Proteolytic Enzyme Activities of Bromelain, Ficin, and Papain from Fruit By-Products and Potential Applications in Sustainable and Functional Cosmetics for Skincare
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
- Bromelain: approximately 31% of the references, focusing on its source (pineapple), its biochemical properties, purification techniques, and cosmetic applications.
- Ficin: approximately 20% of the references, focusing on the extraction of ficin from the fig latex, the unique substrate specificity, and its gentle exfoliating properties.
- Papain: approximately 24% of the references, focusing on the derivation of papain from the papaya latex, its strong proteolytic activity, and its role in deep exfoliation.
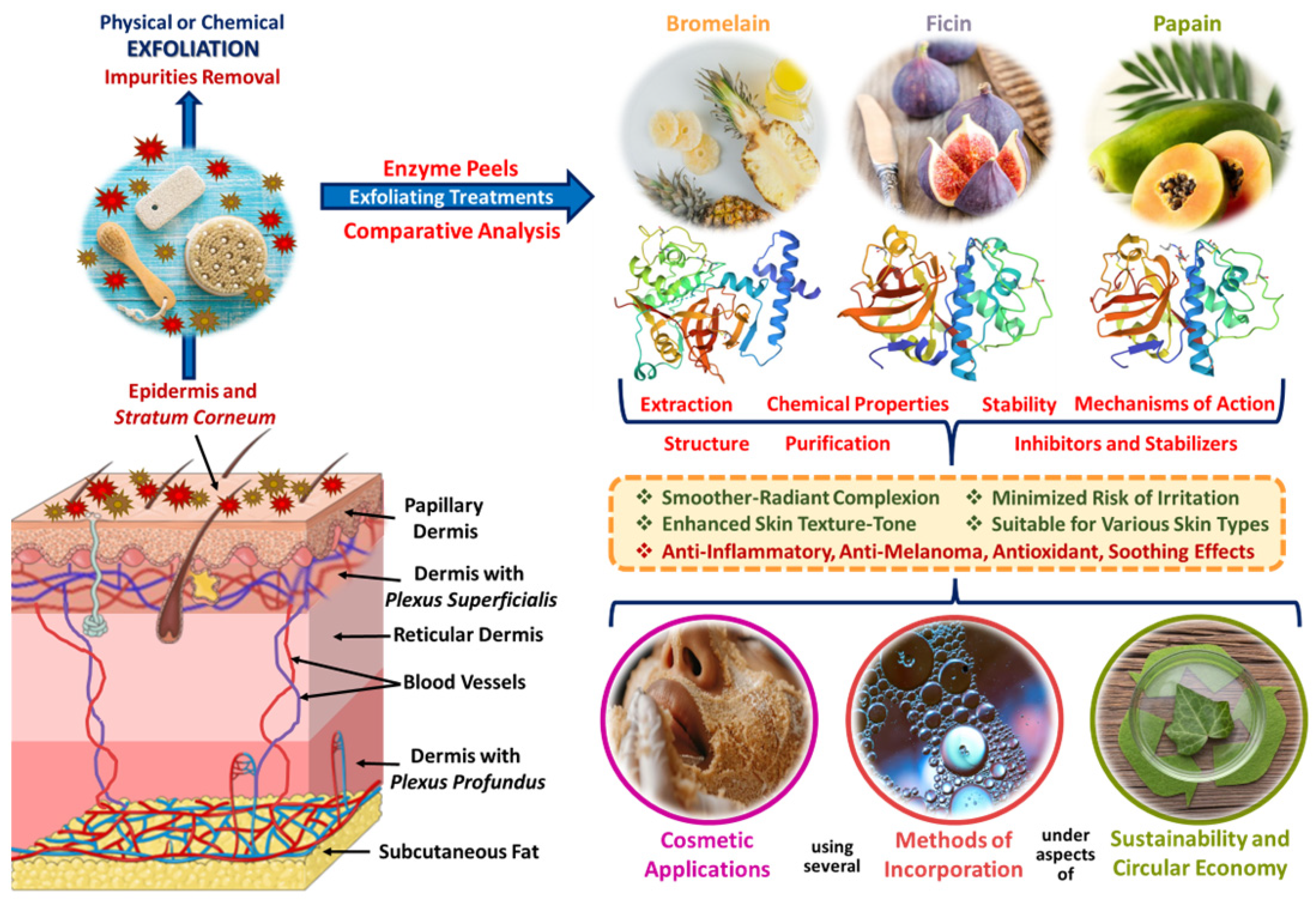
3. Brief Overview of Proteolytic Enzymes: Bromelain, Ficin, and Papain’s Classification
4. Bromelain Use as an Exfoliant Agent in Cosmetic Applications
4.1. General Profile and Source of Bromelain
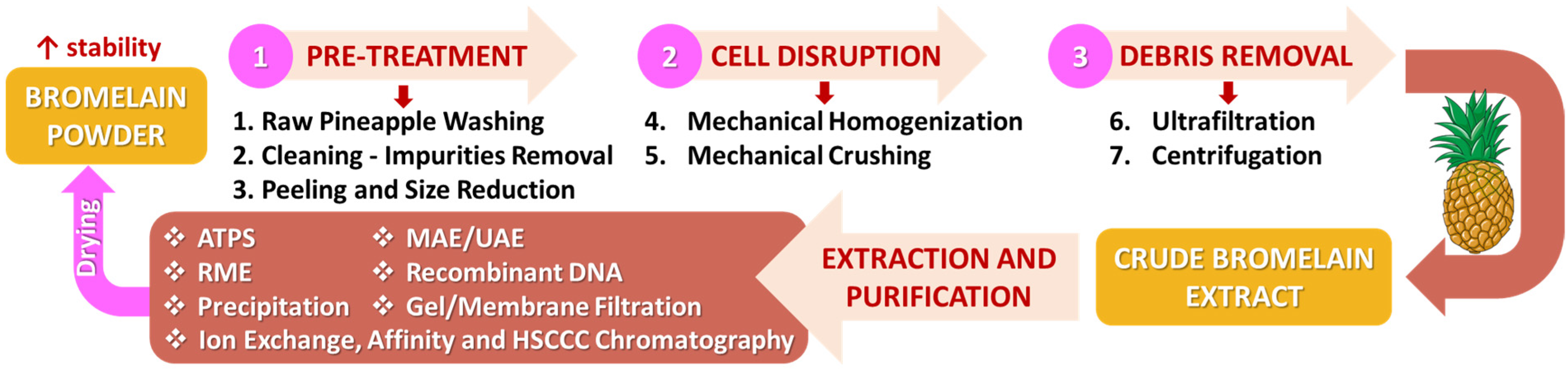
4.2. Methods of Extraction and Purification of Bromelain
4.3. Chemical Properties and Structure of Bromelain
4.3.1. Molecular Structure
4.3.2. Stability and Activity Under Different pH and Temperature Conditions
4.4. Bromelain Stabilizers and Inhibitors in Cosmetic Formulations
5. Ficin Use as an Exfoliant Agent in Cosmetic Applications
5.1. General Profile and Ficin Source
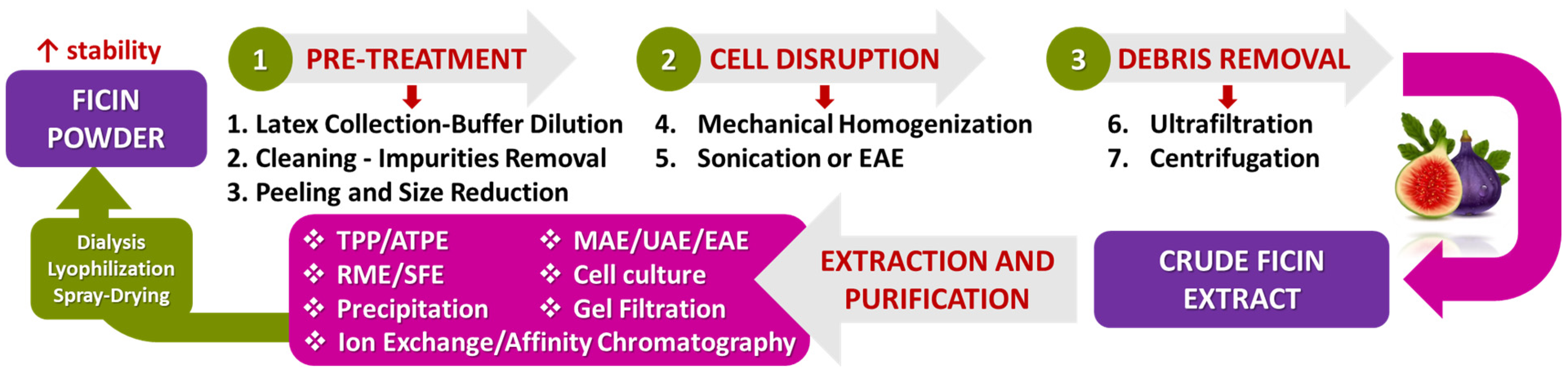
5.2. Methods of Extraction and Purification of Ficin
5.3. Chemical Properties and Structure of Ficin
5.3.1. Molecular Structure
5.3.2. Stability and Activity Under Different pH and Temperature Conditions
5.4. Ficin Stabilizers and Inhibitors in Cosmetic Formulations
6. Papain Use as an Exfoliant Agent in Cosmetic Applications
6.1. General Profile and Source of Papain
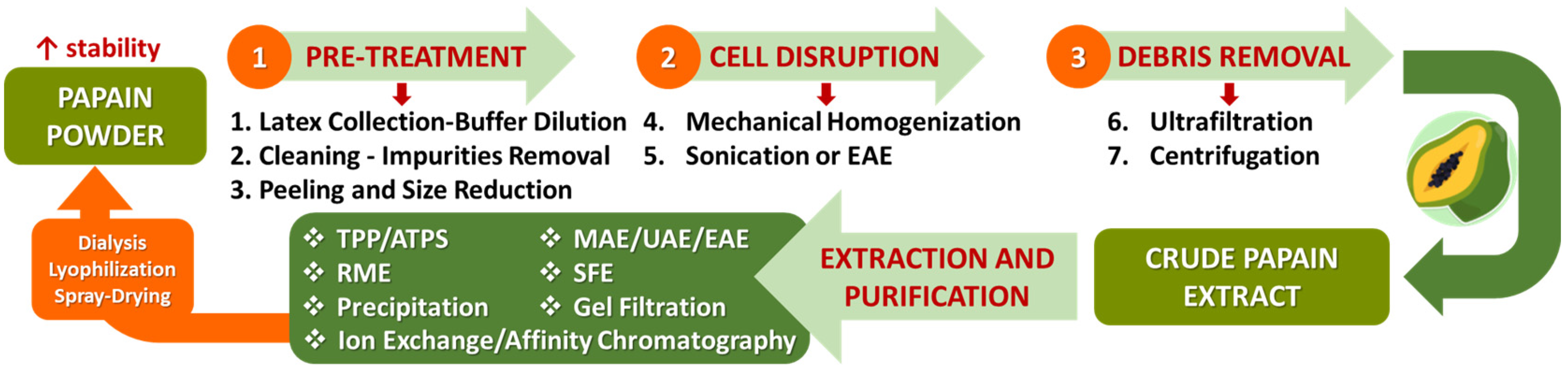
6.2. Methods of Extraction and Purification of Papain
6.3. Chemical Properties and Structure of Papain
6.3.1. Molecular Structure
6.3.2. Stability and Activity Under Different pH and Temperature Conditions
6.4. Papain Stabilizers and Inhibitors in Cosmetic Formulations
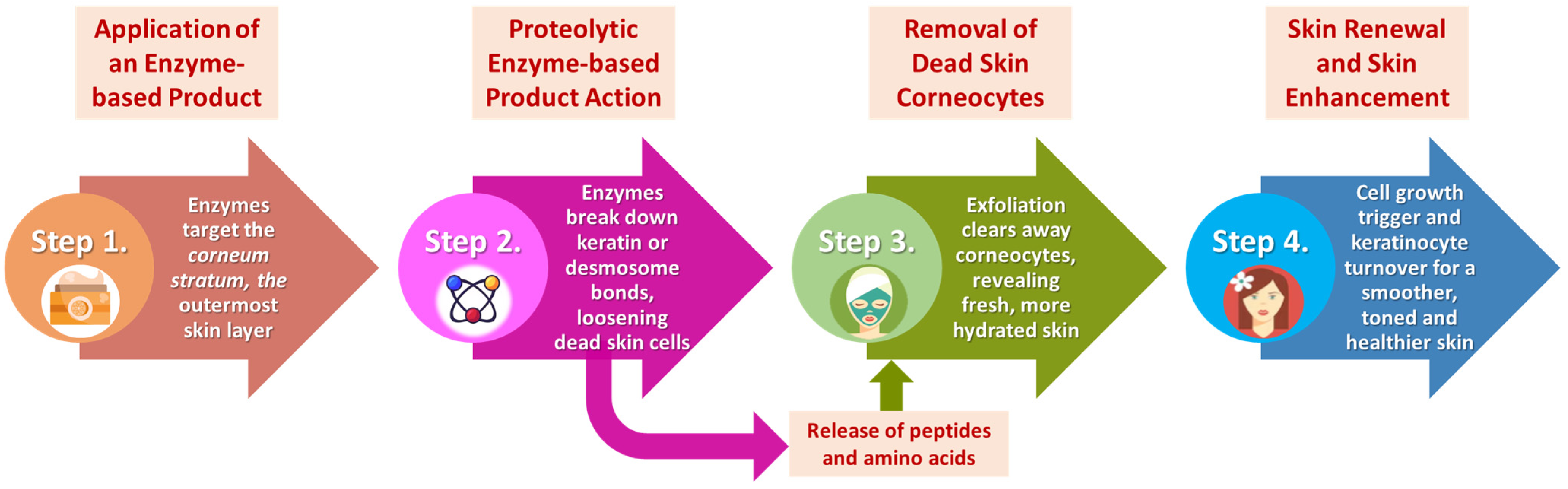
7. Bromelain, Ficin, and Papain Exfoliants in Cosmetic Formulations: Mechanisms of Action and Biochemical Pathways
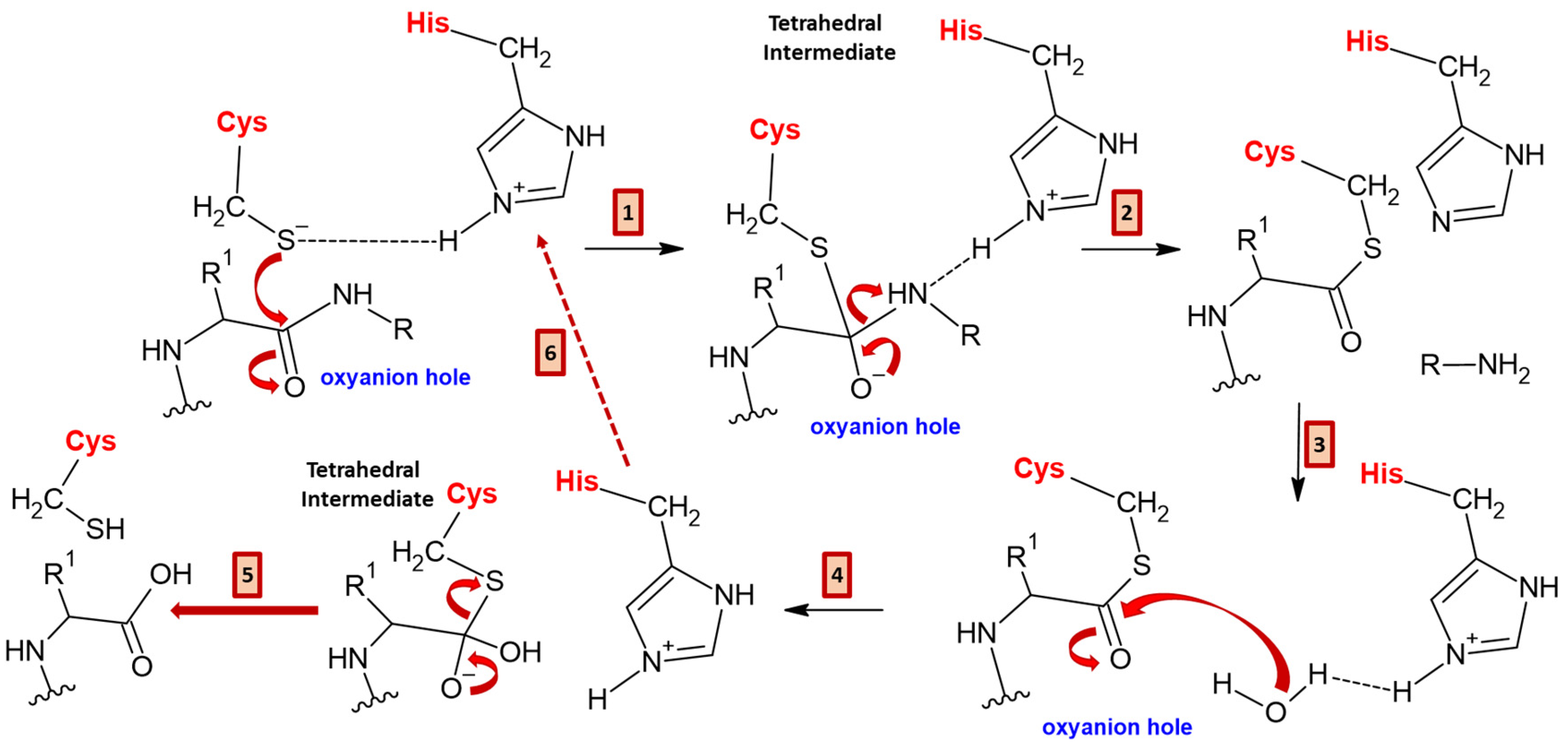
7.1. Bromelain, Ficin, and Papain Mechanisms of Action
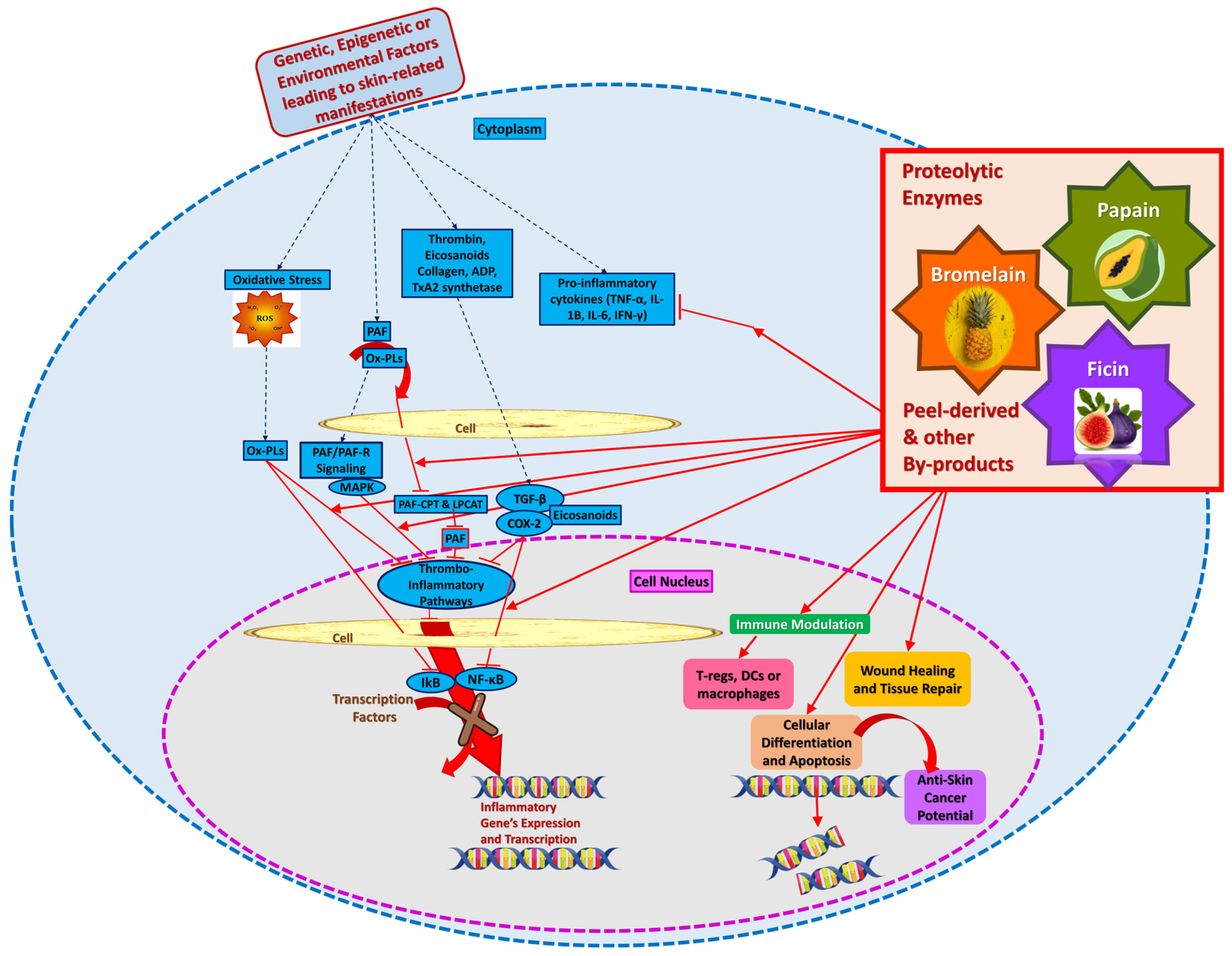
7.2. Bromelain, Ficin, and Papain Biochemical Pathways of Action and Relative Properties
8. Bromelain, Ficin, and Papain Peels By-Products-Based Cosmetic Applications
9. Methods of Incorporation of Bromelain, Ficin, and Papain Peel By-Products-Based Cosmetic Applications
10. Comparative Analysis of Several Aspects Regarding Bromelain, Ficin, and Papain Enzyme Peel By-Products Utilized in Cosmetic Applications
11. Sustainability and Circular Economy of Bromelain, Ficin, and Papain Enzyme Peel By-Products-Based Cosmetic Applications
12. Current Research Gaps, Future Directions, and Perspectives
13. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McMullen, R.L.; Dell’Acqua, G. History of Natural Ingredients in Cosmetics. Cosmetics 2023, 10, 71. [Google Scholar] [CrossRef]
- Sasounian, R.; Martinez, R.M.; Lopes, A.M.; Giarolla, J.; Rosado, C.; Magalhães, W.V.; Velasco, M.V.R.; Baby, A.R. Innovative Approaches to an Eco-Friendly Cosmetic Industry: A Review of Sustainable Ingredients. Clean Technol. 2024, 6, 176–198. [Google Scholar] [CrossRef]
- Gomes, C.; Silva, A.C.; Marques, A.C.; Sousa Lobo, J.; Amaral, M.H. Biotechnology Applied to Cosmetics and Aesthetic Medicines. Cosmetics 2020, 7, 33. [Google Scholar] [CrossRef]
- Linares-Devia, N.; Arrieta-Escobar, J.; Baena, Y.; Orjuela, A.; Osorio, C. Development and Characterization of Emulsions Containing Ground Seeds of Passiflora Species as Biobased Exfoliating Agents. Cosmetics 2022, 9, 15. [Google Scholar] [CrossRef]
- Michalak, M. Plant Extracts as Skin Care and Therapeutic Agents. Int. J. Mol. Sci. 2023, 24, 15444. [Google Scholar] [CrossRef]
- Rostkowska, E.; Poleszak, E.; Wojciechowska, K.; Dos Santos Szewczyk, K. Dermatological Management of Aged Skin. Cosmetics 2023, 10, 55. [Google Scholar] [CrossRef]
- Namkoong, J.; Goswami, S.; Tartar, O.; Diaz, I.; Wu, J. In-Vitro Efficacy Investigation and an Open-Label, Single-Arm Clinical Study of a Gentle Micropeeling Cream for Sensitive and Non-Sensitive Skin. Cosmetics 2022, 9, 138. [Google Scholar] [CrossRef]
- Sołdacka, D.; Barańska-Rybak, W. Evaluation of Safety and Efficacy of Chemical Peels With and Without Sonophoresis on Selected Skin Parameters—A Prospective Comparative Study. Cosmetics 2024, 11, 185. [Google Scholar] [CrossRef]
- Grace, F.; Balu, A.; Kanimozhi, T.; Shanmuganathan, S. Preparation and evaluation of deep cleansing exfoliator. Asian J. Pharm. Clin. Res. 2018, 11, 356–359. [Google Scholar] [CrossRef]
- Baker, P.; Huang, C.; Radi, R.; Moll, S.B.; Jules, E.; Arbiser, J.L. Skin Barrier Function: The Interplay of Physical, Chemical, and Immunologic Properties. Cells 2023, 12, 2745. [Google Scholar] [CrossRef]
- Packianathan, N.; Kandasamy, R. Skin Care with Herbal Exfoliants. Functonal Plant Sci. Biotechnol. 2011, 5, 94–97. [Google Scholar]
- Tuchin, V.V. Tissue Optics and Photonics: Biological Tissue Structures. J. Biomed. Photonics Eng. 2015, 1, 3–21. [Google Scholar] [CrossRef]
- Minich, I.A.; Silyukov, O.I.; Kurnosenko, S.A.; Gak, V.V.; Kalganov, V.D.; Kolonitskiy, P.D.; Zvereva, I.A. Physical–Chemical Exfoliation of n-Alkylamine Derivatives of Layered Perovskite-like Oxide H2K0.5Bi2.5Ti4O13 into Nanosheets. Nanomaterials 2021, 11, 2708. [Google Scholar] [CrossRef]
- Divakaran, D.; Sriariyanun, M.; Suyambulingam, I.; Mavinkere Rangappa, S.; Siengchin, S. Exfoliation and Physico-Chemical Characterization of Novel Bioplasticizers from Nelumbo nucifera Leaf for Biofilm Application. Heliyon 2023, 9, e22550. [Google Scholar] [CrossRef]
- Liu, J.-K. Natural Products in Cosmetics. Nat. Prod. Bioprospect. 2022, 12, 40. [Google Scholar] [CrossRef]
- Tang, S.-C.; Yang, J.-H. Dual Effects of Alpha-Hydroxy Acids on the Skin. Molecules 2018, 23, 863. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-H.; Wang, H.-M.D.; Shieh, C.-J. Enzymes in Biomedical, Cosmetic and Food Application. Catalysts 2024, 14, 162. [Google Scholar] [CrossRef]
- Gonçalves, S. Use of Enzymes in Cosmetics: Proposed Enzymatic Peel Procedure. CosmEthically ACTIVE 2021, 1, 29–35. [Google Scholar]
- Vandorou, M.; Plakidis, C.; Tsompanidou, I.M.; Adamantidi, T.; Panagopoulou, E.A.; Tsoupras, A. A Review on Apple Pomace Bioactives for Natural Functional Food and Cosmetic Products with Therapeutic Health-Promoting Properties. Int. J. Mol. Sci. 2024, 25, 10856. [Google Scholar] [CrossRef]
- Adamantidi, T.; Lafara, M.-P.; Venetikidou, M.; Likartsi, E.; Toganidou, I.; Tsoupras, A. Utilization and Bio-Efficacy of Carotenoids, Vitamin A and Its Vitaminoids in Nutricosmetics, Cosmeceuticals, and Cosmetics’ Applications with Skin-Health Promoting Properties. Appl. Sci. 2025, 15, 1657. [Google Scholar] [CrossRef]
- Tsiapali, O.I.; Ayfantopoulou, E.; Tzourouni, A.; Ofrydopoulou, A.; Letsiou, S.; Tsoupras, A. Unveiling the Utilization of Grape and Winery By-Products in Cosmetics with Health Promoting Properties. Appl. Sci. 2025, 15, 1007. [Google Scholar] [CrossRef]
