Preparation, Characterization and Evaluation of the Antibacterial Activity of Ag Nanoparticles Embedded in Transparent Oxide Matrices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of Films
2.2. Antibacterial Efficiency Tests
3. Results
3.1. Characterization of Physical Properties
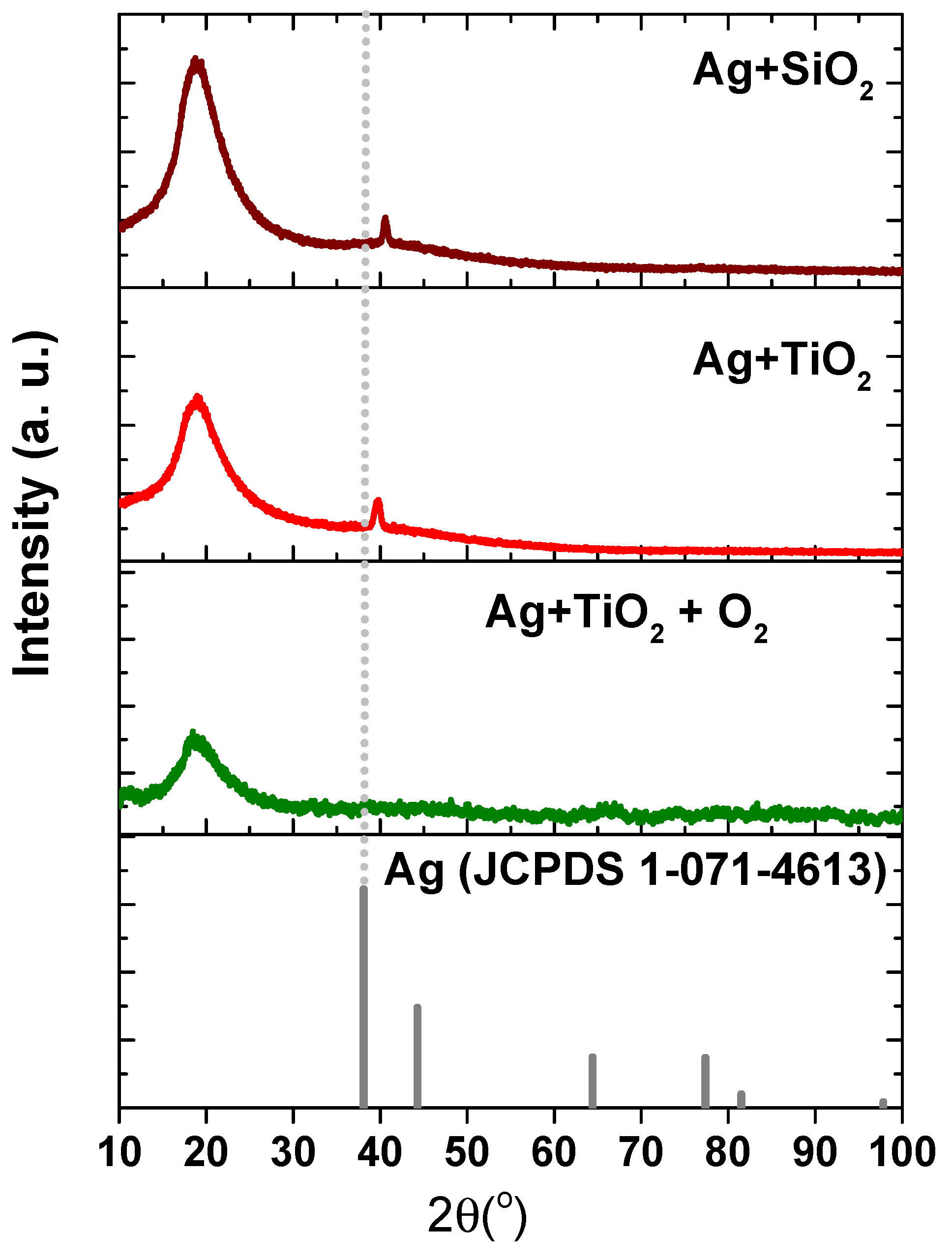
3.1.1. X-Ray Diffraction for Structural Analysis of the Films
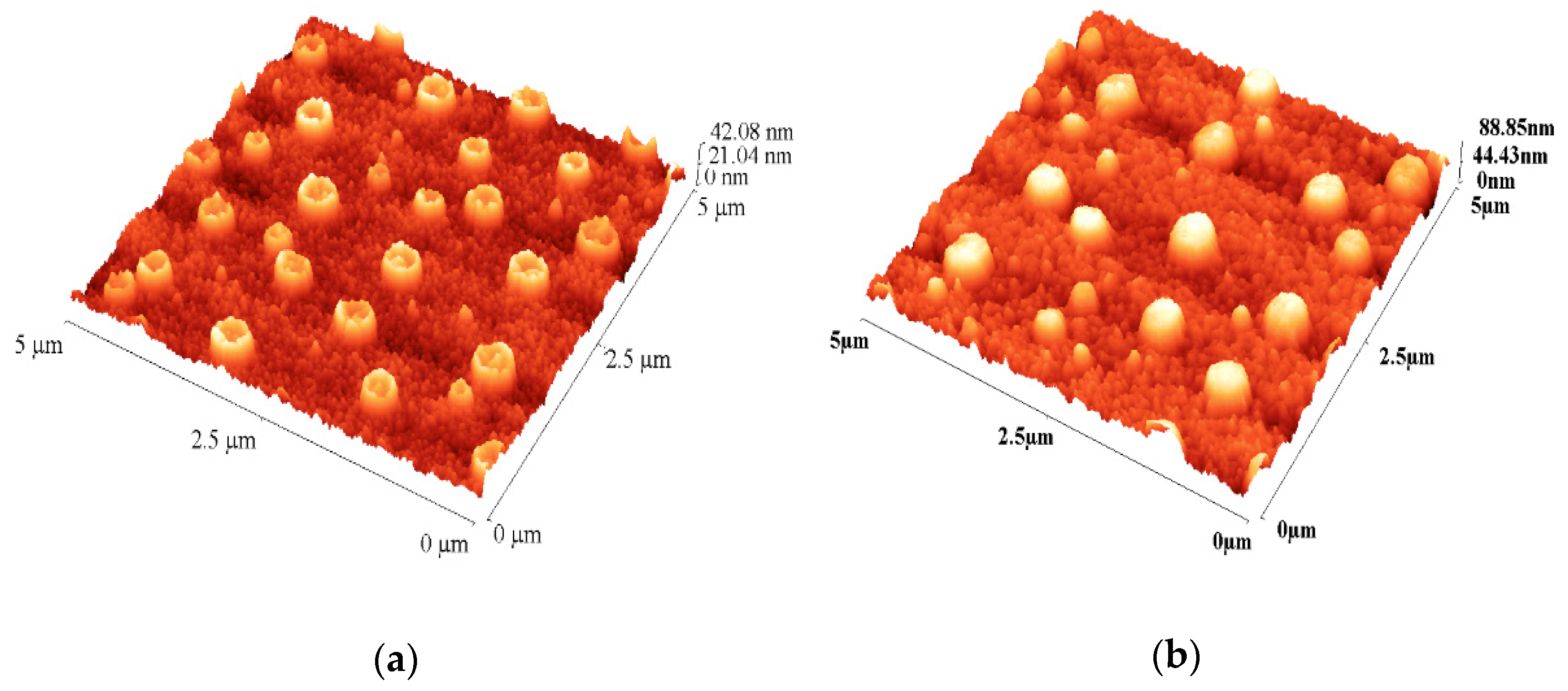
3.1.2. Atomic Force Microscopy—Surface, Topography and Roughness
3.1.3. Energy Dispersive X-Ray Spectroscopy, EDS and Elemental Composition
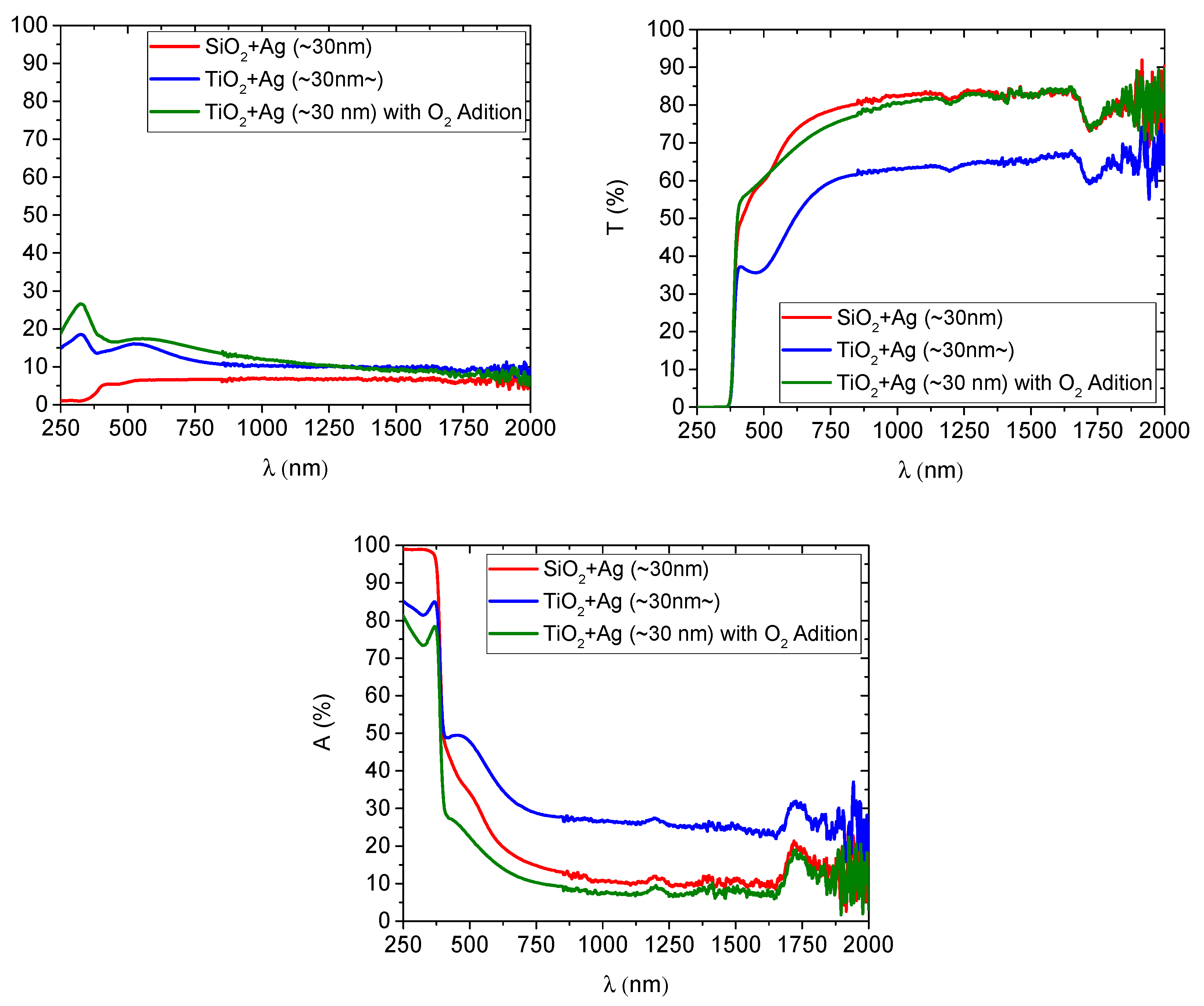
3.1.4. UV–Vis–NIR Spectrophotometry and Optical Properties
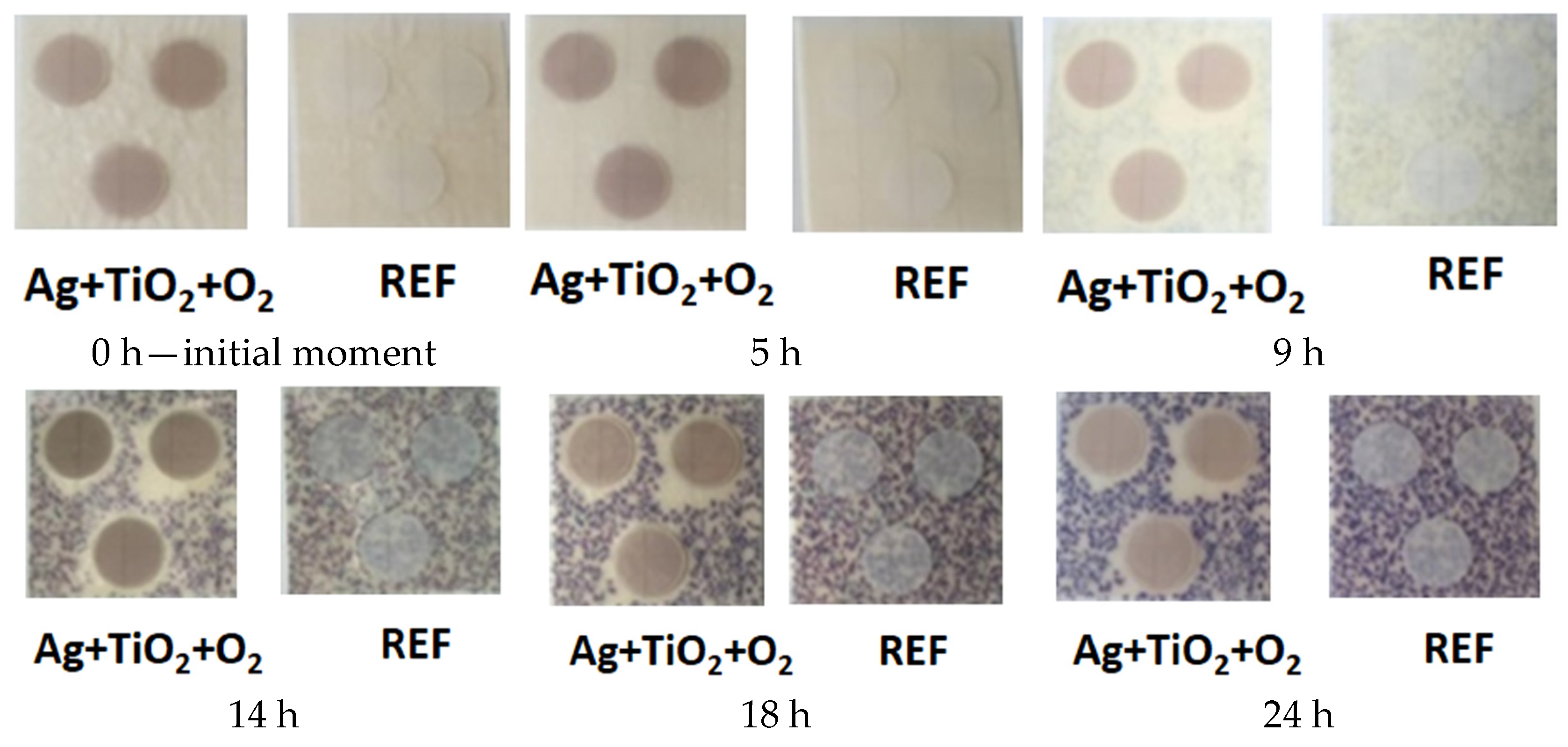
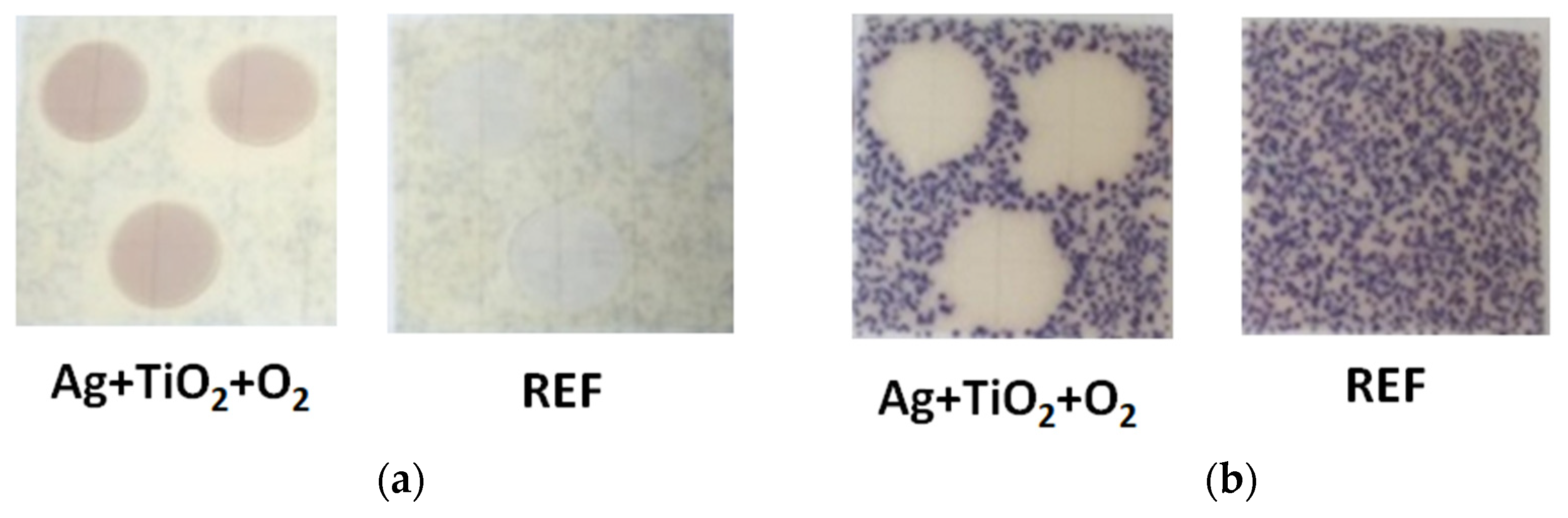
3.2. Antibacterial Activity
3.3. Mechanism of Action of Antibacterial Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mishra, S.K.; Basukala, P.; Basukala, O.; Parajuli, K.; Pokhrel, B.M.; Rijal, B.P. Detection of Biofilm Production and Antibiotic Resistance Pattern in Clinical Isolates from Indwelling Medical Devices. Curr. Microbiol. 2015, 70, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Bereanu, A.-S.; Bereanu, R.; Mohor, C.; Vintilă, B.I.; Codru, I.R.; Olteanu, C.; Sava, M. Prevalence of Infections and Antimicrobial Resistance of ESKAPE Group Bacteria Isolated from Patients Admitted to the Intensive Care Unit of a County Emergency Hospital in Romania. Antibiotics 2024, 13, 400. [Google Scholar] [CrossRef]
- Orapiriyakul, W.; Young, P.S.; Damiati, L.; Tsimbouri, P.M. Antibacterial Surface Modification of Titanium Implants in Orthopaedics. J. Tissue Eng. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Silhavy, T.J.; Kahne, D.; Walker, S. The Bacterial Cell Envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef]
- Li, P.; Li, X.; Saravanan, R.; Li, C.M.; Leong, S.S.J. Antimicrobial Macromolecules: Synthesis Methods and Future Applications. RSC Adv. 2012, 2, 4031–4044. [Google Scholar] [CrossRef]
- Qiu, H.; Si, Z.; Luo, Y.; Feng, P.; Wu, X.; Hou, W.; Zhu, Y.; Chan-Park, M.B.; Xu, L.; Huang, D. The Mechanisms and the Applications of Antibacterial Polymers in Surface Modification on Medical Devices. Front. Bioeng. Biotechnol. 2020, 8, 910. [Google Scholar] [CrossRef]
