Abstract
This study aimed to evaluate the acute effects of consuming in a full-course meal manner over one hour, with intervals between courses, on glycemic and insulin parameters in young healthy women, with a randomized controlled crossover study design. Experiment 1: Fifteen participants consumed a test meal under two eating conditions: fast eating manner for 10 min, and eating in a full-course meal manner for 60 min. In both conditions, the food order was standardized: vegetables first, followed by the main dish, and carbohydrates last. Blood glucose and insulin concentrations were measured at 0, 40, 80, 120, and 180 min on two separate days. Postprandial blood glucose and insulin levels at 40 min, as well as the incremental area under the curve (IAUC) at 40 min for glucose and the IAUC at both 40 and 80 min for insulin, were significantly lower for the full-course meal manner compared to the fast eating manner, due to delayed consumption of the carbohydrate dish in the former condition at these time points. To continuously monitor postprandial blood glucose responses over a 24 h period, Experiment 2 was conducted using an intermittent continuous glucose monitoring system (isCGM). Eighteen participants wore isCGM devices and consumed the same test meals under the two different eating conditions as in Experiment 1. The mean amplitude of glycemic excursions (MAGE; p < 0.05) and IAUC for glucose were significantly lower for the full-course meal manner compared to the fast eating manner. These findings suggest that consuming meals in a full-course meal manner, with intervals between courses, is associated with a reduced MAGE in young healthy women.
1. Introduction
Medical nutrition therapy is the keystone of managing type 2 diabetes (T2DM) and preventing its onset [1,2,3]. Nutritional recommendations have been delivered by several scientific societies to support clinicians and dietitians in their choice of the most suitable medical nutrition therapy for individuals with T2DM [4,5,6]; however, adherence to these recommendations in clinical practice is generally poor [7,8]. In many cases, these nutritional recommendations are based on macro- and micronutrients, which often prevent patients’ understanding and adherence in real life. In the last decade, human nutrition science has shifted to focus not only on “which nutrients to take” or “how much to eat”, but also on to “how to eat”, considering aspects such as food order [9,10,11], eating speed [12], mealtime [13], and intermittent fasting [14].
The Mediterranean diet is considered to be one of the healthiest dietary patterns [15], and is effective in improving both glycemic control and cardiovascular risk in people with T2DM. The characteristics of the Mediterranean diet represent a dietary pattern rich in vegetables, fruits, nuts, wholegrains, and seafood, in addition to highlighting food-related cultural and social activities. These activities help to establish a lifestyle that encourages enjoying cooking and taking a long time to eat with other people [16]. Particularly in the Mediterranean region, people traditionally enjoy full-course meals, consuming one dish at a time, with intervals between courses, over several hours. In contrast, Japanese people are known for eating quickly within a limited timeframe, often without commensality. However, they occasionally share meals with family and friends in a full-course meal manner over extended periods. Many studies have shown that eating in a short period of time increases the risk of diabetes [17], obesity [18,19], cardiovascular diseases [20], and metabolic syndrome [21]. We have previously reported that eating in a short period of time demonstrated significantly higher glycemic and insulin responses [12]. Moreover, our previous study demonstrated no significant differences in glycemic and insulin responses between eating meals in 10 min and eating meals in 20 min when following a food order of consuming vegetables first, followed by the main dish, and carbohydrates last [22]. While various studies have investigated the effects of different eating speeds or times required for meal and food orders [3,9,10,11,12,17,18,19,20,21,22], the impact of consuming a full-course meal, with extended intervals between dishes, on blood glucose and insulin responses remains unknown. This study aimed to evaluate the impact of consuming a meal in a full-course meal manner over one hour, with intervals between courses, on glycemic and insulin responses in young healthy women, using a randomized controlled crossover trial.
2. Materials and Methods
2.1. Experiment
2.1.1. Subjects of Experiment 1 and 2
Students from Kyoto Women’s University voluntarily participated in Experiments 1 and 2. Prior to their involvement, all participants received a detailed explanation of the study’s objectives, procedures, and potential risks. The exclusion criteria for participants were as follows: (1) individuals with type 1 or type 2 diabetes, (2) those taking medications that affect blood glucose levels, and (3) pregnant women. Participants provided written informed consent before enrollment in the study. This study employed an open-label, within-subject, randomized crossover design, and was conducted from September 2023 to July 2024. Participants who failed to adhere to the study protocol were excluded. The study protocol was developed in accordance with the ethical principles outlined in the Declaration of Helsinki, and received approval from the Clinical Research Review Committee of Kyoto Women’s University (Approval Nos. 2023-28 and 2023-39).
2.1.2. Study Design of Experiment 1
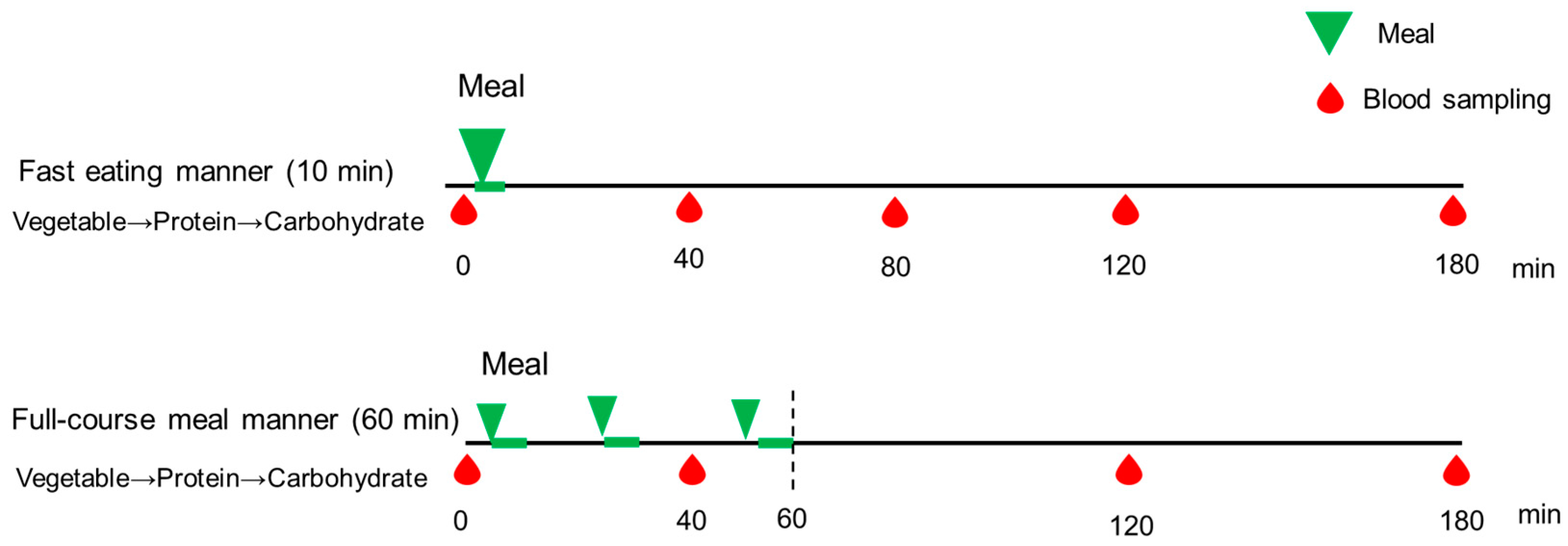
On the study days, participants arrived at Kyoto Women’s University at 8:20 AM, following a 12 h overnight fast. At 9:00 AM, they consumed a test meal identical to the dinner provided in Experiment 2 (Table 1). In both conditions, the food order was standardized: vegetables first, followed by the main dish, and carbohydrates last (Figure 1). In the full-course meal manner condition, with intervals between courses, the meal was consumed over a total duration of 60 min, beginning with a vegetable dish consumed for 10 min, followed by a 15 min interval. The second dish, consisting of protein, was then consumed for 10 min, followed by another 15 min interval. Finally, the third dish, consisting of carbohydrates, was consumed for 10 min. In contrast, in the fast eating manner, participants consumed an identical meal in a total of 10 min, following the same food order. The test meals were served by the research group at Kyoto Women’s University. The nurse collected blood samples from all participants at 0 (baseline), 40, 80, 120, and 180 min after the initiation of test meal consumption, to assess blood glucose and insulin concentrations. Each participant completed both eating conditions on separate days, one week apart, as part of the randomized controlled crossover trial. These parameters were then compared within participants across the two study days. The satiety and satisfaction of the test meals was measured by using visual analog scales (VASs) after consumption [23].