- Bialkowski, K. Proteolytic Activity of Cosmetic Enzyme Peel Products. Acta Biochim. Pol. 2022, 69, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.; Shanbhag, T.; Kothare, A. Applications of Bromelain from Pineapple Waste towards Acne. Saudi J. Biol. Sci. 2021, 28, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Pezzani, R.; Jiménez-Garcia, M.; Capó, X.; Sönmez Gürer, E.; Sharopov, F.; Rachel, T.Y.L.; Ntieche Woutouoba, D.; Rescigno, A.; Peddio, S.; Zucca, P.; et al. Anticancer Properties of Bromelain: State-of-the-Art and Recent Trends. Front. Oncol. 2023, 12, 1068778. [Google Scholar] [CrossRef]
- Cho, U.M.; Choi, D.H.; Yoo, D.S.; Park, S.J.; Hwang, H.S. Inhibitory Effect of Ficin Derived from Fig Latex on Inflammation and Melanin Production in Skin Cells. Biotechnol. Bioproc. E 2019, 24, 288–297. [Google Scholar] [CrossRef]
- Kim, H.-M.; Kang, Y.-M.; Lee, M.; An, H.-J. Papain Suppresses Atopic Skin Inflammation through Anti-Inflammatory Activities Using In Vitro and In Vivo Models. Antioxidants 2024, 13, 928. [Google Scholar] [CrossRef]
- Trevisol, T.C.; Henriques, R.O.; Souza, A.J.A.; Furigo, A., Jr. An Overview of the Use of Proteolytic Enzymes as Exfoliating Agents. J. Cosmet. Dermatol. 2022, 21, 3300–3307. [Google Scholar] [CrossRef]
- Obaha, A.; Novinec, M. Regulation of Peptidase Activity beyond the Active Site in Human Health and Disease. Int. J. Mol. Sci. 2023, 24, 17120. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Barrett, A.J.; Thomas, P.D.; Huang, X.; Bateman, A.; Finn, R.D. The MEROPS Database of Proteolytic Enzymes, Their Substrates and Inhibitors in 2017 and a Comparison with Peptidases in the PANTHER Database. Nucleic Acids Res. 2018, 46, D624–D632. [Google Scholar] [CrossRef]
- Kieliszek, M.; Pobiega, K.; Piwowarek, K.; Kot, A.M. Characteristics of the Proteolytic Enzymes Produced by Lactic Acid Bacteria. Molecules 2021, 26, 1858. [Google Scholar] [CrossRef]
- Gurumallesh, P.; Alagu, K.; Ramakrishnan, B.; Muthusamy, S. A Systematic Reconsideration on Proteases. Int. J. Biol. Macromol. 2019, 128, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, R.; Altier, C.; Oikonomopoulou, K.; Hollenberg, M.D. Proteinases, Their Extracellular Targets, and Inflammatory Signaling. Pharmacol. Rev. 2016, 68, 1110–1142. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Mangla, B.; Javed, S.; Ahsan, W.; Kumar, P.; Garg, V.; Dureja, H. Bromelain: A Review of Its Mechanisms, Pharmacological Effects and Potential Applications. Food Funct. 2023, 14, 8101–8128. [Google Scholar] [CrossRef]
- Vatić, S.; Mirković, N.; Milošević, J.R.; Jovčić, B.; Polović, N.Đ. Broad Range of Substrate Specificities in Papain and Fig Latex Enzymes Preparations Improve Enumeration of Listeria monocytogenes. Int. J. Food Microbiol. 2020, 334, 108851. [Google Scholar] [CrossRef]
- Varilla, C.; Marcone, M.; Paiva, L.; Baptista, J. Bromelain, a Group of Pineapple Proteolytic Complex Enzymes (Ananas comosus) and Their Possible Therapeutic and Clinical Effects. A Summary. Foods 2021, 10, 2249. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.; Nikhade, P.; Patel, A.; Mankar, N.; Sedani, S. Bromelain: A Potent Phytomedicine. Cureus 2022, 14, e27876. [Google Scholar] [CrossRef]
- Masbagusdanta, K.; Setiasih, S.; Handayani, S.; Hudiyono, S. Partial Purification and Evaluation of Bromelain from Pineapple Stem (Ananas comosus) in Cream Based Preparation and Its in Vitro Anti-Inflammatory Activity. AIP Conf. Proc. 2020, 2243, 030012. [Google Scholar] [CrossRef]
- Ketnawa, S.; Chaiwut, P.; Rawdkuen, S. Pineapple Wastes: A Potential Source for Bromelain Extraction. Food Bioprod. Process. 2012, 90, 385–391. [Google Scholar] [CrossRef]
- Misran, E.; Idris, A.; Mat Sarip, S.H.; Ya’akob, H. Properties of Bromelain Extract from Different Parts of the Pineapple Variety Morris. Biocatal. Agric. Biotechnol. 2019, 18, 101095. [Google Scholar] [CrossRef]
- Morea, D.; Fortunati, S.; Martiniello, L. Circular Economy and Corporate Social Responsibility: Towards an Integrated Strategic Approach in the Multinational Cosmetics Industry. J. Clean. Prod. 2021, 315, 128232. [Google Scholar] [CrossRef]
- Machado, M.; Silva, S.; Costa, E.M. Byproducts as a Sustainable Source of Cosmetic Ingredients. Appl. Sci. 2024, 14, 10241. [Google Scholar] [CrossRef]
- Kansakar, U.; Trimarco, V.; Manzi, M.V.; Cervi, E.; Mone, P.; Santulli, G. Exploring the Therapeutic Potential of Bromelain: Applications, Benefits, and Mechanisms. Nutrients 2024, 16, 2060. [Google Scholar] [CrossRef] [PubMed]
- Spir, L.G.; Ataide, J.A.; De Lencastre Novaes, L.C.; Moriel, P.; Mazzola, P.G.; De Borba Gurpilhares, D.; Silveira, E.; Pessoa, A.; Tambourgi, E.B. Application of an Aqueous Two-Phase Micellar System to Extract Bromelain from Pineapple (Ananas comosus) Peel Waste and Analysis of Bromelain Stability in Cosmetic Formulations. Biotechnol. Prog. 2015, 31, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Colletti, A.; Li, S.; Marengo, M.; Adinolfi, S.; Cravotto, G. Recent Advances and Insights into Bromelain Processing, Pharmacokinetics and Therapeutic Uses. Appl. Sci. 2021, 11, 8428. [Google Scholar] [CrossRef]
- Arshad, Z.I.M.; Amid, A.; Yusof, F.; Jaswir, I.; Ahmad, K.; Loke, S.P. Bromelain: An Overview of Industrial Application and Purification Strategies. Appl. Microbiol. Biotechnol. 2014, 98, 7283–7297. [Google Scholar] [CrossRef]
- Chakraborty, A.J.; Mitra, S.; Tallei, T.E.; Tareq, A.M.; Nainu, F.; Cicia, D.; Dhama, K.; Emran, T.B.; Simal-Gandara, J.; Capasso, R. Bromelain a Potential Bioactive Compound: A Comprehensive Overview from a Pharmacological Perspective. Life 2021, 11, 317. [Google Scholar] [CrossRef]
- Krishna, S.H.; Srinivas, N.D.; Raghavarao, K.S.M.S.; Karanth, N.G. Reverse Micellar Extraction for Downstream Processing of Proteins/Enzymes. Adv. Biochem. Eng. Biotechnol. 2002, 75, 119–183. [Google Scholar] [CrossRef]
- Campos, D.A.; Coscueta, E.R.; Valetti, N.W.; Pastrana-Castro, L.M.; Teixeira, J.A.; Picó, G.A.; Pintado, M.M. Optimization of Bromelain Isolation from Pineapple Byproducts by Polysaccharide Complex Formation. Food Hydrocoll. 2019, 87, 792–804. [Google Scholar] [CrossRef]
- Mala, T.; Sadiq, M.B.; Anal, A.K. Comparative Extraction of Bromelain and Bioactive Peptides from Pineapple Byproducts by Ultrasonic- and Microwave-Assisted Extractions. J. Food Process Eng. 2021, 44, e13709. [Google Scholar] [CrossRef]
- Kaushal, R.; Kaur, B.; Panesar, P.S.; Anal, A.K.; Chu-Ky, S. Valorization of Pineapple Rind for Bromelain Extraction Using Microwave Assisted Technique: Optimization, Purification, and Structural Characterization. J. Food Sci. Technol. 2024, 61, 551–562. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ali, H.A.; Zain, N.M. Microwave-Assisted Extraction of Phenolic Compounds from Carica papaya Leaves: An Optimization Study and LC-QTOF-MS Analysis. Future Foods 2021, 3, 100035. [Google Scholar] [CrossRef]
