On the Formation of Carbonaceous By-Product Species in Spray Flame Synthesis of Maghemite Nanoparticles
Abstract
1. Introduction
1.1. By-Product Types in SFS and Their Relevance
- Precursor and solvent residues can be by-products in case these species do not react/disintegrate entirely [13].
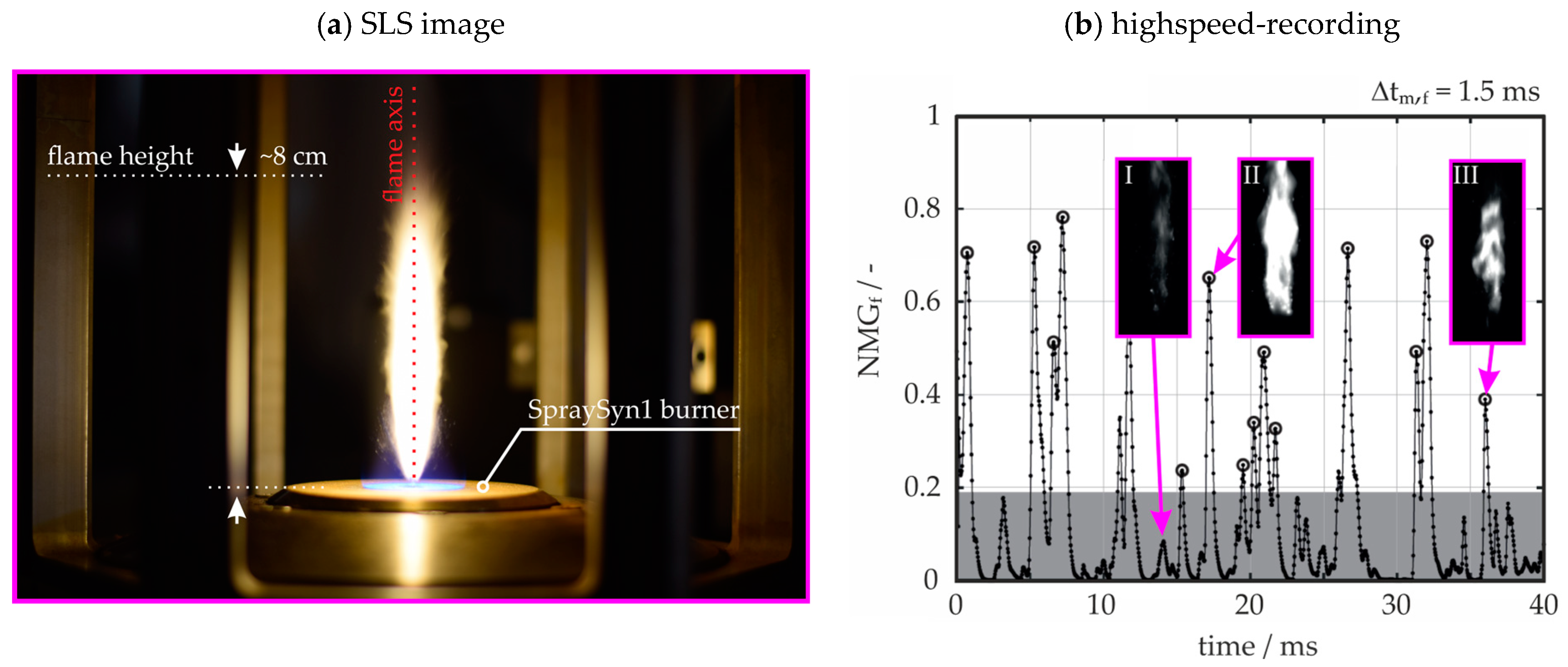
1.2. Study Scope
2. Materials and Methods
2.1. Using Standardized Burner Types

2.2. Precursor Systems and Global Oxygen Availability
2.3. Powder Analyses
- (1)
- Raman spectroscopy is performed using an inVia™ confocal Raman microscope (Renishaw GmbH, Pliezhausen, Germany). Therefore, a low laser intensity of 1% at 633 nm (0.175 mW) is used to avoid any phase transformations that could otherwise occur during Raman measurements [41]. Each measurement consisted of 50 repetitions, with each repetition lasting 10 s, resulting in a total sampling duration of 500 s.