- Negut, I.; Floroian, L.; Ristoscu, C.; Mihailescu, C.N.; Rosca, J.C.M.; Tozar, T.; Badea, M.; Grumezescu, V.; Hapenciuc, C.; Mihailescu, I.N. Functional Bioglass—Biopolymer Double Nanostructure for Natural Antimicrobial Drug Extracts Delivery. Nanomaterials 2020, 10, 385. [Google Scholar] [CrossRef]
- Park, J.; Lim, D.H.; Lim, H.J.; Kwon, T.; Choi, J.S.; Jeong, S.; Choi, I.H.; Cheon, J. Size Dependent Macrophage Responses and Toxicological Effects of Ag Nanoparticles. Chem. Commun. 2011, 47, 4382–4384. [Google Scholar] [CrossRef]
- Francolini, I.; Vuotto, C.; Piozzi, A.; Donelli, G. Antifouling and Antimicrobial Biomaterials: An Overview. APMIS 2017, 125, 392–417. [Google Scholar] [CrossRef]
- Riga, E.K.; Vöhringer, M.; Widyaya, V.T.; Lienkamp, K. Polymer-Based Surfaces Designed to Reduce Biofilm Formation: From Antimicrobial Polymers to Strategies for Long-Term Applications. Macromol. Rapid Commun. 2017, 38, 1700216. [Google Scholar] [CrossRef]
- Jose, A.; Gizdavic-Nikolaidis, M.; Swift, S. Antimicrobial Coatings: Reviewing Options for Healthcare Applications. Appl. Microbiol. 2023, 3, 145–174. [Google Scholar] [CrossRef]
- Le Ouay, B.; Stellacci, F. Antibacterial Activity of Silver Nanoparticles: A Surface Science Insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef]
- Tang, S.; Zheng, J. Antibacterial Activity of Silver Nanoparticles: Structural Effects. Adv. Healthc. Mater. 2018, 7, 1701503. [Google Scholar] [CrossRef]
- Menichetti, A.; Mavridi-Printezi, A.; Mordini, D.; Montalti, M. Effect of Size, Shape and Surface Functionalization on the Antibacterial Activity of Silver Nanoparticles. J. Funct. Biomater. 2023, 14, 244. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.; Dille, J.; Godet, S. Synthesis and Antibacterial Activity of Silver Nanoparticles against Gram-Positive and Gram-Negative Bacteria. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 37–45. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Garavand, F.; Jafari, S.M. Incorporation of Silver Nanoparticles into Active Antimicrobial Nanocomposites: Release Behavior, Analyzing Techniques, Applications and Safety Issues. Adv. Colloid Interface Sci. 2021, 293, 102440. