Change in Right Ventricular Strain After Cone Reconstruction of Ebstein’s Anomaly: A Cardiovascular Magnetic Resonance-Feature Tracking Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. CMR Acquisition Protocol
2.3. CMR Feature Tracking
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Impact of Cone Repair on Right Ventricular Volumes
4.2. Role of CMR-FT in the Perioperative Assessment of Right Ventricular Function
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CMR | Cardiovascular magnetic resonance |
| EA | Ebstein’s anomaly |
| EDVi | Indexed end-diastolic volume |
| EGC | Electrocardiography |
| ESVi | Indexed end-systolic volume |
| FAC | Fractional area change |
| FT | Feature tracking |
| GCS | Global circumferential strain |
| GLS | Global longitudinal strain |
| GRS | Global radial strain |
| LV | Left ventricle |
| PA | Pulmonary artery |
| RV | Right ventricle |
| SD | Standard deviation |
| SSFP | Steady-state-free precession |
| SVi | Indexed stroke volume |
| TR | Tricuspid regurgitation |
| TV | Tricuspid valve |
References
- Jost, C.H.A.; Connolly, H.M.; Dearani, J.A.; Edwards, W.D.; Danielson, G.K. Ebstein’s anomaly. Circulation 2007, 115, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Paranon, S.; Acar, P. Ebstein’s anomaly of the tricuspid valve: From fetus to adult: Congenital heart disease. Heart 2008, 94, 237–243. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.P.; Baumgratz, J.F.; da Fonseca, L.; Franchi, S.M.; Lopes, L.M.; Tavares, G.M.P.; Soares, A.M.; Moreira, L.F.; Barbero-Marcial, M. The cone reconstruction of the tricuspid valve in Ebstein’s anomaly. The operation: Early and midterm results. J. Thorac. Cardiovasc. Surg. 2007, 133, 215–223. [Google Scholar] [CrossRef]