- Sharma, G.; Vimal, A. Bromelain: An Enzyme Expanding Its Horizon from Food to Pharmaceutical Industry. Curr. Pharm. Biotechnol. 2023, 24, 1715–1726. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Bhattacharyya, D. Resistance of Bromelain to SDS Binding. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2009, 1794, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Roy, P.; Sardar, P.S.; Ghosh, S. Addressing the Interaction of Stem Bromelain with Different Anionic Surfactants, below, at and above the Critical Micelle Concentration (Cmc) in Phosphate Buffer at pH 7: Physicochemical, Spectroscopic, & Molecular Docking Study. Int. J. Biol. Macromol. 2024, 271, 132368. [Google Scholar] [CrossRef]
- Rani, A.; Pannuru, V. Unanticipated Behaviour of Sorbitol towards the Stability and Activity of Stem Bromelain: An Outlook through Biophysical Techniques. Process Biochem. 2016, 51, 1028–1039. [Google Scholar] [CrossRef]
- Li, X.; Wu, Y.; Duan, R.; Yu, H.; Liu, S.; Bao, Y. Research Progress in the Extraction, Structural Characteristics, Bioactivity, and Commercial Applications of Oat β-Glucan: A Review. Foods 2024, 13, 4160. [Google Scholar] [CrossRef]
- Ataide, J.A.; Geraldes, D.C.; Gérios, E.F.; Bissaco, F.M.; Cefali, L.C.; Oliveira-Nascimento, L.; Mazzola, P.G. Freeze-Dried Chitosan Nanoparticles to Stabilize and Deliver Bromelain. J. Drug Deliv. Sci. Technol. 2021, 61, 102225. [Google Scholar] [CrossRef]
- Braham, S.A.; Siar, E.-H.; Arana-Peña, S.; Carballares, D.; Morellon-Sterling, R.; Bavandi, H.; de Andrades, D.; Kornecki, J.F.; Fernandez-Lafuente, R. Effect of Concentrated Salts Solutions on the Stability of Immobilized Enzymes: Influence of Inactivation Conditions and Immobilization Protocol. Molecules 2021, 26, 968. [Google Scholar] [CrossRef]
- Feng, Y.; Feng, C.; Wang, Y.; Gao, S.; Sun, P.; Yan, Z.; Su, X.; Sun, Y.; Zhu, Q. Effect of CaCl2 Treatment on Enzymatic Browning of Fresh-Cut Luffa (Luffa cylindrica). Horticulturae 2022, 8, 473. [Google Scholar] [CrossRef]
- Zhou, W.; Ye, C.; Geng, L.; Chen, G.; Wang, X.; Chen, W.; Sa, R.; Zhang, J.; Zhang, X. Purification and Characterization of Bromelain from Pineapple (Ananas comosus L.) Peel Waste. J. Food Sci. 2021, 86, 385–393. [Google Scholar] [CrossRef]
- Bresolin, I.R.A.P.; Bresolin, I.T.L.; Mazzola, P.G.; Tambourgi, E.B. Incorporation of Bromelain into Dermatological Bases: Accelerated Stability Studies. J. Chem. Chem. Eng. 2014, 8, 270–277. [Google Scholar] [CrossRef]
- Rachmawati, H.; Sulastri, E.; Iwo, M.I.; Safitri, D.; Rahma, A. Bromelain Encapsulated in Self Assembly Nanoemulsion Exhibits Better Debridement Effect in Animal Model of Burned Skin. J. Nano Res. 2016, 40, 158–166. [Google Scholar] [CrossRef]
- Sypka, M.; Jodłowska, I.; Białkowska, A.M. Keratinases as Versatile Enzymatic Tools for Sustainable Development. Biomolecules 2021, 11, 1900. [Google Scholar] [CrossRef] [PubMed]
- Hidayani, W.A.; Setiasih, S.; Hudiyono, S. Determination of the Effect of EDTA and PCMB on Purified Bromelain Activity from Pineapple Core and In Vitro Antiplatelet Activity. IOP Conf. Ser. Mater. Sci. Eng. 2020, 763, 012054. [Google Scholar] [CrossRef]
- Meccariello, L.; Bello, A.I.; Bove, G.; Gagliardo, N.; Raffaele, D.; Matera, L. The Ion Resonance and Bromelain-Vitamin C vs Bromelainvitamin C to Prevent Ankle Complications in Post-Operative Bimalleolar Surgery. Med. Glas. 2024, 21, 236–243. [Google Scholar] [CrossRef]
- Kaur, T.; Kaur, A.; Grewal, R.K. Kinetics Studies with Fruit Bromelain (Ananas comosus) in the Presence of Cysteine and Divalent Ions. J. Food Sci. Technol. 2015, 52, 5954–5960. [Google Scholar] [CrossRef]
- Alnajar, L.A.M. Effect of Some Metal Ions on the Activity of Bromalein Enzyme Purified from Pineapple Juice. AIP Conf. Proc. 2023, 2839, 060004. [Google Scholar] [CrossRef]
- Ayuso, M.; Carpena, M.; Taofiq, O.; Albuquerque, T.G.; Simal-Gandara, J.; Oliveira, M.B.P.P.; Prieto, M.A.; Ferreira, I.C.F.R.; Barros, L. Fig “Ficus carica L.” and Its by-Products: A Decade Evidence of Their Health-Promoting Benefits towards the Development of Novel Food Formulations. Trends Food Sci. Technol. 2022, 127, 1–13. [Google Scholar] [CrossRef]
- Gagaoua, M.; Boucherba, N.; Bouanane-Darenfed, A.; Ziane, F.; Nait-Rabah, S.; Hafid, K.; Boudechicha, H.-R. Three-Phase Partitioning as an Efficient Method for the Purification and Recovery of Ficin from Mediterranean Fig (Ficus carica L.) Latex. Sep. Purif. Technol. 2014, 132, 461–467. [Google Scholar] [CrossRef]
- Morellon-Sterling, R.; El-Siar, H.; Tavano, O.L.; Berenguer-Murcia, Á.; Fernández-Lafuente, R. Ficin: A Protease Extract with Relevance in Biotechnology and Biocatalysis. Int. J. Biol. Macromol. 2020, 162, 394–404. [Google Scholar] [CrossRef]
- Milošević, J.; Vrhovac, L.; Đurković, F.; Janković, B.; Malkov, S.; Lah, J.; Polović, N.Đ. Isolation, Identification, and Stability of Ficin 1c Isoform from Fig Latex. New J. Chem. 2020, 44, 15716–15723. [Google Scholar] [CrossRef]
- Shi, Y.; Mon, A.M.; Fu, Y.; Zhang, Y.; Wang, C.; Yang, X.; Wang, Y. The Genus Ficus (Moraceae) Used in Diet: Its Plant Diversity, Distribution, Traditional Uses and Ethnopharmacological Importance. J. Ethnopharmacol. 2018, 226, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Qu, H.; Zhang, L.; Du, S.; Yang, S.; Hao, D.; Wang, X. Purification and Characterization of a Proteolytic Enzyme from Fig Latex. Chem. Res. Chin. Univ. 2008, 24, 348–352. [Google Scholar] [CrossRef]
- Ayodipupo Babalola, B.; Ifeolu Akinwande, A.; Otunba, A.A.; Ebenezer Adebami, G.; Babalola, O.; Nwufo, C. Therapeutic Benefits of Carica papaya: A Review on Its Pharmacological Activities and Characterization of Papain. Arab. J. Chem. 2024, 17, 105369. [Google Scholar] [CrossRef]
- Raskovic, B.; Bozovic, O.; Prodanovic, R.; Niketic, V.; Polovic, N. Identification, Purification and Characterization of a Novel Collagenolytic Serine Protease from Fig (Ficus carica Var. Brown turkey) Latex. J. Biosci. Bioeng. 2014, 118, 622–627. [Google Scholar] [CrossRef]
- Homaei, A.; Stevanato, R.; Etemadipour, R.; Hemmati, R. Purification, Catalytic, Kinetic and Thermodynamic Characteristics of a Novel Ficin from Ficus johannis. Biocatal. Agric. Biotechnol. 2017, 10, 360–366. [Google Scholar] [CrossRef]
- Qurashi, A.A.F.; Jabbar, A.D. Isolation, Purification and Partial Characterization of Ficin from Ficus carica Latex. J. Wasit Sci. Med. 2009, 2, 55–66. [Google Scholar] [CrossRef]
- Łubek-Nguyen, A.; Ziemichód, W.; Olech, M. Application of Enzyme-Assisted Extraction for the Recovery of Natural Bioactive Compounds for Nutraceutical and Pharmaceutical Applications. Appl. Sci. 2022, 12, 3232. [Google Scholar] [CrossRef]
- Lin, Y.; Pi, J.; Jin, P.; Liu, Y.; Mai, X.; Li, P.; Fan, H. Enzyme and Microwave Co-Assisted Extraction, Structural Characterization and Antioxidant Activity of Polysaccharides from Purple-heart radish. Food Chem. 2022, 372, 131274. [Google Scholar] [CrossRef]
- Yang, C.; Liu, W.; Zhu, X.; Zhang, X.; Wei, Y.; Huang, J.; Yang, F.; Yang, F. Ultrasound-Assisted Enzymatic Digestion for Efficient Extraction of Proteins from Quinoa. LWT 2024, 194, 115784. [Google Scholar] [CrossRef]
- Ketnawa, S.; Chaiwut, P.; Rawdkuen, S. Aqueous Two-Phase Extraction of Bromelain from Pineapple Peels (‘Phu Lae’ Cultv.) and Its Biochemical Properties. Food Sci. Biotechnol. 2011, 20, 1219. [Google Scholar] [CrossRef]
- Chen, C.; Tian, H.; Xing, S.; Li, C.; Zeng, X.; He, L. Influence of Different Parameters on Reverse Micelle Extraction Combined with Acetone Precipitation to Purify Sn-1,3 Extracellular Lipase from Aspergillus Niger GZUF36. J. Food Sci. Technol. 2019, 56, 2899–2908. [Google Scholar] [CrossRef]