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Krishnan, S.; Hii, Y.S.; Pan, S.; Chan, Y.S.; Acquah, C.; Danquah, M.K.; Rodrigues, J. Synthesis Approach-Dependent Antiviral Properties of Silver Nanoparticles and Nanocomposites. J. Nanostruct. Chem. 2022, 12, 809–831. [Google Scholar] [CrossRef]
- Mungkalasiri, J.; Bedel, L.; Emieux, F.; Doré, J.; Renaud, F.N.R.; Sarantopoulos, C.; Maury, F. CVD Elaboration of Nanostructured TiO2-Ag Thin Films with Efficient Antibacterial Properties. Chem. Vap. Depos. 2010, 16, 35–41. [Google Scholar] [CrossRef]
- Park, M.S.; Kang, M. The Preparation of the Anatase and Rutile Forms of Ag-TiO2 and Hydrogen Production from Methanol/Water Decomposition. Mater. Lett. 2008, 62, 183–187. [Google Scholar] [CrossRef]
- Herrmann, J.M.; Tahiri, H.; Ait-Ichou, Y.; Lassaletta, G.; González-Elipe, A.R.; Fernandez, A. Characterization and Photocatalytic Activity in Aqueous Medium of TiO2 and Ag-TiO2 Coatings on Quartz. Appl. Catal. B Environ. 1997, 13, 219–228. [Google Scholar] [CrossRef]
- Chang, C.C.; Lin, C.K.; Chan, C.C.; Hsu, C.S.; Chen, C.Y. Photocatalytic Properties of Nanocrystalline TiO2 Thin Film with Ag Additions. Thin Solid Film. 2006, 494, 274–278. [Google Scholar] [CrossRef]
- Chakravadhanula, V.S.K.; Hrkac, T.; Zaporojtchenko, V.; Podschun, R.; Kotnur, V.G.; Kulkarni, A.; Strunskus, T.; Kienle, L.; Faupel, F. Nanostructural and Functional Properties of Ag-TiO2 Coatings Prepared by Co-Sputtering Deposition Technique. J. Nanosci. Nanotechnol. 2011, 11, 4893–4899. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D. Influence of Substrate Temperature and Silver-Doping on the Structural and Optical Properties of TiO2 Films. Thin Solid Films 2016, 598, 204–213. [Google Scholar] [CrossRef]
- Sauthier, G.; Pérez Del Pino, A.; Figueras, A.; György, E. Synthesis and Characterization of Ag Nanoparticles and Ag-Loaded TiO2 Photocatalysts. J. Am. Ceram. Soc. 2011, 94, 3780–3786. [Google Scholar] [CrossRef]
- Hou, Y.; Feng, J.; Wang, Y.; Li, L. Enhanced Antibacterial Activity of Ag-Doped ZnO/Polyaniline Nanocomposites. J. Mater. Sci. Mater. Electron. 2016, 27, 6615–6622. [Google Scholar] [CrossRef]
