Abstract
(1) Background: Individuals with high levels of state depression are hypothesized to have an impairment of attentional control functions necessary for filtering irrelevant information. This study used the event-related potential of early PD, a marker of distractor suppression, and N2pc, an indicator of attentional capture to investigate whether high state depression affects selective attention in ignoring or suppressing distractors. (2) Methods: Thirty-three undergraduate students completed the Depression Anxiety Stress Scale-21 (DASS-21) and performed a modified, delayed match-to-sample task. Participants encoded abstract shapes under low or high perceptual load conditions in the visual working memory while ignoring a lateralized Chinese character as a task-irrelevant singleton distractor. (3) Results: Individuals with high state depression failed to suppress the distractor, as evidenced by the absence of early PD. Under low perceptual loads, they also displayed a significant N2pc component, indicating attentional allocation to the distractor. In contrast, low-state-depression participants successfully suppressed the distractor, showing early PD and the absence of N2pc. (4) Conclusions: These findings suggest that high-state-depression individuals have an impairment in top–down attentional control, particularly in feature-based selective attention. This deficit hinders the ability to filter out irrelevant information, potentially contributing to cognitive difficulties associated with depression.
1. Introduction
Deficits in top–down attention, particularly selective attention, are well documented in individuals with Major Depressive Disorder (MDD) (for a review, see [1]). Top–down attention refers to the guided allocation of attentional resources based on prior knowledge, goals, and plans [2]. A critical subdomain, selective attention, allows individuals to focus on task-relevant information while suppressing irrelevant distractions [3]. Impairments in selective attention can significantly impact daily functioning and provide insight into the cognitive mechanisms underlying MDD.
Research demonstrates that individuals with MDD often exhibit deficits in feature-based selective attention, which involves prioritizing specific attributes (e.g., color or shape) of stimuli while ignoring distractions. For example, slower reaction times and poor performance on the non-emotional Stroop color–word task have been linked to reduced functional connectivity within the frontoparietal attention network, as evaluated with fMRI and a decreased parietal alpha power and as measured with resting state EEG with closed eyes, predisposing individuals with worse attentional focus to developing depression [4]. In contrast, spatial selective attention, which prioritizes specific locations, appears to be relatively preserved in MDD, as evidenced by tasks like the Flanker paradigm showing no significant differences between MDD and healthy controls [5,6]. These findings suggest that attentional impairments in MDD are domain-specific, warranting further investigation into the mechanisms governing feature-based selective attention.
The current study aimed to investigate feature-based selective attention in individuals with depressive tendencies, characterized by persistent negative moods, a lack of enjoyment, low self-esteem, and anhedonia as compared to matched controls. Specifically, the study examined, in high- and low-state-depression individuals, the neurophysiological effects of attentional allocation and distractor processing under varying visual working memory (VWM) loads. VWM is a capacity-limited system that temporarily maintains visual information to support ongoing tasks [7,8].
This study is grounded in Lavie’s Perceptual Load Theory (PLT [9,10]), which posits that the perceptual load of a task, referring to the complexity and numerosity of the physical stimuli, determines the extent of distractor processing. According to the PLT, perception is proposed to be an automatic process that continues until the limited perceptual capacity is fully exhausted. Under low perceptual loads (e.g., encoding and maintaining one complex object in VWM), excess attentional resources may spill over, allowing irrelevant stimuli to capture attention. In contrast, high perceptual loads (e.g., encoding and maintaining four complex objects in VWM) deplete attentional capacity, reducing or eliminating distractor processing. Although PLT has been supported mainly by behavioral studies (e.g., [11]), its neurophysiological underpinnings remain somewhat underexplored.
Event-related potentials (ERPs) provide a time-sensitive method for examining attentional processes at the neural level, making them essential for understanding how individuals with depressive tendencies regulate attention. Traditional behavioral measures, such as reaction times and accuracy, may not fully capture the rapid and automatic nature of attentional mechanisms. ERPs, in contrast, allow researchers to directly measure the temporal dynamics of attentional suppression and selection, offering a more precise investigation of attentional control deficits in depression. A systematic evaluation of ERP studies has reported perceptual load effects as predicted by the PLT at all processing stages. The findings are, however, mixed, and this inconsistency is not only especially observed for earlier ERP components (i.e., C1, P1, N1) but also seen during later stages (i.e., P2, N2) (for a review, see [12]). However, the role of lateralized ERP components, such as PD (distractor positivity) and N2pc (N2-posterior-contralateral), as measures of distinct aspects of attentional selection and control under low and high perceptual load conditions has not been fully investigated in previous studies.
The N2pc is observed as a negative deflection that is larger when contralateral to the attended stimulus and reflects the neural process of orienting and focusing covert attention on peripheral object features among competing distractors, and it is indicative of attentional capture by salient stimuli [13,14,15,16]. Research utilizing magnetoencephalographic (MEG) recordings has identified two distinct neural sources contributing to the N2pc: the Early Parietal Source between 180 and 200 ms after stimulus onset, associated with the initiation of attentional shifts within the visual field, and the Later Occipito-Temporal Source between 220 and 240 ms after stimulus onset, thought to reflect the focusing of attention, implemented by extrastriate areas of the occipital and inferior temporal cortex [17]. Furthermore, numerous studies have identified PD as a lateralized posterior ERP component elicited by salient distractors. While PD shares a similar topography with the N2pc, it exhibits the opposite polarity, presenting as a contralateral positivity rather than contralateral negativity. PD typically occurs within 100 to 500 ms after stimulus onset and reflects neural mechanisms involved in suppressing irrelevant or competing stimuli during tasks that demand focused attention [18,19,20,21]. In prior studies, when contralateral positivity appeared relatively early (e.g., 100–275 ms) before the first shift in attention (i.e., N2pc), it has been classified as early PD, providing supporting evidence for the development of the signal suppression hypothesis (SSH) of controlled attention capture [18,22,23,24,25,26]. According to the SSH, the salience of distractors triggers an automatic signal that, under certain conditions, can be proactively suppressed through top–down attentional control mechanisms to prevent attentional capture, particularly when distractors conflict with task goals (for reviews, see [20,27]).
Previous ERP studies promoting SSH have considered attentional control as a process where either a distractor captures attention or is suppressed, without directly examining for the possible modulatory effect of VWM load on this process. However, an exception is represented in the study of Feldmann-Wüstefeld and Vogel (2019), who examined the processes of enhancement and suppression during the encoding of information into VWM. Using a change detection task, participants were required to memorize certain items while ignoring others. The results showed that to-be-ignored items elicited a PD component, which increased its amplitude with respect to the salient distractor load, suggesting that the PD serves as an index of suppression efficiency [28].
The Current Study
In this investigation, we primarily formulate our hypotheses based on the fundamental assumption of PLT [9,29,30,31,32]. According to PLT, in the low perceptual load condition, spare perceptual capacities should automatically spill over to process the features of the task-irrelevant singleton, leading to a significant N2pc response (i.e., attentional capture). In contrast, under high perceptual load conditions, the perceptual capacity is exhausted by processing task-relevant complex stimuli, resulting in an early selection that ignores the task-irrelevant singleton and, therefore, an absence of N2pc. However, it seems unlikely that processing capacity is ever completely exhausted. Thus, under certain experimental conditions, PLT’s predictions may not always hold. If the N2pc results of our study do not fully support PLT—for instance, by showing attentional capture under high perceptual loads or failing to show attentional capture under low perceptual loads in either participant group—then we should consider whether alternative theories, such as the SSH of controlled attentional capture [18,22,23,25,26,33], play a role.
According to the SSH, in the current study, we hypothesize that if individuals with depressive tendencies, as compared to matched controls, have a relative deficit in top–down attentional control processes in preventing attentional capture by a task-irrelevant singleton, then the electrophysiological results should reveal a weak (or absent) early PD, followed (or replaced) by the N2pc component, which reflects the process of orienting and focusing covert attention on peripheral object features. In particular, we predict that individuals with high state depression, as compared to low state depression, should not show an increased PD amplitude with an increase in perceptual load in the attempt to actively suppress task-irrelevant singleton features.
To test our hypotheses, we employed a modified version of a delayed match-to-sample task [34]. Participants were required to encode and retain either one (low perceptual load) or four (high perceptual load) complex objects (abstract shapes) in VWM while ignoring a lateralized, task-irrelevant complex singleton distractor object (Chinese character). The singleton distractor object, a lateralized stimulus that differed in shape from the other task-irrelevant homogeneous items (circles) in a circular visual array, was presented during the retention interval. Participants’ ability to resist its attentional capture was examined using primarily electrophysiological measures. By elucidating the interplay between VWM, selective attention, and depressive symptomatology, this study contributes to a deeper understanding of cognitive dysfunction in MDD.
2. Materials and Methods
The study was approved by the local ethical committee of the University of Roehampton’s School of Psychology (ethics application reference: PSYC 22/446), in accordance with the Declaration of Helsinki. All participants signed an informed consent form before their participation and received course credits or volunteered without credits for participating. Participants were fully debriefed about the purpose of the study.
