Development of a Metabolic Syndrome Prediction Model Using HOMA-IR and Multivariate Factors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Variables
2.2.1. Analysis of MetS-Related Factors
- Waist circumference: ≥102 cm in men and ≥88 cm in women;
- Hypertriglyceridemia: a blood triglyceride level of ≥150 mg/dL;
- Decreased high-density lipoprotein (HDL) cholesterol levels: HDL cholesterol levels of <40 mg/dL in men and <50 mg/dL in women;
- Hypertension: Blood pressure of ≥130/85 mmHg or treatment for hypertension;
- Hyperglycemia: a fasting blood glucose level of ≥100 mg/dL or treatment for Diabetes Mellitus.
2.2.2. Homeostatic Model Assessment for Insulin Resistance
2.2.3. Building a Predictive Model Using a Logistic Regression Analysis
β4 = 0.649(Diabetes Mellitus = 1, non-Diabetes Mellitus = 0)
2.3. Statistical Analysis
3. Results
3.1. Comparison of Anthropometric, Clinical, and Biochemical Characteristics Between Patients with and Without Diabetes Mellitus
3.2. Multiple Logistic Regression Analysis of Variables Affecting the Occurrence of Metabolic Syndrome
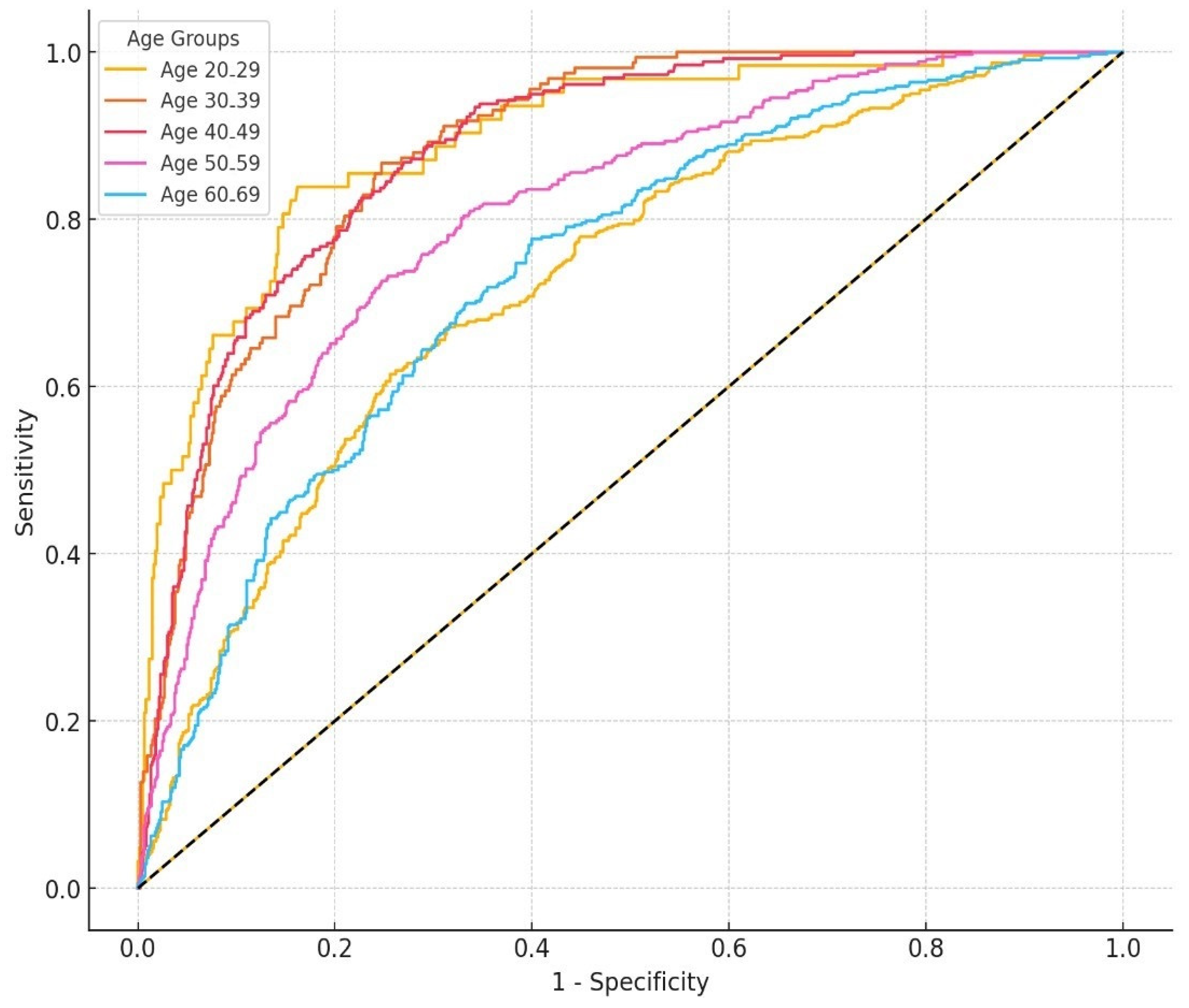
3.3. ROC Curve Based on a Logistic Regression Model and AUC Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kassi, E.; Pervanidou, P.; Kaltsas, G.; Chrousos, G. Metabolic syndrome: Definition and controversies. BMC Med. 2011, 9, 48. [Google Scholar] [CrossRef]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.J.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, L.J.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [PubMed]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Bellanger, T. Epidemiology, trends and morbidities of obesity and the metabolic syndrome. Endocrine 2006, 29, 109–117. [Google Scholar] [CrossRef]
- Danaei, G.; Finucane, M.M.; Lu, Y.; Singh, G.M.; Cowan, M.J.; Paciorek, C.J.; Lin, J.K.; Farzadfar, F.; Khang, Y.-H.; Stevens, G.A.; et al. Global burden of metabolic risk factors of chronic diseases collaborating group (Blood Glucose). National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: Systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 2011, 378, 31–40. [Google Scholar]
- Finucane, M.M.; Stevens, G.A.; Cowan, M.J.; Danaei, G.; Lin, J.K.; Paciorek, C.J.; Singh, G.M.; Gutierrez, H.R.; Lu, Y.; Bahalin, A.N.; et al. Global burden of metabolic risk factors of chronic diseases collaborating group (body mass index). National, regional, and global trends in body-mass index since 1980: Systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 2011, 377, 557–567. [Google Scholar]
- Rader, D.J. Effect of insulin resistance, dyslipidemia, and intra-abdominal adiposity on the development of cardiovascular disease and diabetes mellitus. Am. J. Med. 2007, 120, S1–S8. [Google Scholar] [CrossRef]