- Vitelaru, C.; Parau, A.C.; Kiss, A.E.; Pana, I.; Dinu, M.; Constantin, L.R.; Vladescu, A.; Tonofrei, L.E.; Adochite, C.S.; Costinas, S.; et al. Silver-Containing Thin Films on Transparent Polymer Foils for Antimicrobial Applications. Coatings 2022, 12, 170. [Google Scholar] [CrossRef]
- Adochițe, C.; Vițelaru, C.; Parau, A.C.; Kiss, A.E.; Pană, I.; Vlădescu, A.; Costinaș, S.; Moga, M.; Muntean, R.; Badea, M.; et al. Synthesis and Investigation of Antibacterial Activity of Thin Films Based on TiO2-Ag and SiO2-Ag with Potential Applications in Medical Environment. Nanomaterials 2022, 12, 902. [Google Scholar] [CrossRef]
- Vladkova, T.; Angelov, O.; Stoyanova, D.; Gospodinova, D.; Gomes, L.; Soares, A.; Mergulhao, F.; Ivanova, I. Magnetron Co-Sputtered TiO2/SiO2/Ag Nanocomposite Thin Coatings Inhibiting Bacterial Adhesion and Biofilm Formation. Surf. Coatings Technol. 2020, 384, 125322. [Google Scholar] [CrossRef]
- Floroian, L.; Ristoscu, C.; Mihailescu, N.; Negut, I.; Badea, M.; Ursutiu, D.; Chifiriuc, M.C.; Urzica, I.; Dyia, H.M.; Bleotu, C.; et al. Functionalized Antimicrobial Composite Thin Films Printing for Stainless Steel Implant Coatings. Molecules 2016, 21, 740. [Google Scholar] [CrossRef]
- Floroian, L.; Ristoscu, C.; Candiani, G.; Pastori, N.; Moscatelli, M.; Mihailescu, N.; Negut, I.; Badea, M.; Gilca, M.; Chiesa, R.; et al. Antimicrobial Thin Films Based on Ayurvedic Plants Extracts Embedded in a Bioactive Glass Matrix. Appl. Surf. Sci. 2017, 417, 224–233. [Google Scholar] [CrossRef]
- Bai, L.; Hang, R.; Gao, A.; Zhang, X.; Huang, X.; Wang, Y.; Tang, B.; Zhao, L.; Chu, P.K. Nanostructured Titanium-Silver Coatings with Good Antibacterial Activity and Cytocompatibility Fabricated by One-Step Magnetron Sputtering. Appl. Surf. Sci. 2015, 355, 32–44. [Google Scholar] [CrossRef]
- Fan, X.; Yahia, L.; Sacher, E. Antimicrobial Properties of the Ag, Cu Nanoparticle System. Biology 2021, 10, 137. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, R.; Calderon, S.V.; Fangueiro, R.; Henriques, M.; Carvalho, S. Influence of Oxygen Content on the Antibacterial Effect of Ag-O Coatings Deposited by Magnetron Sputtering. Surf. Coatings Technol. 2016, 305, 1–10. [Google Scholar] [CrossRef]