Table 1.
The composition and macronutrient content of the test meals.
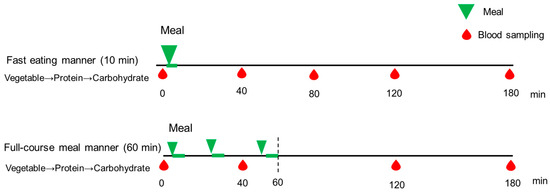
Figure 1.
The study protocol of Experiment 1. A full description can be found in the Study Design of Experiment 1. Blood samples were drawn at 0, 40, 80, 120, and 180 min, and blood glucose and insulin concentrations were examined.
2.1.3. Study Design of Experiment 2
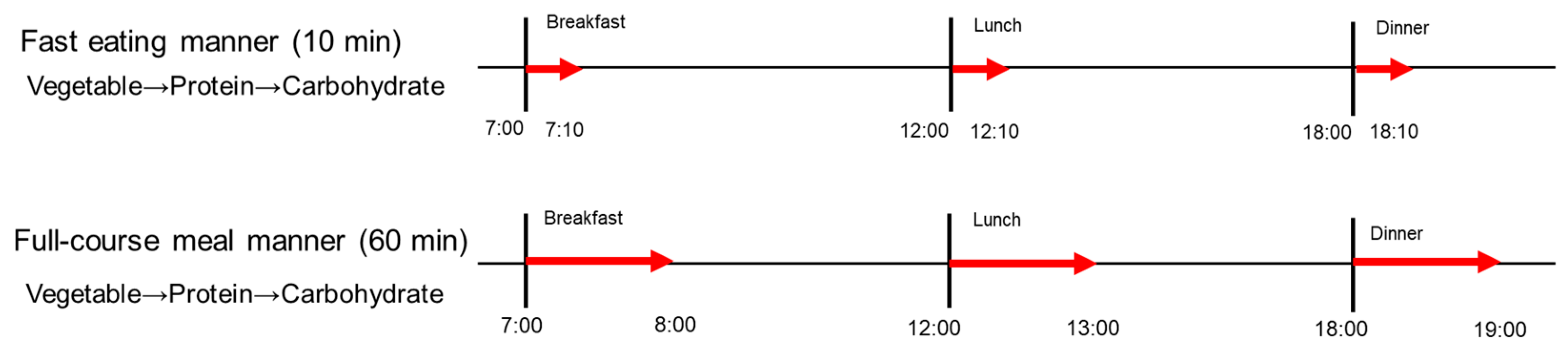
To continuously monitor postprandial blood glucose responses over a 24 h period, we conducted a subsequent experiment utilizing an intermittent continuous glucose monitoring system (isCGM, FreeStye, Libre Pro, Abbott Japan, LLC. Tokyo, Japan). All participants self-applied isCGM devices to the back of their left upper arm, under the supervision of researchers at Kyoto Women’s University. In this randomized controlled crossover clinical trial, participants consumed the same test meals (Table 1) at 07:00 for breakfast, 12:00 for lunch, and 18:00 for dinner on the fourth and fifth study days, with the same meals being consumed in the two different eating manners defined as Experiment 1; in the fast eating manner for 10 min, and in the full-course meal manner for 60 min (Figure 2).
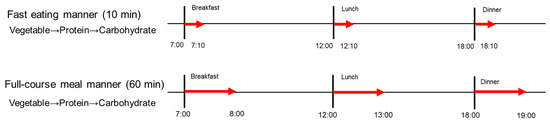
Figure 2.
The study protocol of Experiment 2. A full description can be found in the Study Design of Experiment 2.
The composition and macronutrient content of the test meals are presented in Table 1. The main dishes for lunch and dinner were provided as frozen meal boxes by the research group (Tokatsu Foods, Co., Ltd. Yokohama, Japan), whereas the bread, white rice, tomato, and spinach were prepared by the participants according to the study protocol. The frozen meal boxes were stored in the freezer until consumption, and participants precisely measured 200 g of boiled white rice for lunch and dinner and 90 g of white bread for breakfast, and then heated it in a microwave before eating.
Participants were instructed to refrain from consuming any food or beverages other than the test meals, with the exception of water and unsweetened tea, and to avoid excessive physical activity throughout the study period. They were required to strictly adhere to the study protocol, such as recording their mealtimes, eating manners, and carbohydrate intake. On the sixth day, participants removed their isCGM devices under the guidance of the research group at Kyoto Women’s University. Glycemic parameters from the two study days, during which participants consumed the same test meals in different eating manners, were compared.