- Ibrahim, M.; Tsang, V.T.; Caruana, M.; Hughes, M.L.; Jenkyns, S.; Perdreau, E.; Giardini, A.; Marek, J. Cone reconstruction for Ebstein’s anomaly: Patient outcomes, biventricular function, and cardiopulmonary exercise capacity. J. Thorac. Cardiovasc. Surg. 2015, 149, 1144–1150. [Google Scholar] [CrossRef]
- Lange, R.; Burri, M.; Eschenbach, L.K.; Badiu, C.C.; da Silva, J.P.; Nagdyman, N.; Fratz, S.; Hörer, J.; Kühn, A.; Schreiber, C.; et al. Da Silva’s cone repair for Ebstein’s anomaly: Effect on right ventricular size and function. Eur. J. Cardio-Thorac. Surg. 2015, 48, 316–321, discussion 20-1. [Google Scholar] [CrossRef]
- Holst, K.A.; Dearani, J.A.; Said, S.; Pike, R.B.; Connolly, H.M.; Cannon, B.C.; Sessions, K.L.; O’Byrne, M.M.; O’leary, P.W. Improving Results of Surgery for Ebstein Anomaly: Where Are We After 235 Cone Repairs? Ann. Thorac. Surg. 2018, 105, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Perdreau, E.; Tsang, V.; Hughes, M.L.; Ibrahim, M.; Kataria, S.; Janagarajan, K.; Iriart, X.; Khambadkone, S.; Marek, J. Change in biventricular function after cone reconstruction of Ebstein’s anomaly: An echocardiographic study. Eur. Hear. J. Cardiovasc. Imaging 2018, 19, 808–815. [Google Scholar] [CrossRef]
- Li, D.; Hirata, Y.; Zhou, X.; Masuzawa, A.; Ono, M.; An, Q. Effect of cone reconstruction on right ventricular function in patients with Ebstein’s anomaly: A meta-analysis. Interact. Cardiovasc. Thorac. Surg. 2018, 26, 301–306. [Google Scholar] [CrossRef]
- Schuster, A.; Hor, K.N.; Kowallick, J.T.; Beerbaum, P.; Kutty, S. Cardiovascular Magnetic Resonance Myocardial Feature Tracking: Concepts and Clinical Applications. Circ. Cardiovasc. Imaging 2016, 9, e004077. [Google Scholar] [CrossRef]