- Goodwin, P.; Ennis, M.; Bahl, M. High insulin levels in newly diagnosed breast cancer patients reflect underlying insulin resistance and are associated with components of the insulin resistance syndrome. Breast Cancer Res. Treat. 2009, 114, 517–525. [Google Scholar] [CrossRef]
- Seriolo, B.; Ferrone, C.; Cutolo, M. Long-term anti-tumor necrosis factor α treatment in patients with refractory rheumatoid arthritis: Relationship between insulin resistance and disease activity. J. Rheumatol. 2008, 35, 355–357. [Google Scholar]
- Lann, D.; LeRoith, D. Insulin resistance as the underlying cause for the metabolic syndrome. Med. Clin. N. Am. 2007, 91, 1063–1077. [Google Scholar] [CrossRef] [PubMed]
- Antuna-Puente, B.; Disse, E.; Rabasa-Lhoret, R.; Laville, M.; Capeau, J.; Bastard, J.P. How can we measure insulin sensitivity/resistance? Diabetes Metab. 2011, 37, 179–188. [Google Scholar] [CrossRef]
- Pepe, M.S. The Statistical Evaluation of Medical Tests for Classification and Prediction; Oxford University Press: New York, NY, USA, 2003. [Google Scholar]
- Wilson, P.W.F.; D’Agostino, R.B.; Levy, D.; Belanger, A.M.; Silbershatz, H.; Kannel, W.B. Prediction of coronary heart disease using risk factor categories. Circulation 1998, 97, 1837–1847. [Google Scholar] [CrossRef] [PubMed]
- Lean, M.E.; Han, T.S.; Morrison, C.E. Waist circumference as a measure for indicating need for weight management. BMJ 1995, 311, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Mirmiran, P.; Bahadoran, Z.; Vakili, A.Z.; Navab, M. The association of dietary patterns and the metabolic syndrome. Iran. J. Public Health 2009, 38, 1–2. [Google Scholar]
- Warburton, D.E.R.; Nicol, C.W.; Bredin, S.S.D. Health benefits of physical activity: The evidence. Can. Med. Assoc. J. 2006, 174, 801–809. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Gayoso-Diz, P.; Otero-González, A.; Rodriguez-Alvarez, M.X.; Gude, F.; Cadarso-Suarez, C.; García, F.; De Francisco, A. IR index (HOMA-IR) levels in a general adult population: Curves percentile by gender and age. The EPIRCE study. Diabetes Res. Clin. Pract. 2011, 94, 146–155. [Google Scholar] [CrossRef]
- Otero, A.; De Francisco, A.; Gayoso, P.; Garcia, F. Prevalence of chronic renal disease in Spain: Results of the EPIRCE Study. Nefrologia 2010, 30, 78–86. [Google Scholar]
- Gayoso-Diz, P.; Otero-González, A.; Rodriguez-Alvarez, M.X.; Gude, F.; García, F.; De Francisco, A.; González Quintela, A. Insulin resistance (HOMA-IR) cut-off values and the metabolic syndrome in a general adult population: Effect of gender and age: EPIRCE cross-sectional study. BMC Endocr. Disord. 2013, 13, 47. [Google Scholar] [CrossRef]