2.2. Measurements
Before the study commenced, anthropometric measurements and blood samples were obtained from participants in the morning, following an overnight fast. In Experiment 1, all collected blood samples were sent to a clinical testing laboratory (Nihon Rinsho, Inc., Kyoto, Japan), where plasma glucose concentrations were analyzed using the HK-G6PDH method (Kanto Chemical Co., Inc., Tokyo, Japan). Hemoglobin A1c (HbA1c) levels were measured using the NGSP method (Kyowa Medix Co., Inc., Tokyo, Japan), while serum insulin concentrations were determined by the CLEIA method (Fujirebio Co., Inc., Tokyo, Japan). The incremental area under the curve (IAUC) for glucose and insulin was computed using the trapezoidal method in Experiment 1 [24]. Glycemic and insulin variability was evaluated based on several parameters, including the standard deviation (SD) and glucose peak (GP). In Experiment 2, continuous glucose monitoring data were collected via isCGM, and subsequently analyzed. Several glycemic variability indices, including the IAUC, GP, SD, maximum blood glucose (MAX), large amplitude of glycemic excursions (LAGE), mean amplitude of glycemic excursions (MAGE), and coefficient of variation (%CV), were assessed in Experiment 2 [25].
2.3. Sample Size and Statistical Analysis
A sample size of 15 participants provided 95% power to detect a 5% difference in postprandial glucose concentrations, based on a previous study investigating different food sequences in healthy women (G*Power 3.1, Heinrich-Heine-Universität Düsseldorf, Germany) [11]. In Experiment 1, the primary outcome was the postprandial glucose concentration, and the secondary outcome was the postprandial insulin concentration. In Experiment 2, the primary outcome was the MAGE, while the secondary outcomes were postprandial glucose concentration and the IAUC for glucose. Statistical analyses were performed using paired t-tests or t-tests, with a significance level set at p < 0.05. The results are expressed as the mean ± the standard error of the mean (SEM), unless otherwise specified. All statistical analyses were conducted using SPSS Statistics version 26 (IBM Corp., Armonk, NY, USA).
3. Results
3.1. Results of Experiment 1
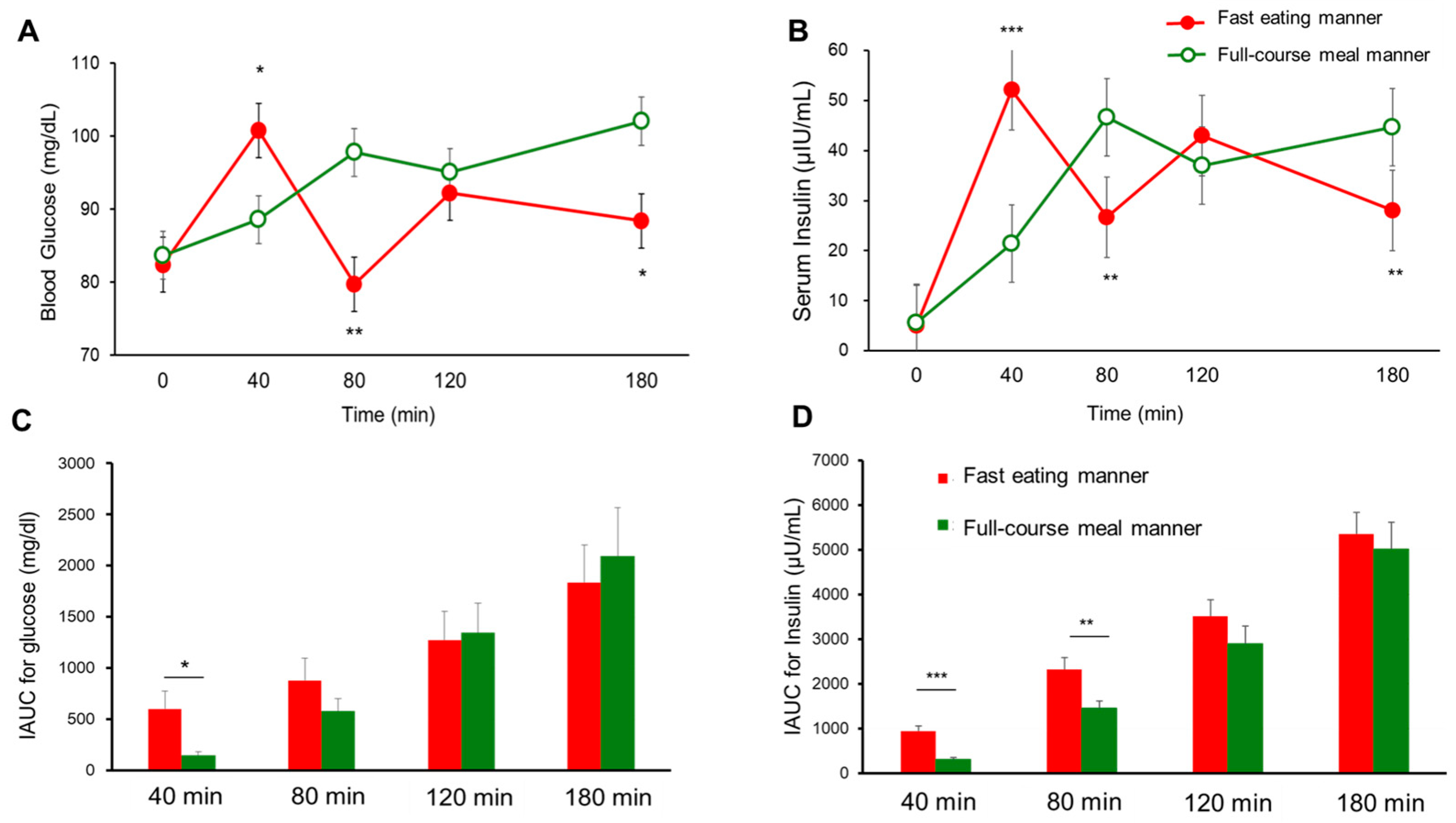
Seventeen participants were initially enrolled in Experiment 1, and fifteen participants completed the study (age: 21.2 ± 0.9 years, BMI: 20.2 ± 2.2 kg/m2, HbA1c: 5.2 ± 0.2%, fasting plasma glucose [FPG]: 85.4 ± 5.0 mg/dL; mean ± SD); two participants withdrew due to COVID-19 infection. Postprandial blood glucose and insulin levels at 40 min (88.6 ± 2.4 vs. 100.8 ± 5.6 mg/dL, p < 0.05 for glucose, and 21.4 ± 2.3 vs. 52.1 ± 5.8 μIU/mL, p < 0.001 for insulin; Figure 3A,B), as well as the IAUC at 40 min for glucose (143.6 ± 42.7 vs. 601.5 ± 175.6 mg/dL, p < 0.05; Figure 3C) and the IAUC at both 40 min (316.2 ± 35.2 vs. 943.1 ± 110.3 μIU/mL, p < 0.001) and 80 min for insulin (1452.8 ± 171.5 vs. 2320.5 ± 265.1 μIU/mL, p < 0.01), were significantly lower for the full-course meal manner compared to the fast eating manner, due to delayed consumption of the carbohydrate dish in the former condition at these time points (Figure 3D). However, at later time points, postprandial blood glucose and insulin concentrations were significantly higher for the full-course meal manner than the fast eating manner. At 80 min, glucose (97.8 ± 3.3 vs. 79.7 ± 4.2 mg/dL, p < 0.01) and insulin (46.6 ± 6.9 vs. 26.7 ± 3.3 μIU/mL, p < 0.01) levels were elevated (Figure 3A,B). Similarly, at 180 min, glucose (102.1 ± 4.8 vs. 88.4 ± 4.6 mg/dL, p < 0.05) and insulin concentrations (44.6 ± 4.6 vs. 28.1 ± 2.0 μIU/mL, p < 0.01) remained significantly higher for the full-course meal manner (Figure 3A,B). Although the VAS for satiety showed no significant difference between the two eating manners, the satisfaction level was significantly higher for the full-course meal manner compared to the fast eating manner (67.2 ± 10.9 vs. 55.0 ± 10.0 mm, mean ± SD, p < 0.01).
