Nitrate and Bacterial Loads in Dairy Cattle Drinking Water and Potential Treatment Options for Pollutants—A Review
Abstract
1. Introduction
2. Materials and Methods
3. Nitrate and Bacterial Contamination in Dairy Cattle Drinking Water
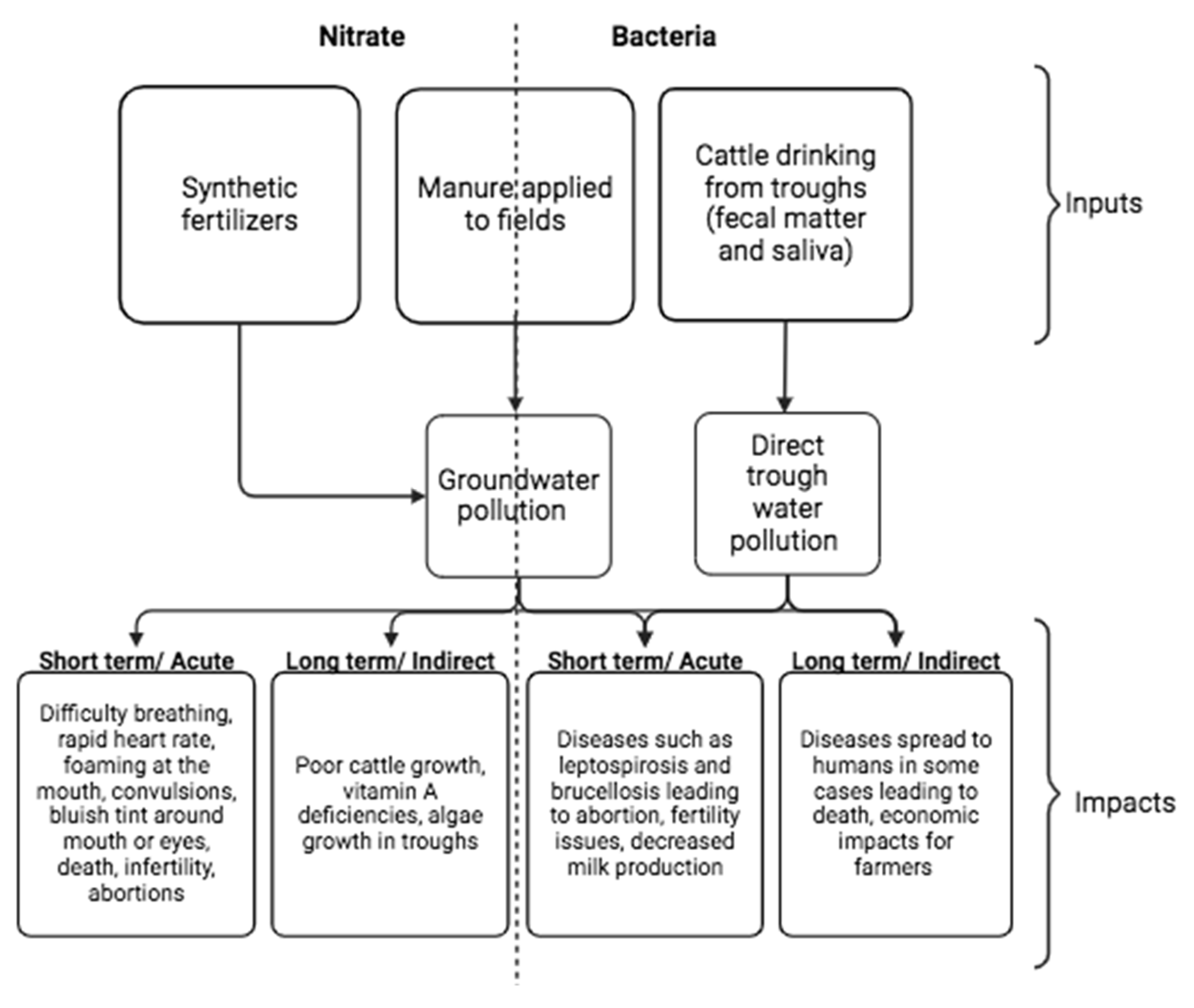
3.1. Nitrate and Microbial Pollution Present in Dairy Cattle Drinking Water: Sources, Recommended Levels, and Health Impacts
3.2. Current Manure Handling Practices
3.3. Water Treatment Options
3.4. Filter Media Options for Adsorption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lowrance, R. Management of Dairy Cattle Manure. Available online: https://sciwheel.com/work/item/16031339/resources/18681202/pdf (accessed on 12 February 2024).