2.1. Participants
A tool to compute statistical power analyses [35] was used to estimate a minimum sample size for the experiment of the current study. This was determined by referring to previous similar research reported in [29], which obtained large effect sizes, as in Experiment 1b (Cohen’s d = 0.62). Setting the alpha level at 0.05 (two-tails) and a power of 0.80 to detect the effects of Experiment 1b [29], the suggested sample size was 23 participants. Overall, 36 volunteers naïve to the objective of the experiment participated. Of the initial sample, one volunteer was removed due to an incomplete questionnaire and two were removed due to an unusually low percentage of correct trials (51% and 53%) in performing the delayed match-to-sample task. Of the remaining 33 participants whose scores on the “Depression” subscale of the Depression Anxiety Stress Scales-21 (DASS-21; [36,37]) were below 14 (i.e., normal or mild), they were classified as low-state-depression (LSD) individuals (N = 19). Those scoring 14 or above (i.e., moderate, severe, or extremely severe) were classified as high-state-depression (HSD) individuals (N = 14). Event-related potential (ERP) and behavioral data from these two groups were analyzed. ERP studies concentrating on early PD and N2pc generally evaluate 12 to 20 participants per subgroup, as demonstrated by an earlier investigation involving two groups with varying levels of trait anxiety [38]. Consequently, our sample size was adequate for detecting variations in early PD or N2pc between the LSD and HSD groups. The specific characteristics of the subjects in both groups are detailed in Table 1. Participants affirmed that they had no medication use, history of chemical dependency, neurological issues, psychiatric/psychological disorders, or closed-head injuries. There were no significant differences in age or gender between the two groups. However, the HSD group scored higher on all three subscales of the DASS-21 questionnaire compared to the LSD group.

Table 1.
Demographic information of subjects with varying levels of state depression.
2.2. Self-Report Measure of State Depression
The DASS-21 is a widely administered self-report measure for assessing the negative emotional states of depression anxiety and stress [37]. The DASS-21 variation of the test, which is the shorter version of the 42-item questionnaire by [36] used in this study, contains 7 questions for each emotional state. This psychometric test has been proven to be reliable [39]. It displayed high internal consistency, with Cronbach’s alpha values of 0.81, 0.89, and 0.78 for the depression anxiety and stress subscales, respectively, and it was tested for validity among both clinical and non-clinical samples [40,41]. The questionnaire assumes that depression, anxiety, and stress all consist of an identical nature of distress, but it also acknowledges that they have distinct individual characteristics [37]. In the case of the depression subscale, it assesses symptoms such as dysphoria, hopelessness, self-worthlessness, and lack of interest. Each item is rated on a 4-point Likert scale (0 = did not apply to me at all; 3 = applied to me very much or most of the time). Scores are summed for each subscale and then multiplied by 2 (to align with the DASS-42 scale).
2.3. Stimuli, Experimental Procedure, and Task
The experiment was carried out on a PC running on a Windows operating system, which was located in a computer room. An open-source Experiment generator software (Working Memory Analyser, version 1.0.2.2) programmed in Lazarus free Pascal (https://www.lazarus-ide.org) using OpenGL and Simple Direct media Layer (SDL2) libraries was used to run the visual paradigm. To promote the replicability of this study across laboratories, the software, including the experimental library of the experiment used in this study, was distributed under the GPL-3.0 license, and it can be downloaded from its GitHub repository (https://github.com/gfuggetta-lab/WorkingMemoryAnalyser/releases (accessed on 1 October 2024). Stimuli were presented on a 24″ (53.1 cm × 29.9 cm) AOC G2460PG LCD monitor with a resolution of 1920 × 1080 pixels and a refresh rate of 59.940 Hz at a viewing distance of 88 cm. To ensure accurate synchronization with millisecond precision between the onset of the visual stimuli on screen and the markers on the EEG recording system, the Trigger Station device (BrainTrends Ltd., Rome, Italy, https://www.braintrends.it/) was used.
For the modified and delayed match-to-sample task, the monitor continuously displayed a 0.1° white fixation dot (21.5 cd/m2) at the center of an 11.84° grey circle (6.5 cd/m2), shown against a black background (see Figure 1). Four empty white rings (i.e., placeholders, 21.5 cd/m2) with an outer diameter of 1.30° and inner diameter of 1.17° (0.065° thickness) were constantly shown in the top-left, top-right, bottom-left, and bottom-right quadrants on the circumference of an imaginary circle of 9.20° diameter around the central fixation dot. The visual paradigm comprised the following stimuli in sequential order: First, a foveally presented fixation, which was a 0.78° × 0.78° grey (21.5 cd/m2) cross, appeared at the beginning of each trial for 500 ms. Second, after an interval of 500 ms, a sample array (i.e., memory set of items) comprising four objects presented in the top-left, top-right, bottom-left, and bottom-right quadrants on the circumference of an imaginary circle of 2.66° diameter around the central fixation dot was shown for 2000 ms. These four objects could either be three grey circles and one grey abstract shape (i.e., low VWM capacity load of 1) or four grey abstract shapes (i.e., high VWM capacity load of 4). All objects had a size of 0.84°. In terms of non-nameable abstract shapes, sixteen stimuli ( ,
, ,
, ,
, ,
, ,
, ,
, ,
, ,
, ,
, ,
, ,
, ,
, ,
, ,
, , and
, and  ) were used, as well as their 180° clockwise-rotated copies, for a total of 32 abstract shapes. These abstract shapes were generated according to method 1 of [42], as in previous studies [43,44]. Third, after an interval of 800 ms, a task-irrelevant singleton array (i.e., prime display) was shown for 200 ms. The distractor singleton was always a task-irrelevant Chinese character (not part of the current task set). Sixteen Chinese characters (
) were used, as well as their 180° clockwise-rotated copies, for a total of 32 abstract shapes. These abstract shapes were generated according to method 1 of [42], as in previous studies [43,44]. Third, after an interval of 800 ms, a task-irrelevant singleton array (i.e., prime display) was shown for 200 ms. The distractor singleton was always a task-irrelevant Chinese character (not part of the current task set). Sixteen Chinese characters ( ,
, ,
, ,
, ,
, ,
, ,
, ,
, ,
, ,
, ,
, ,
, ,
, ,
, ,
, , and
, and  ) selected from set A and set B of the targets of the Shum Visual Learning Test [45] were shown, as well as their 180° clockwise-rotated copies, for a total of 32 characters. Eadie and Shum (1995) demonstrated that non-Chinese speakers do not easily verbalize Chinese characters. They developed a test of visual learning based on the set of Chinese characters with the lowest scores on verbalizability and provided evidence for its construct validity [46]. The task-irrelevant Chinese characters appeared within one of the four placeholders’ positions among fifteen non-target homogenous 0.84° diameter grey circles, spaced evenly on the circumference of an imaginary circle of 9.20° diameter around the central fixation dot. All shapes were isoluminant (30.4 cd/m2). Fourth, after an interval of 800 ms, a memory probe with an abstract shape (i.e., target), which appeared within one of four placeholders’ positions, was shown for 500 ms. This limited visual search relative to the four positions was needed to perform the modified, delayed match-to-sample task. Thus, the 4 distractor positions appeared an equal number of times per trial and were fully counterbalanced with the 4 target locations, making the target position unpredictable. Using a block design, in each of the 8 blocks of 64 trials, the singleton distractor object not part of the task set comprised a Chinese character. Fifth, after a response time interval of 1800 ms, visual feedback of 2.07° with either a green happy face (
) selected from set A and set B of the targets of the Shum Visual Learning Test [45] were shown, as well as their 180° clockwise-rotated copies, for a total of 32 characters. Eadie and Shum (1995) demonstrated that non-Chinese speakers do not easily verbalize Chinese characters. They developed a test of visual learning based on the set of Chinese characters with the lowest scores on verbalizability and provided evidence for its construct validity [46]. The task-irrelevant Chinese characters appeared within one of the four placeholders’ positions among fifteen non-target homogenous 0.84° diameter grey circles, spaced evenly on the circumference of an imaginary circle of 9.20° diameter around the central fixation dot. All shapes were isoluminant (30.4 cd/m2). Fourth, after an interval of 800 ms, a memory probe with an abstract shape (i.e., target), which appeared within one of four placeholders’ positions, was shown for 500 ms. This limited visual search relative to the four positions was needed to perform the modified, delayed match-to-sample task. Thus, the 4 distractor positions appeared an equal number of times per trial and were fully counterbalanced with the 4 target locations, making the target position unpredictable. Using a block design, in each of the 8 blocks of 64 trials, the singleton distractor object not part of the task set comprised a Chinese character. Fifth, after a response time interval of 1800 ms, visual feedback of 2.07° with either a green happy face ( ) for correct responses or a red sad face (
) for correct responses or a red sad face ( ) for incorrect responses or omissions in front of the fixation dot at the center of the screen was shown for 200 ms, followed by a fixed duration of 300 ms before the next trial began.
) for incorrect responses or omissions in front of the fixation dot at the center of the screen was shown for 200 ms, followed by a fixed duration of 300 ms before the next trial began.