- Bernardo-Gil, M.G.; Casquilho, M.; Esquível, M.M.; Ribeiro, M.A. Supercritical Fluid Extraction of Fig Leaf Gourd Seeds Oil: Fatty Acids Composition and Extraction Kinetics. J. Supercrit. Fluids 2009, 49, 32–36. [Google Scholar] [CrossRef]
- Anderson, C.D.; Hall, P.L. Purification of Ficin by Affinity Chromatography. Anal. Biochem. 1974, 60, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shen, D.; Long, Y.; Xie, Z.; Zheng, H. Intrinsic Peroxidase-like Activity of Ficin. Sci. Rep. 2017, 7, 43141. [Google Scholar] [CrossRef] [PubMed]
- Zare, H.; Moosavi-Movahedi, A.A.; Salami, M.; Mirzaei, M.; Saboury, A.A.; Sheibani, N. Purification and Autolysis of the Ficin Isoforms from Fig (Ficus carica cv. Sabz) Latex. Phytochemistry 2013, 87, 16–22. [Google Scholar] [CrossRef]
- Zare, H.; Moosavi-Movahedi, A.A.; Salami, M.; Sheibani, N.; Khajeh, K.; Habibi-Rezaei, M. Autolysis Control and Structural Changes of Purified Ficin from Iranian Fig Latex with Synthetic Inhibitors. Int. J. Biol. Macromol. 2016, 84, 464–471. [Google Scholar] [CrossRef]
- Dini, I.; Falanga, D.; Di Lorenzo, R.; Tito, A.; Carotenuto, G.; Zappelli, C.; Grumetto, L.; Sacchi, A.; Laneri, S.; Apone, F. An Extract from Ficus carica Cell Cultures Works as an Anti-Stress Ingredient for the Skin. Antioxidants 2021, 10, 515. [Google Scholar] [CrossRef]
- Baeyens-Volant, D.; Matagne, A.; El Mahyaoui, R.; Wattiez, R.; Azarkan, M. A Novel Form of Ficin from Ficus carica Latex: Purification and Characterization. Phytochemistry 2015, 117, 154–167. [Google Scholar] [CrossRef]
- Devaraj, K.B.; Kumar, P.R.; Prakash, V. Purification, Characterization, and Solvent-Induced Thermal Stabilization of Ficin from Ficus carica. J. Agric. Food Chem. 2008, 56, 11417–11423. [Google Scholar] [CrossRef]
- Da Lopez, R.E.; Goncalves, R.N. Therapeutic Proteases from Plants: Biopharmaceuticals with Multiple Applications. J. Appl. Biotechnol. Bioeng. 2019, 6, 101–109. [Google Scholar] [CrossRef]
- Tikhonov, S.L.; Tikhonova, N.V.; Kudryashov, L.S.; Kudryashova, O.A.; Moskovenko, N.V.; Tretyakova, I.N. Efficiency of Microencapsulation of Proteolytic Enzymes. Catalysts 2021, 11, 1270. [Google Scholar] [CrossRef]
- Sorokin, A.V.; Olshannikova, S.S.; Lavlinskaya, M.S.; Holyavka, M.G.; Faizullin, D.A.; Zuev, Y.F.; Artukhov, V.G. Chitosan Graft Copolymers with N-Vinylimidazole as Promising Matrices for Immobilization of Bromelain, Ficin, and Papain. Polymers 2022, 14, 2279. [Google Scholar] [CrossRef]
- Nascimento, N.S.; Torres-Obreque, K.M.; Oliveira, C.A.; Rabelo, J.; Baby, A.R.; Long, P.F.; Young, A.R.; Rangel-Yagui, C.d.O. Enzymes for Dermatological Use. Exp. Dermatol. 2024, 33, e15008. [Google Scholar] [CrossRef] [PubMed]
- El Enshasy, H.A.; Abomoelak, B.; Rahman, R.A.; Leng, O.M.; Sukmawati, D.; Rasid, Z.I. Fig Enzymes: Characterization, Biological Roles, and Applications. In Fig (Ficus carica): Production, Processing, and Properties; Ramadan, M.F., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 523–537. ISBN 978-3-031-16493-4. [Google Scholar]
- Manosroi, A.; Chankhampan, C.; Pattamapun, K.; Manosroi, W.; Manosroi, J. Antioxidant and Gelatinolytic Activities of Papain from Papaya Latex and Bromelain from Pineapple Fruits. Chiang Mai J. Sci. 2014, 41, 635–648. [Google Scholar]
- Rashidi, Z.; Homaei, A.; Fernandez-Lafuente, R. Enhanced Stability of Papain at Extreme pHs and Temperatures by Its Immobilization on Cellulose Nanocrystals Coated with Polydopamine. Process Biochem. 2024, 146, 147–159. [Google Scholar] [CrossRef]
- Babalola, B.A.; Akinwande, A.I.; Gboyega, A.E.; Otunba, A.A. Extraction, Purification and Characterization of Papain Cysteine-Proteases from the Leaves of Carica papaya. Sci. Afr. 2023, 19, e01538. [Google Scholar] [CrossRef]
- Choudhary, R.; Kaushik, R.; Chawla, P.; Manna, S. Exploring the Extraction, Functional Properties, and Industrial Applications of Papain from Carica papaya. J. Sci. Food Agric. 2025, 105, 1533–1545. [Google Scholar] [CrossRef]
- Mamboya, F.; Amri, E. Papain, a Plant Enzyme of Biological Importance: A Review. AJBB 2012, 8, 99–104. [Google Scholar] [CrossRef]
- Feijoo-Siota, L.; Villa, T.G. Native and Biotechnologically Engineered Plant Proteases with Industrial Applications. Food Bioprocess. Technol. 2011, 4, 1066–1088. [Google Scholar] [CrossRef]
- Koul, B.; Pudhuvai, B.; Sharma, C.; Kumar, A.; Sharma, V.; Yadav, D.; Jin, J.-O. Carica papaya L.: A Tropical Fruit with Benefits beyond the Tropics. Diversity 2022, 14, 683. [Google Scholar] [CrossRef]
- Ribeiro, L.F.O.; Vitória, E.L.d.; Soprani Júnior, G.G.; Chen, P.; Lan, Y. Impact of Operational Parameters on Droplet Distribution Using an Unmanned Aerial Vehicle in a Papaya Orchard. Agronomy 2023, 13, 1138. [Google Scholar] [CrossRef]
- Iordănescu, O.A.; Băla, M.; Gligor, D.; Zippenfening, S.E.; Cugerean, M.I.; Petroman, M.I.; Hădărugă, D.I.; Hădărugă, N.G.; Riviş, M. A DPPH· Kinetic Approach on the Antioxidant Activity of Various Parts and Ripening Levels of Papaya (Carica papaya L.) Ethanolic Extracts. Plants 2021, 10, 1679. [Google Scholar] [CrossRef] [PubMed]
- Benito-Vázquez, I.; Muñoz-Labrador, A.; Garrido-Romero, M.; Hontoria-Caballo, G.; García-García, C.; Diez-Municio, M.; Moreno, F.J. New Pipeline for Analysing Fruit Proteolytic Products Used as Digestive Health Nutraceuticals. Int. J. Mol. Sci. 2024, 25, 10315. [Google Scholar] [CrossRef]
- Di Giacomo, M.; Bertoni, F.A.; Rocha, M.V.; Nerli, B.B.; Rodríguez, F. Cloud Point Extraction Based on Non-Ionic Surfactants: An Ecofriendly Tool for Recovering Papain from Papaya Latex. J. Environ. Chem. Eng. 2022, 10, 108762. [Google Scholar] [CrossRef]
- Hafid, K.; John, J.; Sayah, T.M.; Domínguez, R.; Becila, S.; Lamri, M.; Dib, A.L.; Lorenzo, J.M.; Gagaoua, M. One-Step Recovery of Latex Papain from Carica papaya Using Three Phase Partitioning and Its Use as Milk-Clotting and Meat-Tenderizing Agent. Int. J. Biol. Macromol. 2020, 146, 798–810. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, H.; Yang, L.; Tian, K. Optimization of Purification Conditions for Papain in a Polyethylene Glycol-Phosphate Aqueous Two-Phase System Using Quaternary Ammonium Ionic Liquids as Adjuvants by BBD-RSM. Protein Expr. Purif. 2019, 156, 8–16. [Google Scholar] [CrossRef]
- Yulirohyami; Hidayat, H.; Wijaya, A.R.; Fatimah, I. Papain Enzyme Assisted Extraction of Virgin Coconut Oil as Candidate In-House Reference Material. Processes 2022, 10, 315. [Google Scholar] [CrossRef]
- Biswas, R.; Sarkar, A.; Alam, M.; Roy, M.; Mahdi Hasan, M.M. Microwave and Ultrasound-Assisted Extraction of Bioactive Compounds from Papaya: A Sustainable Green Process. Ultrason. Sonochem. 2023, 101, 106677. [Google Scholar] [CrossRef]
- Lin, M.; Yu, T.; Wan, J.; Cao, X. Prediction of the Reverse Micellar Extraction of Papain Using Dissipative Particle Dynamics Simulation. Appl. Biochem. Biotechnol. 2017, 181, 1338–1346. [Google Scholar] [CrossRef]
- Chai, Y.H.; Yusup, S.; Ruslan, M.S.H.; Chin, B.L.F. Supercritical Fluid Extraction and Solubilization of Carica papaya Linn. Leaves in Ternary System with CO2 + Ethanol Solvents. Chem. Eng. Res. Des. 2020, 156, 31–42. [Google Scholar] [CrossRef]