- Jensen, V.B.; Darby, J.L.; Seidel, C. Technical Report 6: Drinking Water Treatment for Nitrate. Published online July 2012. Available online: https://ucanr.edu/sites/groundwaternitrate/files/139107.pdf (accessed on 15 August 2023).
- Pfost, D.L.; Fulhage, C.D. Water Quality for Livestock Drinking|MU Extension. Water Quality for Livestock Drinking. February 2001. Available online: https://extension.missouri.edu/publications/eq381 (accessed on 9 March 2025).
- USPHS/FDA USD of H and HS. Grade “A” Pasteurized Milk Ordinance. Published online 2019. Available online: https://www.fda.gov/media/114169/download (accessed on 29 August 2023).
- Dyer, R.; Cattle, E. Water Requirements and Quality Issues for Cattle. Available online: https://secure.caes.uga.edu/extension/publications/files/pdf/SB%2056_5.PDF (accessed on 9 March 2025).
- Rossi, J.; Pence, M. Water Requirements and Quality Issues for Cattle|UGA Cooperative Extension. March 2007. Available online: https://hdl.handle.net/10724/11999 (accessed on 30 July 2024).
- NRCS. Watering Systems for Serious Grazers. Published online August 2006. Available online: https://sciwheel.com/work/item/15304410/resources/17660967/pdf (accessed on 26 August 2023).
- Socha, M. Variability of Water Composition and Potential Impact On Animal Performance. AABP Proc. 2003, 36, 85–96. [Google Scholar]
- Mohammed, A.N. Field study on evaluation of the efficacy and usability of two disinfectants for drinking water treatment at small cattle breeders and dairy cattle farms. Environ. Monit. Assess. 2016, 188, 151. [Google Scholar] [CrossRef]
- Thomas, C.; Beede, D. Michigan Dairy Review: Thumb H20 Project: Water Treatment Options [Part 2]. Published online April 2011. Available online: https://sciwheel.com/work/item/15342684/resources/17735043/pdf (accessed on 11 June 2024).
- Harter, T.; Dzurella, K.; Kourakos, G.; Hollander, A.; Bell, A.; Santos, N.; Hart, Q.; King, A.; Quinn, J.; Lampinen, G.; et al. Nitrogen Loading to Groundwater in the Central Valley. Final Report to the Fertilizer Research Education Program, Projects. 2017 Aug:11-0301. Available online: https://www.cdfa.ca.gov/is/ffldrs/frep/pdfs/CompletedProjects/15-0454_partialFR-Harter.pdf (accessed on 4 March 2025).