 ,
, ,
, ,
, ,
, ,
, ,
, ,
, ,
, ,
, ,
, ,
, ,
, ,
, ,
, , and
, and  ) were used, as well as their 180° clockwise-rotated copies, for a total of 32 abstract shapes. These abstract shapes were generated according to method 1 of [42], as in previous studies [43,44]. Third, after an interval of 800 ms, a task-irrelevant singleton array (i.e., prime display) was shown for 200 ms. The distractor singleton was always a task-irrelevant Chinese character (not part of the current task set). Sixteen Chinese characters (
) were used, as well as their 180° clockwise-rotated copies, for a total of 32 abstract shapes. These abstract shapes were generated according to method 1 of [42], as in previous studies [43,44]. Third, after an interval of 800 ms, a task-irrelevant singleton array (i.e., prime display) was shown for 200 ms. The distractor singleton was always a task-irrelevant Chinese character (not part of the current task set). Sixteen Chinese characters ( ,
, ,
, ,
, ,
, ,
, ,
, ,
, ,
, ,
, ,
, ,
, ,
, ,
, ,
, , and
, and  ) selected from set A and set B of the targets of the Shum Visual Learning Test [45] were shown, as well as their 180° clockwise-rotated copies, for a total of 32 characters. Eadie and Shum (1995) demonstrated that non-Chinese speakers do not easily verbalize Chinese characters. They developed a test of visual learning based on the set of Chinese characters with the lowest scores on verbalizability and provided evidence for its construct validity [46]. The task-irrelevant Chinese characters appeared within one of the four placeholders’ positions among fifteen non-target homogenous 0.84° diameter grey circles, spaced evenly on the circumference of an imaginary circle of 9.20° diameter around the central fixation dot. All shapes were isoluminant (30.4 cd/m2). Fourth, after an interval of 800 ms, a memory probe with an abstract shape (i.e., target), which appeared within one of four placeholders’ positions, was shown for 500 ms. This limited visual search relative to the four positions was needed to perform the modified, delayed match-to-sample task. Thus, the 4 distractor positions appeared an equal number of times per trial and were fully counterbalanced with the 4 target locations, making the target position unpredictable. Using a block design, in each of the 8 blocks of 64 trials, the singleton distractor object not part of the task set comprised a Chinese character. Fifth, after a response time interval of 1800 ms, visual feedback of 2.07° with either a green happy face (
) selected from set A and set B of the targets of the Shum Visual Learning Test [45] were shown, as well as their 180° clockwise-rotated copies, for a total of 32 characters. Eadie and Shum (1995) demonstrated that non-Chinese speakers do not easily verbalize Chinese characters. They developed a test of visual learning based on the set of Chinese characters with the lowest scores on verbalizability and provided evidence for its construct validity [46]. The task-irrelevant Chinese characters appeared within one of the four placeholders’ positions among fifteen non-target homogenous 0.84° diameter grey circles, spaced evenly on the circumference of an imaginary circle of 9.20° diameter around the central fixation dot. All shapes were isoluminant (30.4 cd/m2). Fourth, after an interval of 800 ms, a memory probe with an abstract shape (i.e., target), which appeared within one of four placeholders’ positions, was shown for 500 ms. This limited visual search relative to the four positions was needed to perform the modified, delayed match-to-sample task. Thus, the 4 distractor positions appeared an equal number of times per trial and were fully counterbalanced with the 4 target locations, making the target position unpredictable. Using a block design, in each of the 8 blocks of 64 trials, the singleton distractor object not part of the task set comprised a Chinese character. Fifth, after a response time interval of 1800 ms, visual feedback of 2.07° with either a green happy face ( ) for correct responses or a red sad face (
) for correct responses or a red sad face ( ) for incorrect responses or omissions in front of the fixation dot at the center of the screen was shown for 200 ms, followed by a fixed duration of 300 ms before the next trial began.
) for incorrect responses or omissions in front of the fixation dot at the center of the screen was shown for 200 ms, followed by a fixed duration of 300 ms before the next trial began.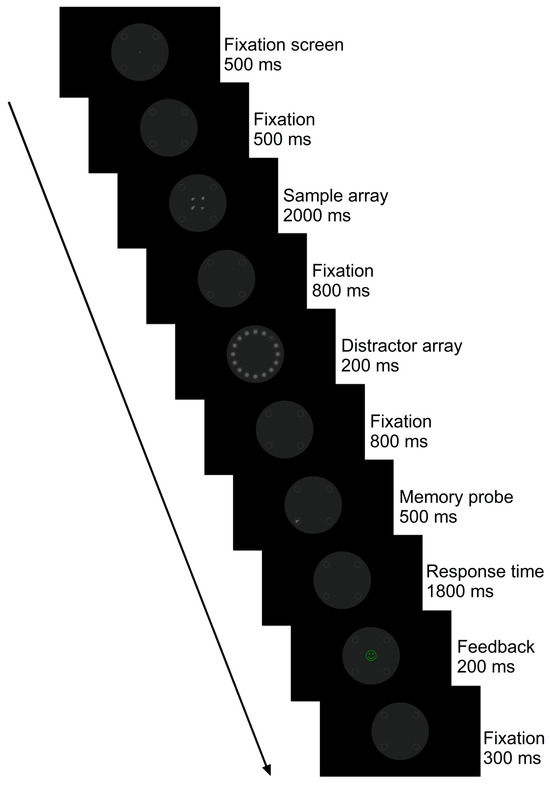
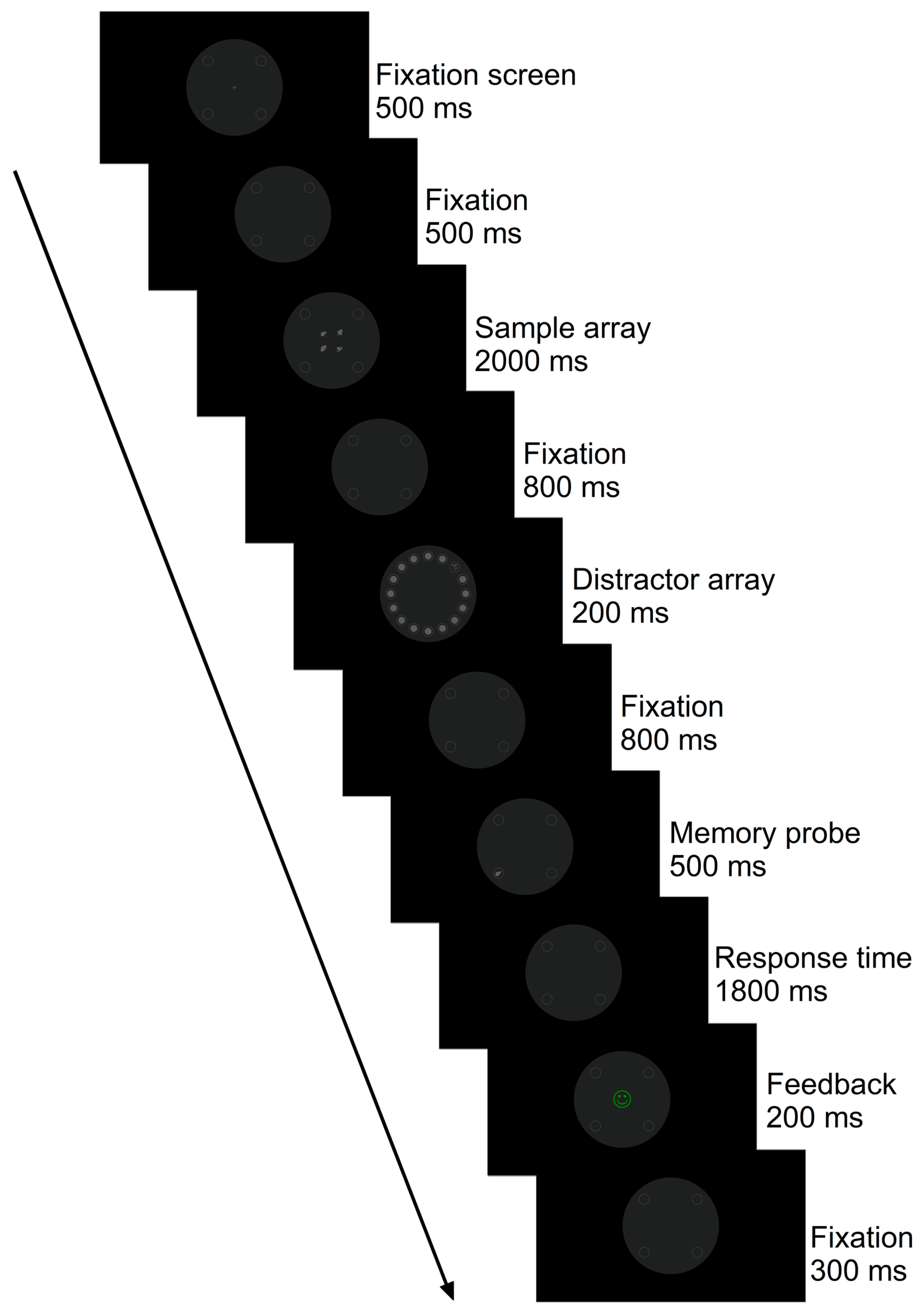
Figure 1.
Example of the stimuli of a trial of the modified, delayed match-to-sample task. Each trial began with the presentation of a fixation cross, followed by a sample array (i.e., memory set). A distractor array was interleaved during the retention interval, showing a task-irrelevant “singleton” item. The singleton was a Chinese character that was not part of the current task set. The trial ended with a memory probe (i.e., target) followed by a response time interval and visual feedback. Participants were asked to hold the memory set in mind during the retention interval and decide whether the memory probe was matching or mismatching any of the items presented as part of the sample array (i.e., match-to-sample task). They were also instructed to ignore the distractor array and fixate on the central fixation dot throughout the experiment. This trial is an example of high-visual-working-memory (VWM) capacity load (i.e., four abstract shapes are encoded in VWM), with a Chinese character as a distractor singleton and spatially incompatible distractor-to-memory-probe positions (i.e., the distractor singleton appeared on the top-right quadrant, and the memory probe appeared on the bottom-left quadrant). This is an example of a match memory probe-to-sample task trial (i.e., the abstract shape shown as a memory probe matches one of the abstract shapes presented as part of the sample array). Stimuli are drawn to scale.