- Miccoli, R.; Biamchi, C.; Odoguardi, L. Prevalence of the metabolic syndrome among Italian adults according to ATPII definition. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Ascaso, J.F.; Romero, P.; Real, J.T.; Priego, A.; Valdecabres, C.; Carmena, R. Insulin resistance quantification by fasting insulin plasma values and HOMA index in a non-diabetic population. Med. Clin. 2001, 117, 530–533. [Google Scholar] [CrossRef]
- Esteghamati, A.; Ashraf, H.; Khalilzadeh, O.; Zandieh, A.; Nakhjavani, M.; Rashidi, A.; Haghazali, M.; Asgari, F. Optimal cut-off of homeostasis model assessment of IR (HOMA-IR) for the diagnosis of metabolic syndrome: Third national surveillance of risk factors of non-communicable diseases in Iran (SuRFNCD-2007). Nutr. Metab. 2010, 7, 26. [Google Scholar] [CrossRef]
- Shiny, A.; Bibin, Y.S.; Shanthirani, C.S.; Regin, B.S.; Anjana, R.M.; Balasubramanyam, M.; Jebarani, S.; Mohan, V. Association of neutrophil-lymphocyte ratio with glucose intolerance: An indicator of systemic inflammation in patients with type 2 diabetes. Diabetes Technol. Ther. 2014, 16, 524–530. [Google Scholar] [CrossRef]
- Feng, Y.-M.; Zhao, D.; Zhang, N.; Yu, C.-G.; Zhang, Q.; Thijs, L.; Staessen, J.A. Insulin resistance in relation to lipids and inflammation in type-2 diabetic patients and non-diabetic people. PLoS ONE 2016, 11, e0153171. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, X.; Guo, S.; Li, R.; Li, Y.; Yu, Y.; Liu, T. Correlation between chronic low-grade inflammation and glucose and lipid metabolism indicators in polycystic ovary syndrome. Gynecol. Endocrinol. 2024, 40, 2302402. [Google Scholar] [CrossRef]
- Panagiotakos, D.; Chrysohoou, C.; Pitsavos, C.; Tsioufis, K. Prediction of 10-year cardiovascular disease risk, by diabetes status and lipoprotein-a levels; the HellenicSCORE II+. Hell. J. Cardiol. 2023, 79, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Tune, J.D.; Goodwill, A.G.; Sassoon, D.J.; Mather, K.J. Cardiovascular consequences of metabolic syndrome. Transl. Res. 2017, 183, 57–70. [Google Scholar] [CrossRef]
- Welty, F.K.; Alfaddagh, A.; Elajami, T.K. Targeting inflammation in metabolic syndrome. Transl. Res. 2016, 167, 257–280. [Google Scholar] [CrossRef]
- Wilson, P.W.; Kannel, W.B.; Silbershatz, H.; D’Agostino, R.B. Clustering of metabolic factors and coronary heart disease. Arch. Intern. Med. 1999, 159, 1104–1109. [Google Scholar] [CrossRef]

| Variable | Excluded (n = 1976) | Included (n = 6134) | p |
|---|---|---|---|
| Men | 990 (50.1) | 3120 (50.8) | 0.45 |
| Women | 986 (49.9) | 3014 (49.2) | |
| Age (years) | 52.31 ± 16.72 | 51.7 ± 16.93 | 0.45 |
| Waist circumference (cm) | 83.52 ± 13.34 | 84.08 ± 10.44 | 0.35 |
| Variable | Diabetes Mellitus (n = 624) | Non-Diabetes Mellitus (n = 5672) | p |
|---|---|---|---|
| Sex | |||
| Men | 316 (50.6) | 2479 (43.7) | <0.001 |
| Women | 308 (49.4) | 3193 (56.3) | |
| Age (years) | 65.69 ± 10.94 | 50.44 ± 16.83 | <0.001 |