- Priya, E.; Kumar, S.; Verma, C.; Sarkar, S.; Maji, P.K. A comprehensive review on technological advances of adsorption for removing nitrate and phosphate from waste water. J. Water Process Eng. 2022, 49, 103159. [Google Scholar] [CrossRef]
- Balazs, C.; Morello-Frosch, R.; Hubbard, A.; Ray, I. Social disparities in nitrate-contaminated drinking water in California’s San Joaquin Valley. Environ. Health Perspect. 2011, 119, 1272–1278. [Google Scholar] [CrossRef]
- Mehrabi, N.; Soleimani, M.; Yeganeh, M.M.; Sharififard, H. Parameter optimization for nitrate removal from water using activated carbon and composite of activated carbon and Fe2 O3 nanoparticles. RSC Adv. 2015, 5, 51470–51482. [Google Scholar] [CrossRef]
- Strickland, G.; Richards, C.; Zhang, H.; Step, D.L. Nitrate Toxicity in Livestock|Oklahoma State University. Nitrate Toxicity in Livestock. February 2017. Available online: https://extension.okstate.edu/fact-sheets/nitrate-toxicity-in-livestock.html (accessed on 29 February 2024).
- National Research Council, Board on Agriculture and Natural Resources, Committee on Animal Nutrition, Subcommittee on Dairy Cattle Nutrition. Nutrient Requirements of Dairy Cattle: Seventh Revised Edition; National Academies Press: Washington, DC, USA, 2001; Available online: https://nap.nationalacademies.org/catalog/9825/nutrient-requirements-of-dairy-cattle-seventh-revised-edition-2001 (accessed on 4 March 2025).
- Kumar, M.; Puri, A. A review of permissible limits of drinking water. Indian J. Occup. Environ. Med. 2012, 16, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Wunderly, M. Maintaining a Clean Water Trough for Cattle. Available online: https://sciwheel.com/work/item/16407823/resources/19153617/pdf (accessed on 30 April 2024).
- Stegal, K.; NCCooperative Extension, K. Reducing the Algae in Your Livestock Water Tanks|N.C. Cooperative Extension. 23 March 2020. Available online: https://stanly.ces.ncsu.edu/wp-content/uploads/2023/06/Summer-2023-Tri-County-Livestock-Newsletter-1.pdf?fwd=no (accessed on 4 March 2025).
- Oun, A.; Kumar, A.; Harrigan, T.; Angelakis, A.; Xagoraraki, I. Effects of biosolids and manure application on microbial water quality in rural areas in the US. Water 2014, 6, 3701–3723. [Google Scholar] [CrossRef]
- Goldfarb, W. The clean water act: Introduction. In Water Law; CRC Press: Boca Raton, FL, USA, 2020; pp. 167–170. [Google Scholar] [CrossRef]
- Estimated Nitrate Concentrations in Groundwater Used for Drinking|US EPA. Estimated Nitrate Concentrations in Groundwater Used for Drinking|US EPA. 5 July 2023. Available online: https://www.epa.gov/nutrient-policy-data/estimated-nitrate-concentrations-groundwater-used-drinking (accessed on 9 August 2023).
- Blake, S.B. Spatial relationships among dairy farms, drinking water quality, and maternal-child health outcomes in the San Joaquin Valley. Public Health Nurs. 2014, 31, 492–499. [Google Scholar] [CrossRef]
- Millner, P.D. Manure Management. In The Produce Contamination Problem; Elsevier: Amsterdam, The Netherlands, 2009; pp. 79–104. [Google Scholar] [CrossRef]
- Lorimor, J.; Fulhage, C.; Zhang, R. Manure management strategies and technologies. In Animal Agriculture and the Environment, National Center for Manure & Animal Waste Management White Papers; Manure Management Strategies and Technologies; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2006. [Google Scholar] [CrossRef]
- Chang, A.; Harter, T.; Letey, J. Managing Dairy Manure in the Central Valley of California. Published online June 2005. Available online: https://groundwater.ucdavis.edu/files/136450.pdf (accessed on 9 March 2025).
- Willers, H.C.; Karamanlis, X.N.; Schulte, D.D. Potential of closed water systems on dairy farms. Water Sci. Technol. 1999, 39, 113–119. [Google Scholar] [CrossRef]
- Harter, T.; Davis, H.; Mathews, M.C.; Meyer, R.D. Shallow groundwater quality on dairy farms with irrigated forage crops. J. Contam. Hydrol. 2002, 55, 287–315. [Google Scholar] [CrossRef]
- Viers, J.H.; Liptzin, D.; Rosenstock, T.S. Nitrogen Sources and Loading to Groundwater. 2012. Available online: https://ucanr.edu/sites/groundwaternitrate/files/139110.pdf (accessed on 4 March 2025).