Participants were presented with the instructions for the delayed match-to-sample task in auditory and visual modalities prior to performing the training trials of the visual paradigm. Thus, a pre-recorded audio file in MP3 format was played (see Supplementary Materials), and a picture in bitmap format was shown on screen alongside the audio instructions given by an AI-generated voice to ensure consistency in delivering the instructions across participants and that participants understood the requirements of the task, including which buttons to press using a response box (see Appendix A). Participants were explicitly instructed to fixate on the central fixation dot throughout the experiment, memorize the initial sample array, ignore the distractor array, and respond only to the memory probe. The participant’s task was to indicate, using the left and right buttons of a response box, whether the abstract shape, as part of the memory probe, was the same or different from the abstract shape(s) shown as part of the sample array (i.e., delayed match-to-sample task) in either low-VWM-capacity-load (1) or high-VWM-capacity-load (4) conditions. The singleton stimulus as part of the distractor array was consistently a Chinese character and not part of the current task set. Importantly, the task-irrelevant Chinese character appeared as a single pop-out item among a homogeneous array of 15 grey circles across all conditions requiring a singleton detection mode, and none of the distractor arrays were altered between VWM load conditions (i.e., they were perceptually identical), preventing any dilution of the salient distractor item. In the experiment, we therefore manipulated the perceptual load whilst maintaining a singleton detection attentional set.
The distractor singleton as a Chinese character was lateralized and could appear unpredictably in one of four possible quadrants on screen either in a compatible (25% of valid trials) (i.e., spatial ignored repetition) or incompatible (75% of invalid trials) spatial position compared to the incoming lateralized target location, which also appeared unpredictably in one of four quadrants. Thus, in only 25% of trials, the spatial position of the distractor singleton in the prime display became the target’s spatial position in the subsequent probe display. These experimental manipulations could lead to either spatial negative priming (SNP) or spatial positive priming (SPP) effects [47,48]. SNP/SPP represents an inhibitory/faciliatory effect that occurs when shifting attention toward a perceived distractor’s spatial position on a prime display, with a subsequent re-orientation of spatial attention, and this results in slower/faster and less/more accurate target detection when it appears at the same distractor stimulus’ spatial location compared to elsewhere due to residual inhibition/facilitation being deployed to that location [47,48]. To keep the target as a single-onset stimulus but clearly demarcate the distractor as a task-irrelevant singleton as part of the distractor array, we presented this singleton distractor (i.e., prime display) in a fixed temporal order of 1000 ms prior to the target onset (i.e., probe display), which is sufficient time to disengage from the distractor [49]. Unlike many previous manipulations of perceptual capacity using the VWM load, which requires a dual-task design (e.g., [29,50,51]), the current design is a single-task design and therefore mitigates any additional cognitive loads from maintaining multiple task rules in mind. Before the main part of the experiment, participants completed 20 practice trials to familiarize themselves with the task and adjust to the requirements. These practice trials were repeated until the average accuracy was greater than 60%. Participants completed 512 trials in eight blocks of 64 trials and were allowed to pause in between blocks. The experiment had the following experimental conditions: (1) VWM capacity load, with two levels of randomly distributed trials: low (256 trials) vs. high (256 trials); (2) distractor-to-memory-probe spatial compatibility, where each level of VWM capacity loads had four levels of randomly distributed distractor-to-memory-probe horizontal and vertical spatial compatibilities: horizontally compatible and vertically compatible (64 trials) vs. horizontally compatible and vertically incompatible (64 trials); horizontally incompatible and vertically compatible (64 trials); horizontally incompatible and vertically incompatible (64 trials). The distractor-to-memory-probe spatial compatibility effects indicate the extent to which participants’ selective attention was biased by the singleton distractor’s features relative to direct electrophysiological measures and indirect behavioral measures in performing the modified, delayed match-to-sample abstract shape task. Speed and accuracy were encouraged. The number of trials where the memory probe was matching or mismatching relative to any of the items presented as part of the sample array was balanced across each combination of the eight experimental conditions: VWM capacity load (low 1 and high 4), horizontal spatial compatibility (incompatible and compatible) and vertical spatial compatibility (incompatible and compatible). Half of the trials within each experimental condition required the same probe-to-sample response, whereas the remaining half of the trials required different probe-to-sample responses. The response button mapping (i.e., left button when the memory probe was different from the sample and right button when the memory probe was the same as the sample or vice versa) was randomized across participants.
2.4. Electrophysiological Data
2.4.1. General Pre-Processing of Electrophysiological Data
Using the 64-channel Biosemi ActiveTwo EEG system (BioSemi, Amsterdam, The Netherlands), continuous EEG data were recorded through a quickcap adhering to the 10/10 system at a sampling rate of 2048 Hz. Flat-type electrodes were positioned 1 cm from the outer canthi of both eyes to capture horizontal electrooculograms (HEOGs). For vertical electrooculograms (VEOGs) and blinks, electrodes were placed above and below the right eye. Additionally, two electrodes were attached to each earlobe. Pin-type electrodes were secured with an elastic cap after applying electrode gel. Unlike other EEG systems, ActiveTwo allows for reference-free EEG signal recording. Instead, the ground reference is managed by two electrodes (DRL/CMS), creating a feedback loop to regulate the current from the participant to the analog–digital (AD) box.
EEG data pre-processing was conducted with BrainVision Analyzer 2.3 software (Brain Products GmbH, Munich, Germany). First, three channels that did not contain data for further analysis were disabled (EXG7, EXG8, and Status). Next, a linear derivation function was applied to compute the bipolar HEOG channel from the outer canthi electrodes of both eyes (i.e., HEOGL and HEOGR) and the VEOG channel using the two electrodes and one electrode placed above and below the right eye (i.e., VEOGU and VEOGL). Next, raw EEG data were band-pass-filtered between 0.3 and 46 Hz with 8th-order rolloffs, with a 50 Hz notch employing zero-phase-shift Butterworth (IIR) filters. Next, automatic raw data inspection was conducted, excluding frontal electrodes that were contaminated by eye movements and blinks (i.e., Fp1, AF7, AF3, F7, F5, F3, F1, FPz, AFz, Fz, Fp2, AF8, AF4, F8, F6, F4, F2). The EEG events are marked as bad from 200 ms before to 200 ms after the event by applying the following exclusion criteria: (1) check the gradient if the maximal allowed voltage step exceeds 50 µV/ms; (2) check the difference (max–min) if the values in 200 ms intervals exceed 200 µV; (3) check the amplitude if the minimal and maximal allowed amplitudes exceed ±200 µV; (4) check for low activity if the values in 100 ms intervals are lower than 0.5 µV). Next, if EEG channels contained intervals with obvious non-ocular artifacts (i.e., muscular contractions and electrode artifacts) over prolonged periods (i.e., >100 s), these were removed. Next, after the exclusion of bad channels, the automatic raw data inspection procedure was re-conducted on the remaining “good” channels to reduce the amount of EEG intervals marked as bad and, therefore, maximize the amount of EEG signals to use in subsequent pre-processing stages. Next, as a pre-processing step for ICA ocular artifact reduction procedures, high-pass filtering at 1 Hz (8th-order rolloffs) with a zero-phase-shift Butterworth (IIR) filter was applied to compute the ICA weights, and this produced good results in terms of the signal-to-noise ratio (SNR) [52]. Next, ocular artifacts were reduced by employing the ICA function within the BrainVision Analyzer software using VEOG/HEOG channels. This function searches for an ocular artifact template in VEOG/HEOG channels and then finds ICA-derived components that account for a user-specified amount of variance (70%) in the template-matched portion of the signal from VEOG/HEOG. Data used for ICA were 328 s long, and the ICA algorithm used was Infomax-restricted. Next, ICA weights for less high-pass-filtered data (i.e., 0.3 Hz, 8th-order rolloffs) were applied using a linear derivation function. So, the ICA components containing artifacts were removed from the EEG signal, which was then reconstructed for further processing. Next, bad EEG channels that were previously removed were topographically spline-interpolated with order 4, degree 10, and lambda 1 × 10−5. Next, the original order of the 64 electrodes plus A1, A2, HEOG, and VEOG was re-established. Then, EEG data were re-referenced relative to the average of earlobe electrodes (A1 and A2). Next, the sampling rate was down-sampled to 1000 Hz based on spline interpolation. Moreover, in the case of ERPs at the distractor singleton array onset, continuous EEG data were epoched from −1500 ms to +1501 ms and baseline-corrected with a 200 ms period before distractor array onset. Next, an artifact rejection–automatic inspection function was applied three times. Epochs within the time interval from −200 ms to 350 ms post-distractor-singleton onset, with eye blinks (exceeding ±60 µV at the FP1, FPz, and FP2 channels), eye movements (exceeding ±30 µV in the HEOG channels), and movement-related artifacts (exceeding ±80 µV in all other channels), were rejected.
2.4.2. Analysis of Event-Related Potentials
Artifact-free epochs were used to compute separate average ERPs for lateral parieto-occipital electrodes. To isolate the magnitude of the PD and N2pc components elicited by the singleton distractor array, using lateral occipital PO7/8 electrodes, we computed difference waves by subtracting ipsilateral electrodes from contralateral electrodes relative to the singleton distractor location. To eliminate any hemispheric asymmetries that were unrelated to attention, we averaged the difference waves across the left and right hemispheres. At post-distractor array onset, the mean amplitude measures of the lateralized ERP components were extracted from a time window between 170 and 230 ms for early PD and between 273 and 293 ms for N2pc at the PO7/8 electrodes site.
