascorbic Acid Metabolism and Functions: A Comparison of Plants and Mammals. Free Radic. Biol. Med. 2018, 122, 116–129. [Google Scholar] [CrossRef]
- Susa, F.; Pisano, R. Advances in Ascorbic Acid (Vitamin C) Manufacturing: Green Extraction Techniques from Natural Sources. Processes 2023, 11, 3167. [Google Scholar] [CrossRef]
- Pullar, J.M.; Carr, A.C.; Vissers, M. The Roles of Vitamin C in Skin Health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef]
- Wargala, E.; Sławska, M.; Zalewska, A.; Toporowska, M. Health Effects of Dyes, Minerals, and Vitamins Used in Cosmetics. Women 2021, 1, 223–237. [Google Scholar] [CrossRef]
- CIR—Cosmetic Ingredient Review. CIR Reports. 2025. Available online: https://cir-reports.cir-safety.org/ (accessed on 14 December 2024).
- Nakazawa, T.; Miyanoki, Y.; Urano, Y.; Uehara, M.; Saito, Y.; Noguchi, N. Effect of Vitamin E on 24(S)-Hydroxycholesterol-Induced Necroptosis-Like Cell Death and Apoptosis. J. Steroid Biochem. Mol. Biol. 2017, 169, 69–76. [Google Scholar] [CrossRef]
- Niki, E. Evidence for Beneficial Effects of Vitamin E. Korean J. Intern. Med. 2015, 30, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Mohn, E.S.; Kuchan, M.J.; Erdman, J.W.; Neuringer, M.; Matthan, N.R.; Chen, C.O.; Johnson, E.J. The Subcellular Distribution of Alpha-Tocopherol in the Adult Primate Brain and Its Relationship with Membrane Arachidonic Acid and Its Oxidation Products. Antioxidants 2017, 6, 97. [Google Scholar] [CrossRef] [PubMed]
- Kong, R.; Cui, Y.; Fisher, G. A Comparative Study of the Effects of Retinol and Retinoic Acid on Histological, Molecular, and Clinical Properties of Human Skin. J. Cosmet. Dermatol. 2015, 15, 49–57. [Google Scholar] [CrossRef]
- Carazo, A.; Macáková, K.; Matoušová, K.; Krčmová, L.K.; Protti, M.; Mladěnka, P. Vitamin A Update: Forms, Sources, Kinetics, Detection, Function, Deficiency, Therapeutic Use and Toxicity. Nutrients 2021, 13, 1703. [Google Scholar] [CrossRef]
- Blaner, W.S.; Shmarakov, I.O.; Traber, M.G. Vitamin A and Vitamin E: Will the Real Antioxidant Please Stand Up? Annu. Rev. Nutr. 2021, 41, 105–131. [Google Scholar] [CrossRef]
- Köpcke, W.; Krutmann, J. Protection from Sunburn with -Carotene—A Meta-analysis. Photochem. Photobiol. 2008, 84, 284–288. [Google Scholar] [CrossRef]
- Darlenski, R.; Surber, C.; Fluhr, J.W. Topical Retinoids in the Management of Photodamaged Skin: From Theory to Evidence-Based Practical Approach. Br. J. Dermatol. 2010, 163, 1157–1165. [Google Scholar] [CrossRef]
- Mukherjee, S.; Date, A.; Patravale, V.; Korting, H.C.; Roeder, A.; Weindl, G. Retinoids in the Treatment of Skin Aging: An Overview of Clinical Efficacy and Safety. Clin. Interv. Aging 2006, 1, 327–348. [Google Scholar] [CrossRef]
- Kafi, R.; Kwak, H.; Schumacher, W. Improvement of Naturally Aged Skin with Vitamin A (Retinol). Arch. Dermatol. 2007, 143, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, M.; Rossetti, D.; Leyden, J.J. One-Year Topical Stabilized Retinol Treatment Improves Photodamaged Skin in a Double-Blind, Vehicle-Controlled Trial. J. Drugs Dermatol. 2015, 14, 271–280. [Google Scholar] [PubMed]
- Zasada, M.; Budzisz, E. Retinoids: Active Molecules Influencing Skin Structure Formation in Cosmetic and Dermatological Treatments. Postępy Dermatol. Alergol. 2019, 36, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Bellemere, G.; Stamatas, G.N.; Bruere, V.; Bertin, C.; Issachar, N.; Oddos, T. Antiaging Action of Retinol: From Molecular to Clinical. Ski. Pharmacol. Physiol. 2009, 22, 200–209. [Google Scholar] [CrossRef]
- Sorg, O.; Kuenzli, S.; Kaya, G.; Saurat, J.H. Proposed Mechanisms of Action for Retinoid Derivatives in the Treatment of Skin Aging. J. Cosmet. Dermatol. 2015, 4, 237–244. [Google Scholar] [CrossRef]
- VanBuren, C.A.; Everts, H.B. Vitamin A in Skin and Hair: An Update. Nutrients 2022, 14, 2952. [Google Scholar] [CrossRef]
- Miallot, R.; Millet, V.; Galland, F.; Naquet, P. The Vitamin B5/Coenzyme A Axis: A Target for Immunomodulation? Eur. J. Immunol. 2023, 53, e2350435. [Google Scholar] [CrossRef]
- Sanvictores, T.; Chauhan, S. Vitamin B5 (Pantothenic Acid). In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK563233/ (accessed on 14 December 2024).
- Draelos, Z.D. Essentials of Hair Care often Neglected: Hair Cleansing. Int. J. Trichol. 2010, 2, 24. [Google Scholar] [CrossRef]
- Moncrieff, G.; Van Onselen, J.; Young, T. The Role of Emollients in Maintaining Skin Integrity. Wounds 2015, 11, 68–74. Available online: https://wounds-uk.com/journal-articles/the-role-of-emollients-in-maintaining-skin-integrity/ (accessed on 14 December 2024).
- Tadi, S.R.R.; Nehru, G.; Sivaprakasam, S. Microbial Production of Pantothenic Acid. In Microbial Production of Food Bioactive Compounds; Jafari, S.M., Harzevili, F.D., Eds.; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Bruno, R.S.; Leonard, S.W.; Atkinson, J.; Montine, T.J.; Ramakrishnan, R.; Bray, T.M.; Traber, M.G. Faster Plasma Vitamin E Disappearance in Smokers is Normalized by Vitamin C Supplementation. Free Radic. Biol. Med. 2006, 40, 689–697. [Google Scholar] [CrossRef]
- Burke, K.E. Interaction of Vitamins C and E as Better Cosmeceuticals. Dermatol. Ther. 2007, 20, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Baran, M.; Miziak, P.; Bonio, K. Characteristics of Carotenoids and their Use in the Cosmetics Industry. J. Educ. Health Sport 2020, 10, 192–196. [Google Scholar] [CrossRef]
- Biskanaki, F.; Kalofiri, P.; Tertipi, N.; Sfyri, E.; Andreou, E.; Kefala, V.; Rallis, E. Carotenoids and Dermoaesthetic Benefits: Public Health Implications. Cosmetics 2023, 10, 120. [Google Scholar] [CrossRef]
- Arct, J.; Mieloch, M. β-Carotene in Skin Care. Pol. J. Cosmetol. 2016, 19, 206–213. Available online: https://scholar.google.com/scholar?oi=bibs&cluster=6458389035210254153&btnI=1&hl=en (accessed on 14 December 2024).
- Antille, C.; Tran, C.; Sorg, O.; Saurat, J.H. Topical Beta-Carotene Is Converted to Retinyl Esters in Human Skin ex Vivo and Mouse Skin in Vivo. Exp. Dermatol. 2004, 13, 558–5621. [Google Scholar] [CrossRef]
- Wang, J.; Ma, W.; Ma, W.; Fang, Z.; Jiang, Y.; Jiang, W.; Kong, X.; Xin, F.; Zhang, W.; Jiang, M. Strategies for the Efficient Biosynthesis of Β-Carotene through Microbial Fermentation. World J. Microbiol. Biotechnol. 2024, 40, 160. [Google Scholar] [CrossRef]
- Shegokar, R.; Mitri, K. Carotenoid Lutein: A Promising Candidate for Pharmaceutical and Nutraceutical Applications. J. Diet. Suppl. 2012, 9, 183–210. [Google Scholar] [CrossRef]
- Fuad, N.I.N.; Sekar, M.; Gan, S.H.; Lum, P.T.; Vaijanathappa, J.; Ravi, S. Lutein: A Comprehensive Review on its Chemical, Biological Activities and Therapeutic Potentials. Pharmacogn. J. 2020, 12, 1769–1778. [Google Scholar] [CrossRef]
- Montuori, E.; Lima, S.; Marchese, A.; Scargiali, F.; Lauritano, C. Lutein Production and Extraction from Microalgae: Recent Insights and Bioactive Potential. Int. J. Mol. Sci. 2024, 25, 2892. [Google Scholar] [CrossRef]
- Rescio, L.; Maio, A.; Cazzola, P. Lycopene, Photoprotection and Skin Care: The Benefits of Organic Quality. J. Plastic Dermatol. 2010, 6, 37–47. Available online: https://www.researchgate.net/publication/286888377_Lycopene_photoprotection_and_skin_care_The_benefits_of_organic_quality (accessed on 14 December 2024).