- Cho, J.C.; Cho, H.B.; Kim, S.J. Heavy contamination of a subsurface aquifer and a stream by livestock wastewater in a stock farming area, Wonju, Korea. Environ. Pollut. 2000, 109, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Urseler, N.; Bachetti, R.; Morgante, V.; Agostini, E.; Morgante, C. Groundwater quality and vulnerability in farms from agricultural-dairy basin of the Argentine Pampas. Environ. Sci. Pollut. Res. Int. 2022, 29, 63655–63673. [Google Scholar] [CrossRef] [PubMed]
- Beede, D.K. Water nutrition and quality for dairy cattle. In Proceedings of the Western Large Herd Dairy Management Conference, Las Vegas, NV, USA, 22–24 April 1993; p. 194. [Google Scholar]
- Hafemeister, G. Providing High-Quality Drinking Water for Dairy Cows. Wisconsin State Farmer. 11 October 2016. Available online: https://www.wisfarmer.com/story/news/national/2016/10/11/providing-high-quality-drinking-water-dairy-cows/91892174/ (accessed on 4 March 2025).
- Distillation for Home Water Treatment. Published online January 1990. Available online: https://www.extension.purdue.edu/extmedia/wq/wq-12.html (accessed on 4 March 2025).
- Beede, D. Solving Bad Water Problems for Thirsty Cows. 2009. Available online: http://wdmc.org/2009/Solving%20Bad%20Water%20Problems%20for%20Thirsty%20Cows.pdf (accessed on 9 October 2023).
- Gan, L.; Wu, Y.; Song, H.; Zhang, S.; Lu, C.; Yang, S.; Wang, Z.; Jiang, B.; Wang, C.; Li, A. Selective removal of nitrate ion using a novel activated carbon composite carbon electrode in capacitive deionization. Sep. Purif. Technol. 2019, 212, 728–736. [Google Scholar] [CrossRef]
- Matei, A. Review of the Technologies for Nitrates Removal from Water Intended for Human Consumption. 2021. Available online: https://iopscience.iop.org/article/10.1088/1755-1315/664/1/012024/pdf (accessed on 9 April 2024).
- CDC. Water Treatment|Public Water Systems|Drinking Water|Healthy Water|CDC. Water Treatment. 16 April 2022. Available online: https://www.cdc.gov/healthywater/drinking/public/water_treatment.html (accessed on 30 July 2024).
- US EPA. Wastewater Technology Fact Sheet: Ultraviolet Disinfection. Published online September 1999. Available online: https://www3.epa.gov/npdes/pubs/uv.pdf (accessed on 9 April 2024).
- Lotfy, H.R.; Roubík, H. Water purification using activated carbon prepared from agriculture waste—Overview of a recent development. Biomass Conv. Biorefinery 2021, 13, 15577–15590. [Google Scholar] [CrossRef]
- California Water Boards. Frequently Asked Questions: Biological Treatment Systems for Nitrate Removal in Drinking Water Applications. Published online 23 June 2020. Available online: https://www.waterboards.ca.gov/drinking_water/certlic/drinkingwater/documents/publicwatersystems/faq_2020_bio_ts.pdf (accessed on 4 March 2025).