2.5. Statistical Analysis
The Kolmogorov–Smirnov test was employed to determine whether parametric tests were appropriate to be used. In the case of the statistical analysis of electrophysiological data at the distractor array onset, the mean amplitudes of lateralized early PD and N2pc were submitted to mixed ANOVAs, which had a between-subject factor relative to the state depression group (low and high) and a within-subject factor relative to VWM capacity loads (low 1 and high 4). To evaluate the presence of a significant magnitude of early PD and N2pc components for each of the two state depression groups at different levels of VWM capacity loads, one-sample t-tests with a test value of 0 were also conducted.
In terms of behavioral data, after removing omissions and response times less than 200 ms and above 2100 ms, the mean accuracy (%) and mean correct response times (RTs; ms) in performing the task were analyzed. The mean accuracy (%) and mean correct RTs (ms) were subjected to a 4-way mixed-design ANOVA with a between-subject factor relative to the state depression group (low and high) and three within-subject factors with respect to the VWM capacity load (low 1 and high 4); the distractor-to-memory-probe with horizontal and spatial compatibility (compatible and incompatible); and the distractor-to-memory probe with vertical and spatial compatibility (compatible and incompatible). In all repeated-measure ANOVAs for lateralized ERPs and behavioral data, Greenhouse–Geisser epsilon adjustments for non-sphericity were applied where appropriate. Post hoc pairwise comparisons of the means were conducted using the Bonferroni method unless differently specified. For all statistical tests, p < 0.05 (2-tailed) was considered significant.
3. Results
3.1. Electrophysiological Results
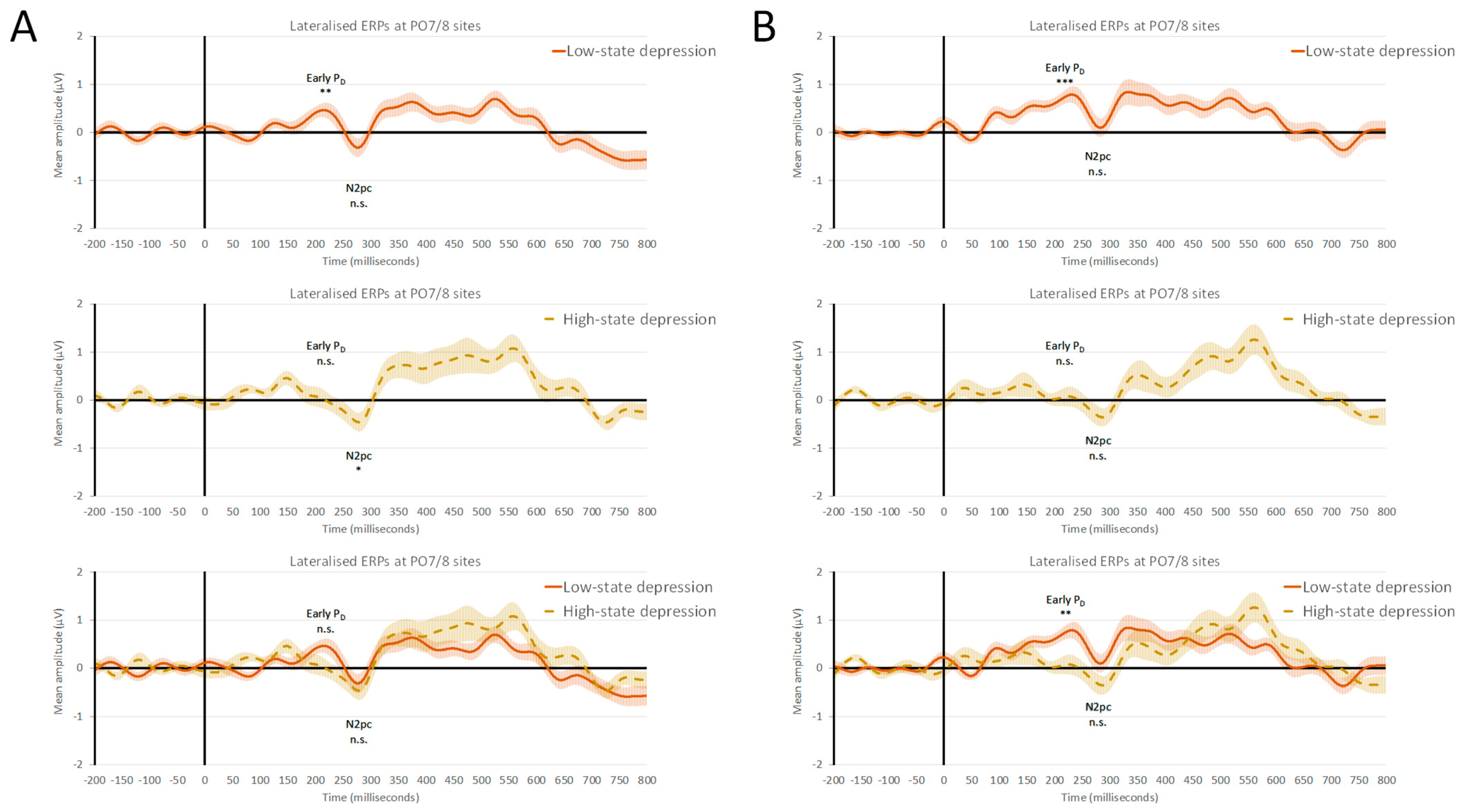
Statistical analyses of the mean amplitude (±SEM) of early PD, with an overall amplitude of 0.28 (±0.07) µV, revealed a non-significant main effect of the VWM capacity load (F(1, 31) = 1.11, p = 0.30, and ηp2 = 0.03) or interactions between the VWM capacity load and the state depression group (F(1, 31) = 1.31, p = 0.26, and ηp2 = 0.04). Crucially, there was a significant main effect of the state depression group, with F(1, 31) = 9.05, p < 0.01, and ηp2 = 0.23. The mean amplitude of the early PD was 0.07 (±0.11) µV in the case of the HSD group and 0.49 (±0.09) µV for the LSD group. One-sample t-tests with a test value of 0 revealed that, in the case of HSD individuals, the magnitude of early PD did not reach statistical significance at both levels of VWM capacity loads: low load (1) of 0.07 (±0.18) µV, with t(13) = 0.42, p = 0.68; and high load (4) of 0.06 (±0.13) µV, with t(13) = 0.47, p = 0.64. In contrast, in the case of LSD individuals, there was a significant magnitude of early PD at both levels of VWM capacity loads: a low load (1) of 0.35 (±0.12) µV, with t(18) = 2.95, p < 0.01; and high load (4) of 0.62 (±0.11) µV, with t(18) = 5.87, p < 0.001.
Statistical analyses of the mean amplitude (±SEM) of N2pc, with an overall amplitude of −0.23 (±0.11) µV, revealed a non-significant main effect of the VWM capacity load, (F(1, 31) = 2.14, p = 0.15, and ηp2 = 0.06) or interactions between the VWM capacity load and the state depression group (F(1, 31) = 0.84, p = 0.36, and ηp2 = 0.03). Lastly, the main effect of the state depression group was not significant, with F(1, 31) = 1.96, p = 0.17, and ηp2 = 0.06. The mean amplitude of N2pc was −0.38 (±0.17) µV in the case of the HSD group and −0.07 (±0.14) µV for the LSD group. One-sample t-tests with a test value of 0 revealed that, in the case of HSD individuals, in the low-VWM-capacity-load (1) condition, there was a significant N2pc magnitude of −0.42 (±0.19) µV, with t(13) = −2.21 and p < 0.05. However, in the high VWM capacity load (4), the N2pc magnitude of −0.33 (±0.19) µV did not reach statistical significance, with t(13) = −1.76 and p = 0.10. In the case of LSD individuals, the magnitude of N2pc was −0.27 (±0.19) µV at low (1) and −0.12 (±0.19) µV at high (4) VWM capacity load conditions and did not reach statistical significance (t(18) = −1.44, p = 0.17; and t(18) = 0.64, p = 0.53, respectively). The output file of the statistical analysis conducted on ERP data can be found in the Supplementary Materials. The grand averages of the lateralized early PD and N2pc at PO7/8 electrodes comparing the two groups of participants for low and high VWM loads are shown in Figure 2.
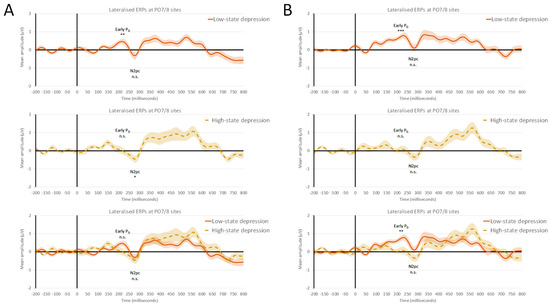
Figure 2.