- Marchena, A.M.; Franco, L.; Romero, A.M.; Barriga, C.; Rodríguez, A.B. Lycopene and Melatonin: Antioxidant Compounds in Cosmetic Formulations. Ski. Pharmacol. Physiol. 2020, 33, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, Z.; Jiang, H.; Mao, X. Biotechnological Production of Lycopene by Microorganisms. Appl. Microbiol. Biotechnol. 2020, 104, 10307–10324. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, J.; Yang, Q.; Yang, J. Metabolic Engineering Escherichia coli for the Production of Lycopene. Molecules 2020, 25, 3136. [Google Scholar] [CrossRef] [PubMed]
- Sztretye, M.; Dienes, B.; Gönczi, M.; Czirják, T.; Csernoch, L.; Dux, L.; Szentesi, P.; Keller-Pintér, A. Astaxanthin: A Potential Mitochondrial-Targeted Antioxidant Treatment in Diseases and with Aging. Oxid. Med. Cell Longev. 2019, 2019, 31814873. [Google Scholar] [CrossRef]
- Higuera-Ciapara, I.; Félix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A Review of its Chemistry and Applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef]
- Dutta, S.K.; Kumar, S.P.J.; Banerjee, R. A Comprehensive Review on Astaxanthin Sources, Structure, Biochemistry and Applications in the Cosmetic Industry. Algal Res. 2023, 74, 103168. [Google Scholar] [CrossRef]
- Komatsu, T.; Sasaki, S.; Manabe, Y.; Hirata, T.; Sugawara, T. Preventive Effect of Dietary Astaxanthin on UVA-Induced Skin Photoaging in Hairless Mice. PLoS ONE 2017, 12, e0171178. [Google Scholar] [CrossRef]
- Davinelli, S.; Nielsen, M.E.; Scapagnini, G. Astaxanthin in Skin Health, Repair, and Disease: A Comprehensive Review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef]
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus Astaxanthin: Applications for Human Health and Nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- Janiczek, M. Astaxanthin—A Carotenoid Antioxidant with Anti-Aging Properties. Zesz. Nauk. Akad. Górnośląskiej 2023, 11, 44–53. [Google Scholar]
- Suárez-Rivero, J.M.; Pastor-Maldonado, C.J.; Povea-Cabello, S.; Álvarez-Córdoba, M.; Villalón-García, I.; Munuera-Cabeza, M.; Suárez-Carrillo, A.; Talaverón-Rey, M.; Sánchez-Alcázar, J.A. Coenzyme Q10 Analogues: Benefits and Challenges for Therapeutics. Antioxidants 2021, 10, 236. [Google Scholar] [CrossRef] [PubMed]
- Zeifer, A. Coenzyme Q10. Structure, Role, Sources and Application in Cosmetology. Aesth. Cosmetol. Med. 2021, 10, 205–208. [Google Scholar]
- Lain, E.T.; Agrawal, N.; Ruvolo, E.; Weise, J.M.; Callender, V.D. The Role of Coenzyme Q10 in Skin Aging and Opportunities for Topical Intervention: A Review. J. Clin. Aesth. Dermatol. 2024, 17, 50–55. Available online: https://pubmed.ncbi.nlm.nih.gov/39148958/ (accessed on 14 December 2024).
- Potagrowicz, E.; Staniszewska, M.; Szerszenowicz, E. Coenzyme Q10—Properties and Application in Cosmetics. Pol. J. Cosmetol. 2006, 9, 2–6. Available online: http://www.kosmet.pl/pjc.php?opc=AR&lng=en&art=291 (accessed on 14 December 2024).
- Fan, J.; Xu, W.; Xu, X.; Wang, Y. Production of Coenzyme Q10 by Microbes: An Update. World J. Microbiol. Biotechnol. 2022, 38, 194. [Google Scholar] [CrossRef]
- Temova Rakuša, Ž.; Roškar, R. Quality Control of Vitamins A and E and Coenzyme Q10 in Commercial Anti-Ageing Cosmetic Products. Cosmetics 2021, 8, 61. [Google Scholar] [CrossRef]
- Shi, L.; Zhao, W.; Yang, Z.; Subbiah, V.; Suleria, H.A.R. Extraction and Characterization of Phenolic Compounds and their Potential Antioxidant Activities. Environ. Sci. Pollut. Res. 2022, 29, 81112–81129. [Google Scholar] [CrossRef]
- Foss, K.; Przybyłowicz, K.E.; Sawicki, T. Antioxidant Activity and Profile of Phenolic Compounds in Selected Herbal Plants. Plant Foods Hum. Nutr. 2022, 77, 383–389. [Google Scholar] [CrossRef]
- Rodrigues, C.V.; Pintado, M. Hesperidin from Orange Peel as a Promising Skincare Bioactive: An Overview. Int. J. Mol. Sci. 2024, 25, 1890. [Google Scholar] [CrossRef]
- Greensky Biological Tech. Co. Hesperidin Production: A Complete Guide for Consumers and Manufacturers. Available online: http://www.greenskybio.com/blog5/hesperidin-production-a-complete-guide-for-consumers-and-manufacturers.html (accessed on 14 December 2024).
- Girisa, S.; Saikia, Q.; Bordoloi, D.; Banik, K.; Monisha, J.; Daimary, U.D.; Verma, E.; Ahn, K.S.; Kunnumakkara, A.B. Xanthohumol from Hop: Hope For Cancer Prevention and Treatment. IUBMB Life 2021, 73, 1016–1044. [Google Scholar] [CrossRef]
- Żołnierczyk, A.K.; Baczyńska, D.; Potaniec, B.; Kozłowska, J.; Grabarczyk, M.; Woźniak, E.; Anioł, M. Antiproliferative and Antioxidantactivity of Xanthohumol Acyl Derivatives. Med. Chem. Res. 2017, 26, 1764–1771. [Google Scholar] [CrossRef]
- Kołodziejczak, A.; Dziedzic, M.; Algiert-Zielińska, B.; Mucha, P.; Rotsztejn, H. A Novel Look at Mechanisms and Applications of Xanthohumol (XN) in Dermatology and Cosmetology. Int. J. Mol. Sci. 2024, 25, 11938. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Hansen, P.E.; Wang, G.; Qiu, L.; Dong, J.; Yin, H.; Qian, Z.; Yang, M.; Miao, J. Pharmacological Profile of Xanthohumol, a Prenylated Flavonoid from Kops (Humulus lupulus). Molecules 2015, 20, 754–790. [Google Scholar] [CrossRef] [PubMed]
- Grudniewska, A.; Pastyrczyk, N. New Insight for Spent Hops Utilization: Simultaneous Extraction of Protein and Xanthohumol Using Deep Eutectic Solvents. Biomass Conversion Biorefin. 2023, 13, 14975–14986. [Google Scholar] [CrossRef]
- Das, A.; Baidya, R.; Chakraborty, T.; Samanta, A.K.; Roy, S. Pharmacological Basis and New Insights of Taxifolin: A Comprehensive Review. Biomed. Pharmacother. 2021, 142, 112004. [Google Scholar] [CrossRef]
- Topal, F.; Nar, M.; Gocer, H.; Kalin, P.; Kocyigit, U.M.; Gülçin, İ.; Alwasel, S.H. Antioxidant Activity of Taxifolin: An Activity–Structure Relationship. J. Enzym. Inhib. Med. Chem. 2016, 31, 674–683. [Google Scholar] [CrossRef]
- Micek, I.; Nawrot, J.; Seraszek-Jaros, A.; Jenerowicz, D.; Schroeder, G.; Spizewski, T.; Suchan, A.; Pawlaczyk, M.; Gornowicz-Porowska, J. Taxifolin as a Promising Ingredient of Cosmetics for Adult Skin. Antioxidants 2021, 10, 1625. [Google Scholar] [CrossRef]
- Sui, Y.; Han, Y.; Qiu, Z.; Yan, B.; Zhao, G.-R. Heterologous Biosynthesis of Taxifolin in Yarrowia lipolytica: Metabolic Engineering and Genome-Scale Metabolic Modeling. Appl. Biochem. Biotechnol. 2024, 1–23. [Google Scholar] [CrossRef]
- An, H.J.; Lee, Y.; Liu, L.; Lee, S.; Lee, J.D.; Yi, Y. Physical and Chemical Stability of Formulations Loaded with Taxifolin Tetra-octanoate. Chem. Pharm. Bull. 2019, 67, 985–991. [Google Scholar] [CrossRef]
- Li, D.; Rui, Y.X.; Guo, S.D.; Luan, F.; Liu, R.; Zeng, N. Ferulic Acid: A Review of its Pharmacology, Pharmacokinetics and Derivatives. Life Sci. 2021, 284, 119921. [Google Scholar] [CrossRef]
- Lambruschini, C.; Demori, I.; El Rashed, Z.; Rovegno, L.; Canessa, E.; Cortese, K.; Grasselli, E.; Moni, L. Synthesis, Photoisomerization, Antioxidant Activity, and Lipid-Lowering Effect of Ferulic Acid and Feruloyl Amides. Molecules 2020, 26, 89. [Google Scholar] [CrossRef]
- Zduńska-Pęciak, K.; Dębowska, R.; Kołodziejczak, A.; Rotsztejn, H. Ferulic Acid—A Novel Topical Agent in Reducing Signs of Photoaging. Dermatol. Ther. 2022, 35, e15543. [Google Scholar] [CrossRef] [PubMed]
- Hahn, H.J.; Kim, K.B.; Bae, S.; Choi, B.G.; An, S.; Ahn, K.J.; Kim, S.J. Pretreatment of Ferulic Acid Protects Human Dermal Fibroblasts Against Ultraviolet a Irradiation. Ann. Dermatol. 2016, 28, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Ambothi, K.; Nagarajan, R.P. Ferulic Acid Prevents Ultraviolet-B Radiation Induced Oxidative DNA Damage in Human Dermal Fibroblasts. Int. J. Nutr. Pharmacol. Neurol. Dis. 2014, 4, 203–213. [Google Scholar] [CrossRef]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Ferulic Acid Properties and Application. Ski. Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Truong, H.T.; Do Van, M.; Duc Huynh, L.; Thi Nguyen, L.; Do Tuan, A.; Le Xuan Thanh, T.; Duong Phuoc, H.; Takenaka, N.; Imamura, K.; Maeda, Y. A Method for Ferulic Acid Production from Rice Bran Oil Soapstock Using a Homogenous System. Appl. Sci. 2017, 7, 796. [Google Scholar] [CrossRef]
- Lv, H.; Zhang, Y.; Shao, J.; Liu, H.; Wang, Y. Ferulic Acid Production by Metabolically Engineered Escherichia coli. Bioresour. Bioprocess. 2021, 8, 70. [Google Scholar] [CrossRef]
- Blanchard, N.; Sutterlin, W.R.; Long, R.A. Methods for the Production of Ferulic Acid. US WO 2021138549A1, 31 December 2020. Available online: https://patents.google.com/patent/WO2021138549A1/en (accessed on 14 December 2024).
- Mlakić, M.; Fodor, L.; Odak, I.; Horváth, O.; Lovrić, M.J.; Barić, D.; Milašinović, V.; Molčanov, K.; Marinić, Ž.; Lasić, Z.; et al. Resveratrol-Maltol and Resveratrol-Thiophene Hybrids as Cholinesterase Inhibitors and Antioxidants: Synthesis, Biometal Chelating Capability and Crystal Structure. Molecules 2022, 27, 6379. [Google Scholar] [CrossRef]
- Farris, P.; Yatskayer, M.; Chen, N. Evaluation of Efficacy and Tolerance of a Nighttime Topical Antioxidant Containing Resveratrol, Baicalin, and Vitamin E for Treatment of Mild to Moderately Photodamaged Skin. J. Drugs Dermatol. 2014, 13, 1467–1472. Available online: https://www.semanticscholar.org/paper/Evaluation-of-efficacy-and-tolerance-of-a-nighttime-Farris-Yatskayer/c4644112a07ad3718738e6320b9d1b486699a5d2 (accessed on 14 December 2024).