- Gizaw, A.; Zewge, F.; Kumar, A.; Mekonnen, A.; Tesfaye, M. A comprehensive review on nitrate and phosphate removal and recovery from aqueous solutions by adsorption. J. Water Supply Res. Technol.-AQUA Publ. 2021, 70, 921–947. [Google Scholar] [CrossRef]
- Aktar, J. Batch adsorption process in water treatment. In Intelligent Environmental Data Monitoring for Pollution Management; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–24. [Google Scholar] [CrossRef]
- Razali, M.C.; Wahab, N.A.; Sunar, N.; Shamsudin, N.H. Existing filtration treatment on drinking water process and concerns issues. Membranes 2023, 13, 285. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Abou-Zeid, R.; Hassan, E.; Berglund, L.; Aitomäki, Y.; Oksman, K. Membranes based on cellulose nanofibers and activated carbon for removal of Escherichia coli bacteria from water. Polymers 2017, 9, 335. [Google Scholar] [CrossRef]
- CPL Puragen. What Is Granular Activated Carbon?|CPL Activated Carbons. Granular Activated Carbon. 10 June 2022. Available online: https://activated-carbon.com/news/granular-activated-carbon/ (accessed on 4 March 2025).
- Bhatnagar, A.; Hogland, W.; Marques, M.; Sillanpää, M. An overview of the modification methods of activated carbon for its water treatment applications. Chem. Eng. J. 2013, 219, 499–511. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Hameed, B.H.; Khan, M.A. Recent advances on activated carbon-based materials for nitrate adsorption: A review. J. Anal. Appl. Pyrolysis 2023, 169, 105856. [Google Scholar] [CrossRef]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for preparation and activation of activated carbon: A review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Schmidt, H.-P.; Hagemann, N.; Draper, K.; Kammann, C. The use of biochar in animal feeding. PeerJ 2019, 7, e7373. [Google Scholar] [CrossRef] [PubMed]
- Phillips, B.M.; Fuller, L.B.M.; Siegler, K.; Deng, X.; Tjeerdema, R.S. Treating Agricultural Runoff with a Mobile Carbon Filtration Unit. Arch. Environ. Contam. Toxicol. 2022, 82, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Hoslett, J.; Massara, T.M.; Malamis, S.; Ahmad, D.; Boogaert, I.v.D.; Katsou, E.; Ahmad, B.; Ghazal, H.; Simons, S.; Wrobel, L.; et al. Surface water filtration using granular media and membranes: A review. Sci Total Environ. 2018, 639, 1268–1282. [Google Scholar] [CrossRef] [PubMed]
- Azis, K.; Mavriou, Z.; Karpouzas, D.G.; Ntougias, S.; Melidis, P. Evaluation of Sand Filtration and Activated Carbon Adsorption for the Post-Treatment of a Secondary Biologically-Treated Fungicide-Containing Wastewater from Fruit-Packing Industries. Processes 2021, 9, 1223. [Google Scholar] [CrossRef]
- Huno, S.K.M.; Rene, E.R.; van Hullebusch, E.D.; Annachhatre, A.P. Nitrate removal from groundwater: A review of natural and engineered processes. J. Water Supply Res. Technol.-AQUA 2018, 67, 885–902. [Google Scholar] [CrossRef]
- Aslan, S. Biological nitrate removal in a laboratory-scale slow sand filter. WSA 2018, 34, 99. [Google Scholar] [CrossRef]
- Gatti, M.N.; Fernández, L.G.; Sánchez, M.P.; Parolo, M.E. Aminopropyltrimethoxysilane- and aminopropyltrimethoxysilane-silver-modified montmorillonite for the removal of nitrate ions. Environ. Technol. 2016, 37, 2658–2668. [Google Scholar] [CrossRef]
- Hussain, Z.; Cheng, T.; Irshad, M.; Khattak, R.A.; Yao, C.; Song, D.; Mohiuddin, M. Bentonite clay with different nitrogen sources can effectively reduce nitrate leaching from sandy soil. PLoS ONE 2022, 17, e0278824. [Google Scholar] [CrossRef]
- Bekele, W.; Faye, G.; Fernandez, N. Removal of nitrate ion from aqueous solution by modified ethiopian bentonite clay. Int. J. Res. Pharm. Chem. 2014, 192–201. Available online: https://www.semanticscholar.org/paper/REMOVAL-OF-NITRATE-ION-FROM-AQUEOUS-SOLUTION-BY-Bekele-Faye/2e8362266a64c394b9c10eadd78a270fe6a7c964 (accessed on 4 March 2025).