- Baessato, F.; Furtmüller, C.; Shehu, N.; Ferrari, I.; Reich, B.; Nagdyman, N.; Martinoff, S.; Stern, H.; Ewert, P.; Meierhofer, C. Detection of early signs of right ventricular systolic impairment in unoperated Ebstein’s anomaly by cardiac magnetic resonance feature tracking. Cardiovasc. Diagn. Ther. 2022, 12, 278–288. [Google Scholar] [CrossRef]
- Erley, J.; Tanacli, R.; Genovese, D.; Tapaskar, N.; Rashedi, N.; Bucius, P.; Kawaji, K.; Karagodin, I.; Lang, R.M.; Kelle, S.; et al. Myocardial strain analysis of the right ventricle: Comparison of different cardiovascular magnetic resonance and echocardiographic techniques. J. Cardiovasc. Magn. Reson. 2020, 22, 51. [Google Scholar] [CrossRef] [PubMed]
- Prati, G.; Vitrella, G.; Allocca, G.; Muser, D.; Buttignoni, S.C.; Piccoli, G.; Morocutti, G.; Delise, P.; Pinamonti, B.; Proclemer, A.; et al. Right Ventricular Strain and Dyssynchrony Assessment in Arrhythmogenic Right Ventricular Cardiomyopathy: Cardiac Magnetic Resonance Feature-Tracking Study. Circ. Cardiovasc. Imaging 2015, 8, e003647, discussion e. [Google Scholar] [CrossRef]
- Ouyang, R.; Leng, S.; Sun, A.; Wang, Q.; Hu, L.; Zhao, X.; Yan, Q.; Tan, R.-S.; Zhong, L.; Zhong, Y. Detection of persistent systolic and diastolic abnormalities in asymptomatic pediatric repaired tetralogy of Fallot patients with preserved ejection fraction: A CMR feature tracking study. Eur. Radiol. 2021, 31, 6156–6168. [Google Scholar] [CrossRef]
- Alfakih, K.; Plein, S.; Bloomer, T.; Jones, T.; Ridgway, J.; Sivananthan, M. Comparison of right ventricular volume measurements between axial and short axis orientation using steady-state free precession magnetic resonance imaging. J. Magn. Reson. Imaging 2003, 18, 25–32. [Google Scholar] [CrossRef]