- Kusumasari, C.; Meidyawati, R.; Megantoro, A.; Tiara, R.; Meiskya, A.; Darwish, K.M.; Abdou, A. Development of a Novel Papain Gel Formulation: Exploring Different Concentrations for Smear-Layer Deproteinization and Enhanced Dentin Bonding. Heliyon 2024, 10, e39035. [Google Scholar] [CrossRef] [PubMed]
- Sim, Y.-C.; Lee, S.-G.; Lee, D.-C.; Kang, B.-Y.; Park, K.-M.; Lee, J.-Y.; Kim, M.-S.; Chang, I.-S.; Rhee, J.-S. Stabilization of Papain and Lysozyme for Application to Cosmetic Products. Biotechnol. Lett. 2000, 22, 137–140. [Google Scholar] [CrossRef]
- Vasconcelos, N.F.; Cunha, A.P.; Ricardo, N.M.P.S.; Freire, R.S.; Vieira, L.d.A.P.; Brígida, A.I.S.; Borges, M.d.F.; Rosa, M.d.F.; Vieira, R.S.; Andrade, F.K. Papain Immobilization on Heterofunctional Membrane Bacterial Cellulose as a Potential Strategy for the Debridement of Skin Wounds. Int. J. Biol. Macromol. 2020, 165, 3065–3077. [Google Scholar] [CrossRef] [PubMed]
- Trevisol, T.C.; Henriques, R.O.; Souza, A.J.A.; Cesca, K.; Furigo, A. Starch- and Carboxymethyl Cellulose-Based Films as Active Beauty Masks with Papain Incorporation. Int. J. Biol. Macromol. 2023, 231, 123258. [Google Scholar] [CrossRef]
- Boonkerd, S.; Wantha, L. Antisolvent Crystallization of Papain. ChemEngineering 2024, 8, 4. [Google Scholar] [CrossRef]
- Mesquita, M.d.S.; Santos, P.D.d.F.; Holkem, A.T.; Thomazini, M.; Favaro-Trindade, C.S. Encapsulation of Formosa Papaya (Carica papaya L.) Seed Extract: Physicochemical Characteristics of Particles, and Study of Stability and Release of Encapsulated Phenolic Compounds. Processes 2023, 11, 27. [Google Scholar] [CrossRef]
- Lima, C.S.A.d.; Varca, J.P.R.O.; Nogueira, K.M.; Fazolin, G.N.; Freitas, L.F.d.; Souza, E.W.d.; Lugão, A.B.; Varca, G.H.C. Semi-Solid Pharmaceutical Formulations for the Delivery of Papain Nanoparticles. Pharmaceutics 2020, 12, 1170. [Google Scholar] [CrossRef]
- LaLonde, J.M.; Zhao, B.; Smith, W.W.; Janson, C.A.; DesJarlais, R.L.; Tomaszek, T.A.; Carr, T.J.; Thompson, S.K.; Oh, H.J.; Yamashita, D.S.; et al. Use of Papain as a Model for the Structure-Based Design of Cathepsin K Inhibitors: Crystal Structures of Two Papain-Inhibitor Complexes Demonstrate Binding to S’-Subsites. J. Med. Chem. 1998, 41, 4567–4576. [Google Scholar] [CrossRef]
- David Troncoso, F.; Alberto Sánchez, D.; Luján Ferreira, M. Production of Plant Proteases and New Biotechnological Applications: An Updated Review. ChemistryOpen 2022, 11, e202200017. [Google Scholar] [CrossRef]
- Kordiš, D.; Turk, V. Origin and Early Diversification of the Papain Family of Cysteine Peptidases. Int. J. Mol. Sci. 2023, 24, 11761. [Google Scholar] [CrossRef]
- Guo, J.-T.; Xiang, Y.; Guan, Z.; He, Y.-H. Papain-Catalyzed Aldol Reaction for the Synthesis of Trifluoromethyl Carbinol Derivatives. J. Mol. Catal. B Enzym. 2016, 131, 55–64. [Google Scholar] [CrossRef]
- Harrison, M.J.; Burton, N.A.; Hillier, I.H. Catalytic Mechanism of the Enzyme Papain: Predictions with a Hybrid Quantum Mechanical/Molecular Mechanical Potential. J. Am. Chem. Soc. 1997, 119, 12285–12291. [Google Scholar] [CrossRef]
- Chiarelli, P.G.; Martinez, B.; Nakamura, T.; Mis Solval, K. Enhancing Bromelain Recovery from Pineapple By-Products: A Sustainable Approach for Value Addition and Waste Reduction. Foods 2024, 13, 589. [Google Scholar] [CrossRef] [PubMed]
- Errasti, M.E.; Prospitti, A.; Viana, C.A.; Gonzalez, M.M.; Ramos, M.V.; Rotelli, A.E.; Caffini, N.O. Effects on Fibrinogen, Fibrin, and Blood Coagulation of Proteolytic Extracts from Fruits of Pseudananas Macrodontes, Bromelia Balansae, and B. Hieronymi (Bromeliaceae) in Comparison with Bromelain. Blood Coagul. Fibrinolysis 2016, 27, 441–449. [Google Scholar] [CrossRef]
- de Lencastre Novaes, L.C.; Jozala, A.F.; Lopes, A.M.; de Carvalho Santos-Ebinuma, V.; Mazzola, P.G.; Pessoa Junior, A. Stability, Purification, and Applications of Bromelain: A Review. Biotechnol. Prog. 2016, 32, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Aider, M. Potential Applications of Ficin in the Production of Traditional Cheeses and Protein Hydrolysates. JDS Commun. 2021, 2, 233–237. [Google Scholar] [CrossRef]
- Min, K.H.; Kim, D.H.; Ki, M.-R.; Pack, S.P. Recent Progress in Flocculation, Dewatering, and Drying Technologies for Microalgae Utilization: Scalable and Low-Cost Harvesting Process Development. Bioresour. Technol. 2022, 344, 126404. [Google Scholar] [CrossRef]
- Guengerich, F.P. Mechanisms of Enzyme Catalysis and Inhibition. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2023; ISBN 978-0-12-801238-3. [Google Scholar]
- Qiu, J.; Wilkens, C.; Barrett, K.; Meyer, A.S. Microbial Enzymes Catalyzing Keratin Degradation: Classification, Structure, Function. Biotechnol. Adv. 2020, 44, 107607. [Google Scholar] [CrossRef]
- Lopes, F.C.; Silva, L.A.D.E.; Tichota, D.M.; Daroit, D.J.; Velho, R.V.; Pereira, J.Q.; Corrêa, A.P.F.; Brandelli, A. Production of Proteolytic Enzymes by a Keratin-Degrading Aspergillus Niger. Enzyme Res. 2011, 2011, 487093. [Google Scholar] [CrossRef]
- Lourenço, C.B.; Ataide, J.A.; Cefali, L.C.; Novaes, L.C.d.L.; Moriel, P.; Silveira, E.; Tambourgi, E.B.; Mazzola, P.G. Evaluation of the enzymatic activity and stability of commercial bromelain incorporated in topical formulations. Int. J. Cosmet. Sci. 2016, 38, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Pavan, R.; Jain, S.; Shraddha; Kumar, A. Properties and Therapeutic Application of Bromelain: A Review. Biotechnol. Res. Int. 2012, 2012, 976203. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.-C.; Ng, M.Y.; Liao, Y.-W.; Maekawa, S.; Lin, T.; Yu, C.-C. Bromelain Inhibits the Inflammation and Senescence Effect in Diabetic Periodontitis: A Preliminary in Vitro Study. J. Dent. Sci. 2023, 18, 659–665. [Google Scholar] [CrossRef]
- Seyedain-Ardabili, M.; Azizi, M.-H. Effect of Ficin-Hydrolyzed Wheat Gluten on Bread Quality and in Vitro Antioxidant Activity before and after Simulated Gastrointestinal Digestion. Food Sci. Nutr. 2024, 12, 1768–1778. [Google Scholar] [CrossRef]
- Garmidolova, A.; Desseva, I.; Mihaylova, D.; Fidan, H.; Terziyska, M.; Pavlov, A. Papain Hydrolysates of Lupin Proteins with Antioxidant, Antimicrobial, and Acetylcholinesterase Inhibitory Activities. Appl. Sci. 2022, 12, 12370. [Google Scholar] [CrossRef]
- Kong, Y.R.; Jong, Y.X.; Balakrishnan, M.; Bok, Z.K.; Weng, J.K.K.; Tay, K.C.; Goh, B.H.; Ong, Y.S.; Chan, K.G.; Lee, L.H.; et al. Beneficial Role of Carica papaya Extracts and Phytochemicals on Oxidative Stress and Related Diseases: A Mini Review. Biology 2021, 10, 287. [Google Scholar] [CrossRef] [PubMed]
- Baidamshina, D.R.; Trizna, E.Y.; Holyavka, M.G.; Bogachev, M.I.; Artyukhov, V.G.; Akhatova, F.S.; Rozhina, E.V.; Fakhrullin, R.F.; Kayumov, A.R. Targeting Microbial Biofilms Using Ficin, a Nonspecific Plant Protease. Sci. Rep. 2017, 7, 46068. [Google Scholar] [CrossRef]
- Baidamshina, D.R.; Trizna, E.Y.; Goncharova, S.S.; Sorokin, A.V.; Lavlinskaya, M.S.; Melnik, A.P.; Gafarova, L.F.; Kharitonova, M.A.; Ostolopovskaya, O.V.; Artyukhov, V.G.; et al. The Effect of Ficin Immobilized on Carboxymethyl Chitosan on Biofilms of Oral Pathogens. Int. J. Mol. Sci. 2023, 24, 16090. [Google Scholar] [CrossRef]