- Zuo, J.; Keil, P.; Grundmeier, G. Synthesis and Characterization of Photochromic Ag-Embedded TiO2 Nanocomposite Thin Films by Non-Reactive RF-Magnetron Sputter Deposition. Appl. Surf. Sci. 2012, 258, 7231–7237. [Google Scholar] [CrossRef]
- Chakhtouna, H.; Benzeid, H.; Zari, N.; Qaiss, A.e.k.; Bouhfid, R. Recent Progress on Ag/TiO2 Photocatalysts: Photocatalytic and Bactericidal Behaviors. Environ. Sci. Pollut. Res. 2021, 28, 44638–44666. [Google Scholar] [CrossRef]
- Yong, S.S.; Lee, J.I.; Kang, D.H. TiO2-Based Photocatalyst Generated Reactive Oxygen Species Cause Cell Membrane Disruption of Staphylococcus Aureus and Escherichia Coli O157:H7. Food Microbiol. 2022, 109, 104119. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Visible-Light Active Titanium Dioxide Nanomaterials with Bactericidal Properties. Nanomaterials 2020, 10, 124. [Google Scholar] [CrossRef]
- Ouyang, B.; Wei, D.; Wu, B.; Yan, L.; Gang, H.; Cao, Y.; Chen, P.; Zhang, T.; Wang, H. In the View of Electrons Transfer and Energy Conversion: The Antimicrobial Activity and Cytotoxicity of Metal-Based Nanomaterials and Their Applications. Small 2024, 20, e2303153. [Google Scholar] [CrossRef]
- Lok, C.N.; Ho, C.M.; Chen, R.; He, Q.Y.; Yu, W.Y.; Sun, H.; Tam, P.K.H.; Chiu, J.F.; Che, C.M. Silver Nanoparticles: Partial Oxidation and Antibacterial Activities. J. Biol. Inorg. Chem. 2007, 12, 527–534. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Batista, J.G.S.; Rodrigues, M.Á.V.; Thipe, V.C.; Minarini, L.A.R.; Lopes, P.S.; Lugão, A.B. Advances in Silver Nanoparticles: A Comprehensive Review on Their Potential as Antimicrobial Agents and Their Mechanisms of Action Elucidated by Proteomics. Front. Microbiol. 2024, 15, 1440065. [Google Scholar] [CrossRef]
- Anees Ahmad, S.; Sachi Das, S.; Khatoon, A.; Tahir Ansari, M.; Afzal, M.; Saquib Hasnain, M.; Kumar Nayak, A. Bactericidal Activity of Silver Nanoparticles: A Mechanistic Review. Mater. Sci. Energy Technol. 2020, 3, 756–769. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef] [PubMed]