Grand averages of lateralized early PD and N2pc ERP components at parieto-occipital PO7/8 electrode sites from the distractor array onset comparing low-state-depression (LSD) and high-state-depression (HSD) groups. (A) Lateralized ERPs at low-visual-working-memory capacity loads (i.e., one abstract shape encoded in VWM); (B) lateralized ERPs at high-visual-working-memory capacity loads (i.e., four abstract shapes encoded in VWM). Overall, LSD individuals successfully suppressed the singleton distractor, showing early PD and the absence of N2pc. In contrast, HSD participants were demonstrated to have an impairment in the top–down proactive control of features gained to prevent attentional capture via the task-irrelevant singleton, showing an absence of early PD and the presence of N2pc in the low-VWM load conditions. n.s. denotes a non-significant difference, whereas *, **, and *** indicate a significant difference with p < 0.05, p < 0.01, and p < 0.001, respectively. Error bars represent (±SEM).
3.2. Behavioral Results
The overall mean accuracy (±SEM) in performing the modified, delayed match-to-sample task was 76.00 (±1.65) %. There was a significant main effect relative to the VWM capacity load, with F(1,31) = 201.31, p < 0.001, and ηp2 = 0.87. The accuracy in performing the task was significantly reduced at low (1) compared to high (4) VWM loads (87.83 vs. 64.17%). There was a significant two-way interaction VWM load x state depression group, with F(1, 31) = 4.23, p < 0.05, and ηp2 = 0.12. Post hoc pairwise comparisons revealed that this significant interaction was driven by the VWM experimental manipulation rather than the individual differences in state depression. Indeed, in the case of the HSD group, there was a reduction in accuracy comparing low and high VWM loads (89.04 vs. 61.95%, p < 0.001). A similar VWM load effect was found for LSD individuals (86.61 vs. 66.38%, p < 0.001). However, the mean accuracy in performing the task did not differ significantly comparing the HSD with LSD group at low (89.04 vs. 86.61%, p = 0.53) and high (61.95 vs. 66.38%, p = 0.24) VWM loads. There was also a significant two-way VWM load x distractor interaction to target vertical spatial compatibility, with F(1, 31) = 4.27, p < 0.05, and ηp2 = 0.12. However, post hoc pairwise comparisons did not reveal a significant difference when comparing vertically spatially compatible to vertically spatially incompatible distractor-to-memory-probe conditions at both low (87.13 vs. 88.52%, p = 0.06) and high VWM loads (64.81 vs. 63.52%, p = 0.19). No other main effects or interactions were statistically significant.
The overall mean correct response time (±SEM) in performing the voluntary task was 928.39 (±35.86) ms. There was a significant main effect relative to the VWM capacity load, with F(1,31) = 82.40, p < 0.001, and ηp2 = 0.73, revealing significantly faster responses at low (1) compared to high (4) VWM loads (851.70 vs. 1005.08 ms). There was a significant main effect regarding the distractor in targeting horizontal spatial compatibility, with F(1, 31) = 5.27, p < 0.05, and ηp2 = 0.15. The mean correct response time was significantly faster for the same conditions compared to different distractors in targeting horizontal positions (922.41 vs. 934.37 ms), suggesting the presence of a spatial positive priming (SPP) effect. No other main effects or interactions were statistically significant. In particular, there was no significant difference in mean correct responses when comparing the HSD with LSD groups at low (837.99 vs. 865.40 ms, p = 0.73) and high (976.38 vs. 1033.78 ms, p = 0.42) VWM loads. The output file of the statistical analysis conducted on behavioral data can be found in the Supplementary Materials.
4. Discussion
This electrophysiological study aimed at investigating whether individuals experiencing high state depression exhibit weakened top–down attentional control processes, especially relative to feature-based selective attention. Two groups of participants with different levels of state depression, as evaluated with DASS-21 [36,37], performed a modified version of a delayed match-to-sample task [34], which required ignoring a task-irrelevant singleton as part of a distractor array under low- and high-VWM-capacity loads.
The first ERP component evaluated at the distractor array onset was the early PD, which is thought to measure a process associated with proactive attentional suppression that is applied to salient distractors to prevent attentional capture [19,25,27], whereas the second ERP component under scrutiny was the N2pc, which reflects the process of orienting and focusing covert attention on peripheral stimulus features and is indicative of attentional capture [13,14,15,16].
Two main theories were considered in examining the ERP results of the current study. The PLT [9,10,32] assumes that the early selection process of task-irrelevant distractors is governed by an automatic mechanism (i.e., if perceptual capacity is exhausted in processing a large amount of relevant information, then no attentional resources for perceiving irrelevant stimuli are left). Thus, according to the PLT, in the high (vs. low)-perceptual-load condition, due to perceptual capacity exhaustion, there should be ERP evidence of automatic rejection of the distractor with the absence of both early PD and N2pc components. On the contrary, according to the SSH of controlled attentional capture [18,23,25], in the high (vs. low)-perceptual-load condition, with the intervention of top–down attentional control mechanisms, there should be ERP evidence of a proactive suppression of task-irrelevant distractor features with an enhanced amplitude of early PD and the absence of N2pc components in order to promote the performance of the current task at hand in the face of distraction.
The ERP results highlight distinct patterns in early PD and N2pc amplitudes between the state depression groups and under varying perceptual loads. The individuals belonging to the LSD group demonstrated a significant magnitude of early PD for both low- and high-VWM-capacity loads. These findings for the LSD individuals support the predictions of the SSH of controlled attention capture, revealing that salient stimuli elicit a bottom–up “attend to me” signal, which under some task conditions, such as when they are incongruent with the current attentional set, can be successfully suppressed (see review in [27]). Interestingly, LSD individuals exhibited an overall significantly larger early PD amplitude compared to HSD individuals. Crucially, there was no evidence of early PD for either low- or high-VWM-capacity loads in the HSD group. Overall, these findings support the main hypothesis of this study, suggesting that individuals exhibiting high levels of depressive symptoms have an impairment in the top–down mechanism of the proactive control of feature gains, a cognitive strategy that involves maintaining goal-relevant information before a task-irrelevant stimulus biases attention and perception [53].
In the current study, the reduced negativity for contralateral (vs. ipsilateral) singletons was associated with early PD, a measure of proactive attentional distractor suppression (for a review, see [27]). However, in the experiment, the distractor array has a unilateral singleton distractor. Thus, it may be the case that the presence of an early lateralized waveform could instead be the positivity posterior contralateral (Ppc) component, which may not only be related to a low-level sensory imbalance in the physical structure of search displays by presenting a feature singleton in one hemifield but not the other [54,55,56] but also the initial activation on salience maps with a generalized salience signal [55,57,58,59]. Crucially, the results of the early lateralized positive component in the current study confirm that it was indeed the early PD that was present, rather than the Ppc because the low-level imbalance of the distractor array was identical in both groups of participants regardless of their differences in state depression. Thus, the only remaining explanation as to why the LSD group only showed a significant early effect of reduced negativity for contralateral (vs. ipsilateral) singletons in both low and high VWM loads is that the efficiency of the top–down mechanism operates to actively suppress the singleton distractor in this group compared to the HSD group.
To complement the findings of the early PD, the distractor was found to trigger N2pc in the HSD group but not in the LSD group under low-perceptual-load conditions. These findings suggest that individuals exhibiting high levels of depressive symptoms oriented and focused covert attention on the peripheral task-irrelevant singleton features. Therefore, HSD participants, due to their relative deficits in top–down attentional control mechanisms, did not successfully prevent the stimulus-driven attention capture relative to the task-irrelevant singleton’s features, which were not part of the current attentional set, compared to their LSD counterparts. Interestingly, individuals with higher depression states did not show a significant magnitude of N2pc in the high-perceptual-load condition, suggesting an absence of attentional bias toward the task-irrelevant singleton’s features when VWM was strained. Overall, the N2pc findings for the HSD group demonstrate the influence of perceptual loads on distractor processing, consistent with the predictions of the PLT [9,10]. PLT posits that perception is an automatic process that is dependent on limited perceptual capacity. Consequently, when the perceptual load is low (e.g., encoding one complex object in VWM), the surplus capacity allows for the processing of irrelevant stimuli (i.e., a Chinese character’s shape). However, when the perceptual load is high (e.g., encoding four complex objects in VWM), attentional capacity is depleted, effectively preventing distractor processing.
Contrary to the direct electrophysiological findings of the current study, our behavioral data did not support the hypothesis that HSD individuals have an impairment in attentional control by hindering the suppression of attentional capture via irrelevant stimuli. We propose that individuals who experience high levels of depressive symptoms may compensate for potential top–down attentional control deficits by allocating more attentional resources to the primary match-to-sample task, enabling them to perform as well as individuals with LSD. This compensatory mechanism has been suggested in previous research, which observed impaired executive control in individuals with high anxiety levels, highlighting the potential limitations of relying solely on behavioral performance measures to identify specific attentional deficits [38,60]. The main behavioral finding of the current study was that regardless of state depression, correct response times in performing match-to-sample tasks were significantly faster for the same distractor positions (compatible) compared to different (incompatible) distractor-to-target horizontal positions, which is suggests the presence of a spatially positive priming (SPP) effect [47]. Thus, it seems that participants’ visual–spatial attention was somewhat drawn to the task-irrelevant singleton on the prime display. This led to faster target detection when it appeared in the same location as the distractor, presumably due to residual attentional activation at that location.
4.1. Limitations of the Current Study
Despite providing valuable insights into the relationship between state depression and attentional control mechanisms, the present study has several limitations that should be acknowledged.
First, while ERP measures (early PD and N2pc) reveal the neural markers of attentional suppression and capture, the lack of behavioral differences between groups limits generalizability. HSD individuals may have used compensatory strategies to maintain performance, suggesting that ERP findings alone do not fully capture attentional control deficits. Future research should incorporate additional behavioral measures, such as eye-tracking, for a more comprehensive assessment.