- Leis, K.; Pisanko, K.; Jundziłł, A.; Mazur, E.; Mêcińska-Jundziłł, K.; Witmanowski, H. Resveratrol as a Factor Preventing Skin Aging and Affecting its Regeneration. Postepy Dermatol. Alergol. 2022, 39, 439–445. [Google Scholar] [CrossRef]
- Brinke, A.S.; Janssens-Bocker, C.; Kerscher, M. Skin Anti-Aging Benefits of a 2% Resveratrol Emulsion. JCDSA 2021, 11, 155–157. [Google Scholar] [CrossRef]
- Thapa, S.B.; Jeon, J.; Park, B.G.; Shim, D.; Lee, C.S.; Sohng, J.K. Production of Resveratrol Glucosides and Its Cosmetic Activities. Cosmetics 2023, 10, 98. [Google Scholar] [CrossRef]
- Ratz-Łyko, A.; Arct, J. Resveratrol as an Active Ingredient for Cosmetic and Dermatological Applications: A Review. J. Cosmet. Laser Ther. 2018, 21, 84–90. [Google Scholar] [CrossRef]
- Mehta, G.; Nayak, U.R.; Dev, S. Bakuchiol, a Novel Monoterpenoid. Tetrahedron Lett. 1966, 7, 4561–4567. [Google Scholar] [CrossRef]
- Labbé, C.; Faini, F.; Coll, J.; Connolly, J.D. Bakuchiol Derivatives from the Leaves of Psoralea glandulosa. Phytochemistry 1996, 42, 1299–1303. [Google Scholar] [CrossRef]
- Haraguchi, H.; Inoue, J.; Tamura, Y.; Mizutani, K. Antioxidative Components of Psoralea corylifolia (Leguminosae). Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2002, 16, 539–544. [Google Scholar] [CrossRef]
- Adhikari, S.; Joshi, R.; Patro, B.S.; Ghanty, T.K.; Chintalwar, G.J.; Sharma, A.; Chattopadhyay, S.; Mukherjee, T. Antioxidant Activity of Bakuchiol: Experimental Evidences and Theoretical Treatments on the Possible Involvement of the Terpenoid Chain. Chem. Res. Toxicol. 2003, 16, 1062–1069. [Google Scholar] [CrossRef]
- Chaudhuri, R.K.; Bojanowski, K. Bakuchiol: A Retinol-Like Functional Compound Revealed by Gene Expression Profiling and Clinically Proven to Have Anti-Aging Effects. Int. J. Cosmet. Sci. 2014, 36, 221–230. [Google Scholar] [CrossRef]
- Dhaliwal, S.; Rybak, I.; Ellis, S.R.; Notay, M.; Trivedi, M.; Burney, W.; Vaughn, A.R.; Nguyen, M.; Reiter, P.; Bosanac, S.; et al. Prospective, Randomized, Double-Blind Assessment of Topical Bakuchiol and Retinol for Facial Photoageing. Br. J. Dermatol. 2019, 180, 289–296. [Google Scholar] [CrossRef]
- Bacqueville, D.; Maret, A.; Noizet, M.; Duprat, L.; Coutanceau, C.; Georgescu, V.; Bessou-Touya, S.; Duplan, H. Efficacy of a Dermocosmetic Serum Combining Bakuchiol and Vanilla tahitensis Extract to Prevent Skin Photoaging In Vitro and to Improve Clinical Outcomes for Naturally Aged Skin. Clin. Cosmet. Investig. Dermatol. 2020, 13, 359–370. [Google Scholar] [CrossRef]
- Puyana, C.; Chandan, N.; Tsoukas, M. Applications of Bakuchiol in Dermatology: Systematic Review of the Literature. J. Cosmet. Dermatol. 2022, 21, 6636–6643. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.H. A Comprehensive Review of Topical Bakuchiol for the Treatment of Photoaging. J. Integr. Derm. 2022, 1–22. Available online: https://www.jintegrativederm.org/article/38079-a-comprehensive-review-of-topical-bakuchiol-for-the-treatment-of-photoaging (accessed on 14 December 2024).
- Mascarenhas-Melo, F.; Ribeiro, M.M.; Kahkesh, K.H.; Parida, S.; Pawar, K.D.; Velsankar, K.; Jha, N.K.; Damiri, F.; Costa, G.; Veiga, F.; et al. Comprehensive Review of the Skin Use of Bakuchiol: Physicochemical Properties, Sources, Bioactivities, Nanotechnology Delivery Systems, Regulatory and Toxicological Concerns. Phytochem. Rev. 2024, 23, 1377–1413. [Google Scholar] [CrossRef]
- Apel, A.R.; Kumar, A.; Kalbarczyk, K.; Thacker, D.F. Engineered Enzymes and Bioproduction of Bakuchiol. WO 2023168043A1, 3 March 2023. Available online: https://patents.google.com/patent/WO2023168043A1/en (accessed on 14 December 2024).
- Barchielli, G.; Capperucci, A.; Tanini, D. The Role of Selenium in Pathologies: An Updated Review. Antioxidants 2022, 11, 251. [Google Scholar] [CrossRef] [PubMed]
- Lemire, M.; Philibert, A.; Fillion, M.; Passos, C.J.S.; Guimarães, J.R.D.; Barbosa, F.; Mergler, D. No Evidence of Selenosis from a Selenium-Rich Diet in the Brazilian Amazon. Environ. Int. 2012, 40, 128–136. [Google Scholar] [CrossRef]
- Bjørklund, G.; Shanaida, M.; Lysiuk, R.; Antonyak, H.; Klishch, I.; Shanaida, V.; Peana, M. Selenium: An Antioxidant with a Critical Role in Anti-Aging. Molecules 2022, 27, 6613. [Google Scholar] [CrossRef]
- Zaporowska, H.; Szymanowski, R. Selen in Cosmetics, Nutricosmetics and Cosmetology. Pol. J. Cosmetol. 2014, 17, 197–202. Available online: http://www.kosmet.pl/pjc.php?opc=AR&lng=en&art=641 (accessed on 14 December 2024).
- Krzysik, M.; Biernat, J.; Grajeta, H. The influence of Chosen Nutrients on Immune System Functioning Part II. Immunomodulatory Effects of Vitamins and Trace Elements on the Human Body. Adv. Clin. Exp. Med. 2007, 16, 123–133. Available online: https://advances.umw.edu.pl/en/article/2007/16/1/123/ (accessed on 14 December 2024).
- Mai, W.; Wang, F.; He, S.; Wen, Y.; Yu, G.; Zhang, L.; Dong, H. Zinc Contents in Foods and Estimates of Dietary Intakes in Guangzhou, Guangdong Province, China. Front. Nutr. 2024, 11, 1364033. [Google Scholar] [CrossRef]
- Rostan, E.F.; DeBuys, H.V.; Madey, D.L.; Pinnell, S.R. Evidence Supporting Zinc as an Important Antioxidant for Skin. Int. J. Dermatol. 2002, 41, 606–611. [Google Scholar] [CrossRef]
- Marreiro, D.D.N.; Cruz, K.J.C.; Morais, J.B.S.; Beserra, J.B.; Severo, J.S.; De Oliveira, A.R.S. Zinc and Oxidative Stress: Current Mechanisms. Antioxidants 2017, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Frydrych, A.; Arct, J.; Kasiura, K. Zinc: A Criticai lmportance Element in Cosmetology. J. Appl. Cosmetol. 2004, 22, 1–13. Available online: https://api.semanticscholar.org/CorpusID:53871011 (accessed on 14 December 2024).
- Abendrot, M.; Kalinowska-Lis, U. Zinc-containing Compounds for Personal Care Applications. Int. J. Cosmet. Sci. 2018, 40, 319–327. [Google Scholar] [CrossRef]
- Nie, C.; Zou, Y.; Liao, S.; Gao, Q.; Li, Q. Peptides as Carriers of Active Ingredients: A Review. Curr. Res. Food Sci. 2023, 7, 100592. [Google Scholar] [CrossRef]
- Pickart, L.; Margolina, A. Regenerative and Protective Actions of the GHK-Cu Peptide in the Light of the New Gene Data. Int. J. Mol. Sci. 2018, 19, 1987. [Google Scholar] [CrossRef]
- Badenhorst, T.; Svirskis, D.; Marrilees, M.; Bolke, L.; Wu, Z. Effects of GHK-Cu on MMP and TIMP Expression, Collagen and Elastin Production, and Facial Wrinkle Parameters. J. Aging Sci. 2016, 4, 166. Available online: https://api.semanticscholar.org/CorpusID:55701310 (accessed on 14 December 2024). [CrossRef]
- Hussain-Łukaszewicz, A. The Role of Glutathione and Glutathione-Related Enzymes in Antioxidative Process. Med. Prac. 2003, 54, 473–479. Available online: https://pubmed.ncbi.nlm.nih.gov/14978897/ (accessed on 14 December 2024).
- Zhang, Z.; Apse, K.; Pang, J.; Stanton, R.C. High Glucose Inhibits Glucose-6-Phosphate Dehydrogenase via Camp in Aortic Endothelial Cells. J. Biol. Chem. 2000, 275, 40042–40047. [Google Scholar] [CrossRef]
- Villarama, C.D.; Maibach, H.I. Glutathione as a Depigmenting Agent: An Overview. Int. J. Cosm. Sci. 2005, 27, 147–153. [Google Scholar] [CrossRef]
- Mohan, S.; Mohan, L.; Sangal, R.; Singh, N. Glutathione for skin lightening for dermatologists and cosmetologists. Int. J. Res. Dermatol. 2020, 6, 284–287. [Google Scholar] [CrossRef]
- Li, Y.; Wei, G.; Chen, J. Glutathione: A Review on Biotechnological Production. Appl. Microbiol. Biotechnol. 2004, 66, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Schmacht, M.; Lorenz, E.; Senz, M. Microbial Production of Glutathione. World J. Microbiol. Biotechnol. 2017, 33, 106. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Tao, R.; Shen, Z.; Sun, L.; Zhu, F.; Yang, S. Enzymatic Production of Glutathione by Bifunctional γ-Glutamylcysteine Synthetase/Glutathione Synthetase Coupled with In Vitro Acetate Kinase-Based ATP Generation. Appl. Biochem. Biotechnol. 2016, 180, 1446–1455. [Google Scholar] [CrossRef] [PubMed]
- Dean, O.; Giorlando, F.; Berk, M. N-Acetylcysteine in Psychiatry: Current Therapeutic Evidence and Potential Mechanisms of Action. J. Psychiatry Neurosci. 2011, 36, 78–86. [Google Scholar] [CrossRef]
- Holdiness, M. Clinical Pharmokinetics of N-acetylcysteine. Clin. Pharmacokinet. 1991, 20, 123–134. [Google Scholar] [CrossRef]
- Janeczek, M.; Moy, L.; Riopelle, A.; Vetter, O.; Reserva, J.; Tung, R.; Swan, J. The Potential Uses of N-acetylcysteine in Dermatology: A Review. J. Clin. Aesthet. Dermatol. 2019, 12, 20–26. Available online: https://www.semanticscholar.org/paper/The-Potential-Uses-of-N-acetylcysteine-in-A-Review.-Janeczek-Moy/8fe0191240d3ff79b13509aaaa58e4c787b2aabc (accessed on 14 December 2024).