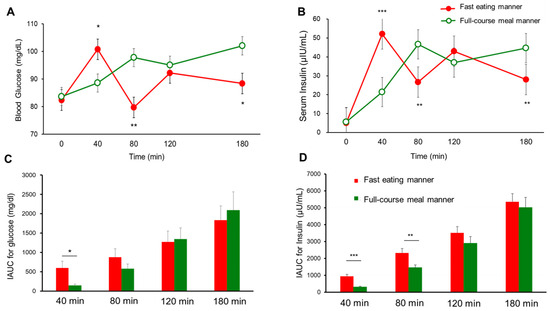
Figure 3.
The postprandial blood glucose (A), insulin (B), IAUC for glucose (C), and IAUC for insulin (D) in young healthy women (n = 15) for the full-course meal manner and fast eating manner in Experiment 1. The data are the mean ± SEM. Full-course meal manner vs. fast eating manner; p < 0.05 *, p < 0.01 **, p < 0.001 *** by a paired t-test.
3.2. Results of Experiment 2
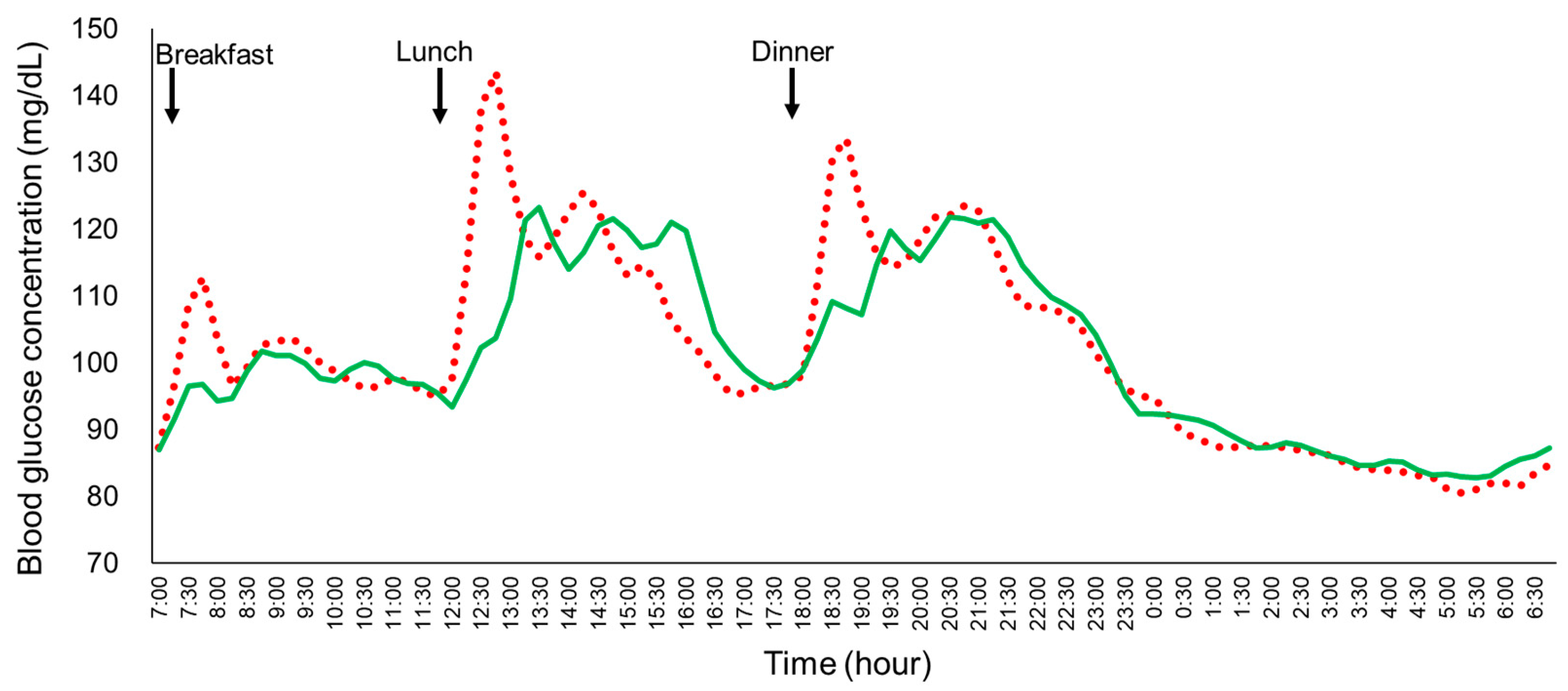
Twenty-two participants were initially enrolled in Experiment 2. Four participants were excluded from the final analysis due to COVID-19 infection (n = 2) or malfunction of the isCGM device (n = 2). As a result, data from eighteen participants were included in the final analysis (age: 21.5 ± 0.6 years, BMI: 20.3 ± 2.3 kg/m2, HbA1c: 5.2 ± 0.2%, FPG: 94.6 ± 12.3 mg/dL; mean ± SD). The mean blood glucose profiles in the young, healthy women under the two different eating manners were analyzed (Figure 4). Key parameters, including the SD, MAGE, MAX, LAGE, %CV, GP during lunch and dinner, and IAUC for glucose at 30, 60, 120, and 180 min, were all significantly lower for the full-course meal manner compared to the fast eating manner (Table 2). Despite the significant reduction in glycemic variability and related parameters, no significant differences were observed in the mean blood glucose and the 24 h IAUC between the two eating manners.