- More, P.R.; Pandit, S.; Filippis, A.D.; Franci, G.; Mijakovic, I.; Galdiero, M. Silver Nanoparticles: Bactericidal and Mechanistic Approach against Drug Resistant Pathogens. Microorganisms 2023, 11, 369. [Google Scholar] [CrossRef] [PubMed]
- Mikhailova, E.O. Green Silver Nanoparticles: An Antibacterial Mechanism. Antibiotics 2025, 14, 5. [Google Scholar] [CrossRef]
- Teramura, H.; Ogura, A.; Everis, L.; Betts, G. MC-Media Pad EC for Enumeration of Escherichia Coli and Coliforms in a Variety of Foods. J. AOAC Int. 2019, 102, 1502–1515. [Google Scholar] [CrossRef]
- Kang, J.Y.; Lee, S.H.; Jo, A.H.; Park, E.J.; Bak, Y.S.; Kim, J.B. Improving the Accuracy of Coliform Detection in Meat Products Using Modified Dry Rehydratable Film Method. Food Sci. Biotechnol. 2020, 29, 1289–1294. [Google Scholar] [CrossRef]
- Adochite, R.C.; Munteanu, D.; Torrell, M.; Cunha, L.; Alves, E.; Barradas, N.P.; Cavaleiro, A.; Riviere, J.P.; Le Bourhis, E.; Eyidi, D.; et al. The Influence of Annealing Treatments on the Properties of Ag:TiO2 Nanocomposite Films Prepared by Magnetron Sputtering. Appl. Surf. Sci. 2012, 258, 4028–4034. [Google Scholar] [CrossRef]
- Duhan, S.; Devi, S.; Srivastava, M. Characterization of Nanocrystalline Ag/SiO2 Nanocomposites and Synthesis by Wet Chemical Method. Indian J. Pure Appl. Phys. 2010, 48, 271. [Google Scholar]
- Okumu, J.; Dahmen, C.; Sprafke, A.N.; Luysberg, M.; Von Plessen, G.; Wuttig, M. Photochromic Silver Nanoparticles Fabricated by Sputter Deposition. J. Appl. Phys. 2005, 97, 094305. [Google Scholar] [CrossRef]
- Veiseh, M.; Zhang, M. Effect of Silicon Oxidation on Long-Term Cell Selectivity of Cell-Patterned Au/SiO2 Platforms. J. Am. Chem. Soc. 2006, 128, 1197–1203. [Google Scholar] [CrossRef]
- Chen, Z.; Li, J.; Wang, S.; Zhao, J.; Liu, J.; Shen, J.; Qi, C.; Yang, P. Structure-Property-Performance Relationship of Transition Metal Doped WO3 Mixed Oxides for Catalytic Degradation of Organic Pollutants. Chemosphere 2023, 316, 137797. [Google Scholar] [CrossRef] [PubMed]
- Farooq, U.; Ahmad, T.; Naaz, F.; Islam, S. Review on Metals and Metal Oxides in Sustainable Energy Production: Progress and Perspectives. Energy Fuels 2023, 37, 1577–1632. [Google Scholar] [CrossRef]