- (2)
- TEM samples are analyzed using a JEOL JEM-ARM200F high-resolution microscope with Cs correction and field emission HRTEM-STEM capabilities sourced from JEOL (Germany) GmbH (Freising, Germany). Therefore, conventional carbon-coated Cu grids (type S160, PLANO GmbH, Wetzlar, Germany) are used. TEM samples are either obtained from thermophoretic sampling or dipping sample grids into the powder. No organic solvents are used for preparation, which can otherwise cause biases in TEM examinations [11].
- (3)
- Thermogravimetric analysis (TGA) coupled with differential scanning calorimetry (DSC) and mass spectrometry (MS) is conducted using an STA 449 F1 Jupiter® device obtained from NETZSCH GmbH (Selb, Germany). Therefore, powders are pressed into pellets and heated from 25 to 800 °C in a silicon carbide oven, while the gas atmosphere was monitored by MS using a QMS 403 Aëolos® quadrupole device also sourced from NETZSCH GmbH (Selb, Germany). Mass-to-charge (m/z) ratios of 18 (H2O), 44 (CO2), and 32 (O2) are monitored while a rather low-temperature ramp of 7.5 K min−1 is applied to mitigate possible superpositions of thermal events. Measurements are carried out in synthetic air and argon. Altering the atmosphere in this way is valuable for revealing the origin of thermal events, i.e., oxidative decomposition events are sensitive to the O2 availability, while physical mechanisms, such as physical gas desorption and evaporation, are not.
- (4)