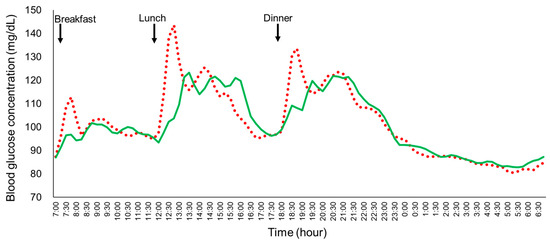
Figure 4.
The mean plasma glucose profiles of the young healthy women (n = 18) under the fast eating manner and the full-course meal manner in Experiment 2. Each participant consumed the same meals in the fast eating manner (10 min) or the full-course meal manner (60 min) on the fourth and the fifth day in a randomized crossover study. Solid green line: full-course meal manner; red dotted line: fast eating manner.

Table 2.
The glycemic parameters of the young healthy women under the full-course meal manner and fast eating manner in Experiment 2, using an isCGM (n = 18).
4. Discussion
In Experiment 1, the postprandial blood glucose and insulin levels at 40 min, as well as the IAUC at 40 min for glucose and the IAUC at both 40 and 80 min for insulin, were significantly lower for the full-course meal manner compared to the fast eating manner. This difference was due to the delayed consumption of the carbohydrate dish in the full-course meal manner at these time points. Thus, the design of Experiment 1 did not allow for an assessment of the prolonged effects of consuming meals in a certain manner, nor did it facilitate continuous monitoring of postprandial glucose excursions over an extended period, thereby limiting its ability to capture long-term glycemic variations.
To address this limitation, Experiment 2 was conducted using an isCGM to track blood glucose fluctuations over a 24 h period. The results demonstrated that glycemic parameters, including the SD, MAGE, MAX, LAGE, %CV, and IAUC for glucose at 30, 60, 120, and 180 min, were all significantly lower for the full-course meal manner compared to the fast eating manner. Conversely, no significant differences were observed in mean blood glucose levels or the 24 h IAUC between the two eating manners. This finding is consistent with previous studies investigating the effects of different eating speeds or food orders, likely because the participants consumed the same test meals on both study days [11,12,22]. Notably, in Experiment 1, the improved postprandial glucose and insulin levels observed at 40 and 80 min, and in Experiment 2, the reductions in the IAUC for glucose at 30 and 60 min, are clearly attributable to the delayed or incomplete consumption of carbohydrates at those time points. However, the results findings in Experiment 2 underscore the importance of promoting a full-course meal approach, in which each dish is served sequentially, with intervals between courses.
Numerous epidemiological studies have revealed that eating in a short period of time shows a positive correlation with increments in body weight [18,19], blood glucose, insulin resistance [26], the risk of T2DM [17], cardiovascular diseases [20], and metabolic syndrome [21]. We previously reported that eating meals in 10 min was associated with higher glycemic and insulin responses than eating meals in 20 min [12]. However, previous research showed that if a meal was consumed with the food order of vegetables first and carbohydrates last, amelioration could be achieved in postprandial blood glucose and insulin concentrations, even if the meal was consumed in 10 min [22].
One possible explanation for the improvement in glycemic and insulin parameters observed for the full-course meal manner is the dietary fiber contained in the vegetables consumed first, which slows the digestion and absorption of carbohydrates [27]. Some researchers suggest that individuals who eat quickly tend to consume meals in a short period and are more likely to choose ultra-processed foods, which are low in dietary fiber and require minimal chewing, rather than fiber-rich foods that necessitate thorough mastication [28]. These studies indicate that eating manner and chewing frequency may influence insulin, glucagon-like peptide-1 (GLP-1), and satiety-related hormones [29,30,31]. Thus, different eating manners may be associated with meal satisfaction related to GLP-1 and satiety hormones that delay gastric emptying rate and regulate appetite and satiety. Additionally, the effectiveness of the full-course meal manner observed in this study can be explained by the small portions and intervals of the meal taken during one hour of mealtime. Because the amount of nutrients in each bite when eating over a long period of time was smaller than that when eating fast, the concentration of early postprandial glucose and insulin was reduced, while GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) might have been induced intermittently in the one-hour mealtime, as we have reported in the paper [13,32].
A key strength of this study lies in its design as the first randomized controlled crossover interventional trial to demonstrate the amelioration of the MAGE through the consumption of a meal in a full-course meal manner, over one hour. This eating pattern effectively replicates the traditional full-course meal approach, in which dishes are served sequentially over an extended period. The observed reductions in glycemic responses may be beneficial for individuals both with and without T2DM, as higher MAGE levels have been associated with an increased risk of T2DM, arteriosclerosis, cardiovascular diseases [33], and dementia [34], even before the onset of T2DM [35]. Therefore, reducing MAGE may contribute to lowering the risk of developing impaired glucose tolerance, T2DM, and cardiovascular diseases in the long term [36,37,38,39].
Furthermore, the delayed consumption of the carbohydrate dish, which resulted in reduced early postprandial insulin secretion, may be particularly beneficial for East Asian populations, including Japanese individuals. This is because early-phase and total insulin secretion in East Asians is significantly lower than in Caucasians [40]. The suppression of excessive insulin may help to maintain the function of β cells in the pancreas, consequently helping to lower the risk of T2DM [41]. Insulin modulates vascular function through its effects on vasoreactivity, lipid metabolism, and inflammation, and through these multiple pathways, insulin dysregulation could contribute to neurodegeneration [42]. Because insulin plays a critical role in proteostasis, it has been reported to influence the clearance of amyloid β peptides and the phosphorylation of tau, both of which are hallmarks of Alzheimer’s disease [42]. Additionally, hyperinsulinemia is a key etiological factor in the development of metabolic syndrome, type 2 diabetes mellitus (T2DM), cardiovascular disease, and cancer [43].
One of the key characteristics of traditional Japanese cuisine (Washoku), recognized as an Intangible Cultural Heritage by UNESCO, is its emphasis on home-cooked meals and the practice of spending extended periods enjoying food with others [44]. However, traditional dietary patterns have increasingly shifted toward Western-style eating habits, which prioritize fast food and ultra-processed products over the enjoyment and sharing of home-cooked meals. Modern lifestyles and globalization have further contributed to changes in eating behaviors, resulting in meals being consumed quickly and with diminished social interaction in many countries, including Japan. Meal satisfaction is not solely determined by portion size or caloric intake [45]; instead, the act of taking time to eat and sharing food and beverages with others are critical components. In contrast, consuming fast food in a short period of time, with limited social engagement, is often associated with reduced meal satisfaction [46,47]. Our findings underscore the importance of not only the composition of macro- and micronutrients, but also different eating manners, in maintaining optimal metabolic responses. Occasionally taking the time to enjoy each dish individually, with friends and family, can be considered a factor that enriches life. In the present study, the specific effects of food order on glycemic response were not directly evaluated, as numerous studies have already demonstrated that consuming vegetables first and carbohydrates last plays a significant role in mitigating glycemic and insulin excursions [9,11,12,22]. However, it is important to emphasize that food order—consuming vegetables first and carbohydrates last—is a crucial factor. Even when eating in a full-course meal manner, consuming carbohydrates first elevates postprandial glucose and insulin responses, thereby diminishing the beneficial effects of eating over a long period of time [11,12,22].