- Chauhan, T.; Szőri-Dorogházi, E.; Muránszky, G.; Kecskés, K.; Finšgar, M.; Szabó, T.; Leskó, M.; Németh, Z.; Hernadi, K. Application of modified clays in the removal of phosphates and E. coli from aqueous solution. Environ. Nanotechnol. Monit. Manag. 2024, 22, 100965. [Google Scholar] [CrossRef]
- Álvarez-Chávez, E.; Godbout, S.; Rousseau, A.N.; Brassard, P.; Fournel, S. Performance of Various Filtering Media for the Treatment of Cow Manure from Exercise Pens—A Laboratory Study. Water 2022, 14, 1912. [Google Scholar] [CrossRef]
- Robertson, W.D.; Ford, G.I.; Lombardo, P.S. Wood-based filter for nitrate removal in septic systems. Trans. ASAE 2005, 48, 121–128. [Google Scholar] [CrossRef]
- Lopez-Ponnada, E.V.; Lynn, T.J.; Peterson, M.; Ergas, S.J.; Mihelcic, J.R. Application of denitrifying wood chip bioreactors for management of residential non-point sources of nitrogen. J. Biol. Eng. 2017, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Schipper, L.A.; Robertson, W.D.; Gold, A.J.; Jaynes, D.B.; Cameron, S.C. Denitrifying bioreactors—An approach for reducing nitrate loads to receiving waters. Ecol. Eng. 2010, 36, 1532–1543. [Google Scholar] [CrossRef]
- Soupir, M.L.; Hoover, N.L.; Moorman, T.B.; Law, J.Y.; Bearson, B.L. Impact of temperature and hydraulic retention time on pathogen and nutrient removal in woodchip bioreactors. Ecol. Eng. 2018, 112, 153–157. [Google Scholar] [CrossRef]
- Shukla, A.; Zhang, Y.-H.; Dubey, P.; Margrave, J.L.; Shukla, S.S. The role of sawdust in the removal of unwanted materials from water. J. Hazard. Mater. 2002, 95, 137–152. [Google Scholar] [CrossRef]
- Xi, Y.; Mallavarapu, M.; Naidu, R. Preparation, characterization of surfactants modified clay minerals and nitrate adsorption. Appl. Clay Sci. 2010, 48, 92–96. [Google Scholar] [CrossRef]
- Charki, A.; Ahari, M.; Hadoudi, N.; Benyoub, B.; Zaki, N.; Fraiha, O.; El Hammoudani, Y.; Elyoussfi, A.; Salhi, A.; El Ouarghi, H. Natural Moroccan bentonite clay as an adsorbent for nutrient removal from synthetic leachate: Performance and evaluation. Desalination Water Treat. 2024, 318, 100381. [Google Scholar] [CrossRef]
- Synder, J.W.; Mains, C.N.; Anderson, R.E.; Bissonnette, G.K. Effect of point-of-use, activated carbon filters on the bacteriological quality of rural groundwater supplies. Appl. Environ. Microbiol. 1995, 61, 4291–4295. [Google Scholar] [CrossRef]
- Mazarji, M.; Aminzadeh, B.; Baghdadi, M.; Bhatnagar, A. Removal of nitrate from aqueous solution using modified granular activated carbon. J. Mol. Liq. 2017, 233, 139–148. [Google Scholar] [CrossRef]
- Wu, C.-C.; Love, N.G.; Olson, T.M. Bacterial transmission and colonization in activated carbon block (ACB) point-of-use (PoU) filters. Environ. Sci. Water Res. Technol. 2021, 7, 1114–1124. [Google Scholar] [CrossRef]
- Schipper, L.; Vojvodić-Vuković, M. Nitrate Removal from Groundwater Using a Denitrification Wall Amended with Sawdust: Field Trial. J. Environ. Qual. 1998, 27, 664–668. [Google Scholar] [CrossRef]
- Soltanpour, P.N.; Raley, W.L. Livestock Drinking Water Quality. Colorado State University Extension Service. 1999. Available online: https://ucanr.edu/sites/Rangelands/files/294869.pdf (accessed on 4 March 2025).