- Ishfaq, M.; Kousar, T.; Hussain, M.; Somaily, H.; Mubeen, S.; Potrich, E.; Panduro-Tenazoa, N.M.; Salam, M.A.; Ejaz, S.R.; Aadil, M. Synergistic Effect of Binary Metal Doping and Nanotechnology to Boost the Light-Harvesting Properties of Rare Earth Metal Oxide. Ceram. Int. 2023, 49, 745. [Google Scholar] [CrossRef]
- Zarzzeka, C.; Goldoni, J.; de Paula de Oliveira, J.d.R.; Lenzi, G.G.; Bagatini, M.D.; Colpini, L.M.S. Photocatalytic Action of Ag/TiO2 Nanoparticles to Emerging Pollutants Degradation: A Comprehensive Review. Sustain. Chem. Environ. 2024, 8, 100177. [Google Scholar] [CrossRef]
- Martínez-Castañón, G.; Martínez, J.R.; Zarzosa, G.O.; Ruiz, F.; Sánchez-Loredo, M.G. Optical Absorption of Ag Particles Dispersed in a SiO2 Amorphous Matrix. J. Sol-Gel Sci. Technol. 2005, 36, 137–145. [Google Scholar] [CrossRef]
- Mosquera, A.A.; Albella, J.M.; Navarro, V.; Bhattacharyya, D.; Endrino, J.L. Effect of Silver on the Phase Transition and Wettability of Titanium Oxide Films. Sci. Rep. 2016, 6, 32171. [Google Scholar] [CrossRef]
- Usha, K.; Kumbhakar, P.; Mondal, B. Effect of Ag-Doped TiO2 Thin Film Passive Layers on the Performance of Photo-Anodes for Dye-Sensitized Solar Cells. Mater. Sci. Semicond. Process. 2016, 43, 17–24. [Google Scholar] [CrossRef]
- Balasubramaniam, B.; Prateek; Ranjan, S.; Saraf, M.; Kar, P.; Singh, S.P.; Thakur, V.K.; Singh, A.; Gupta, R.K. Antibacterial and Antiviral Functional Materials: Chemistry and Biological Activity toward Tackling COVID-19-like Pandemics. ACS Pharmacol. Transl. Sci. 2021, 4, 8–54. [Google Scholar] [CrossRef]
- Mueller, M.; Tainter, C.R. Escherichia Coli Infection; StatPearls Publishing LLC: Treasure Island, FL, USA, 2025. [Google Scholar]
- Cattaruzza, E.; Gonella, F.; Ali, S.; Sada, C.; Quaranta, A. Silver and Gold Doping of SiO2 Glass by Solid-State Field-Assisted Diffusion. J. Non. Cryst. Solids 2009, 355, 1136–1139. [Google Scholar] [CrossRef]
- Huang, K.S.; Yang, C.H.; Huang, S.L.; Chen, C.Y.; Lu, Y.Y.; Lin, Y.S. Recent Advances in Antimicrobial Polymers: A Mini-Review. Int. J. Mol. Sci. 2016, 17, 1578. [Google Scholar] [CrossRef]