Several limitations of the present study should be acknowledged. First, this study was designed as an acute interventional trial to examine the effects of consuming a meal in a full-course meal manner, replicating the prolonged mealtimes characteristic of social gatherings, such as parties or banquets. However, the study protocol did not include the consumption of alcohol, which is typically present during parties or banquets, nor did it incorporate social interactions, such as conversations with others. As a result, the findings may not fully capture the metabolic responses associated with these additional factors, limiting the generalizability of the results. Second, the study population consisted of small number of young, healthy Japanese women; therefore, it is uncertain whether these results are applicable to individuals with T2DM, different genders, elderly people, or other racial groups. Third, GLP-1 and other incretin hormones may play a role in the regulation of blood glucose and insulin levels, as well as in delaying gastric emptying, in individuals both with and without T2DM, when meals are consumed one dish at a time, with intervals between courses. However, GLP-1, GIP, and gastric emptying rate were not investigated in this study; the role of incretin hormones when eating in a full-course meal manner remains unclear. Therefore, future investigations, particularly large-scale clinical studies, are worth conducting to confirm the overall mechanisms and effectiveness of eating in a full-course meal manner regarding glycemic and hormone responses in individuals with and without T2DM.
5. Conclusions
Our findings indicate that consuming meals in a full-course meal manner for one hour, with intervals between courses, improves the MAGE in young healthy women. However, future studies, particularly large-scale clinical trials, are warranted to further elucidate the underlying mechanisms and evaluate the efficacy of consuming meals in a full-course meal manner regarding glycemic and hormonal responses in individuals with and without T2DM.
Author Contributions
S.I. designed the study, recruited participants, conducted experiments, performed the data analysis, and wrote the article. S.K. (Shizuo Kajiyama) conducted the experiments, contributed to the discussion, and reviewed the article. Y.H. (Yuki Higuchi). and S.I. contributed to recruiting participants, performed the data analysis, and contributed to the writing of the article. K.K. and T.M. performed the data analysis and contributed to the writing of the article. S.M., N.O., S.K. (Shintaro Kajiyama), Y.H. (Yoshitaka Hashimoto), and M.F. contributed to the discussion and reviewed the article. S.I. and S.K. (Shizuo Kajiyama) are guarantors of this work, and, as such, have full access to all the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Kyoto Women’s University.
Institutional Review Board Statement
The study was conducted in accordance with the guidelines of Declaration of Helsinki and approved by the Institutional Review Board of Kyoto Women’s University on 1 August 2023 for Approval Nos. 2023-28 and on 16 January 2024 for Approval. Nos. 2023-39.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the participants to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.
Acknowledgments
We thank all the investigators and volunteers for participating in this study.
Conflicts of Interest
Y.H. reports grants from Asahi Kasei Pharma and personal fees from Mitsubishi Tanabe Pharma Corp., Novo Nordisk Pharma Ltd., Sanofi K.K., and Daiichi Sankyo Co., Ltd., outside of the submitted work. M.F. received grants from Takeda Pharma Co., Ltd., Sanofi K.K., Kissei Phama Co., Ltd., Mitsubishi Tanabe Pharma Corp, Astellas Pharma Inc., Nippon Boehringer Ingelheim Co., Ltd., Daiichi Sankyo Co., Ltd., MSD K.K., Sanwa Kagagu Kenkyusho Co., Ltd., Kowa Pharma Co., Ltd., Kyowa Kirin Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Novo Nordisk Pharma Ltd., Ono Pharma Co., Ltd., Eli Lilly Japan K.K., Taisho Pharma Co., Ltd., Tejin Pharma LtD., Nippon Chemiphar Co., Ltd., Johnson & Johnson k.k. Medical Co., Abbott japan Co., Ltd., and Terumo Corp., and personal fees from Teijin Pharma Ltd., Arkray Inc., Kissei Pharma Co., Ltd., Novo Nordisk Pharma Ltd., Sanofi K.K., Takeda Pharma Co., Ltd., Astellas Pharma Inc., MSD K.K., Kyowa Kirin Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Daiichi Sankyo Co., Ltd., Kowa Pharma Co., Ltd., Ono Pharma Co., Ltd., Sanwa Kagaku Kenkyusho Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Taisho Pharma Co., Ltd., Bayer Ya-kuhin, Ltd., AstraZeneca K.K., Mochida Pharma Co., Ltd., Abbott japan Co., Ltd., Eli Lilly Japan K.K., Medtronic Japan Co., Ltd., and Nipro Corp., outside of the submitted work. The sponsors were not involved in the study design; in the collection, analysis, or interpretation of data; in the writing of this manuscript; or in the decision to submit the article for publication. The authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article. The authors declare that although they are affiliated with a department that is supported financially by a pharmaceutical company, the authors received no current funding for this study, and this does not alter their adherence to all the journal policies on sharing data and materials. The other authors have nothing to disclose.
References
- Ajala, O.; English, P.; Pinkney, J. Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am. J. Clin. Nutr. 2013, 97, 505–516. [Google Scholar] [CrossRef] [PubMed]
- The DCCT Research Group; Delahanty, L.; Simkins, S.W.; Camelon, K. Expanded role of the dietitian in the Diabetes Control and Complications Trial: Implications for clinical practice. J. Am. Diet. Assoc. 1993, 93, 758–764. [Google Scholar] [CrossRef]
- Nitta, A.; Imai, S.; Kajiayama, S.; Matsuda, M.; Miyawaki, T.; Matsumoto, S.; Kajiyama, S.; Hashimoto, Y.; Ozasa, N.; Fukui, M. Impact of Dietitian-Led Nutrition Therapy of Food Order on 5-Year Glycemic Control in Outpatients with Type 2 Diabetes at Primary Care Clinic: Retrospective Cohort Study. Nutrients 2022, 14, 2865. [Google Scholar] [CrossRef] [PubMed]
- Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD). Evidence-based European recommendations for the dietary management of diabetes. Diabetologia 2023, 66, 965–985. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Overview of Nutrition and Diabetes. Available online: https://diabetes.org/food-nutrition (accessed on 6 November 2024).