Second, the use of a unilateral distractor array raises concerns that the observed early lateralized waveform might reflect not only proactive suppression (early PD) but also a posterior contralateral positivity (Ppc) component. Although our comparison between the two groups ruled out this possibility, future studies could employ bilateral distractor designs and experimental control conditions to better differentiate these effects.
Finally, categorizing participants using DASS-21 may not fully capture the complexity of depressive symptoms, as it assesses transient emotional states rather than clinical depression. Additionally, factors like trait anxiety and fatigue were not controlled, despite their known influence on attentional control and ERP responses. Future studies should account for these variables to refine our understanding of attentional deficits in depression.
4.2. Conclusions
Overall, the electrophysiological results of the current investigation suggest that state-depressed individuals have an impairment in top–down attentional control and particularly in feature-based selective attention, as has been previously documented in MDD [1,4]. This impairment makes it challenging for them to filter out irrelevant, distracting information, potentially hindering their ability to maintain focus on goal-oriented tasks and contributing to the cognitive difficulties linked to depression.
In terms of the implications of the findings of this study and future research directions, the modified, delayed match-to-sample task may be used in clinical applied research to evaluate the efficacy of targeted training programs developed to strengthen attentional control and selective attention mechanisms in individuals with depression. Alternatively, it can be used as part of attention-based cognitive training tools designed to help individuals filter out irrelevant stimuli and enhance their ability to focus on goal-relevant information. This can be particularly useful in educational settings or workplace environments, where distractions impair performance, and in clinical treatments for depression to reduce cognitive interference and improve daily functioning.
Moreover, longitudinal studies should be conducted to understand how attentional control deficits evolve over the course of depression, providing insights into whether these impairments are a consequence or a potential precursor of the disorder. By tracking individuals over time, researchers can examine how fluctuations in depressive symptoms influence attentional suppression and selection mechanisms, as measured through behavioral tasks and neurophysiological markers such as ERPs. This approach allows for the identification of causal relationships between depression severity and attentional control deficits. Additionally, longitudinal studies can assess the impact of therapeutic interventions, such as cognitive training or antidepressant treatments, on attentional control over time, offering valuable information on whether improvements in mood correlate with enhanced top–down attention regulation. Understanding these dynamics could help refine models of depression and guide the development of targeted interventions aimed at mitigating attentional dysfunction in affected individuals.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/app15063069/s1, The Supplementary Materials include the following: the audio (.mp3) and video (.mp4) instructions of the modified, delayed match-to-sample task; a video showing an example of a few trails of the modified, delayed match-to-sample task; and the output files of the statistical analysis of this study. The installer for the Working Memory Analyser (WMA) software for Windows and macOS, including the visual paradigm (i.e., modified, delayed match-to-sample task) employed in this study, is available for download from the GitHub repository: https://github.com/gfuggetta-lab/WorkingMemoryAnalyser/releases (accessed on 1 October 2024).
Author Contributions
Conceptualization, G.F.; methodology, G.F.; software, G.F. and P.A.D.; formal analysis, G.F.; investigation, G.F., R.C., P.M., J.C., and E.D.; resources, G.F.; data curation, G.F. and R.C.; writing—original draft preparation, G.F.; writing—review and editing, G.F.; visualization, G.F.; supervision, G.F.; project administration, G.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of University of Roehampton (ethics application reference code: PSYC 22/446. Date of approval: 6 February 2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author, [G.F.].
Acknowledgments
Giorgio Fuggetta wishes to thank Dmitry Boyarintsev for his support in programming the software Working Memory Analyser (WMA) for Windows and MacOS using Lazarus, the professional Free Pascal cross-platform Integrated Development Environment (IDE) for Rapid Application Development (RAD) (https://www.lazarus-ide.org/). This research is dedicated to the memory of Enea Francesco Pavone (1972–2018), Howard David Page-Clark (1953–2023), Giorgio Fuggetta’s loving mother-in-law, Sadhana ‘Bani’ Purkayastha (1945–2024) and father-in-law, Prodyut Kumar Purkayastha (1937–2025).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| MDD | Major depressive disorder; |
| DASS | Depression Anxiety Stress Scale; |
| VWM | Visual working memory; |
| ERP | Event-related potential; |
| PD | Distractor positivity; |
| PLT | Perceptual load theory; |
| SSH | Signal suppression hypothesis; |
| LSD | Low state depression; |
| HSD | High state depression; |
| MEG | Magnetoencephalographic; |
| SNP | Spatial negative priming; |
| SPP | Spatial positive priming. |
Appendix A
Participants received visual instructions for the delayed match-to-sample task while listening to a pre-recorded audio file in MP3 format containing the instructions delivered by an AI voice.

References
- Keller, A.S.; Leikauf, J.E.; Holt-Gosselin, B.; Staveland, B.R.; Williams, L.M. Paying attention to attention in depression. Transl. Psychiatry 2019, 9, 279. [Google Scholar] [CrossRef] [PubMed]
- Katsuki, F.; Constantinidis, C. Bottom-Up and Top-Down Attention:Different Processes and Overlapping Neural Systems. Neuroscientist 2014, 20, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Nobre, A.C.; Kastner, S. 1201Attention: Time Capsule 2013. In The Oxford Handbook of Attention; ANobre, C., Kastner, S., Eds.; Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- Keller, A.S.; Ball, T.M.; Williams, L.M. Deep phenotyping of attention impairments and the ‘Inattention Biotype’ in Major Depressive Disorder. Psychol. Med. 2020, 50, 2203–2212. [Google Scholar] [CrossRef] [PubMed]
- Ladouceur, C.D.; Slifka, J.S.; Dahl, R.E.; Birmaher, B.; Axelson, D.A.; Ryan, N.D. Altered error-related brain activity in youth with major depression. Dev. Cogn. Neurosci. 2012, 2, 351–362. [Google Scholar] [CrossRef]
- Olvet, D.M.; Klein, D.N.; Hajcak, G. Depression symptom severity and error-related brain activity. Psychiatry Res. 2010, 179, 30–37. [Google Scholar] [CrossRef]
- Luck, S.J.; Vogel, E.K. The capacity of visual working memory for features and conjunctions. Nature 1997, 390, 279–281. [Google Scholar] [CrossRef]
- Luck, S.J.; Vogel, E.K. Visual working memory capacity: From psychophysics and neurobiology to individual differences. Trends Cogn. Sci. 2013, 17, 391–400. [Google Scholar] [CrossRef]
- Lavie, N. Perceptual load as a necessary condition for selective attention. J. Exp. Psychol. Hum. Percept. Perform. 1995, 21, 451–468. [Google Scholar] [CrossRef]
- Lavie, N.; Tsal, Y. Perceptual load as a major determinant of the locus of selection in visual attention. Percept. Psychophys. 1994, 56, 183–197. [Google Scholar] [CrossRef]
- Lavie, N.; Fox, E. The role of perceptual load in negative priming. J. Exp. Psychol. Hum. Percept. Perform. 2000, 26, 1038–1052. [Google Scholar] [CrossRef]
- Brockhoff, L.; Schindler, S.; Bruchmann, M.; Straube, T. Effects of perceptual and working memory load on brain responses to task-irrelevant stimuli: Review and implications for future research. Neurosci. Biobehav. Rev. 2022, 135, 104580. [Google Scholar] [CrossRef]
- Drisdelle, B.L.; Eimer, M. PD components and distractor inhibition in visual search: New evidence for the signal suppression hypothesis. Psychophysiology 2021, 58, e13878. [Google Scholar] [CrossRef] [PubMed]
- Luck, S.J. Electrophysiological correlates of the focusing of attention within complex visual scenes: N2pc and related ERP components. In The Oxford Handbook of Event-Related Potential Components; Oxford University Press: New York, NY, USA, 2012; pp. 329–360. [Google Scholar]
- Luck, S.J.; Hillyard, S.A. Spatial filtering during visual search: Evidence from human electrophysiology. J. Exp. Psychol. Hum. Percept. Perform. 1994, 20, 1000–1014. [Google Scholar] [CrossRef] [PubMed]
- Luck, S.J.; Hillyard, S.A. Electrophysiological correlates of feature analysis during visual search. Psychophysiology 1994, 31, 291–308. [Google Scholar] [CrossRef]