- Yu, J.; Wang, F.; Shen, Y.; Yu, F.; Qiu, L.; Zhang, L.; Chen, Y.; Yuan, Q.; Zhang, H.; Sun, Y.; et al. Inhibitory Effect of Ficin on Candida Albicans Biofilm Formation and Pre-Formed Biofilms. BMC Oral Health 2022, 22, 350. [Google Scholar] [CrossRef]
- Song, Y.J.; Yu, H.H.; Kim, Y.J.; Lee, N.-K.; Paik, H.-D. The Use of Papain for the Removal of Biofilms Formed by Pathogenic Staphylococcus aureus and Campylobacter jejuni. LWT 2020, 127, 109383. [Google Scholar] [CrossRef]
- Baidamshina, D.R.; Koroleva, V.A.; Olshannikova, S.S.; Trizna, E.Y.; Bogachev, M.I.; Artyukhov, V.G.; Holyavka, M.G.; Kayumov, A.R. Biochemical Properties and Anti-Biofilm Activity of Chitosan-Immobilized Papain. Mar. Drugs 2021, 19, 197. [Google Scholar] [CrossRef] [PubMed]
- dos Anjos, M.M.; da Silva, A.A.; de Pascoli, I.C.; Mikcha, J.M.G.; Machinski, M.; Peralta, R.M.; de Abreu Filho, B.A. Antibacterial Activity of Papain and Bromelain on Alicyclobacillus Spp. Int. J. Food Microbiol. 2016, 216, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chen, W. The Candida Albicans Inhibitory Activity of the Extract from Papaya (Carica papaya L.) Seed Relates to Mitochondria Dysfunction. Int. J. Mol. Sci. 2017, 18, 1858. [Google Scholar] [CrossRef]
- Krieger, Y.; Rubin, G.; Schulz, A.; Rosenberg, N.; Levi, A.; Singer, A.J.; Rosenberg, L.; Shoham, Y. Bromelain-Based Enzymatic Debridement and Minimal Invasive Modality (Mim) Care of Deeply Burned Hands. Ann. Burns Fire Disasters 2017, 30, 198–204. [Google Scholar]
- Baidamshina, D.R.; Koroleva, V.A.; Trizna, E.Y.; Pankova, S.M.; Agafonova, M.N.; Chirkova, M.N.; Vasileva, O.S.; Akhmetov, N.; Shubina, V.V.; Porfiryev, A.G.; et al. Anti-Biofilm and Wound-Healing Activity of Chitosan-Immobilized Ficin. Int. J. Biol. Macromol. 2020, 164, 4205–4217. [Google Scholar] [CrossRef] [PubMed]
- Moreira Filho, R.N.F.; Vasconcelos, N.F.; Andrade, F.K.; Rosa, M.d.F.; Vieira, R.S. Papain Immobilized on Alginate Membrane for Wound Dressing Application. Colloids Surf. B Biointerfaces 2020, 194, 111222. [Google Scholar] [CrossRef]
- Dutra, J.A.P.; Carvalho, S.G.; Zampirolli, A.C.D.; Daltoé, R.D.; Teixeira, R.M.; Careta, F.P.; Cotrim, M.A.P.; Oréfice, R.L.; Villanova, J.C.O. Papain Wound Dressings Obtained from Poly(Vinyl Alcohol)/Calcium Alginate Blends as New Pharmaceutical Dosage Form: Preparation and Preliminary Evaluation. Eur. J. Pharm. Biopharm. 2017, 113, 11–23. [Google Scholar] [CrossRef]
- Rodrigues, A.L.S.; de Oliveira, B.G.R.B.; Futuro, D.O.; Secoli, S.R. Effectiveness of Papain Gel in Venous Ulcer Treatment: Randomized Clinical Trial. Rev. Lat. Am. Enferm. 2015, 23, 458–465. [Google Scholar] [CrossRef]
- Rosenberg, L.; Singer, A.J.; Shoham, Y. Basal Cell Carcinoma Destruction by a Concentrate of Proteolytic Enzymes Enriched in Bromelain: A Preliminary Report. Open Dermatol. J. 2021, 15, 39–44. [Google Scholar] [CrossRef]
- Boyacıoğlu, O.; Kara, B.; Tecimen, S.; Kılıç, M.; Delibaş, M.; Erdoğan, R.; Özdemir, E.; Bahadır, A.; Örenay-Boyacıoğlu, S. Antiproliferative Effect of Ficus carica Latex on Cancer Cell Lines Is Not Solely Linked to Peroxidase-like Activity of Ficin. Eur. J. Integr. Med. 2021, 45, 101348. [Google Scholar] [CrossRef]
- Morovati, M.R.; Ghanbari-Movahed, M.; Barton, E.M.; Farzaei, M.H.; Bishayee, A. A Systematic Review on Potential Anticancer Activities of Ficus carica L. with Focus on Cellular and Molecular Mechanisms. Phytomedicine 2022, 105, 154333. [Google Scholar] [CrossRef] [PubMed]
- Stremnitzer, C.; Manzano-Szalai, K.; Willensdorfer, A.; Starkl, P.; Pieper, M.; König, P.; Mildner, M.; Tschachler, E.; Reichart, U.; Jensen-Jarolim, E. Papain Degrades Tight Junction Proteins of Human Keratinocytes In Vitro and Sensitizes C57BL/6 Mice via the Skin Independent of Its Enzymatic Activity or TLR4 Activation. J. Investig. Dermatol. 2015, 135, 1790–1800. [Google Scholar] [CrossRef]
- Bhogal, R.K.; Mouser, P.E.; Higgins, C.A.; Turner, G.A. Protease Activity, Localization and Inhibition in the Human Hair Follicle. Int. J. Cosmet. Sci. 2014, 36, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.P.; Bishop, M.; Gillis, G.; Maibach, H. Topical Proteolytic Enzymes Affect Epidermal and Dermal Properties. Int. J. Cosmet. Sci. 2007, 29, 15–21. [Google Scholar] [CrossRef]
- Patra, J.K.; Shin, H.-S.; Yang, I.-J.; Nguyen, L.T.H.; Das, G. Sustainable Utilization of Food Biowaste (Papaya Peel) Extract for Gold Nanoparticle Biosynthesis and Investigation of Its Multi-Functional Potentials. Antioxidants 2024, 13, 581. [Google Scholar] [CrossRef]
- Barbulova, A.; Colucci, G.; Apone, F. New Trends in Cosmetics: By-Products of Plant Origin and Their Potential Use as Cosmetic Active Ingredients. Cosmetics 2015, 2, 82–92. [Google Scholar] [CrossRef]
- Wu, S.-Y.; Hu, W.; Zhang, B.; Liu, S.; Wang, J.-M.; Wang, A.-M. Bromelain Ameliorates the Wound Microenvironment and Improves the Healing of Firearm Wounds. J. Surg. Res. 2012, 176, 503–509. [Google Scholar] [CrossRef]
- Ajlia, S.A.S.H.; Majid, F.A.A.; Suvik, A.; Effendy, M.A.W.; Nouri, H.S. Efficacy of Papain-Based Wound Cleanser in Promoting Wound Regeneration. Pak. J. Biol. Sci. 2010, 13, 596–603. [Google Scholar] [CrossRef] [PubMed]
- da Silva Melo, A.E.C.; de Sousa, F.S.R.; dos Santos-Silva, A.M.; do Nascimento, E.G.; Fernandes-Pedrosa, M.F.; de Medeiros, C.A.C.X.; da Silva-Junior, A.A. Immobilization of Papain in Chitosan Membranes as a Potential Alternative for Skin Wounds. Pharmaceutics 2023, 15, 2649. [Google Scholar] [CrossRef]
- Mendes, M.-L.-T.; do Nascimento-Júnior, E.-M.; Reinheimer, D.-M.; Martins-Filho, P.-R.-S. Efficacy of Proteolytic Enzyme Bromelain on Health Outcomes after Third Molar Surgery. Systematic Review and Meta-Analysis of Randomized Clinical Trials. Med. Oral Patol. Oral Cir. Bucal 2019, 24, e61–e69. [Google Scholar] [CrossRef]
- de la Fuente, M.; Lombardero, L.; Gómez-González, A.; Solari, C.; Angulo-Barturen, I.; Acera, A.; Vecino, E.; Astigarraga, E.; Barreda-Gómez, G. Enzyme Therapy: Current Challenges and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 9181. [Google Scholar] [CrossRef]
- Morellon-Sterling, R.; Bolivar, J.M.; Fernandez-Lafuente, R. Switch off/Switch on of a Cysteinyl Protease as a Way to Preserve the Active Catalytic Group by Modification with a Reversible Covalent Thiol Modifier: Immobilization of Ficin on Vinyl-Sulfone Activated Supports. Int. J. Biol. Macromol. 2022, 220, 1155–1162. [Google Scholar] [CrossRef]
- Sorokin, A.V.; Goncharova, S.S.; Lavlinskaya, M.S.; Holyavka, M.G.; Faizullin, D.A.; Zuev, Y.F.; Kondratyev, M.S.; Artyukhov, V.G. Complexation of Bromelain, Ficin, and Papain with the Graft Copolymer of Carboxymethyl Cellulose Sodium Salt and N-Vinylimidazole Enhances Enzyme Proteolytic Activity. Int. J. Mol. Sci. 2023, 24, 11246. [Google Scholar] [CrossRef]
- Holyavka, M.G.; Goncharova, S.S.; Artyukhov, V.G. Various Options for Covalent Immobilization of Cysteine Proteases—Ficin, Papain, Bromelain. Int. J. Mol. Sci. 2025, 26, 547. [Google Scholar] [CrossRef] [PubMed]
- Bialas, F.; Reichinger, D.; Becker, C.F.W. Biomimetic and Biopolymer-Based Enzyme Encapsulation. Enzym. Microb. Technol. 2021, 150, 109864. [Google Scholar] [CrossRef] [PubMed]