- Pfost, D.L.; Fulhage, C.D.; Casteel, S. Water Quality for Livestock Drinking. MU Ext. Univ. Missouri-Columbia. EQ381. 2001. Available online: https://core.ac.uk/download/pdf/62768476.pdf (accessed on 4 March 2025).
- Olkowski, A.A. Livestock Water Quality: A Field Guide for Cattle, Horses, Poultry, and Swine; Agriculture and Agri-Food Canada: Ottawa, ON, Canada, 2009; 157p, Available online: https://www.ag.ndsu.edu/waterquality/livestock/Livestock_Water_QualityFINALweb.pdf (accessed on 4 March 2025).
- California State Water Resources Control Board. Nitrate Project|California State Water Resources Control Board. 7 July 2015. Available online: https://www.waterboards.ca.gov/water_issues/programs/nitrate_project/ (accessed on 16 August 2023).
- LeJeune, J.T.; Besser, T.E.; Merrill, N.L.; Rice, D.H.; Hancock, D.D. Livestock drinking water microbiology and the factors influencing the quality of drinking water offered to cattle. J. Dairy Sci. 2001, 84, 1856–1862. [Google Scholar] [CrossRef] [PubMed]
- Münster, P.; Kemper, N. Long-term analysis of drinking water quality in poultry and pig farms in Northwest Germany. Front. Anim. Sci. 2024, 5, 1467287. [Google Scholar] [CrossRef]
- Mohamed, A.; Khalil, M.; Soliman, F.; El-Sabrout, K. The Effect of Drinking Ionized Water on the Productive Performance, Physiological Status, and Carcass Characteristics of Broiler Chicks. Animals 2025, 15, 229. [Google Scholar] [CrossRef] [PubMed]
- Pokle, S.B.; Bhardwaj, T.; Goldar, A.; Pande, A.; Wagh, S. Smart water dispenser for animals: Automated filling, monitoring, water quality assessment. In Technological Innovations & Applications in Industry 4.0; CRC Press: Boca Raton, FL, USA, 2025; pp. 268–273. [Google Scholar]
- Grossi, S.; Rossi, L.; Dell’Anno, M.; Biffani, S.; Rossi, C.A. Effects of heated drinking water on the growth performance and rumen functionality of fattening charolaise beef cattle in winter. Animals 2021, 11, 2218. [Google Scholar] [CrossRef]
- Ward, M.H.; Jones, R.R.; Brender, J.D.; De Kok, T.M.; Weyer, P.J.; Nolan, B.T.; Villanueva, C.M.; Van Breda, S.G. Drinking water nitrate and human health: An updated review. Int. J. Environ. Res. Public Health 2018, 15, 1557. [Google Scholar] [CrossRef]

| Media Type | Targeted Pollutant (NO3− or E. coli) | Methods/Conditions | Effectiveness | Reference |
|---|---|---|---|---|
| Bentonite clay | NO3− | Varying percentages (0, 2, 4%) of bentonite added to sandy soils and leachate of fertilizer measured after filtering through columns | Increasing amounts of bentonite decreased nitrate leaching and increased NO3− retention in soil (4% bentonite decreased leachate concentrations by 12–19%) | [57,66] |
| NO3− | Bentonite clay is modified with HDTMA and then mixed and centrifuged with nitrate solution (100 mg/L) | The modification method modified the surface to be positive which greatly increased the nitrate adsorption of bentonite as compared to the unmodified form which was essentially ineffective | [66,67,68] | |
| NO3− | Batch experimentation with unmodified bentonite and synthetic landfill leachate mixed together for varying durations and in different ambient temperatures with the mixture filtered and analyzed for nitrate (and other forms of N) concentrations | Longer mixing times and higher temperatures lead to higher adsorption rates. A maximum reduction of 17.33% nitrate was achieved with bentonite | [67,69] | |