- Fratz, S.; Schuhbaeck, A.; Buchner, C.; Busch, R.; Meierhofer, C.; Martinoff, S.; Hess, J.; Stern, H. Comparison of accuracy of axial slices versus short-axis slices for measuring ventricular volumes by cardiac magnetic resonance in patients with corrected tetralogy of fallot. Am. J. Cardiol. 2009, 103, 1764–1769. [Google Scholar] [CrossRef] [PubMed]
- Fratz, S.; Janello, C.; Müller, D.; Seligmann, M.; Meierhofer, C.; Schuster, T.; Schreiber, C.; Martinoff, S.; Hess, J.; Kühn, A.; et al. The functional right ventricle and tricuspid regurgitation in Ebstein’s anomaly. Int. J. Cardiol. 2013, 167, 258–261. [Google Scholar] [CrossRef]
- O’leary, P.W.; Qureshi, M.Y.; Cetta, F.; Nelson, T.J.; Holst, K.A.; Dearani, J.A.; Breuer, A.; Martineau, S.E.; Miller, A.R.; Miller, K.S.; et al. Cone Reconstruction for Ebstein Anomaly: Ventricular Remodeling and Preliminary Impact of Stem Cell Therapy. Mayo Clin. Proc. 2021, 96, 3053–3061. [Google Scholar] [CrossRef] [PubMed]
- Kresoja, K.-P.; Rommel, K.-P.; Lücke, C.; Unterhuber, M.; Besler, C.; von Roeder, M.; Schöber, A.R.; Noack, T.; Gutberlet, M.; Thiele, H.; et al. Right Ventricular Contraction Patterns in Patients Undergoing Transcatheter Tricuspid Valve Repair for Severe Tricuspid Regurgitation. JACC Cardiovasc. Interv. 2021, 14, 1551–1561. [Google Scholar] [CrossRef]
- Buechel, E.R.V.; Mertens, L.L. Imaging the right heart: The use of integrated multimodality imaging. Eur. Heart J. 2012, 33, 949–960. [Google Scholar] [CrossRef]
- Lee, C.M.; Sheehan, F.H.; Bouzas, B.; Chen, S.S.; Gatzoulis, M.A.; Kilner, P.J. The shape and function of the right ventricle in Ebstein’s anomaly. Int. J. Cardiol. 2013, 167, 704–710. [Google Scholar] [CrossRef]
- Okamoto, F.; Karino, K.; Ohori, K.; Abe, T.; Komatsu, S. Effect of coenzyme Q10 on hypertrophied ischemic myocardium during aortic cross clamping for 2 h, from the aspect of energy metabolism. Adv. Myocardiol. 1983, 4, 559–566. [Google Scholar] [CrossRef] [PubMed]