| Waist circumference (cm) | 90.63 ± 9.75 | 83.35 ± 10.28 | <0.001 |
| Triglycerides (mg/dL) | 154.48 ± 127.39 | 129.28 ± 96.87 | <0.001 |
| HDL-cholesterol (md/dL) | 48.05 ± 12.00 | 53.27 ± 12.84 | <0.001 |
| Systolic blood pressure (mmHg) | 127.68 ± 17.04 | 119.06 ± 16.37 | <0.001 |
| Diastolic blood pressure (mmHg) | 73.37 ± 9.76 | 75.87 ± 9.79 | <0.001 |
| Fasting blood glucose (md/dL) | 137.91 ± 41.52 | 97.65 ± 16.46 | <0.001 |
| HOMA-IR (units) | 3.85 ± 4.96 | 2.30 ± 3.41 | <0.001 |
| Metabolic syndrome (ATP III) | |||
| Yes | 339 (57.4) | 1364 (24.8) | <0.001 |
| No | 252 (42.6) | 4139 (75.2) |
| Variable | B | Odds Ratio (95%) |
|---|---|---|
| HOMA-IR | 0.448 | 1.566 (1.504–1.630) |
| Sex | 0.391 | 1.479 (1.303–1.678) |
| Age | 0.033 | 1.033 (1.029–1.037) |
| Diabetes Mellitus | 0.649 | 1.914 (1.572–2.330) |
| Intercept | −4.067 |
| Age | Model * | Cut-Off Value * | Sensitivity | Specificity | AUC (95%CI) | p |
|---|---|---|---|---|---|---|
| Overall (n = 6134) | Combined | 0.241 | 0.800 | 0.689 | 0.819 (0.807–0.829) | <0.001 |
| HOMA-IR only | 1.935 | 0.757 | 0.645 | 0.761 (0.748–0.773) | <0.001 | |
| 20–29 years (n = 742) | Combined | 0.142 | 0.839 | 0.838 | 0.864 (0.853–0.935) | <0.001 |
| HOMA-IR only | 2.745 | 0.806 | 0.803 | 0.860 (0.820–0.920) | <0.001 | |
| 30–39 years (n = 912) | Combined | 0.150 | 0.867 | 0.753 | 0.882 (0.857–0.906) | <0.001 |
| HOMA-IR only | 2.190 | 0.778 | 0.731 | 0.827 (0.806–0.868) | <0.001 | |
| 40–49 years (n = 1103) | Combined | 0.191 | 0.826 | 0.773 | 0.882 (0.861–0.903) | <0.001 |
| HOMA-IR only | 2.210 | 0.806 | 0.754 | 0.840 (0.814–0.866) | <0.001 | |
| 50–59 years (n = 1160) | Combined | 0.243 | 0.726 | 0.752 | 0.805 (0.778–0.831) | <0.001 |
| HOMA-IR only | 1.435 | 0.826 | 0.536 | 0.786 (0.771–0.821) | <0.001 | |
| ≥60 years (n = 2217) | Combined | 0.282 | 0.776 | 0.600 | 0.737 (0.708–0.767) | <0.001 |
| HOMA-IR only | 2.025 | 0.703 | 0.653 | 0.727 (0.705–0.748) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heo, A.-S.; Lee, J.-C. Development of a Metabolic Syndrome Prediction Model Using HOMA-IR and Multivariate Factors. Appl. Sci. 2025, 15, 2985. https://doi.org/10.3390/app15062985
Heo A-S, Lee J-C. Development of a Metabolic Syndrome Prediction Model Using HOMA-IR and Multivariate Factors. Applied Sciences. 2025; 15(6):2985. https://doi.org/10.3390/app15062985
Chicago/Turabian StyleHeo, An-Sik, and Jung-Chul Lee. 2025. "Development of a Metabolic Syndrome Prediction Model Using HOMA-IR and Multivariate Factors" Applied Sciences 15, no. 6: 2985. https://doi.org/10.3390/app15062985
APA StyleHeo, A.-S., & Lee, J.-C. (2025). Development of a Metabolic Syndrome Prediction Model Using HOMA-IR and Multivariate Factors. Applied Sciences, 15(6), 2985. https://doi.org/10.3390/app15062985