| NO3− | Bentonite clay activated with HCL used in batch experimentation with mixing and centrifugation to assess nitrate adsorption | The activated bentonite is able to achieve an 80% nitrate ion reduction from aqueous solution with a maximum adsorption of 7.5 mg/g under ideal conditions | [58,68,70] | |
| Activated carbon | NO3− | GAC treated with both sodium hydroxide and a cationic surfactant used to assess nitrate adsorption within batch experiments | The maximum adsorption capacity for the modified GAC found to be 21.51 mg/g | [69] |
| NO3− | A review of modified, composite, and raw forms of AC and its ability to adsorb nitrate | Modified and composite forms of AC generally have higher adsorption capacity for nitrate than raw forms | [48,66,69] | |
| E. coli | Point-of-use AC filtration units were installed at homes with private wells, and influent and effluent bacterial concentrations were counted and compared | AC filter effluent bacteria levels were elevated as compared to influent numbers but only if left stagnant overnight; with flushing (2 min), the AC filtered effluent had lower bacteria counts compared to influent | [68,70,71] | |
| E. coli | Commercial activated carbon block filters were tested for their ability to filter E. coli under simulated household usage patterns for 29 days | As compared to the spiked influent level, the effluent bacteria counts of E. coli varied from 24 to 60% | [70,71] | |
| E. coli | AC modified with six different antimicrobial chemicals used for column filtration. Filter media post filtration are inoculated on agar plates, and colonies are counted after incubation | All six filter media showed >6 log removal, the recommended USEPA level for human drinking water, even with extended use | [45] | |
| Wood-based material (i.e., woodchips) | NO3− | Sawdust mixed into soil (30% by volume) and constructed into a denitrification wall to intercept groundwater with nitrate removal measured and calculated | Over the study duration of 1 year, it was determined that the denitrification wall can effectively reduce nitrate concentrations in groundwater | [71] |
| NO3− and E. coli | Upflow woodchip bioreactor columns designed to test the E. coli and nitrate removal capacity at different temperatures and hydraulic retention times | At the higher ambient temperature (21.5 °C), the reactor achieved an 87% reduction and at 10 °C achieved a 75% reduction in E. coli levels compared to influent levels; the longer (24 h) HRT achieved a 96% reduction in nitrate | [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Douglas, C.; Pandey, P. Nitrate and Bacterial Loads in Dairy Cattle Drinking Water and Potential Treatment Options for Pollutants—A Review. Appl. Sci. 2025, 15, 3017. https://doi.org/10.3390/app15063017
Douglas C, Pandey P. Nitrate and Bacterial Loads in Dairy Cattle Drinking Water and Potential Treatment Options for Pollutants—A Review. Applied Sciences. 2025; 15(6):3017. https://doi.org/10.3390/app15063017
Chicago/Turabian StyleDouglas, Ceilidh, and Pramod Pandey. 2025. "Nitrate and Bacterial Loads in Dairy Cattle Drinking Water and Potential Treatment Options for Pollutants—A Review" Applied Sciences 15, no. 6: 3017. https://doi.org/10.3390/app15063017
APA StyleDouglas, C., & Pandey, P. (2025). Nitrate and Bacterial Loads in Dairy Cattle Drinking Water and Potential Treatment Options for Pollutants—A Review. Applied Sciences, 15(6), 3017. https://doi.org/10.3390/app15063017