- Casanova, F.; Santos, L. Encapsulation of Cosmetic Active Ingredients for Topical Application—A Review. J. Microencapsul. 2016, 33, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Y.; Lu, Y.-H.; Ma, C.-H.; Tao, W.-W.; Zhu, J.-J.; Zhang, X. A Novel Elastic Liposome for Skin Delivery of Papain and Its Application on Hypertrophic Scar. Biomed. Pharmacother. 2017, 87, 82–91. [Google Scholar] [CrossRef]
- Park, H.; Choi, S.; Kang, B.S.; Yu, H.; Kim, J.; Jung, H.-S.; Jeong, H.E.; Chang, P.-S. Bromelain-Decorated Nanoscale Liposomes for Mucus Permeation and Intestinal Absorption in Oral Drug Delivery. ACS Appl. Nano Mater. 2024, 7, 348–357. [Google Scholar] [CrossRef]
- Higashi, T.; Kogo, T.; Sato, N.; Hirotsu, T.; Misumi, S.; Nakamura, H.; Iohara, D.; Onodera, R.; Motoyama, K.; Arima, H. Efficient Anticancer Drug Delivery for Pancreatic Cancer Treatment Utilizing Supramolecular Polyethylene-Glycosylated Bromelain. ACS Appl. Bio Mater. 2020, 3, 3005–3014. [Google Scholar] [CrossRef]
- Wang, D.-Y.; Yang, G.; Zhang, X.-X.; van der Mei, H.C.; Ren, Y.; Busscher, H.J.; Shi, L. Proton-Mediated Burst of Dual-Drug Loaded Liposomes for Biofilm Dispersal and Bacterial Killing. J. Control. Release 2022, 352, 460–471. [Google Scholar] [CrossRef]
- Duskey, J.T.; da Ros, F.; Ottonelli, I.; Zambelli, B.; Vandelli, M.A.; Tosi, G.; Ruozi, B. Enzyme Stability in Nanoparticle Preparations Part 1: Bovine Serum Albumin Improves Enzyme Function. Molecules 2020, 25, 4593. [Google Scholar] [CrossRef] [PubMed]
- Heidari, F.; Raoufi, Z.; Abdollahi, S.; Chamchangi, M.A.; Asl, H.Z. Evaluation of Sodium Alginate Sponge Infused Bromelain Spray and Helichrysum Italicum Nanoemulsion to Accelerate Wound Healing. Int. J. Biol. Macromol. 2024, 283, 137799. [Google Scholar] [CrossRef] [PubMed]
- da, S. Pereira, A.; Souza, C.P.L.; Moraes, L.; Fontes-Sant’Ana, G.C.; Amaral, P.F.F. Polymers as Encapsulating Agents and Delivery Vehicles of Enzymes. Polymers 2021, 13, 4061. [Google Scholar] [CrossRef]
- Lei, H.; Wang, W.; Chen, L.-L.; Li, X.-C.; Yi, B.; Deng, L. The Preparation and Catalytically Active Characterization of Papain Immobilized on Magnetic Composite Microspheres. Enzym. Microb. Technol. 2004, 35, 15–21. [Google Scholar] [CrossRef]
- Tang, Y.; Scher, H.B.; Jeoh, T. Microencapsulation of Bromelain from Pineapple Extract Powder by Industrially Scalable Complex Coacervation. LWT 2022, 167, 113775. [Google Scholar] [CrossRef]
- Han, J.; Wang, L.; Wang, L.; Li, C.; Mao, Y.; Wang, Y. Fabrication of a Core-Shell-Shell Magnetic Polymeric Microsphere with Excellent Performance for Separation and Purification of Bromelain. Food Chem. 2019, 283, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pertiwi, I.; Setiasih, S.; Handayani, S.; Hudiyono, S. Bromelain Nanoemulsion Formulation Resulting from Partial Purification of Pineapple Core (Ananas comosus [L.] Merr) and in Vitro Testing as Antiinflammation. AIP Conf. Proc. 2020, 2243, 030017. [Google Scholar]
- Redko, Y.A.; Goncharova, S.S.; Holyavka, M.G.; Lavlinskya, M.S.; Sorokin, A.V.; Mikhaylova, A.A.; Artyukhov, V.G. Development of a Production Method for Ficin Associates with Microand Nanoparticles of Carboxymethyl Chitosan. Pharm. Chem. J. 2024, 58, 1267–1272. [Google Scholar] [CrossRef]
- Nhani, G.B.B.; Di Filippo, L.D.; de Paula, G.A.; Mantovanelli, V.R.; da Fonseca, P.P.; Tashiro, F.M.; Monteiro, D.C.; Fonseca-Santos, B.; Duarte, J.L.; Chorilli, M. High-Tech Sustainable Beauty: Exploring Nanotechnology for the Development of Cosmetics Using Plant and Animal By-Products. Cosmetics 2024, 11, 112. [Google Scholar] [CrossRef]
- Fazolin, G.N.; Varca, G.H.C.; de Freitas, L.F.; Rokita, B.; Kadlubowski, S.; Lugão, A.B. Simultaneous Intramolecular Crosslinking and Sterilization of Papain Nanoparticles by Gamma Radiation. Radiat. Phys. Chem. 2020, 171, 108697. [Google Scholar] [CrossRef]
- Žiemytė, M.; Escudero, A.; Díez, P.; Ferrer, M.D.; Murguía, J.R.; Martí-Centelles, V.; Mira, A.; Martínez-Máñez, R. Ficin–Cyclodextrin-Based Docking Nanoarchitectonics of Self-Propelled Nanomotors for Bacterial Biofilm Eradication. Chem. Mater. 2023, 35, 4412–4426. [Google Scholar] [CrossRef]
- Croisfelt, F.M.; Ataide, J.A.; Tundisi, L.L.; Cefali, L.C.; Rebelo, M.d.A.; Sánchez, J.L.D.; da Costa, T.G.; Lima, R.; Jozala, A.F.; Chaud, M.V.; et al. Characterization of PNIPAAm-Co-AAm Hydrogels for Modified Release of Bromelain. Eur. Polym. J. 2018, 105, 48–54. [Google Scholar] [CrossRef]
- Peanparkdee, M.; Janchai, P.; Aksornsri, T.; Vaithanomsat, P. Design and Evaluation of Bromelain-Encapsulated Alginate Beads Reinforced with Gum Arabic: Formulation, Characterization, and Stability in Simulated Gastrointestinal Conditions. J. Agric. Food Res. 2025, 19, 101698. [Google Scholar] [CrossRef]
- Bayat, S.; Rabbani Zabihi, A.; Amel Farzad, S.; Movaffagh, J.; Hashemi, E.; Arabzadeh, S.; Hahsemi, M. Evaluation of Debridement Effects of Bromelain-Loaded Sodium Alginate Nanoparticles Incorporated into Chitosan Hydrogel in Animal Models. Iran. J. Basic Med. Sci. 2021, 24, 1404–1412. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Pan, Y.; Zheng, W.; Yi, D.; Zheng, H. Supramolecular Hydrogel-Immobilized Enzyme Ficin as Peroxidase Mimics for Colorimetric Detection of Glucose. Microchem. J. 2020, 158, 105276. [Google Scholar] [CrossRef]
- Liu, Z.; Li, D.; Dai, H.; Huang, H. Preparation and Characterization of Papain Embedded in Magnetic Cellulose Hydrogels Prepared from Tea Residue. J. Mol. Liq. 2017, 232, 449–456. [Google Scholar] [CrossRef]
- Wang, L.; Li, W.; Liu, Y.; Zhi, W.; Han, J.; Wang, Y.; Ni, L. Green Separation of Bromelain in Food Sample with High Retention of Enzyme Activity Using Recyclable Aqueous Two-Phase System Containing a New Synthesized Thermo-Responsive Copolymer and Salt. Food Chem. 2019, 282, 48–57. [Google Scholar] [CrossRef]
- Hu, R.; Chen, G.; Li, Y. Production and Characterization of Antioxidative Hydrolysates and Peptides from Corn Gluten Meal Using Papain, Ficin, and Bromelain. Molecules 2020, 25, 4091. [Google Scholar] [CrossRef]
- Kortt, A.A.; Hinds, J.A.; Zerner, B. Specificity and pH Dependence of Ficin-Catalyzed Hydrolyses. Comparisons with Bromelain Specificity. Biochemistry 1974, 13, 2029–2037. [Google Scholar] [CrossRef]
- Dini, I.; Laneri, S. The New Challenge of Green Cosmetics: Natural Food Ingredients for Cosmetic Formulations. Molecules 2021, 26, 3921. [Google Scholar] [CrossRef]
- He, X.; Gao, X.; Guo, Y.; Xie, W. Research Progress on Bioactive Factors against Skin Aging. Int. J. Mol. Sci. 2024, 25, 3797. [Google Scholar] [CrossRef]
- Bahari, A.N.; Saari, N.; Salim, N.; Ashari, S.E. Response Factorial Design Analysis on Papain-Generated Hydrolysates from Actinopyga Lecanora for Determination of Antioxidant and Antityrosinase Activities. Molecules 2020, 25, 2663. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, S.; Tesaki, S.; Watanabe, M.; Yanagihara, Y. [Cross-reactivity between bromelain and soluble fraction from wheat flour]. Arerugi 1997, 46, 1170–1173. [Google Scholar]
- Leelakanok, N.; Petchsomrit, A.; Janurai, T.; Saechan, C.; Sunsandee, N. Efficacy and Safety of Bromelain: A Systematic Review and Meta-Analysis. Nutr. Health 2023, 29, 479–503. [Google Scholar] [CrossRef] [PubMed]