| Concentration of Escherichia coli (CFU/mL) | Sample | Bacterial Growth Inhibition Diameter (cm) (Average ± stdev) |
|---|---|---|
| 107 | TiO2+Ag | 1.21 ± 0.032 |
| SiO2+Ag | 0.90 ± 0.037 | |
| 2 × 106 | TiO2+Ag | 1.39 ± 0.020 |
| SiO2+Ag | 1.08 ± 0.111 | |
| 106 | TiO2+Ag | 1.26 ± 0.0110 |
| SiO2+Ag | 0.97 ± 0.025 | |
| 2 × 105 | TiO2+Ag | 1.55 ± 0.004 |
| SiO2+Ag | 1.59 ± 0.013 | |
| 105 | TiO2+Ag | 1.54 ± 0.020 |
| SiO2+Ag | 1.51 ± 0.104 | |
| 2 × 104 | TiO2+Ag | 1.71 ± 0.033 |
| SiO2+Ag | 1.60 ± 0.092 | |
| 104 | TiO2+Ag | 1.79 ± 0.101 |
| SiO2+Ag | 1.84 ± 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gălbau, C.-Ș.; Idomir, M.; Vițelaru, C.; Kiss, A.E.; Parau, A.C.; Constantin, L.R.; Dinu, M.; Pana, I.; Vlădescu, A.; Gaman, E.L.; et al. Preparation, Characterization and Evaluation of the Antibacterial Activity of Ag Nanoparticles Embedded in Transparent Oxide Matrices. Appl. Sci. 2025, 15, 2599. https://doi.org/10.3390/app15052599
Gălbau C-Ș, Idomir M, Vițelaru C, Kiss AE, Parau AC, Constantin LR, Dinu M, Pana I, Vlădescu A, Gaman EL, et al. Preparation, Characterization and Evaluation of the Antibacterial Activity of Ag Nanoparticles Embedded in Transparent Oxide Matrices. Applied Sciences. 2025; 15(5):2599. https://doi.org/10.3390/app15052599
Chicago/Turabian StyleGălbau, Cristina-Ștefania, Mihaela Idomir, Cătălin Vițelaru, Adrian Emil Kiss, Anca Constantina Parau, Lidia Ruxandra Constantin, Mihaela Dinu, Iulian Pana, Alina Vlădescu (Dragomir), Elena Laura Gaman, and et al. 2025. "Preparation, Characterization and Evaluation of the Antibacterial Activity of Ag Nanoparticles Embedded in Transparent Oxide Matrices" Applied Sciences 15, no. 5: 2599. https://doi.org/10.3390/app15052599
APA StyleGălbau, C.-Ș., Idomir, M., Vițelaru, C., Kiss, A. E., Parau, A. C., Constantin, L. R., Dinu, M., Pana, I., Vlădescu, A., Gaman, E. L., Moga, M. A., Mișarcă, C., Vârciu, M., Irimie, C. A., & Badea, M. (2025). Preparation, Characterization and Evaluation of the Antibacterial Activity of Ag Nanoparticles Embedded in Transparent Oxide Matrices. Applied Sciences, 15(5), 2599. https://doi.org/10.3390/app15052599






.jpg)