- Hopf, J.-M.; Luck, S.J.; Girelli, M.; Hagner, T.; Mangun, G.R.; Scheich, H.; Heinze, H.-J. Neural Sources of Focused Attention in Visual Search. Cereb. Cortex 2000, 10, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Gaspelin, N.; Luck, S.J. Combined Electrophysiological and Behavioral Evidence for the Suppression of Salient Distractors. J. Cogn. Neurosci. 2018, 30, 1265–1280. [Google Scholar] [CrossRef]
- Hickey, C.; Di Lollo, V.; McDonald, J.J. Electrophysiological Indices of Target and Distractor Processing in Visual Search. J. Cogn. Neurosci. 2009, 21, 760–775. [Google Scholar] [CrossRef]
- Luck, S.J.; Gaspelin, N.; Folk, C.L.; Remington, R.W.; Theeuwes, J. Progress Toward Resolving the Attentional Capture Debate. Vis Cogn 2021, 29, 1–21. [Google Scholar] [CrossRef]
- Sawaki, R.; Luck, S.J. Active suppression of distractors that match the contents of visual working memory. Vis. Cogn. 2011, 19, 956–972. [Google Scholar] [CrossRef]
- Gaspelin, N.; Leonard, C.J.; Luck, S.J. Suppression of overt attentional capture by salient-but-irrelevant color singletons. Atten. Percept. Psychophys. 2017, 79, 45–62. [Google Scholar] [CrossRef]
- Gaspelin, N.; Luck, S.J. The Role of Inhibition in Avoiding Distraction by Salient Stimuli. Trends Cogn. Sci. 2018, 22, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Ipata, A.E.; Gee, A.L.; Gottlieb, J.; Bisley, J.W. LIP responses to a popout stimulus are reduced if it is overtly ignored. Nat. Neurosci. 2006, 9, 1071–1076. [Google Scholar] [CrossRef]
- Sawaki, R.; Luck, S.J. Capture versus suppression of attention by salient singletons: Electrophysiological evidence for an automatic attend-to-me signal. Atten. Percept. Psychophys. 2010, 72, 1455–1470. [Google Scholar] [CrossRef] [PubMed]
- Vatterott, D.B.; Vecera, S.P. Experience-dependent attentional tuning of distractor rejection. Psychon. Bull. Rev. 2012, 19, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Gaspelin, N.; Lamy, D.; Egeth, H.E.; Liesefeld, H.R.; Kerzel, D.; Mandal, A.; Müller, M.M.; Schall, J.D.; Schubö, A.; Slagter, H.A.; et al. The Distractor Positivity Component and the Inhibition of Distracting Stimuli. J. Cogn. Neurosci. 2023, 35, 1693–1715. [Google Scholar] [CrossRef]
- Feldmann-Wüstefeld, T.; Vogel, E.K. Neural Evidence for the Contribution of Active Suppression During Working Memory Filtering. Cereb. Cortex 2018, 29, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Konstantinou, N.; Lavie, N. Dissociable roles of different types of working memory load in visual detection. J. Exp. Psychol. Hum. Percept. Perform. 2013, 39, 919–924. [Google Scholar] [CrossRef]
- Lavie, N. Distracted and confused?: Selective attention under load. Trends Cogn. Sci. 2005, 9, 75–82. [Google Scholar] [CrossRef]
- Lavie, N.; Cox, S. On the Efficiency of Visual Selective Attention: Efficient Visual Search Leads to Inefficient Distractor Rejection. Psychol. Sci. 1997, 8, 395–396. [Google Scholar] [CrossRef]
- Lavie, N.; Dalton, P. Load Theory of Attention and Cognitive Control. In The Oxford Handbook of Attention; Nobre, A.C., Kastner, S., Eds.; Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- Gaspelin, N.; Leonard, C.J.; Luck, S.J. Direct Evidence for Active Suppression of Salient-but-Irrelevant Sensory Inputs. Psychol. Sci. 2015, 26, 1740–1750. [Google Scholar] [CrossRef]
- Fuggetta, G.; Duke, P.A. Enhancing links between visual short term memory, visual attention and cognitive control processes through practice: An electrophysiological insight. Biol. Psychol. 2017, 126, 48–60. [Google Scholar] [CrossRef][Green Version]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Lovibond, P.F.; Lovibond, S.H. The structure of negative emotional states: Comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav. Res. Ther. 1995, 33, 335–343. [Google Scholar] [CrossRef]
- Zanon, C.; Brenner, R.E.; Baptista, M.N.; Vogel, D.L.; Rubin, M.; Al-Darmaki, F.R.; Gonçalves, M.; Heath, P.J.; Liao, H.-Y.; Mackenzie, C.S.; et al. Examining the Dimensionality, Reliability, and Invariance of the Depression, Anxiety, and Stress Scale–21 (DASS-21) Across Eight Countries. Assessment 2021, 28, 1531–1544. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, J.M.; McDonald, J.J. High Level of Trait Anxiety Leads to Salience-Driven Distraction and Compensation. Psychol. Sci. 2018, 29, 2020–2030. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.; Wong, J.L.; Bagge, C.L.; Freedenthal, S.; Gutierrez, P.M.; Lozano, G. The Depression Anxiety Stress Scales—21 (DASS-21): Further Examination of Dimensions, Scale Reliability, and Correlates. J. Clin. Psychol. 2012, 68, 1322–1338. [Google Scholar] [CrossRef]
- Clara, I.P.; Cox, B.J.; Enns, M.W. Confirmatory Factor Analysis of the Depression–Anxiety–Stress Scales in Depressed and Anxious Patients. J. Psychopathol. Behav. Assess. 2001, 23, 61–67. [Google Scholar] [CrossRef]
- Sinclair, S.J.; Siefert, C.J.; Slavin-Mulford, J.M.; Stein, M.B.; Renna, M.; Blais, M.A. Psychometric Evaluation and Normative Data for the Depression, Anxiety, and Stress Scales-21 (DASS-21) in a Nonclinical Sample of U.S. Adults. Eval. Health Prof. 2012, 35, 259–279. [Google Scholar] [CrossRef]
- Attneave, F.; Arnoult, M.D. The quantitative study of shape and pattern perception. Psychol. Bull. 1956, 53, 452–471. [Google Scholar] [CrossRef]
- Hautzel, H.; Mottaghy, F.M.; Specht, K.; Müller, H.-W.; Krause, B.J. Evidence of a modality-dependent role of the cerebellum in working memory? An fMRI study comparing verbal and abstract n-back tasks. NeuroImage 2009, 47, 2073–2082. [Google Scholar] [CrossRef]
- Thürling, M.; Hautzel, H.; Küper, M.; Stefanescu, M.; Maderwald, S.; Ladd, M.; Timmann, D. Involvement of the cerebellar cortex and nuclei in verbal and visuospatial working memory: A 7T fMRI study. NeuroImage 2012, 62, 1537–1550. [Google Scholar] [CrossRef] [PubMed]
- Shum, D.H.; O’Gorman, J.G.; Eadie, K. Normative data for a new memory test: The Shum Visual Learning Test. Clin Neuropsychol 1999, 13, 121–135. [Google Scholar] [CrossRef]
- Eadie, K.; Shum, D. Assessment of visual memory: A comparison of Chinese characters and geometric figures as stimulus materials. J. Clin. Exp. Neuropsychol. 1995, 17, 731–739. [Google Scholar] [CrossRef]
- Maljkovic, V.; Nakayama, K. Priming of pop-out: II. Role Position. Percept. Psychophys. 1996, 58, 977–991. [Google Scholar] [CrossRef]
- Neill, W.T.; Kleinsmith, A.L. Spatial negative priming: Location or response? Atten. Percept. Psychophys. 2016, 78, 2411–2419. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fukuda, K.; Vogel, E.K. Individual differences in recovery time from attentional capture. Psychol. Sci. 2011, 22, 361–368. [Google Scholar] [CrossRef]
- Konstantinou, N.; Beal, E.; King, J.-R.; Lavie, N. Working memory load and distraction: Dissociable effects of visual maintenance and cognitive control. Atten. Percept. Psychophys. 2014, 76, 1985–1997. [Google Scholar] [CrossRef]
- Roper, Z.J.J.; Vecera, S.P. Visual short-term memory load strengthens selective attention. Psychon. Bull. Rev. 2014, 21, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Winkler, I.; Debener, S.; Müller, K.R.; Tangermann, M. On the influence of high-pass filtering on ICA-based artifact reduction in EEG-ERP. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015. [Google Scholar]
- Braver, T.S. The variable nature of cognitive control: A dual mechanisms framework. Trends Cogn. Sci. 2012, 16, 106–113. [Google Scholar] [CrossRef]
- Corriveau, I.; Fortier-Gauthier, U.; Pomerleau, V.J.; McDonald, J.; Dell’Acqua, R.; Jolicoeur, P. Electrophysiological evidence of multitasking impairment of attentional deployment reflects target-specific processing, not distractor inhibition. Int. J. Psychophysiol. 2012, 86, 152–159. [Google Scholar] [CrossRef]
- Fortier-Gauthier, U.; Moffat, N.; Dell’Acqua, R.; McDonald, J.J.; Jolicœur, P. Contralateral cortical organisation of information in visual short-term memory: Evidence from lateralized brain activity during retrieval. Neuropsychologia 2012, 50, 1748–1758. [Google Scholar] [CrossRef]
- Leblanc, É.; Prime, D.J.; Jolicoeur, P. Tracking the Location of Visuospatial Attention in a Contingent Capture Paradigm. J. Cogn. Neurosci. 2008, 20, 657–671. [Google Scholar] [CrossRef] [PubMed]
- Barras, C.; Kerzel, D. Active suppression of salient-but-irrelevant stimuli does not underlie resistance to visual interference. Biol. Psychol. 2016, 121, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Barras, C.; Kerzel, D. Salient-but-irrelevant stimuli cause attentional capture in difficult, but attentional suppression in easy visual search. Psychophysiology 2017, 54, 1826–1838. [Google Scholar] [CrossRef] [PubMed]
- Jannati, A.; Gaspar, J.M.; McDonald, J.J. Tracking target and distractor processing in fixed-feature visual search: Evidence from human electrophysiology. J. Exp. Psychol. Hum. Percept. Perform. 2013, 39, 1713–1730. [Google Scholar] [CrossRef]
- Eysenck, M.W.; Derakshan, N.; Santos, R.; Calvo, M.G. Anxiety and cognitive performance: Attentional control theory. Emotion 2007, 7, 336–353. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).