- Goodson, A.G.; Cotter, M.A.; Cassidy, P.; Wade, M.; Florell, S.R.; Liu, T.; Boucher, K.M.; Grossman, D. Use of oral N-acetylcysteine for protection of melanocytic nevi against UV-induced oxidative stress: Towards a novel paradigm for melanoma chemoprevention. Clin. Cancer Res. 2009, 15, 7434–7440. [Google Scholar] [CrossRef]
- Wuhan Grand Hoyo. Production method of N-acetyl-L-cysteine. CN 104844488A, 25 March 2015. Available online: https://patents.google.com/patent/CN104844488A/en (accessed on 14 December 2024).
- Gałecka, E.; Jacewicz, R.; Mrowicka, M.; Florkowski, A.; Gałecki, P. Antioxidative Enzymes—Structure, Properties, Functions. Pol. Merk. Lek. 2008, 147, 266–267. Available online: https://pubmed.ncbi.nlm.nih.gov/19112846/ (accessed on 14 December 2024).
- Zheng, M.; Liu, Y.; Zhang, G.; Yang, Z.; Xu, W.; Chen, Q. The Applications and Mechanisms of Superoxide Dismutase in Medicine, Food, and Cosmetics. Antioxidants 2023, 12, 1675. [Google Scholar] [CrossRef]
- McCord, J.M.; Edeas, M.A. SOD, Oxidative Stress and Human Pathologies: A Brief History and a Future Vision. Biomed. Pharmacother. 2005, 59, 139–142. [Google Scholar] [CrossRef]
- Altobelli, G.G.; Van Noorden, S.; Balato, A.; Cimini, V. Copper/Zinc Superoxide Dismutase in Human Skin: Current Knowledge. Front. Med. 2020, 7, 183. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, S.; Rao, P.; Bradshaw, J.; Weller, R. Topical Application of Superoxide Dismutase Mediated by HIV-TAT Peptide Attenuates UVB-Induced Damages in Human Skin. Eur. J. Pharm. Biopharm. 2016, 107, 286–294. [Google Scholar] [CrossRef] [PubMed][Green Version]
- AQUAMER. Microalgal Culture Extract with Superoxide Dismutase-Like Activity Useful for Preparing Cosmetic, Dietetic, Pharmaceutical and Veterinary Products for Preventing or Treating Aging and Degeneration. FR 2785909A1, 16 November 1998. Available online: https://patents.google.com/patent/FR2785909A1/en (accessed on 14 December 2024).
- Kudari, S.; Patrana, M. Production and Characterization of Recombinant Superoxide Dismutase Protein Expressed in E.coli. Int. J. Adv. Res. Publ. 2017, 1, 81–83. Available online: https://www.ijarp.org/published-research-papers/oct2017/Production-And-Characterization-Of-Recombinant-Superoxide-Dismutase-Protein-Expressed-In-Ecoli.pdf (accessed on 14 December 2024).
- Suntory. Process for Production of Superoxide Dismutase. JP 0218472A2, 2 October 1985. Available online: https://patents.google.com/patent/EP0218472A2/en (accessed on 14 December 2024).
- Galano, A.; Tan, D.X.; Reiter, R.J. Melatonin: A Versatile Protector against Oxidative DNA Damage. Molecules 2018, 23, 530. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.B.; Ali, A.; Bilal, M.; Rashid, S.M.; Wani, A.B.; Bhat, R.R.; Rehman, M.U. Melatonin and Health: Insights of Melatonin Action, Biological Functions, and Associated Disorders. Cell. Mol. Neurobiol. 2023, 43, 2437–2458. [Google Scholar] [CrossRef]
- Monteiro, K.K.A.C.; Shiroma, M.E.; Damous, L.L.; Simões, M.d.J.; Simões, R.d.S.; Cipolla-Neto, J.; Baracat, E.C.; Soares, J.M., Jr. Antioxidant Actions of Melatonin: A Systematic Review of Animal Studies. Antioxidants 2024, 13, 439. [Google Scholar] [CrossRef]
- Greco, G.; Di Lorenzo, R.; Ricci, L.; Di Serio, T.; Vardaro, E.; Laneri, S. Clinical Studies Using Topical Melatonin. Int. J. Mol. Sci. 2024, 25, 5167. [Google Scholar] [CrossRef]
- Yang, L.; Palsson, B.O. Biomanufacturing of Melatonin Is Now Possible. Melatonin Res. 2023, 6, 72–78. [Google Scholar] [CrossRef]
- Aragno, M.; Cutrin, J.C.; Mastrocola, R.; Perrelli, M.G.; Restivo, F.; Poli, G.; Danni, O.; Boccuzzi, G. Oxidative Stress and Kidney Dysfunction Due to Ischemia/Reperfusion in Rat: Attenuation by Dehydroepiandrosterone. Kidney Int. 2003, 64, 836–843. [Google Scholar] [CrossRef]
- Nouveau, S.; Bastien, P.; Baldo, F.; de Lacharriere, O. Effects of Topical DHEA on Aging Skin: A Pilot Study. Maturitas 2008, 59, 174–181. [Google Scholar] [CrossRef]
- Zhou, P.; Fang, Y.-K.; Yao, H.-K.; Li, H.; Wang, G.; Liu, Y.-P. Efficient Biotransformation of Phytosterols to Dehydroepiandrosterone by Mycobacterium sp. Appl. Biochem. Biotechnol. 2018, 186, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Hunan Kerey Biotechnology. Synthesis Method for Dehydroepiandrosterone. CN 102212099A, 2 April 2011. Available online: https://patents.google.com/patent/CN102212099A/en (accessed on 14 December 2024).
- Yehye, W.A.; Rahman, N.A.; Ariffin, A.; Abd Hamid, S.B.; Alhadi, A.A.; Kadir, F.A.; Yaeghoobi, M. Understanding the Chemistry behind the Antioxidant Activities of Butylated Hydroxytoluene (BHT): A Review. Eur. J. Med. Chem. 2015, 101, 295–312. [Google Scholar] [CrossRef] [PubMed]
- Lanigan, R.S.; Yamarik, T.A. Final Report on the Safety Assessment of BHT(1). Int. J. Toxicol. 2002, 21, 19–94. [Google Scholar] [CrossRef]
- Flieger, J.; Flieger, W.; Baj, J.; Maciejewski, R. Antioxidants: Classification, Natural Sources, Activity/Capacity Measurements, and Usefulness for Synthesis of Nanoparticles. Materials 2021, 14, 4135. [Google Scholar] [CrossRef] [PubMed]
- Chandra, P.; Sharma, R.K.; Arora, D.S. Antioxidant Compounds from Microbial Sources: A Review. Food Res. Int. 2020, 129, 108849. [Google Scholar] [CrossRef]
- Ślusarczyk, J.; Adamska, E.; Czerwik-Marcinkowska, J. Fungi and Algae as Sources of Medicinal and Other Biologically Active Compounds: A Review. Nutrients 2021, 13, 3178. [Google Scholar] [CrossRef]
- Mukherjee, A.; Das, S.; Chakraborty, D.; Pal, N.; Das, N. Fungi’s Treasure in Cosmeceuticals—A Comprehensive Chemical Approach. S. Afr. J. Bot. 2024, 166, 311–331. [Google Scholar] [CrossRef]
- Muszyńska, B.; Sułkowska-Ziaja, K.; Ekiert, H. Phenolic Acids in Selected Edible Basidiomycota Species: Armillaria mellea, Boletus badius, Boletus edulis, Cantharellus cibarius, Lactarius deliciosus and Pleurotus ostreatus. Acta Sci. Pol. Hortorum Cultus 2013, 12, 107–116. Available online: https://agro.icm.edu.pl/agro/element/bwmeta1.element.agro-96d16b8e-9828-48c3-bb54-989b8cd870c2 (accessed on 11 December 2024).