- Attenuated total reflection Fourier transform infrared spectroscopy (ATR-FTIR) measurements are conducted using a Vertex® 70 spectroscope manufactured by Bruker Corporation (Billerica, MA, USA). The absorbance is measured in the wavelength range of 370–4500 cm−1 with a resolution of 4 cm−1. A platinum holder is used for the ATR measurements.
- (5)
- Macroscopic magnetic properties are investigated with the vibrating sample magnetometer (VSM) option of a PPMS DynaCool obtained from Quantum Design GmbH (Pfungstadt, Germany). Therefore, field-dependent M(H) magnetization loops are recorded at 300 K up to a maximum field of 7 kOe.
- (6)
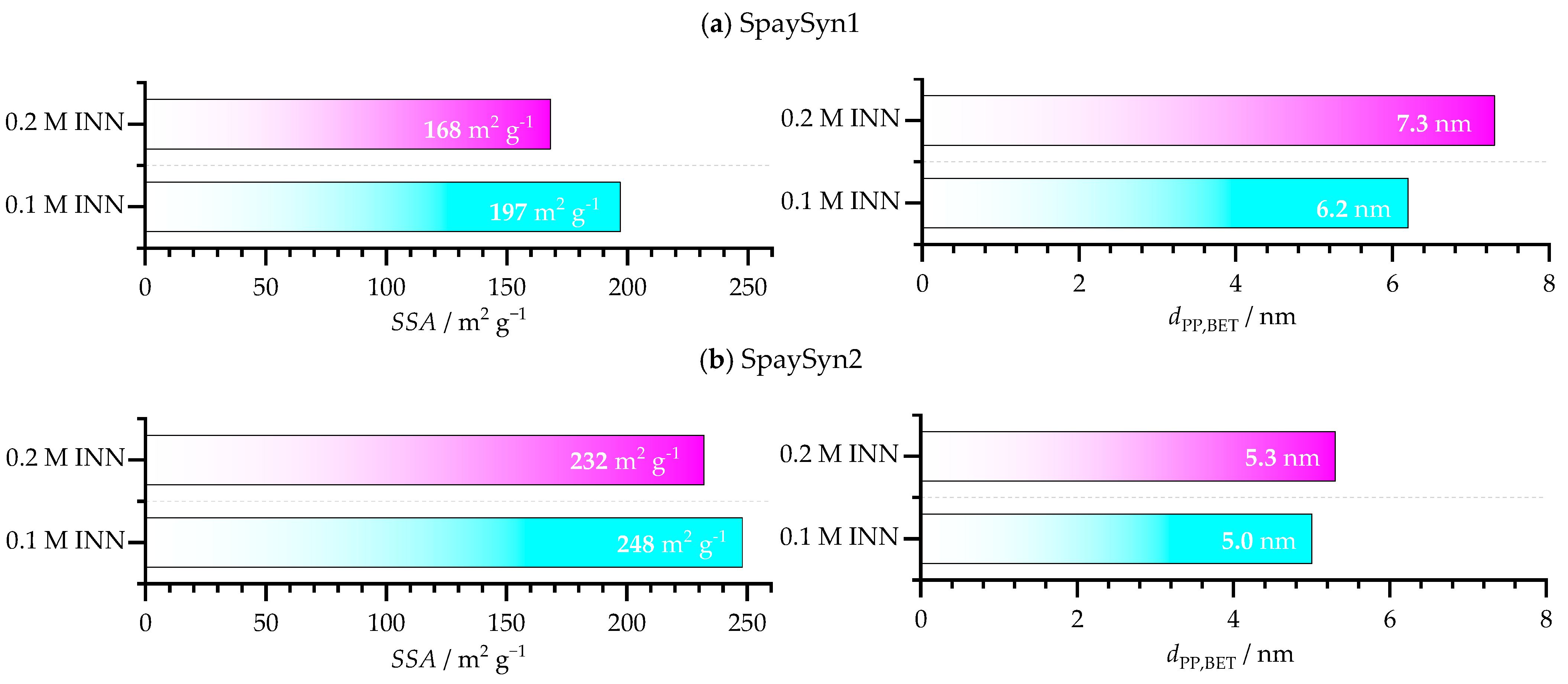
- Brunauer-Emmet-Teller (BET) N2 physisorption is conducted at −196 °C utilizing a Quantachrome Autosorb 6 from Anton Paar Germany GmbH (Ostfildern-Scharnhausen, Germany) to record sorption