- The Japan Diabetes Society. Japanese Clinical Practice Guideline for Doiabetes 2024; Nankodo: Tokyo, Japan, 2024; pp. 37–66. [Google Scholar]
- Rivellese, A.A.; Boemi, M.; Cavalot, F.; Costagliola, L.; De Feo, P.; Miccoli, R.; Patti, L.; Trovati, M.; Vaccaro, O.; Zavaroni, I.; et al. Dietary habits in type II diabetes mellitus: How is adherence to dietary recommendations? Eur. J. Clin. Nutr. 2008, 62, 660–664. [Google Scholar] [CrossRef]
- Vitale, M.; Masulli, M.; Cocozza, S.; Anichini, R.; Babini, A.C.; Boemi, M.; Bonora, E.; Buzzetti, R.; Carpinteri, R.; Caselli, C.; et al. Sex differences in food choices, adherence to dietary recommendations and plasma lipid profile in type 2 diabetes—The TOSCA.IT study. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 879–885. [Google Scholar] [CrossRef]
- Shukla, A.P.; Mauer, E.; Igel, L.I.; Truong, W.; Casper, A.; Kumar, R.B.; Saunders, K.H.; Aronne, L.J. Effect of Food Order on Ghrelin Suppression. Diabetes Care 2018, 41, e76–e77. [Google Scholar] [CrossRef]
- Kuwata, H.; Iwasaki, M.; Shimizu, S.; Minami, K.; Maeda, H.; Seino, S.; Nakada, K.; Nosaka, C.; Murotani, K.; Kurose, T.; et al. Meal sequence and glucose excursion, gastric emptying and incretin secretion in type 2 diabetes: A randomised, controlled crossover, exploratory trial. Diabetologia 2016, 59, 453–461. [Google Scholar] [CrossRef]
- Imai, S.; Fukui, M.; Ozasa, N.; Ozeki, T.; Kurokawa, M.; Komatsu, T.; Kajiyama, S. Eating vegetables before carbohydrates improves postprandial glucose excursions. Diabet. Med. 2013, 30, 370–372. [Google Scholar] [CrossRef]
- Saito, Y.; Kajiyama, S.; Nitta, A.; Miyawaki, T.; Matsumoto, S.; Ozasa, N.; Kajiyama, S.; Hashimoto, Y.; Fukui, M.; Imai, S. Eating Fast Has a Significant Impact on Glycemic Excursion in Healthy Women: Randomized Controlled Cross-Over Trial. Nutrients 2020, 12, 2767. [Google Scholar] [CrossRef]
- Imai, S.; Kajiyama, S.; Hashimoto, Y.; Yamane, C.; Miyawaki, T.; Ozasa, N.; Tanaka, M.; Fukui, M. Divided consumption of late-night-dinner improves glycemic excursions in patients with type 2 diabetes: A randomized cross-over clinical trial. Diabetes Res. Clin. Pract. 2017, 129, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Obermayer, A.; Tripolt, N.J.; Pferschy, P.N.; Kojzar, H.; Aziz, F.; Müller, A.; Schauer, M.; Oulhaj, A.; Aberer, F.; Sourij, C.; et al. Efficacy and Safety of Intermittent Fasting in People with Insulin-Treated Type 2 Diabetes (INTERFAST-2)-A Randomized Controlled Trial. Diabetes Care 2023, 46, 463–468. [Google Scholar] [CrossRef]
- Dinu, M.; Pagliai, G.; Casini, A.; Sofi, F. Mediterranean diet and multiple health outcomes: An umbrella review of meta-analyses of observational studies and randomised trials. Eur. J. Clin. Nutr. 2018, 72, 30–43. [Google Scholar] [CrossRef]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, M.; Nakamura, K.; Miura, K.; Takamura, T.; Yoshita, K.; Nagasawa, S.Y.; Morikawa, Y.; Ishizaki, M.; Kido, T.; Naruse, Y.; et al. Self-reported speed of eating and 7-year risk of type 2 diabetes mellitus in middle-aged Japanese men. Metabolism 2012, 61, 1566–1571. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, R.; Tamakoshi, K.; Yatsuya, H.; Murata, C.; Sekiya, A.; Wada, K.; Zhang, H.M.; Matsushita, K.; Sugiura, K.; Takefuji, S.; et al. Eating fast leads to obesity: Findings based on self-administered questionnaires among middle-aged Japanese men and women. J. Epidemiol. 2006, 16, 117–124. [Google Scholar] [CrossRef]
- Leong, S.L.; Madden, C.; Gray, A.; Waters, D.; Horwath, C. Faster self-reported speed of eating is related to higher body mass index in a nationwide survey of middle-aged women. J. Am. Diet. Assoc. 2011, 111, 1192–1197. [Google Scholar] [CrossRef]
- Paz-Graniel, I.; Babio, N.; Mendez, I.; Salas-Salvadó, J. Association between Eating Speed and Classical Cardiovascular Risk Factors: A Cross-Sectional Study. Nutrients 2019, 11, 83. [Google Scholar] [CrossRef]
- Nagahama, S.; Kurotani, K.; Pham, N.M.; Nanri, A.; Kuwahara, K.; Dan, M.; Nishiwaki, Y.; Mizoue, T. Self-reported eating rate and metabolic syndrome in Japanese people: Cross-sectional study. BMJ Open 2014, 4, e005241. [Google Scholar] [CrossRef]
- Imai, S.; Kajiyama, S.; Kitta, K.; Miyawaki, T.; Matsumoto, S.; Ozasa, N.; Kajiyama, S.; Hashimoto, Y.; Fukui, M. Eating Vegetables First Regardless of Eating Speed Has a Significant Reducing Effect on Postprandial Blood Glucose and Insulin in Young Healthy Women: Randomized Controlled Cross-Over Study. Nutrients 2023, 15, 1174. [Google Scholar] [CrossRef]
- Flint, A.; Raben, A.; Blundell, J.E.; Astrup, A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Allison, D.B.; Paultre, F.; Maggio, C.; Mezzitis, N.; Pi-Sunyer, F.X. The use of areas under curves in diabetes research. Diabetes Care 1995, 18, 245–250. [Google Scholar] [CrossRef]
- Baghurst, P.A. Calculating the mean amplitude of glycemic excursion from continuous glucose monitoring data: An automated algorithm. Diabetes Technol. Ther. 2011, 13, 296–302. [Google Scholar] [CrossRef]