- Reis, F.S.; Martins, A.; Barros, L.; Ferreira, I.C. Antioxidant Properties and Phenolic Profile of the Most Widely Appreciated Cultivated Mushrooms: A Comparative Study Between In Vivo and In Vitro Samples. Food Chem. Toxicol. 2012, 50, 1201–1207. [Google Scholar] [CrossRef]
- Muszyńska, B.; Sułkowska-Ziaja, K.; Ekiert, H. Indole Compounds in Fruiting Bodies of Some Edible Basidiomycota Species. Food Chem. 2011, 125, 1306–1308. [Google Scholar] [CrossRef]
- Arslan, N.P.; Dawar, P.; Albayrak, S.; Doymus, M.; Azad, F.; Esim, N.; Taskin, M. Fungi-derived Natural Antioxidants. Crit. Rev. Food Sci. Nutr. 2023, 29, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Mago, P.; Sharma, R.; Hafeez, I.; Nawaz, I.; Joshi, M.; Mehrotra, R. Mushroom based Cosmeceuticals: An Upcoming Biotechnology Sector. Biosci. Biotechnol. Res. Asia 2023, 20. [Google Scholar] [CrossRef]
- Fernández-Moriano, C.; Gómez-Serranillos, M.P.; Crespo, A. Antioxidant Potential of Lichen Species and their Secondary Metabolites. A Systematic Review. Pharm. Biol. 2016, 54, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kosanić, M.; Ranković, B. Lichens as Possible Sources of Antioxidants. Pak. J. Pharm. Sci. 2011, 24, 165–170. Available online: https://pubmed.ncbi.nlm.nih.gov/21454165/ (accessed on 11 December 2024). [PubMed]
- Urbanska, N.; Simko, P.; Leskanicova, A.; Karasova, M.; Jendzelovska, Z.; Jendzelovsky, R.; Rucova, D.; Kolesarova, M.; Goga, M.; Backor, M.; et al. Atranorin, a Secondary Metabolite of Lichens, Exhibited Anxiolytic/Antidepressant Activity in Wistar Rats. Life 2022, 12, 1850. [Google Scholar] [CrossRef]
- Studzińska-Sroka, E.; Galanty, A.; Bylka, W. Atranorin—An Interesting Lichen Secondary Metabolite. Mini Rev. Med. Chem. 2017, 17, 1633–1645. [Google Scholar] [CrossRef]
- Sepahvand, A.; Studzińska-Sroka, E.; Ramak, P.; Karimian, V. Usnea sp.: Antimicrobial Potential, Bioactive Compounds, Ethnopharmacological Uses and Other Pharmacological Properties; A Review Article. J. Ethnopharmacol. 2021, 268, 113656. [Google Scholar] [CrossRef]
- Ranković, B.; Ranković, D.; Kosanić, M.; Marić, D. Antioxidant and Antimicrobial Properties of the Lichens Anaptychya ciliaris, Nephroma parile, Ochrolechia tartarea and Parmelia centrifuga. Open Life Sci. 2010, 5, 649–655. [Google Scholar] [CrossRef]
- Gulluce, M.; Aslan, A.; Sokmen, M.; Sahin, F.; Adiguzel, A.; Agar, G.; Sokmen, A. Screening the Antioxidant and Antimicrobial Properties of the Lichens Parmelia saxatilis, Platismatia glauca, Ramalina pollinaria, Ramalina polymorpha and Umbilicaria Nylanderiana. Phytomedicine 2006, 13, 515–521. [Google Scholar] [CrossRef]
- Thomas, N.V.; Kim, S.K. Beneficial Effects of Marine Algal Compounds in Cosmeceuticals. Mar. Drugs 2013, 11, 146–164. [Google Scholar] [CrossRef]
- Vega, J.; Bonomi-Barufi, J.; Gómez-Pinchetti, J.L.; Figueroa, F.L. Cyanobacteria and Red Macroalgae as Potential Sources of Anti-oxidants and UV Radiation-Absorbing Compounds for Cosmeceutical Applications. Mar. Drugs 2020, 18, 659. [Google Scholar] [CrossRef] [PubMed]
- Aslam, A.; Bahadar, A.; Liaqat, R.; Saleem, M.; Waqas, A.; Zwawi, M. Algae as an Attractive Source for Cosmetics to Counter Environmental Stress. Sci. Total Environ. 2021, 772, 144905. [Google Scholar] [CrossRef] [PubMed]
- Guehaz, K.; Boual, Z.; Abdou, I.; Telli, A.; Belkhalfa, H. Microalgae’s Polysaccharides, Are They Potent Antioxidants? Critical Review. Arch. Microbiol. 2023, 206, 14. [Google Scholar] [CrossRef] [PubMed]
- Hoang, H.T.; Moon, J.-Y.; Lee, Y.-C. Natural Antioxidants from Plant Extracts in Skincare Cosmetics: Recent Applications, Challenges and Perspectives. Cosmetics 2021, 8, 106. [Google Scholar] [CrossRef]
- Balboa, E.M.; Conde, E.; Moure, A.; Falqué, E.; Domínguez, H. In Vitro Antioxidant Properties of Crude Extracts and Compounds from Brown Algae. Food Chem. 2013, 138, 1764–1785. [Google Scholar] [CrossRef]
- Gupta, S.; Abu-Ghannam, N. Bioactive Potential and Possible Health Effects of Edible Brown Seaweeds. Trends Food Sci. Technol. 2011, 22, 315–320. [Google Scholar] [CrossRef]
- Yang, G.; Cozad, M.A.; Holland, D.A.; Zhang, Y.; Luesch, H.; Ding, Y. Photosynthetic Production of Sunscreen Shinorine Using an Engineered Cyanobacterium. ACS Synth. Biol. 2018, 7, 664–671. [Google Scholar] [CrossRef]
- Hartmann, A.; Holzinger, A.; Ganzera, M.; Karsten, U. Prasiolin, a New UV-Sunscreen Compound in the Terrestrial Green Macroalga Prasiola calophylla (Carmichael ex Greville) Kützing (Trebouxiophyceae, Chlorophyta). Planta 2016, 243, 161–169. [Google Scholar] [CrossRef]
- Heo, S.J.; Ko, S.C.; Cha, S.H.; Kang, D.H.; Park, H.S.; Choi, Y.U.; Kim, D.; Jung, W.K.; Jeon, Y.J. Effect of Phlorotannins Isolated from Ecklonia cava on Melanogenesis and their Protective Effect Against Photo-Oxidative Stress Induced by UV-B Radiation. Toxicol. Vitr. 2009, 23, 1123–1130. [Google Scholar] [CrossRef]
- Heo, S.J.; Jeon, Y.J. Protective Effect of Fucoxanthin Isolated from Sargassum siliquastrum on UV-B Induced Cell Damage. J. Photochem. Photobiol. B 2009, 95, 101–107. [Google Scholar] [CrossRef]
- Thiyagarasaiyar, K.; Goh, B.H.; Jeon, Y.J.; Yow, Y.Y. Algae Metabolites in Cosmeceutical: An Overview of Current Applications and Challenges. Mar. Drugs 2020, 18, 323. [Google Scholar] [CrossRef] [PubMed]
- Budzianowska, A. Protection against Tobacco Smoke—Cosmetic Preparations from Algae. Farm. Współ. 2013, 6, 1–4. Available online: https://www.akademiamedycyny.pl/wp-content/uploads/2016/05/201303_Farmacja_005.pdf (accessed on 10 December 2024).
- Guerreiro, A.; Andrade, M.A.; Menezes, C.; Vilarinho, F.; Dias, E. Antioxidant and Cytoprotective Properties of Cyanobacteria: Potential for Biotechnological Applications. Toxins 2020, 12, 548. [Google Scholar] [CrossRef]
- Posz, E.; Pinkowska, A.; Stebel, A. Usefulness of Chlorophyta in Cosmetology. Pol. J. Cosmetol. 2016, 19, 36–41. Available online: http://www.kosmet.pl/pjc.php?opc=AR&lng=pl&art=730 (accessed on 10 December 2024).
- Zhao, Y.; Guo, L. A Bioactive Substance Derived from Brown Seaweeds: Phlorotannins. Mar. Drugs 2022, 20, 742. [Google Scholar] [CrossRef]
- Shah, M.M.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-Producing Green Microalga Haematococcus pluvialis: From Single Cell to High Value Commercial Products. Front. Plant Sci. 2016, 7, 531. [Google Scholar] [CrossRef]
- Duval, B.; Shetty, K.; Thomas, W.H. Phenolic Compounds and Antioxidant Properties in the Snow Alga Chlamydomonas nivalis after Exposure to UV Light. J. Appl. Phycol. 1999, 11, 559–566. [Google Scholar] [CrossRef]
- Kasanah, N.; Ulfah, M.; Imania, O.; Hanifah, A.N.; Marjan, M.I.D. Rhodophyta as Potential Sources of Photoprotectants, Antiphotoaging Compounds, and Hydrogels for Cosmeceutical Application. Molecules 2022, 27, 7788. [Google Scholar] [CrossRef]
- Corchete, P. Silybum marianum (L.) Gaertn: The Source of Silymarin. In Bioactive Molecules and Medicinal Plants; Ramawat, K.G., Merillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 123–148. [Google Scholar] [CrossRef]
- Sowińska, M.; Szpunar, M. Silybum marianum—Properties and Application in Medicine—A Review. Eur. J. Clin. Exp. Med. 2022, 20, 266–271. [Google Scholar] [CrossRef]
- Emadi, S.A.; Ghasemzadeh Rahbardar, M.; Mehri, S.; Hosseinzadeh, H. A Review of Therapeutic Potentials of Milk Thistle (Silybum marianum L.) and its Main Constituent, Silymarin, on Cancer, and Their Related Patents. Iran. J. Basic Med. Sci. 2022, 25, 1166–1176. [Google Scholar] [CrossRef]
- Nawaz, A.; Zaib, S.; Khan, I.; Ahmed, A.; Shahzadi, K.; Riaz, H. Silybum marianum: An Overview of its Phytochemistry and Pharmacological Activities with Emphasis on Potential Anticancer Properties. Antican. Agents Med Chem. 2023, 23, 1519–1534. [Google Scholar] [CrossRef] [PubMed]
- Surai, P.F. Silymarin as a Natural Antioxidant: An Overview of the Current Evidence and Perspectives. Antioxidants 2015, 4, 204–247. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Agarwal, R. Cosmeceuticals and Silibinin. Clin. Dermatol. 2009, 27, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Kucharska, E.; Sarpong, R.; Bobkowska, A.; Ryglewicz, J.; Nowak, A.; Kucharski, Ł.; Muzykiewicz-Szymańska, A.; Duchnik, W.; Pełech, R. Use of Silybum marianum Extract and Bio-Ferment for Biodegradable Cosmetic Formulations to Enhance Antioxidant Potential and Effect of the Type of Vehicle on the Percutaneous Absorption and Skin Retention of Silybin and Taxifolin. Appl. Sci. 2024, 14, 169. [Google Scholar] [CrossRef]
- Zhao, T.; Li, C.; Wang, S.; Song, X. Green Tea (Camellia sinensis): A Review of Its Phytochemistry, Pharmacology, and Toxicology. Molecules 2022, 27, 3909. [Google Scholar] [CrossRef]
- Lambert, J.D.; Elias, R.J. The Antioxidant and Pro-oxidant Activities of Green Tea Polyphenols: A Role in Cancer Prevention. Arch. Biochem. Biophys. 2010, 501, 65–72. [Google Scholar] [CrossRef]
- Zhao, C.; Li, C.; Liu, S.; Yang, L. The Galloyl Catechins Contributing to Main Antioxidant Capacity of Tea Made from Camellia sinensis in China. Sci. World J. 2014, 2014, 863984. [Google Scholar] [CrossRef]
- Koch, W.; Zagórska, J.; Marzec, Z.; Kukula-Koch, W. Applications of Tea (Camellia sinensis) and its Active Constituents in Cosmetics. Molecules 2019, 24, 4277. [Google Scholar] [CrossRef]
- Laayouni, Y.; Tlili, I.; Henane, I.; Ali, A.B.; Égei, M.; Takács, S.; Azam, M.; Siddiqui, M.W.; Daood, H.; Pék, Z.; et al. Phytochemical Profile and Antioxidant Activity of Some Open-Field Ancient-Tomato (Solanum lycopersicum L.) Genotypes and Promising Breeding Lines. Horticulturae 2023, 9, 1180. [Google Scholar] [CrossRef]
- Nowak, K.; Żmudzińska-Żurek, B. Tomatoes—The Best Source of Lycopene. Przem. Spoż. 2009, 6, 26–29. Available online: https://yadda.icm.edu.pl/baztech/element/bwmeta1.element.baztech-article-LOD1-0018-0026?q=12bb5e26-21be-4d91-97f6-a2997a4c36e6$4&qt=IN_PAGE (accessed on 14 December 2024).