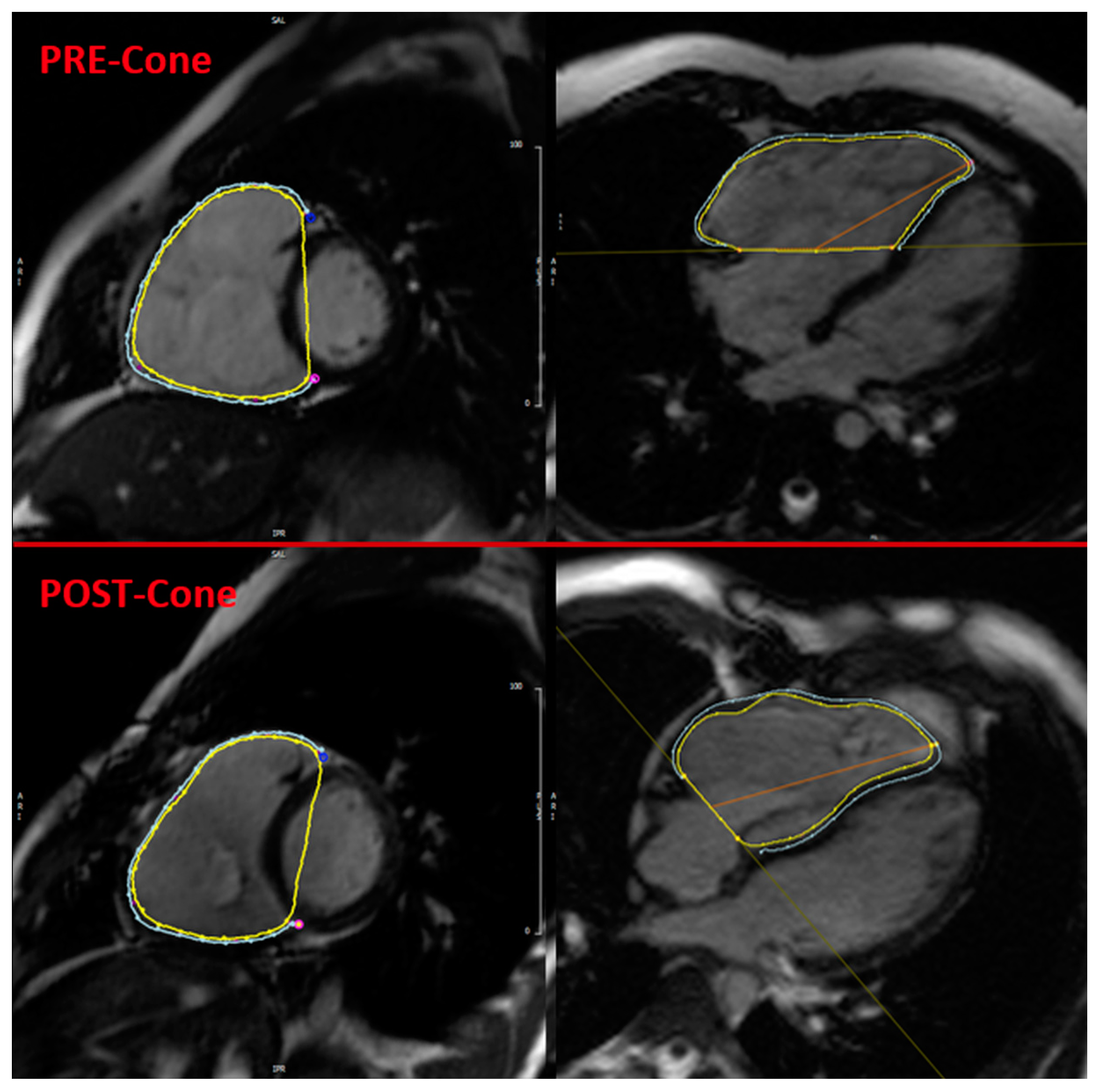
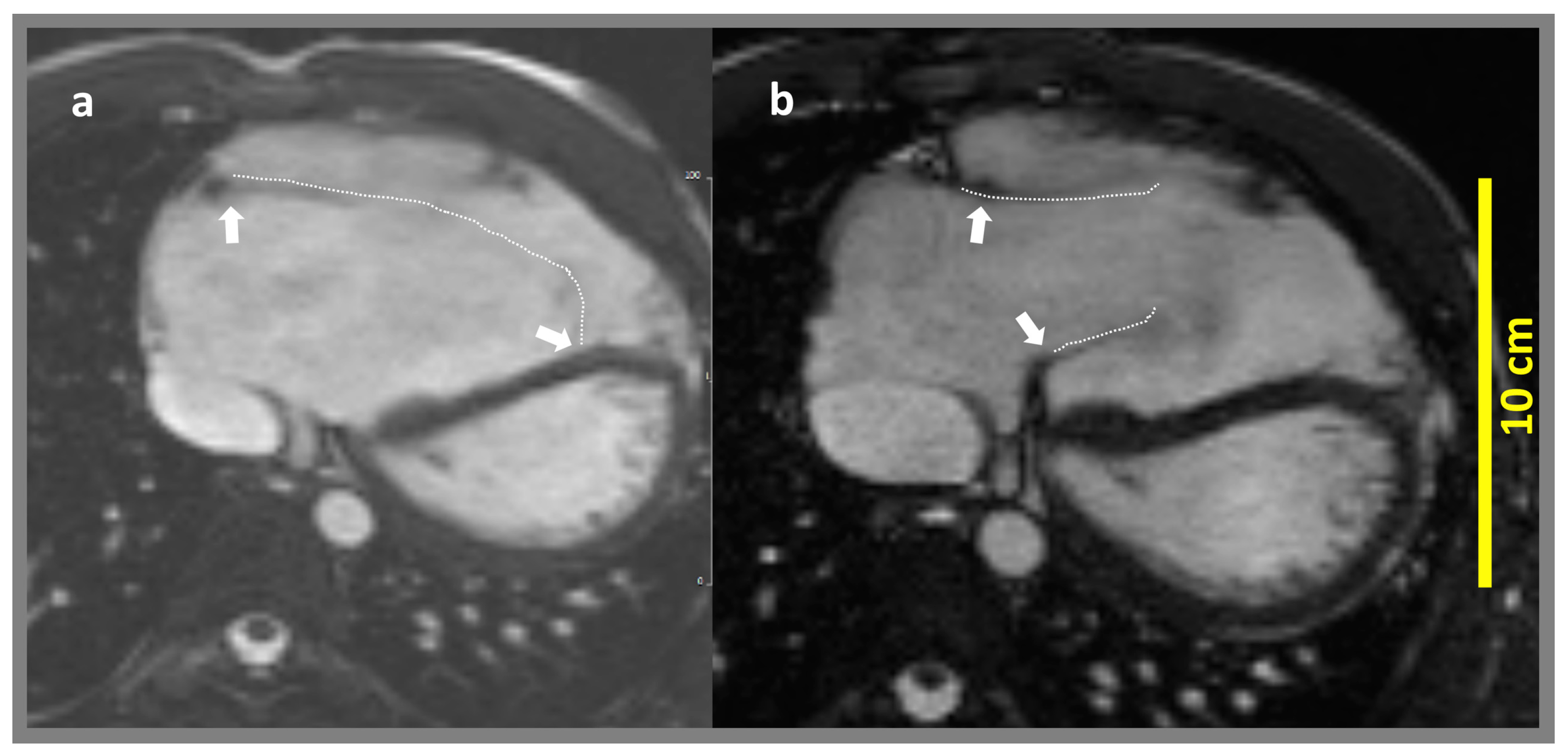
| Parameter | Min | Median | Max | Mean | SD |
|---|---|---|---|---|---|
| Age at Cone procedure (years) | 2.37 | 14.90 | 40.27 | 18.97 | 10.33 |
| Height pre Cone CMR (m) | 0.77 | 1.69 | 1.83 | 1.58 | 0.27 |
| Weight pre Cone CMR (kg) | 9 | 59.5 | 77 | 54.78 | 20.2 |
| Body surface area (cm/m2) | 0.44 | 1.69 | 1.93 | 1.54 | 0.43 |
| Time interval pre Cone CMR to Cone repair (days) | 1 | 229 | 1156 | 280 | 294 |
| Time interval Cone repair to post Cone CMR (days) | 94 | 387 | 575 | 377 | 120 |
| CMR Parameters | Preoperative (n = 18 *) | Postoperative (n = 18 *) | p-Value |
|---|---|---|---|
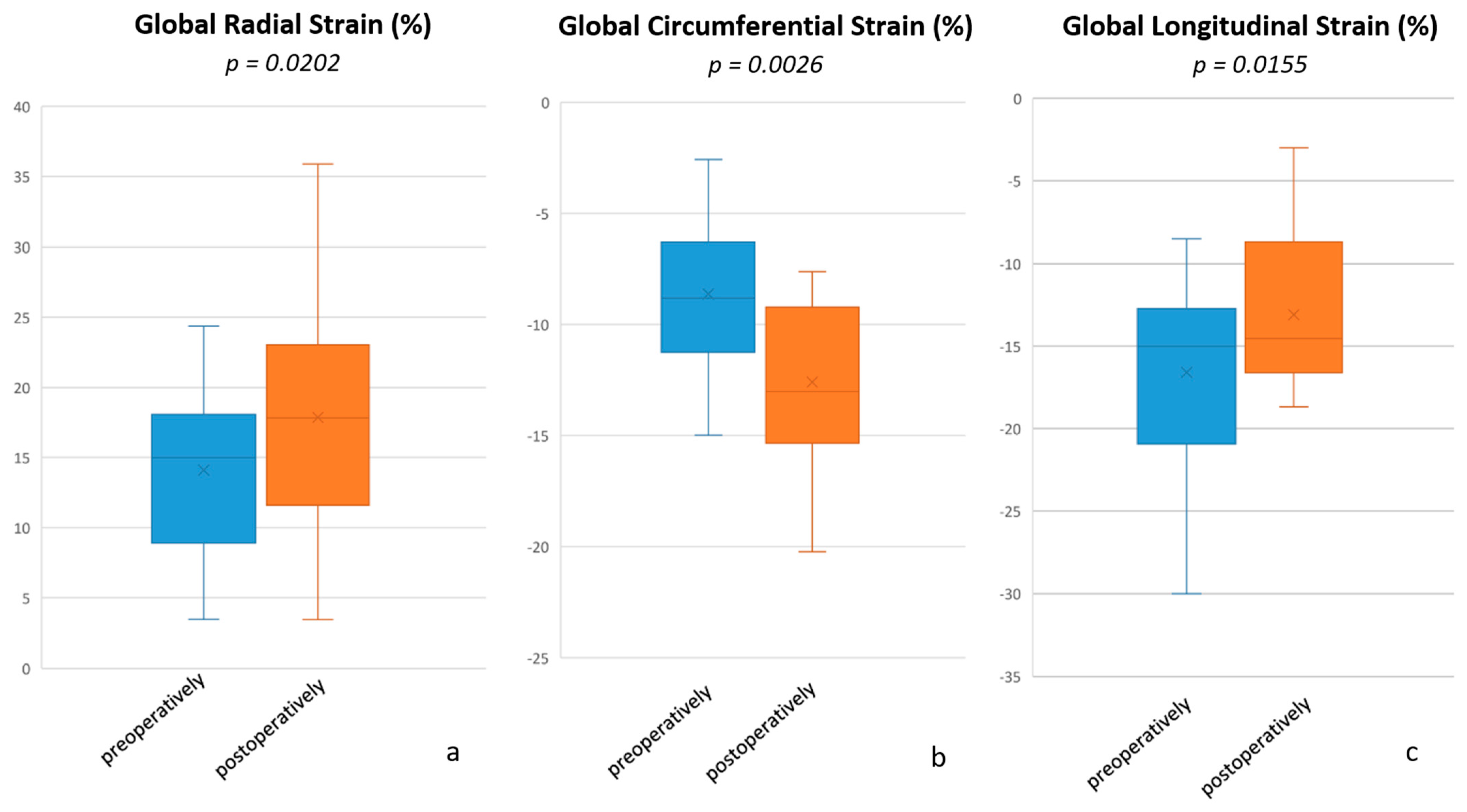
| RV global radial strain (%) | 15.00 (3.47–24.37) | 17.83 (3.46–35.88) | 0.0202 |
| RV global circumferential strain (%) | −8.82 ((−14.99)–(−2.58)) | −13.02 ((−20.22)–(−7.62)) | 0.0026 |
| RV global longitudinal strain (%) | −15.01 ((−30.01)–(−8.53)) | −14.53 ((−18.67)–(−3.00)) | 0.0155 |
| RVEF (%) | 51 (21–61) | 33 (13–50) | 0.0002 |
| RVEDVi (mL/m2) | 161 (62–292) | 122 (87–253) | 0.3465 |
| RVESVi (mL/m2) | 74 (42–152) | 75 (47–196) | 0.3520 |
| RVSVi (mL/m2) | 74 (17–161) | 43 (19–57) | 0.0003 |
| LVEF (%) | 56 (28–67) | 61 (43–70) | 0.0859 |
| LVEDVi (mL/m2) | 63 (40–198) | 65 (38–91) | 0.4878 |
| LVESVi (mL/m2) | 28 (19–142) | 26 (14–39) | 0.4747 |
| LVSVi (mL/m2) | 35 (19–56) | 37 (23–52) | 0.2383 |
| TI direct (%) | 48 (16–90) | 6 (0–19) | 0.0001 |
| TI indirect (%) | 55 (0–80) | 8 (0–20) | 0.0039 |
| PA flow netto indexed (mL/m2) | 38 (18–52) | 41 (20–52) | 0.2897 |
| PA flow antegrade indexed (mL/m2) | 39 (20–54) | 42 (21–55) | 0.2480 |
| Cardiac index PA (L/min/m2) | 2.8 (1.7–4.3) | 2.8 (1.8–3.9) | 0.9678 |