- Giangrieco, I.; Ciardiello, M.A.; Tamburrini, M.; Tuppo, L.; Rafaiani, C.; Mari, A.; Alessandri, C. Comparative Analysis of the Immune Response and the Clinical Allergic Reaction to Papain-like Cysteine Proteases from Fig, Kiwifruit, Papaya, Pineapple and Mites in an Italian Population. Foods 2023, 12, 2852. [Google Scholar] [CrossRef] [PubMed]
- Banchhor, M.; Saraf, S.; Saraf, S.; Saraf, S. Potentiality of Papain as an Antiaging Agent in Cosmetic Formulation. Pharmacogn. Rev. 2008, 2, 266–270. [Google Scholar]
- Lei, H.; Pei, Z.; Jiang, C.; Cheng, L. Recent Progress of Metal-Based Nanomaterials with Anti-Tumor Biological Effects for Enhanced Cancer Therapy. Exploration 2023, 3, 20220001. [Google Scholar] [CrossRef]
- Isa, M.M.; Jaafar, M.N.; Kasim, K.F.; Mutalib, M.F.A. Cultivation of Fig (Ficus carica L.) As An Alternative High Value Crop In Malaysia: A Brief Review. IOP Conf. Ser. Mater. Sci. Eng. 2020, 864, 012134. [Google Scholar] [CrossRef]
- Abraham, R.A.; Joshi, J.T.; Abdullah, S. A Comprehensive Review of Pineapple Processing and Its By-Product Valorization in India. Food Chem. Adv. 2023, 3, 100416. [Google Scholar] [CrossRef]
- Meena, L.; Sengar, A.S.; Neog, R.; Sunil, C.K. Pineapple Processing Waste (PPW): Bioactive Compounds, Their Extraction, and Utilisation: A Review. J. Food Sci. Technol. 2022, 59, 4152–4164. [Google Scholar] [CrossRef]
- Castillo-González, E.; Giraldi-Díaz, M.R.; De Medina-Salas, L.; Sánchez-Castillo, M.P. Pre-Composting and Vermicomposting of Pineapple (Ananas comosus) and Vegetable Waste. Appl. Sci. 2019, 9, 3564. [Google Scholar] [CrossRef]
- Rabinovich, V.A.; Linnenberg, C.; Theilen, U.; Weigand, H. Biogas Production Potential of Mixed Banana and Pineapple Waste as Assessed by Long-Term Laboratory-Scale Anaerobic Digestion. Fermentation 2024, 10, 261. [Google Scholar] [CrossRef]
- Osorio, L.L.D.R.; Flórez-López, E.; Grande-Tovar, C.D. The Potential of Selected Agri-Food Loss and Waste to Contribute to a Circular Economy: Applications in the Food, Cosmetic and Pharmaceutical Industries. Molecules 2021, 26, 515. [Google Scholar] [CrossRef]
- Martins, A.M.; Marto, J.M. A Sustainable Life Cycle for Cosmetics: From Design and Development to Post-Use Phase. Sustain. Chem. Pharm. 2023, 35, 101178. [Google Scholar] [CrossRef]
- Milošević, J.; Janković, B.; Prodanović, R.; Polović, N. Comparative Stability of Ficin and Papain in Acidic Conditions and the Presence of Ethanol. Amino Acids 2019, 51, 829–838. [Google Scholar] [CrossRef]
- Wijayanti, S.D.; Tsvik, L.; Haltrich, D. Recent Advances in Electrochemical Enzyme-Based Biosensors for Food and Beverage Analysis. Foods 2023, 12, 3355. [Google Scholar] [CrossRef]
- Escalé-Besa, A.; Vidal-Alaball, J.; Miró Catalina, Q.; Gracia, V.H.G.; Marin-Gomez, F.X.; Fuster-Casanovas, A. The Use of Artificial Intelligence for Skin Disease Diagnosis in Primary Care Settings: A Systematic Review. Healthcare 2024, 12, 1192. [Google Scholar] [CrossRef]
- Boukid, F.; Ganeshan, S.; Wang, Y.; Tülbek, M.Ç.; Nickerson, M.T. Bioengineered Enzymes and Precision Fermentation in the Food Industry. Int. J. Mol. Sci. 2023, 24, 10156. [Google Scholar] [CrossRef]
- Dahal, R.H.; Nguyen, T.M.; Shim, D.S.; Kim, J.Y.; Lee, J.; Kim, J. Development of Multifunctional Cosmetic Cream Using Bioactive Materials from Streptomyces Sp. T65 with Synthesized Mesoporous Silica Particles SBA-15. Antioxidants 2020, 9, 278. [Google Scholar] [CrossRef]
- Ricardo, P.C.; Serudo, R.L.; Ţălu, Ş.; Lamarão, C.V.; da Fonseca Filho, H.D.; de Araújo Bezerra, J.; Sanches, E.A.; Campelo, P.H. Encapsulation of Bromelain in Combined Sodium Alginate and Amino Acid Carriers: Experimental Design of Simplex-Centroid Mixtures for Digestibility Evaluation. Molecules 2022, 27, 6364. [Google Scholar] [CrossRef]
- Na, G.H.; Kim, S.; Jung, H.M.; Han, S.H.; Han, J.; Koo, Y.K. Skin Anti-Aging Efficacy of Enzyme-Treated Supercritical Caviar Extract: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2023, 16, 137. [Google Scholar] [CrossRef] [PubMed]
- Pickar-Oliver, A.; Gersbach, C.A. The next Generation of CRISPR–Cas Technologies and Applications. Nat. Rev. Mol. Cell Biol. 2019, 20, 490–507. [Google Scholar] [CrossRef] [PubMed]

| Enzymes | Water Solubility (mg/L, 25 °C) * | logKow * | pKa * |
|---|---|---|---|
| Bromelain | 1000 | −11.6 | 6.4; 7.1 |
| Ficin | 10,000 | 2 | 8–9 |
| Papain | 10,000 | −4.5 | 8.3 |
| Aspect/Feature * | Proteolytic Enzyme | References | ||
|---|---|---|---|---|
| Bromelain | Ficin | Papain | ||
| Source, Origin, and General Profile |
|
|
| [23,35,69,70,74,91,98,100,121] |
| Extraction and Purification |
|
|
| [43,45,47,49,50,51,68,70,74,78,79,81,98,106,107,108,111,161,189,190] |
| Molecular Structure |
|
|
| [69,89,90,94,96,127] |
| Optimal pH |
|
|
| [69,74,89,90,91,94] |
| Optimal Temperature |
|
|
| [35,44,69,75,91,97,101,107] |
| Aspect/Feature * | Proteolytic Enzyme | References | ||
|---|---|---|---|---|
| Bromelain | Ficin | Papain | ||
| Substrate Specificity |
|
|
| [22,42,77,125,126,127,128,129,131,137,176,191] |
| Exfoliation Efficacy |
|
|
| [13,22,27,116,156] |
| Mechanism of Action |
|
|
| [22,29,32,74,91,122,124,125,126,127,128,129,130] |
| Biochemical Pathways and Properties |
|
|
| [25,26,35,42,68,70,74,85,96,101,106,107,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154] |
| Aspect/Feature * | Proteolytic Enzyme | References | ||
|---|---|---|---|---|
| Bromelain | Ficin | Papain | ||
| Skin Type Suitability and Corresponding Cosmetic Applications |
|
|
| [23,26,35,42,46,88,119,165,192,193,194] |
| Incorporation Methods in Cosmetic Applications |
|
|
| [33,44,57,93,97,116,119,129,148,157,161,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188] |
| Potential Side Effects |
|
|
| [42,46,195,196,197] |
| Stability |
|
|
| [35,44,57,69,90,91,97,98,101,107,133] |
| Major Stabilizers and Inhibitors |
|
|
| [46,69,89,94,100,114,115,116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venetikidou, M.; Lykartsi, E.; Adamantidi, T.; Prokopiou, V.; Ofrydopoulou, A.; Letsiou, S.; Tsoupras, A. Proteolytic Enzyme Activities of Bromelain, Ficin, and Papain from Fruit By-Products and Potential Applications in Sustainable and Functional Cosmetics for Skincare. Appl. Sci. 2025, 15, 2637. https://doi.org/10.3390/app15052637
Venetikidou M, Lykartsi E, Adamantidi T, Prokopiou V, Ofrydopoulou A, Letsiou S, Tsoupras A. Proteolytic Enzyme Activities of Bromelain, Ficin, and Papain from Fruit By-Products and Potential Applications in Sustainable and Functional Cosmetics for Skincare. Applied Sciences. 2025; 15(5):2637. https://doi.org/10.3390/app15052637
Chicago/Turabian StyleVenetikidou, Maria, Eleni Lykartsi, Theodora Adamantidi, Vasileios Prokopiou, Anna Ofrydopoulou, Sophia Letsiou, and Alexandros Tsoupras. 2025. "Proteolytic Enzyme Activities of Bromelain, Ficin, and Papain from Fruit By-Products and Potential Applications in Sustainable and Functional Cosmetics for Skincare" Applied Sciences 15, no. 5: 2637. https://doi.org/10.3390/app15052637
APA StyleVenetikidou, M., Lykartsi, E., Adamantidi, T., Prokopiou, V., Ofrydopoulou, A., Letsiou, S., & Tsoupras, A. (2025). Proteolytic Enzyme Activities of Bromelain, Ficin, and Papain from Fruit By-Products and Potential Applications in Sustainable and Functional Cosmetics for Skincare. Applied Sciences, 15(5), 2637. https://doi.org/10.3390/app15052637