- Zalewska-Korona, M.; Jabłońska–Ryś, E.; Michalak-Majewska, M. Nutritional and Health-Enhancing Value of Outdoor Tomato Fruits. Bromat. Chem. Toksykol. 2013, 2, 200–205. Available online: https://ptfarm.pl/pub/File/Bromatologia/2013/2/BR%202-2013%20-%20s.%20200-205.pdf (accessed on 14 December 2024).
- Čeryová, N.; Lidiková, J.; Ivanišová, E.; Bobko, M.; Bobková, A.; Ňorbová, M.; Slivková, B. Total Polyphenol Content and Antioxidant Activity of Solanum lycopersicum L. Agrobiodivers. Improv. Nutr. Health Life Qua. 2022, 6. [Google Scholar] [CrossRef]
- Brandt, S.; Pek, Z.; Barna, E.; Lugasi, A.; Helyes, L. Lycopene Content and Colour of Ripening Tomatoes as Affected by Environmental Conditions. J. Sci. Food Agric. 2006, 86, 568–572. [Google Scholar] [CrossRef]
- Skiepko, N.; Chwastowska-Siwiecka, I.; Kondratowicz, J. Properties of Lycopene and Utilizing it to Produce Functional Foods. Żywność Nauka Technologia Jakość 2015, 6, 20–32. Available online: https://journal.pttz.org/wp-content/uploads/2016/01/02_Skiepko.pdf (accessed on 14 December 2024).
- Henderson, A.H.; Lister, I.N.E.; Fachrial, E.; Girsang, E. Antioxidant and Anti-Elastase Activity of Ethanol Extract of Tomato (Solanum lycopersicum L.). Bul. Penelit. Tanam. Rempah Dan Obat 2020, 31, 67–74. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, S.; Wang, Y.; Ni, J.; Xiao, G. The Efficacy of a Novel Tomato Extracts Formulation on Skin Aging and Pigmentation: A Randomized, Double-blind, Parallel-controlled Trial. J. Dermatol. Sci. Cosmet. Technol. 2024, 1, 100005. [Google Scholar] [CrossRef]
- Nowak, R. Nature—The Underestimated Source of Asorbic Acid. Post. Fitoter. 2004, 1, 14–18. Available online: https://www.czytelniamedyczna.pl/2528,natura-niedoceniane-rldo-kwasu-askorbinowego.html (accessed on 14 December 2024).
- Meiyanto, E.; Hermawan, A. Natural Products for Cancer-Targeted Therapy: Citrus Flavonoids as Potent Chemopreventive Agents. Asian Pac. J. Cancer Prev. 2012, 13, 427–436. [Google Scholar] [CrossRef]
- Dini, I.; Laneri, S. The New Challenge of Green Cosmetics: Natural Food Ingredients for Cosmetic Formulations. Molecules 2021, 26, 3921. [Google Scholar] [CrossRef]
- Sharafan, M.; Malinowska, M.A.; Ekiert, H.; Kwaśniak, B.; Sikora, E.; Szopa, A. Vitis vinifera (Vine Grape) as a Valuable Cosmetic Raw Material. Pharmaceutics 2023, 15, 1372. [Google Scholar] [CrossRef]
- Astray, G.; Gullón, P.; Gullón, B.; Munekata, P.E.S.; Lorenzo, J.M. Humulus lupulus L. as a Natural Source of Functional Biomolecules. Appl. Sci. 2020, 10, 5074. [Google Scholar] [CrossRef]
- Przybyś, M.; Skomra, U. Hops as a Source of Biologically Active Compounds. Pol. J. Agron. 2020, 43, 83–102. [Google Scholar] [CrossRef]
- Cieśliński, M.; Idowski, P. Application of Common Hop in Medicine and Cosmetology. Pol. J. Cosmetol. 2003, 6, 188–192. Available online: http://www.kosmet.pl/pjc.php?opc=AR&lng=pl&art=206 (accessed on 14 December 2024).
- Hęś, M.; Dziedzic, K.; Górecka, D.; Jędrusek-Golińska, A.; Gujska, E. Aloe vera (L.) Webb.: Natural Sources of Antioxidants—A Review. Plant Foods Hum. Nutr. 2019, 74, 255–265. [Google Scholar] [CrossRef]
- Catalano, A.; Ceramella, J.; Iacopetta, D.; Marra, M.; Conforti, F.; Lupi, F.R.; Gabriele, D.; Borges, F.; Sinicropi, M.S. Aloe vera—An ExtensiveCReview Focused on Recent Studies. Foods 2024, 13, 2155. [Google Scholar] [CrossRef]
- Xie, M.; Jiang, Z.; Lin, X.; Wei, X. Application of Plant Extracts Cosmetics in the Field of Anti-Aging. J. Dermatol. Sci. Cosmet. Technol. 2024, 1, 100014. [Google Scholar] [CrossRef]
- Mohite, K.; Kamble, T.; Nangare, K.; Payghan, V.; Payghan, S. A Review Article on: Aloe vera: Extraction of Gel and Extraction of Aloin From Aloe vera Gel by Ultrasonic Assisted Method. Int. J. Creat. Res. Thoughts 2021, 9. Available online: www.ijcrt.org (accessed on 14 December 2024).
- Wang, Z.L.; Wang, S.; Kuang, Y.; Hu, Z.M.; Qiao, X.; Ye, M. A Comprehensive Review on Phytochemistry, Pharmacology, and Flavonoid Biosynthesis of Scutellaria baicalensis. Pharm. Biol. 2018, 56, 465–484. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Z.; Zhao, Y.; Kong, L.; Ji, X.; Wu, J.; Gao, Z. Exploring Bioactive Constituents and Pharmacological Effects of Scutellaria baicalensis Georgi: A Review. Nat. Prod. Commun. 2024, 19, 1934578X241266692. [Google Scholar] [CrossRef]
- Gabrielska, J.; Oszmiańsk, J.; Żyłka, R.; Komorowska, M. Antioxidant Activity of Flavones from Scutellaria baicalensis in Lecithin Liposomes. Z. Naturforschung C J. Biosci. 1997, 52, 817–823. Available online: https://pubmed.ncbi.nlm.nih.gov/9463939/ (accessed on 14 December 2024). [CrossRef]
- Seok, J.K.; Kwak, J.Y.; Choi, G.W.; An, S.M.; Kwak, J.H.; Seo, H.H.; Suh, H.J.; Boo, Y.C. Scutellaria radix Extract as a Natural UV Protectant for Human Skin. Phytother. Res. 2016, 30, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Zahid, H.F.; Cottrell, J.J.; Dunshea, F.R. A Comparative Study for Nutritional and Phytochemical Profiling of Coffea arabica (C. arabica) from Different Origins and Their Antioxidant Potential and Molecular Docking. Molecules 2022, 27, 5126. [Google Scholar] [CrossRef] [PubMed]
- Cangeloni, L.; Bonechi, C.; Leone, G.; Consumi, M.; Andreassi, M.; Magnani, A.; Rossi, C.; Tamasi, G. Characterization of Extracts of Coffee Leaves (Coffea arabica L.) by Spectroscopic and Chromatographic/Spectrometric Techniques. Foods 2022, 11, 2495. [Google Scholar] [CrossRef]
- Ruse, G.; Jîjie, A.-R.; Moacă, E.-A.; Pătrașcu, D.; Ardelean, F.; Jojic, A.-A.; Ardelean, S.; Tchiakpe-Antal, D.-S. Coffea arabica: An Emerging Active Ingredient in Dermato-Cosmetic Applications. Pharmaceuticals 2025, 18, 171. [Google Scholar] [CrossRef]
- Censi, R.; Vargas Peregrina, D.; Lacava, G.; Agas, D.; Lupidi, G.; Sabbieti, M.G.; Di Martino, P. Cosmetic Formulation Based on an Açai Extract. Cosmetics 2018, 5, 48. [Google Scholar] [CrossRef]
- Chang, S.K.; Alasalvar, C.; Shahidi, F. Superfruits: Phytochemicals, Antioxidant Efficacies, and Health Effects—A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2018, 59, 1580–1604. [Google Scholar] [CrossRef]
- Pacheco-Palencia, L.A.; Mertens-Talcott, S.; Talcott, S.T. Chemical Composition, Antioxidant Properties, and Thermal Stability of a Phytochemical Enriched Oil from Açaí (Euterpe oleracea Mart.). J. Agric. Food Chem. 2008, 56, 4631–4636. [Google Scholar] [CrossRef]
- Laurindo, L.F.; Barbalho, S.M.; Araújo, A.C.; Guiguer, E.L.; Mondal, A.; Bachtel, G.; Bishayee, A. Açaí (Euterpe oleracea Mart.) in Health and Disease: A Critical Review. Nutrients 2023, 15, 989. [Google Scholar] [CrossRef]
- de Souza Silva, A.P.; de Camargo, A.C.; Lazarini, J.G.; Franchin, M.; Sardi, J.d.C.O.; Rosalen, P.L.; de Alencar, S.M. Phenolic Profile and the Antioxidant, Anti-Inflammatory, and Antimicrobial Properties of Açaí (Euterpe oleracea) Meal: A Prospective Study. Foods 2023, 12, 86. [Google Scholar] [CrossRef]
- Schürch, C.; Blum, P.; Zülli, F. Potential of Plant Cells in Culture for Cosmetic Application. Phytochem. Rev. 2008, 7, 599–605. [Google Scholar] [CrossRef]
- Smetanska, I. Production of Secondary Metabolites Using Plant Cell Cultures. Food Biotechnol. 2008, 111, 187–228. [Google Scholar]
- Barbulova, A.; Apone, F.; Colucci, G. Plant Cell Cultures as Source of Cosmetic Active Ingredients. Cosmetics 2014, 1, 94–104. [Google Scholar] [CrossRef]
- Hermosaningtyas, A.A.; Chanaj-Kaczmarek, J.; Kikowska, M.; Gornowicz-Porowska, J.; Budzianowska, A.; Pawlaczyk, M. Potential of Plant Stem Cells as Helpful Agents for Skin Disorders—A Narrative Review. Appl. Sci. 2024, 14, 7402. [Google Scholar] [CrossRef]
- Moruś, M.; Baran, M.; Rost-Roszkowska, M.; Skotnicka-Graca, U. Plant Stem Cells as Innovation in Cosmetics. Acta Pol. Pharm. Drug Res. 2014, 71, 701–707. Available online: https://pubmed.ncbi.nlm.nih.gov/25362798/ (accessed on 14 December 2024).