| Aorta flow netto indexed (mL/m2) | 38 (23–48) | 40 (20–54) | 0.1066 |
| Aorta flow antegrade indexed (mL/m2) | 38 (23–48) | 41 (20–54) | 0.1058 |
| Cardiac index aorta (L/min/m2) | 2.8 (1.8–4.7) | 2.8 (1.6–5.0) | 0.5619 |
| Heart rate | 82 (56–98) | 73 (59–98) | 0.1099 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furtmüller, C.; Baessato, F.; Ferrari, I.; Shehu, N.; Martinoff, S.; Reich, B.; Ewert, P.; Nagdyman, N.; Cleuziou, J.; Stern, H.; et al. Change in Right Ventricular Strain After Cone Reconstruction of Ebstein’s Anomaly: A Cardiovascular Magnetic Resonance-Feature Tracking Study. Appl. Sci. 2025, 15, 2659. https://doi.org/10.3390/app15052659
Furtmüller C, Baessato F, Ferrari I, Shehu N, Martinoff S, Reich B, Ewert P, Nagdyman N, Cleuziou J, Stern H, et al. Change in Right Ventricular Strain After Cone Reconstruction of Ebstein’s Anomaly: A Cardiovascular Magnetic Resonance-Feature Tracking Study. Applied Sciences. 2025; 15(5):2659. https://doi.org/10.3390/app15052659
Chicago/Turabian StyleFurtmüller, Claudia, Francesca Baessato, Irene Ferrari, Nerejda Shehu, Stefan Martinoff, Bettina Reich, Peter Ewert, Nicole Nagdyman, Julie Cleuziou, Heiko Stern, and et al. 2025. "Change in Right Ventricular Strain After Cone Reconstruction of Ebstein’s Anomaly: A Cardiovascular Magnetic Resonance-Feature Tracking Study" Applied Sciences 15, no. 5: 2659. https://doi.org/10.3390/app15052659
APA StyleFurtmüller, C., Baessato, F., Ferrari, I., Shehu, N., Martinoff, S., Reich, B., Ewert, P., Nagdyman, N., Cleuziou, J., Stern, H., & Meierhofer, C. (2025). Change in Right Ventricular Strain After Cone Reconstruction of Ebstein’s Anomaly: A Cardiovascular Magnetic Resonance-Feature Tracking Study. Applied Sciences, 15(5), 2659. https://doi.org/10.3390/app15052659