isotherms, and the SSA is determined considering five points in the relative pressure region 0.1–0.3. Therefore, samples are vacuum-degassed overnight at 120 °C. Since the SSA represents the ratio of the cumulative surface of all particles, to their cumulative mass, , an equivalent primary particle diameter is obtained by Equation (1), assuming all particles to be spherical and perfectly monodisperse. Hence, represents a surface equivalent mass-weighted average particle diameter, with as the particles’ surface and as the particles’ volume, the total number of primary particles, and the material density, which is ~4.88 g cm−3 for γ-Fe2O3:
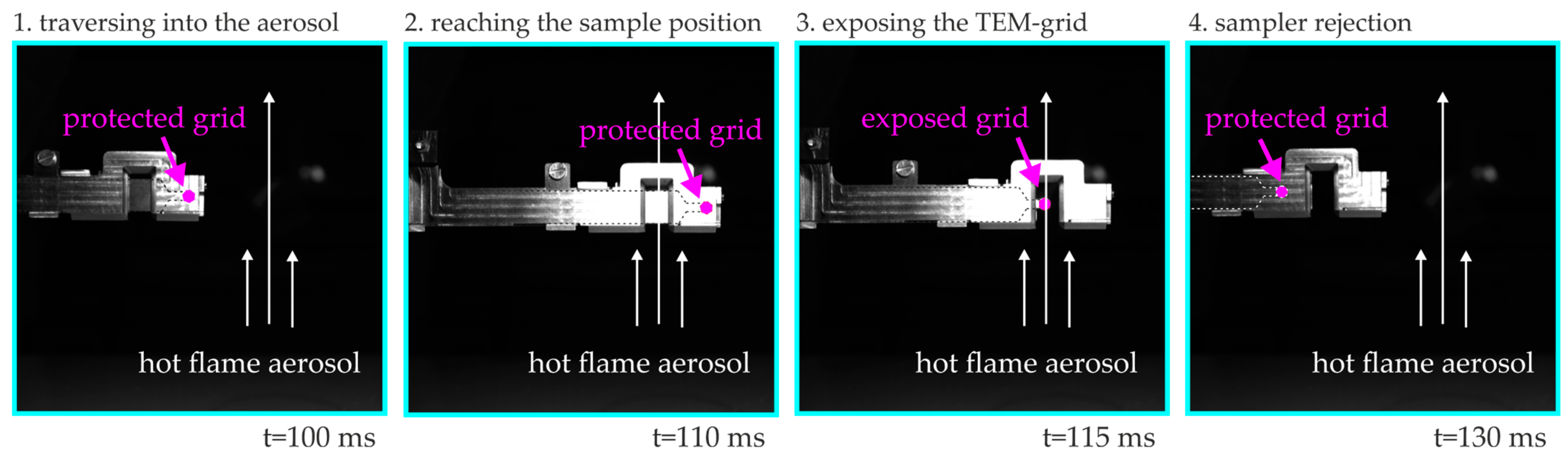
2.4. Thermophoretic Sampling for Transmission Electron Microscopy (TS-TEM)
3. Results and Discussion