- ECHA—European Chemicals Agency. Cosmetic Products Regulation, Annex III—Restricted Substances. 2024. Available online: https://echa.europa.eu/cosmetics-restricted-substances/-/legislationlist/details/EU-COSM_PROD-ANX_III_RESTRIC-100.007.203-VSK-G54WCW (accessed on 14 December 2024).
- Wang, W.; Xiong, P.; Zhang, H.; Zhu, Q.; Liao, C.; Jiang, G. Analysis, Occurrence, Toxicity and Environmental Health Risks of Synthetic Phenolic Antioxidants: A Review. Environ. Res. 2021, 201, 111531. [Google Scholar] [CrossRef]
- Böger, B.R.; Mori, A.L.; Viegas, M.C.; Benassi, M.T. Quality attributes of roasted Arabica coffee oil extracted by pressing: Composition, antioxidant activity, sun protection factor and other physical and chemical parameters. Grasas Aceites 2021, 72, e394. [Google Scholar] [CrossRef]
- Phan, D.; Ha, H.; Ho, T. An Extract and Fractions from Coffea arabica Sediment on Antioxidant and Anti-Tyrosinase Activities, and on the Quality of Whiteleg Shrimp (Litopenaeus vannamei) During Refrigerated Storage. Prev. Nutr. Food Sci. 2021, 26, 346–356. [Google Scholar] [CrossRef]
- Shen, X.-J.; Zhou, Z.-B.; Nie, F.-Q.; Zi, C.-T.; Fan, J.-P. Progress in Phytochemical and Bioactivities of Coffea arabica L. Med. Res. 2020, 4, 200012. [Google Scholar] [CrossRef]
- de Mello, V.; de Mesquita, G.A., Jr.; Alvim, J.G.; Costa, J.D.; Vilela, F.M. Recent Patent Applications for Coffee and Coffee By-Products as Active Ingredients in Cosmetics. Int. J. Cosmet. Sci. 2023, 45, 267–287. [Google Scholar] [CrossRef]
- SpecialChem. The Material Selection Platform. Cosmetic Ingredients. Ganoderma lucidum. 2025. Available online: https://cosmetics.specialchem.com/searchsites/searchproducts?indexpage=1&q=Ganoderma%20lucidum (accessed on 14 December 2024).
- Prospector. Personal Care & Cosmetics. Ingredients. Ganoderma lucidum. 2025. Available online: https://www.ulprospector.com/en/na/PersonalCare/search?k=Ganoderma+lucidum&st=1 (accessed on 14 December 2024).
- Studzińska-Sroka, E.; Bylka, W. Iceland Moss—Active Compounds, Biological Properties. Farm. Przegląd Nauk. 2010, 7, 23–27. Available online: https://www.researchgate.net/publication/279943644_Iceland_Moss_-_active_compounds_biological_properties (accessed on 14 December 2024).
- Xu, M.; Heidmarsson, S.; Olafsdottir, E.S.; Buonfiglio, R.; Kogej, T.; Omarsdottir, S. Secondary Metabolites from Cetrarioid Lichens: Chemotaxonomy, Biological Activities and Pharmaceutical Potential. Phytomedicine 2016, 23, 441–459. [Google Scholar] [CrossRef] [PubMed]
- SpecialChem. The Material Selection Platform. Cosmetic Ingredients. Cetraria islandica. 2025. Available online: https://cosmetics.specialchem.com/searchsites/searchproducts?indexpage=1&q=Cetraria%20islandica (accessed on 14 December 2024).
- Prospector. Personal Care & Cosmetics. Ingredients. Cetraria islandica. 2025. Available online: https://www.ulprospector.com/en/na/PersonalCare/search?k=Cetraria+islandica&st=1 (accessed on 14 December 2024).
- Prateeksha, P.; Paliya, B.S.; Bajpai, R.; Jadaun, V.; Kumar, J.; Kumar, S.; Upreti, D.K.; Singh, B.R.; Nayaka, S.; Joshi, Y.; et al. The Genus Usnea: A Potent Phytomedicine with Multifarious Ethnobotany, Phytochemistry and Pharmacology. RSC Adv. 2016, 6, 21672–21696. [Google Scholar] [CrossRef]
- Zugić, A.; Tadić, V.; Kundaković, T.; Savić, S. Chemical Composition and Biological Activities of the Extracts and Secondary Metabolites of Lichens Belonging to the Genus Usnea, Parmeliaceae. Lek. Sirovine 2018, 38, 68–80. [Google Scholar] [CrossRef]
- Dash, S.; Pradhan, S.; Sahoo, B.; Rath, B. Characterization of Selected Marine Cyanobacteria as a Source of Compounds with Antioxidant Activity. Discov. Plants 2024, 1, 65. [Google Scholar] [CrossRef]
- Gunes, S.; Tamburaci, S.; Dalay, M.C.; Gurhan, I.D. In Vitro Evaluation of Spirulina Platensis Extract Incorporated Skin Cream with its Wound Healing and Antioxidant Activities. Pharm. Biol. 2017, 55, 1824–1832. [Google Scholar] [CrossRef]
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L. The Potential Use of Marine Microalgae and Cyanobacteria in Cosmetics and Thalassotherapy. Cosmetics 2017, 4, 46. [Google Scholar] [CrossRef]
- SpecialChem. The Material Selection Platform. Cosmetic Ingredients. Laminaria digitata. 2025. Available online: https://cosmetics.specialchem.com/searchsites/searchproducts?indexpage=2&q=Laminaria+digitata+ (accessed on 14 December 2024).
- Prospector. Personal Care & Cosmetics. Ingredients. Laminaria digitata. 2025. Available online: https://www.ulprospector.com/en/na/PersonalCare/search?k=Laminaria+digitata&st=1 (accessed on 14 December 2024).
- Mendes, A.R.; Spínola, M.P.; Lordelo, M.; Prates, J.A.M. Chemical Compounds, Bioactivities, and Applications of Chlorella vulgaris in Food, Feed and Medicine. Appl. Sci. 2024, 14, 23. [Google Scholar] [CrossRef]
- Vega, J.; Schneider, G.; Moreira, B.R.; Herrera, C.; Bonomi-Barufi, J.; Figueroa, F.L. Mycosporine-Like Amino Acids from Red Macroalgae: UV-Photoprotectors with Potential Cosmeceutical Applications. Appl. Sci. 2021, 11, 5112. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, A.P.; Singh, V.K.; Singh, P.R.; Jaiswal, J.; Kumari, N.; Upadhye, V.; Singh, S.C.; Sinha, R.P. Natural Sun-Screening Compounds and DNA-Repair Enzymes: Photoprotection and Photoaging. Catalysts 2023, 13, 4. [Google Scholar] [CrossRef]
- Patravale, V.B.; Mandawgade, S.D. Novel Cosmetic Delivery Systems: An Application Update. Int. J. Cosmet. Sci. 2008, 30, 19–33. [Google Scholar] [CrossRef]
- Kaur, I.P.; Kapila, M.; Agrawal, R. Role of Novel Delivery Systems in Developing Topical Antioxidants as Therapeutics to Combat Photoageing. Ageing Res. Rev. 2007, 6, 271–288. [Google Scholar] [CrossRef] [PubMed]
- Laouini, A.; Jaafar-Maalej, C.; Limayem-Blouza, I.; Sfar, S.; Charcosset, C.; Fessi, H. Preparation, Characterization and Applications of Liposomes: State of the Art. J. Colloid Sci. Biotechnol. 2012, 1, 147–168. [Google Scholar] [CrossRef]
- Wagner, A.; Vorauer-Uhl, K. Liposome Technology for Industrial Purposes. J. Drug Deliv. 2011, 591325. [Google Scholar] [CrossRef]
- Marianecci, C.; Di Marzio, L.; Rinaldi, F.; Celia, C.; Paolino, D.; Alhaique, F.; Esposito, S.; Carafa, M. Niosomes from 80s to Present: The State of the Art. Adv. Colloid Interface Sci. 2014, 205, 187–206. [Google Scholar] [CrossRef]
- Kumar, G.P.; Rajeshwarrao, P. Nonionic Surfactant Vesicular Systems for Effective Drug Delivery—An Overview. Acta Pharm. Sin. B 2011, 1, 208–219. [Google Scholar] [CrossRef]
- Chaudhary, H.; Kohli, K.; Kumar, V. Nano-transfersomes as a Novel Carrier for Transdermal Delivery. Int. J. Pharm. 2013, 454, 367–380. [Google Scholar] [CrossRef]
- Verma, P.; Pathak, K. Therapeutic and Cosmeceutical Potential of Ethosomes: An Overview. J. Adv. Pharm. Technol. Res. 2010, 1, 274. [Google Scholar] [CrossRef]
- Rakesh, R.; Anoop, K.R. Ethosomes for Transdermal and Topical Drug Delivery. Int. J. Pharm. Pharm. Sci. 2012, 4, 17–24. Available online: https://www.semanticscholar.org/paper/ETHOSOMES-FOR-TRANSDERMAL-AND-TOPICAL-DRUG-DELIVERY-Rakesh-Anoop/da540f1af3c85e8f400ebe3d457f9fafd403669b (accessed on 14 December 2024).