- Otsuka, R.; Tamakoshi, K.; Yatsuya, H.; Wada, K.; Matsushita, K.; OuYang, P.; Hotta, Y.; Takefuji, S.; Mitsuhashi, H.; Sugiura, K.; et al. Eating fast leads to insulin resistance: Findings in middle-aged Japanese men and women. Prev. Med. 2008, 46, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Livesey, G.; Taylor, R.; Livesey, H.F.; Buyken, A.E.; Jenkins, D.J.A.; Augustin, L.S.A.; Sievenpiper, J.L.; Barclay, A.W.; Liu, S.; Wolever, T.M.S.; et al. Dietary Glycemic Index and Load and the Risk of Type 2 Diabetes: Assessment of Causal Relations. Nutrients 2019, 11, 1436. [Google Scholar] [CrossRef] [PubMed]
- Karl, J.P.; Young, A.J.; Rood, J.C.; Montain, S.J. Independent and combined effects of eating rate and energy density on energy intake, appetite, and gut hormones. Obesity 2013, 21, E244–E252. [Google Scholar] [CrossRef]
- Kokkinos, A.; le Roux, C.W.; Alexiadou, K.; Tentolouris, N.; Vincent, R.P.; Kyriaki, D.; Perrea, D.; Ghatei, M.A.; Bloom, S.R.; Katsilambros, N. Eating slowly increases the postprandial response of the anorexigenic gut hormones, peptide YY and glucagon-like peptide-1. J. Clin. Endocrinol. Metab. 2010, 95, 333–337. [Google Scholar] [CrossRef]
- Takahara, M.; Fukuda, M.; Matsuzawa, Y.; Shimomura, I. Effect of tasteless calorie-free gum chewing before meal on postprandial plasma glucose, insulin, glucagon, and gastrointestinal hormones in Japanese men without diagnosed glucose metabolism disorder: A pilot randomized crossover trial. Diabetol. Int. 2020, 11, 394–402. [Google Scholar] [CrossRef]
- Xu, J.; Xiao, X.; Li, Y.; Zheng, J.; Li, W.; Zhang, Q.; Wang, Z. The effect of gum chewing on blood GLP-1 concentration in fasted, healthy, non-obese men. Endocrine 2015, 50, 93–98. [Google Scholar] [CrossRef][Green Version]
- Imai, S.; Saito, Y.; Kajiyama, S.; Nitta, A.; Miyawaki, T.; Matsumoto, S.; Ozasa, N.; Kajiyama, S.; Hashimoto, Y.; Fukui, M. Late-night-dinner deteriorates postprandial glucose and insulin whereas consuming dinner dividedly ameliorates them in patients with type 2 diabetes: A randomized crossover clinical trial. Asia Pac. J. Clin. Nutr. 2020, 29, 68–76. [Google Scholar] [CrossRef]
- Yapanis, M.; James, S.; Craig, M.E.; O’Neal, D.; Ekinci, E.I. Complications of Diabetes and Metrics of Glycemic Management Derived from Continuous Glucose Monitoring. J. Clin. Endocrinol. Metab. 2022, 107, e2221–e2236. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Zhang, X.Y.; Miao, Y.; Zhu, J.H.; Yan, H.; Wang, B.Y.; Jin, J.; Hu, T.J.; Jia, W.P. The relationship between glucose excursion and cognitive function in aged type 2 diabetes patients. Biomed. Environ. Sci. 2012, 25, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Akasaka, T.; Sueta, D.; Tabata, N.; Takashio, S.; Yamamoto, E.; Izumiya, Y.; Tsujita, K.; Kojima, S.; Kaikita, K.; Matsui, K.; et al. Effects of the Mean Amplitude of Glycemic Excursions and Vascular Endothelial Dysfunction on Cardiovascular Events in Nondiabetic Patients with Coronary Artery Disease. J. Am. Heart Assoc. 2017, 6, e004841. [Google Scholar] [CrossRef]
- Cederberg, H.; Saukkonen, T.; Laakso, M.; Jokelainen, J.; Härkönen, P.; Timonen, M.; Keinänen-Kiukaanniemi, S.; Rajala, U. Postchallenge glucose, A1C, and fasting glucose as predictors of type 2 diabetes and cardiovascular disease: A 10-year prospective cohort study. Diabetes Care 2010, 33, 2077–2083. [Google Scholar] [CrossRef]
- Ceriello, A. The post-prandial state and cardiovascular disease: Relevance to diabetes mellitus. Diabetes Metab. Res. Rev. 2000, 16, 125–132. [Google Scholar] [CrossRef]
- Fujishima, M.; Kiyohara, Y.; Kato, I.; Ohmura, T.; Iwamoto, H.; Nakayama, K.; Ohmori, S.; Yoshitake, T. Diabetes and cardiovascular disease in a prospective population survey in Japan: The Hisayama Study. Diabetes 1996, 45, S14–S16. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Mi, S.H.; Tao, H.; Li, Z.; Yang, H.X.; Zheng, H.; Zhou, Y.; Tian, L. Impact of admission glycemic variability, glucose, and glycosylated hemoglobin on major adverse cardiac events after acute myocardial infarction. Diabetes Care 2013, 36, 1026–1032. [Google Scholar] [CrossRef]
- Fukushima, M.; Suzuki, H.; Seino, Y. Insulin secretion capacity in the development from normal glucose tolerance to type 2 diabetes. Diabetes Res. Clin. Pract. 2004, 66, S37–S43. [Google Scholar] [CrossRef]
- Huising, M.O. Paracrine regulation of insulin secretion. Diabetologia 2020, 63, 2057–2063. [Google Scholar] [CrossRef]
- Kellar, D.; Craft, S. Brain insulin resistance in Alzheimer’s disease and related disorders: Mechanisms and therapeutic approaches. Lancet Neurol. 2020, 19, 758–766. [Google Scholar] [CrossRef]
- Janssen, J.A.M.J.L. Hyperinsulinemia and Its Pivotal Role in Aging, Obesity, Type 2 Diabetes, Cardiovascular Disease and Cancer. Int. J. Mol. Sci. 2021, 22, 7797. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.maff.go.jp/j/keikaku/syokubunka/ich/ (accessed on 3 December 2024).
- Phull, S.; Wills, W.J.; Dickinson, A.M. Is It a Pleasure to Eat Together? Theoretical Reflections on Conviviality and the Mediterranean Diet. Sociol. Compass 2015, 9, 977–986. [Google Scholar] [CrossRef]
- Skeer, M.R.; Ballard, E.L. Are family meals as good for youth as we think they are? A review of the literature on family meals as they pertain to adolescent risk prevention. J. Youth Adolesc. 2013, 42, 943–963. [Google Scholar] [CrossRef] [PubMed]
- Phull, S. The Mediterranean Diet: Socio-Cultural Relevance for Contemporary Health Promotion. Open Public Health J. 2015, 8, 35–40. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).