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) in Cosmetic and Dermatological Preparations. Adv. Drug Deliv. Rev. 2002, 54, S131–S155. [Google Scholar] [CrossRef]
- Müller, R.; Souto, E.; Mehnert, W. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) for Dermal Delivery. In Percutaneous Absorption Drugs, Cosmetics, Mechanisms, Methods, 4th ed.; Bronaugh, R.L., Dragicevic, N., Maibach, H.I., Eds.; CRC Press: Boca Raton, FL, USA, 2005; pp. 719–738. [Google Scholar] [CrossRef]
- Kusumawati, I.; Indrayanto, G. Chapter 15—Natural Antioxidants in Cosmetics. Stud. Nat. Prod. Chem. 2013, 40, 485–505. [Google Scholar] [CrossRef]
- Ledwoń, P.; Errante, F.; Papini, A.M.; Rovero, P.; Latajka, R. Peptides as Active Ingredients: A Challenge for Cosmeceutical Industry. Chem. Biodiver. 2021, 1818, e2000833. [Google Scholar] [CrossRef] [PubMed]


| Group of Ingredients | Ingredient | Classification | Source | Mechanism of Action | Effect in Cosmetics | Dosage | References |
| vitamins | vitamin C | natural | exogenous |
|
| up to 10% | [7,23,24,25,26,27,28,29,30,31,32] |
| vitamin E |
|
| up to 5.4% | [32,33,34,35] | |||
| vitamin A |
|
| 0.1–1.0% | [36,37,38,39,40,41,42,43,44,45,46,47] | |||
| vitamin B5 |
|
| up to 5.3% | [31,32,48,49,50,51] | |||
| carotenoids | β-carotene | natural | exogenous |
|
| not reported | [55,57,58] |
| lutein |
|
| not reported | [56,60,61] | |||
| lycopene |
|
| not reported | [55,63,64] | |||
| astaxantin |
|
| not reported | [67,68,69,70,71,72,73] | |||
| coenzyme Q10 |
|
| not reported | [74,75,76,77,78,79] | |||
| phenolic compounds/flavonoids | hesperidin | natural | exogenous |
|
| not reported | [82,83] |
| xanthohumol |
|
| not reported | [84,85,86,87,88] | |||
| taxifolin |
|
| not reported | [89,90,91,92,93] | |||
| phenolic compounds/phenolic acids | ferulic acid | natural | exogenous |
|
| not reported | [94,95,96,97,98,99,100,101,102] |
| phenolic compounds | resveratrol | natural | exogenous |
| not reported | [104,105,106,107,108] | |
| bakuchiol | natural | exogenous |
|
| 0.25–1% | [109,110,111,112,113,114,115,116,117,118] | |
| trace elements | selenium | natural | exogenous |
| not reported | [120,121,122,123] | |
| zinc |
|
| not reported | [31,124,125,126,127,128,129] | |||
| peptides, amino acids, enzymes | peptides | natural | endogenous/non-enzymatic | [130,131,132] | |||
| glutathione |
|
| not reported | [133,134,135,136,137,138,139] | |||
| N-acetyl-L-cysteine |
|
| not reported | [140,141,142,143,144] | |||
| superoxide dismutase | natural | endogenous/enzymatic |
|
| not reported | [145,146,147,148,149] | |
| hormones | melatonin | natural | endogenous/non-enzymatic |
|
| not reported | [153,154,155,156,157] |
| dehydroepiandrosterone (DHEA) |
|
| not reported | [158,159,160,161] | |||
| fungi-derived antioxidants | kojic acid | natural | exogenous |
|
| 0.1–2% up to 1% | [167,246] |
| Synthetic antioxidants | t-Butylated hydroxytoluene (BHT) | artificial | chemical synthesis |
|
| up to 0.5% | [32,162,163,247] |
| Plant Species/Family | Raw Material Used/Compounds | Active Compounds | Effect in Cosmetics | Dosage | References |
|---|---|---|---|---|---|
| Silybum marianum (L.) Gaertn Asteraceae | seed extract silibinin | flavonolignans (silymarin), quercetin, thymine, histamine, phytosterols, mucus, tannins, mineral compounds, organic acids, and vitamins C and K |
| not reported | [199,200,201,202,203,204] |
| Camellia sinensis (L.) Kuntze Theaceae | leaf extract seed extract polyphenols | vitamins, amino acids, fiber and minerals, polyphenols, flavonoids (catechins-epigallocatechin 3-gallate), tannins, and purine alkaloids |
| up to 2% (leaf extract), up to 7% (leaf powder), up to 30% (leaf water), up to 0.1% (seed extract) | [32,206,207,208,209] |
| Solanum lycopersicum L. Solanaceae | pulp fruit extract lycopene | vitamins, phenolic compounds such as anthocyanins, phenolic acids, flavonoids and carotenoids, including lycopene |
| not reported | [210,211,212,213,214,215,216,217] |
| Citrus limon (L.) Burm. Citrus x paradisi L. Citrus reticulata L. Citrus aurantifolia hort. ex Tanaka Rutaceae | fruit extract hesperidin | flavanones, flavanols, flavones, and vitamin C |
| not reported | [218,219,220] |
| Vitis vinifera L. Vitaceae | seed extract fruit powder juice juice extract skin extract resveratrol | proanthocyanidins, condensed tannins, and leucocyanidins |
| up to 3% (leaf extract), up to 2% (fruit extract and juice) | [32,220,221] |
| Humulus lupulus L. Cannabaceae | hops extract hops oil | resins, essential oils, proteins and polyphenols: quercetin, quercitrin, kaempferol, rutin, xanthohumol, and ferulic acid |
| up to 0.2% | [32,222,223,224] |
| Aloe vera (L.) Webb. Asphodelaceae | flower extract leaf leaf extract leaf juice leaf polysaccharides leaf water | flavonoids, terpenoids, lectins, fatty acids, anthraquinones, mono- and polysaccharides, tannins, sterols (notably campesterol and β-sitosterol), enzymes, salicylic acid, essential minerals, and vitamins |
| up to 20% | [32,220,225,226,227] |
| Scutellaria baicalensis Georgi Lamiaceae | root extract | free flavonoids, flavonoid glycosides, phenylethanoid glycosides, and various other small molecules |
| up to 0.5% | [32,229,230,231,232] |
| Coffea arabica L. Rubiaceae | leaf extract seed extract chlorogenic acid | alkaloids (caffeine, theophylline, theobromine, trigonelline), phenolic acids (chlorogenic acid, caffeic acid), flavonoids (quercetin, rutin, and kaempferol), xanthones, tannins, diterpenes (cafestol and kahweol), carbohydrates, organic acids, amino acids, and fatty acids |
| not reported | [233,248,249,250,251] |
| Euterpe oleracea Mart. (Açaí) Arecaceae | pulp powder juice | unsaturated fatty acids, anthocyanins, proanthocyanidins, other flavonoids (luteolin, quercetin, dihydrokaempferol, and chrysoerial), and carotenoids (carotene, lycopene, astaxanthin, lutein, and zeaxanthin) |
| up to 3% | [32,236,237,238,239,240] |
| Ganoderma lucidum (Curtis) P. Karst, (linghzi, reishi) Ganodermataceae, Basidiomycota, Fungi | fruiting-body stipe stem extract spores extract | triterpenes (ganoderic acids), water-soluble polysaccharides, proteins, amino acids, mannitol, coumarins, sterols (ergosterol), and unsaturated fatty acids |
| not reported | [166,167,172,252,253] |
| Cetraria islandica (L.) Ach. Parmeliaceae, Ascomycota, Fungi (lichen) | thallus extract | lichen acids (protolichesterinic acid, protocetraric acid), carotenoids, and polysaccharides (lichenan, isolichenan, galactomannans) |
| not reported | [254,255,256,257] |
| Usnea barbata (L.) Weber ex F.H. Wigg. Parmeliaceae, Ascomycota, Fungi (lichen) | thallus extact | lichen acids (usnic acid), polysaccharides (lichenan), and phenolic acids |
| not reported | [177,258,259] |
| Arthrospira platensis Gomont (spirulina) (blue-green algae) | thallus powder thallus extract thallus hydrolysate | phycocyanin, proteins (all essential amino acids), minerals, vitamins (B, C, E), trace elements, and unsaturated fatty acids |
| not reported | [191,260,261,262] |
| Fucus vesiculosus L. Fucaceae, Phaeophyceae (brown algae) | thallus powder thallus extract | phlorotanins, alginic acid, fucoidan, fucoxanthin, carbohydrates, and iodine |
| 0.00002–5% | [32,185,191,195] |
| Laminaria digitata (Huds.) Lamouroux, Laminariaceae, Phaeophyceae (brown algae) | thallus powder thallus extract | alginic acid, fucoidan, carbohydrates (laminarin), iodine, and γ-linolenic acid |
| 0.00004–5% (extract), 40% (powder) | [32,185,195,263,264] |
| Haematococcus pluvialis Haematococcaceae, Chlorophyta (green algae) | thallus | carotenoids (astaxanthin), lipids (polyunsaturated fatty acids), proteins, and carbohydrates |
| not reported | [191,196,262] |
| Chlorella vulgaris Beijer. Chlorellaceae, Chlorophyta (green algae) | thallus extract | MAAs, proteins, lipids, carbohydrates, vitamins, chlorophyll, and carotenoids |
| not reported | [191,194,262,265] |
| Porphyra umbilicalis (L.) Kützing Bangiaceae, Rhodophyta (red algae) | thallus extract | mycosporine-like amino acids (MAAs, including porphyra-334), phenolic compounds (phlorotannin and taurine), vitamins (ascorbic acid), polysaccharides (porphyrans), and phycobiliproteins (phycoerythrin and phycocyanin) |
| not reported | [181,191,198,266,267] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Budzianowska, A.; Banaś, K.; Budzianowski, J.; Kikowska, M. Antioxidants to Defend Healthy and Youthful Skin—Current Trends and Future Directions in Cosmetology. Appl. Sci. 2025, 15, 2571. https://doi.org/10.3390/app15052571
Budzianowska A, Banaś K, Budzianowski J, Kikowska M. Antioxidants to Defend Healthy and Youthful Skin—Current Trends and Future Directions in Cosmetology. Applied Sciences. 2025; 15(5):2571. https://doi.org/10.3390/app15052571
Chicago/Turabian StyleBudzianowska, Anna, Katarzyna Banaś, Jaromir Budzianowski, and Małgorzata Kikowska. 2025. "Antioxidants to Defend Healthy and Youthful Skin—Current Trends and Future Directions in Cosmetology" Applied Sciences 15, no. 5: 2571. https://doi.org/10.3390/app15052571
APA StyleBudzianowska, A., Banaś, K., Budzianowski, J., & Kikowska, M. (2025). Antioxidants to Defend Healthy and Youthful Skin—Current Trends and Future Directions in Cosmetology. Applied Sciences, 15(5), 2571. https://doi.org/10.3390/app15052571