3.1. Final Particle Characteristics of Samples Obtained by SpraySyn1 and SpraySyn2
3.1.1. TEM and Raman Spectroscopy
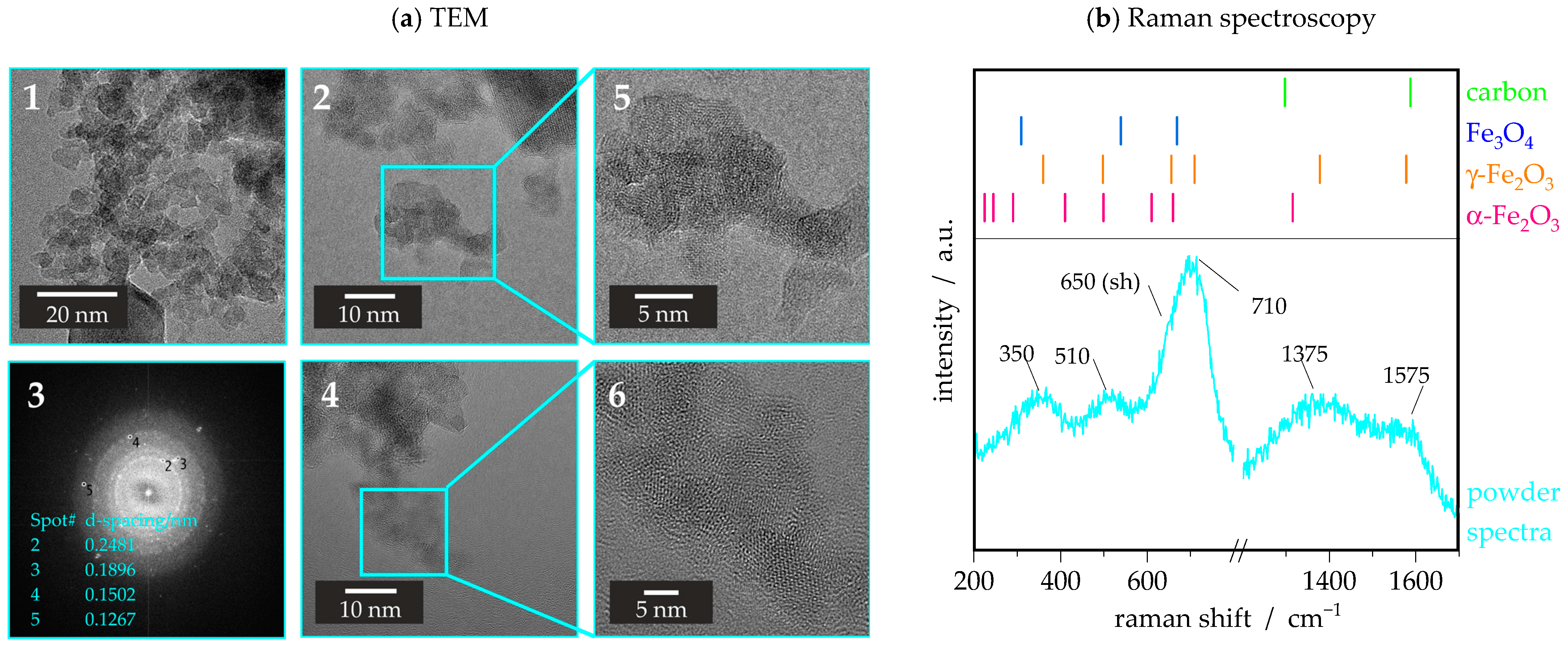
3.1.2. BET and Magnetic Properties
3.1.3. ATR-FTIR
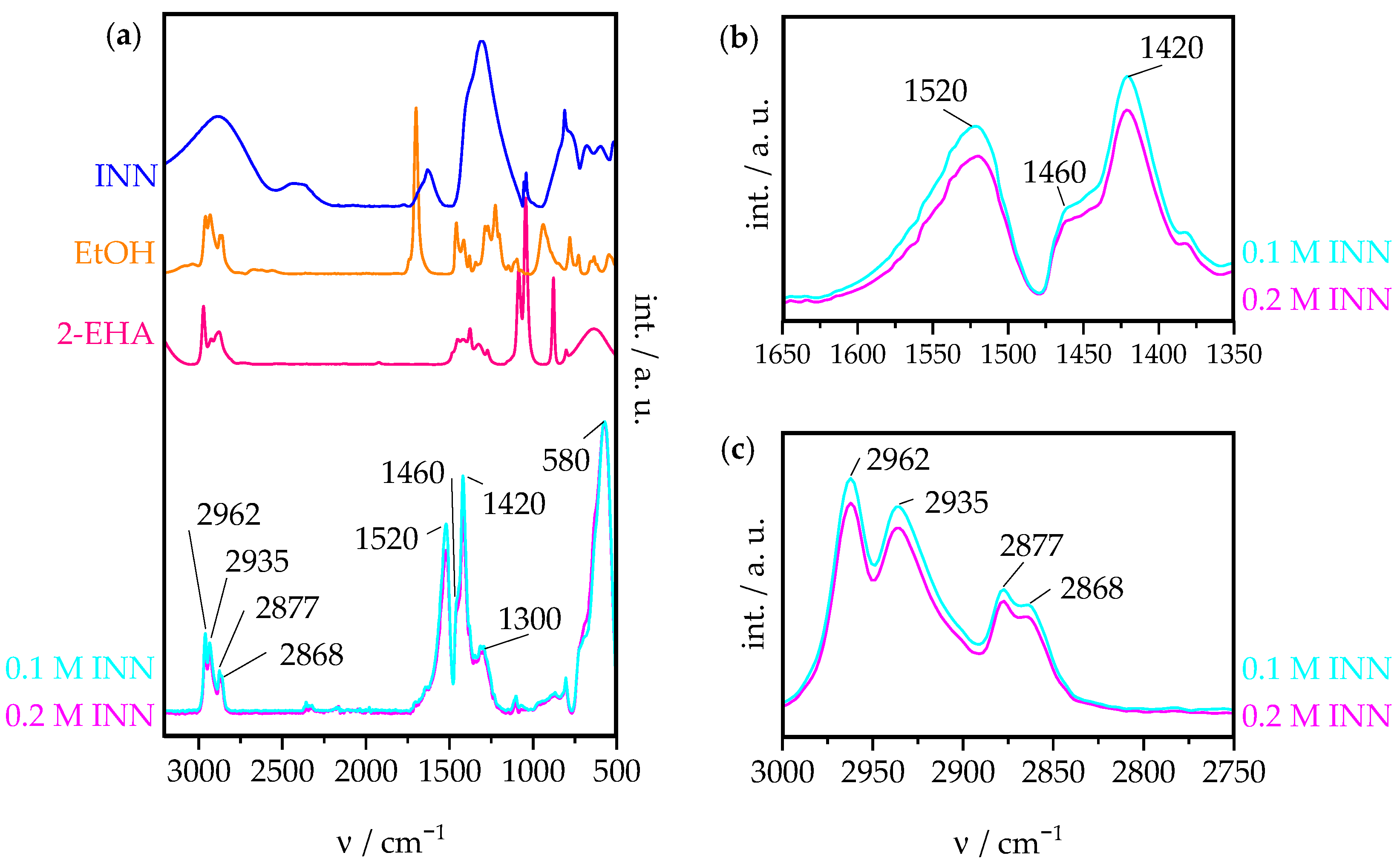
3.1.4. TGA-DSC-MS

- (1)
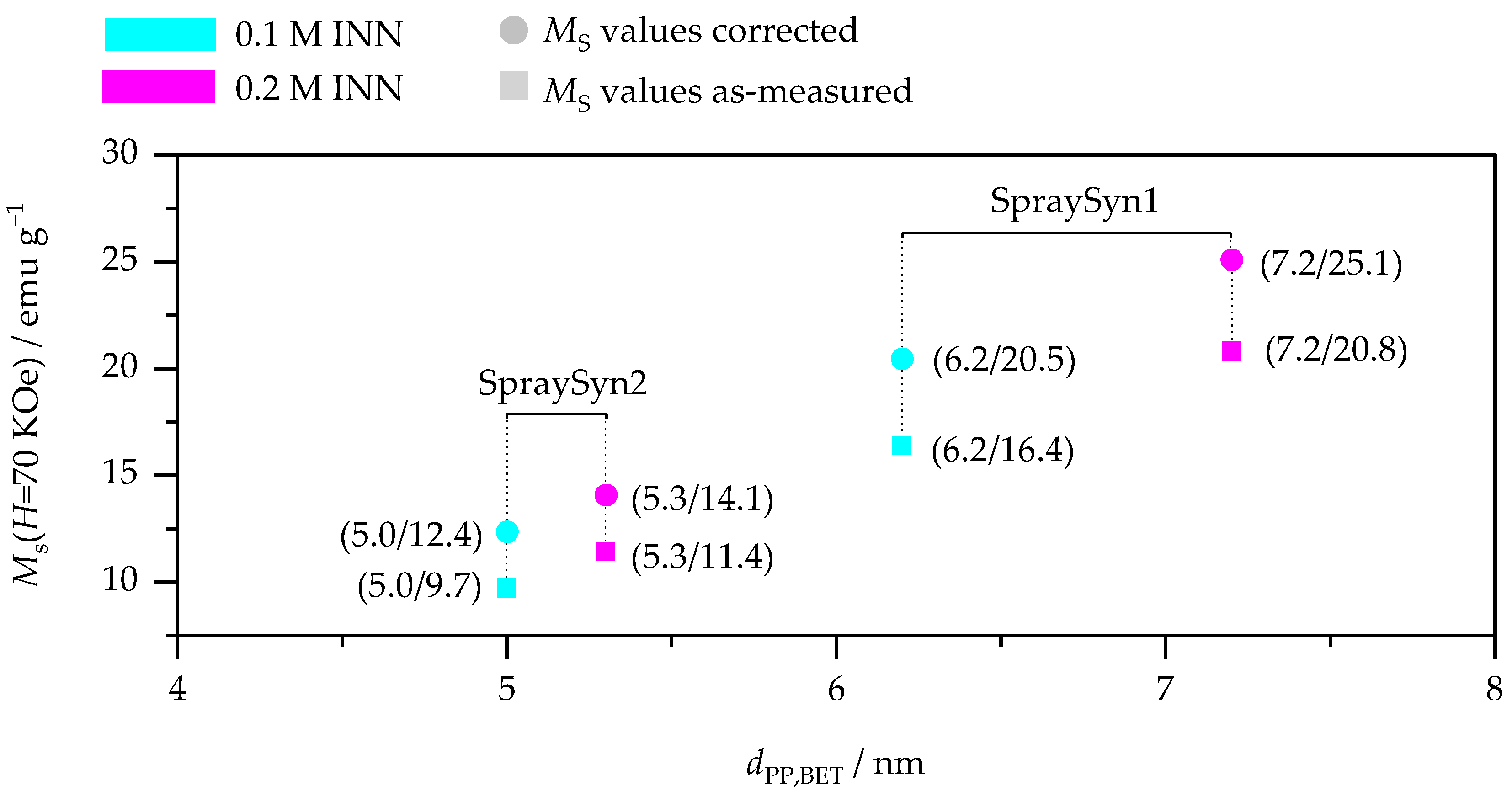
- Since γ-Fe2O3 represents the dominant particle phase, each sample’s total γ-Fe2O3 content is given by the final steady-state TGA value in air, and all four contents of γ-Fe2O3 are presented in Table 3. As shown, the materials exhibit considerable mass losses (~17–21 wt.%) since by-products are present. The fact that solely four-fifths of the overall material mass belonging to γ-Fe2O3 is of particular relevance for weight-related particle applications, e.g., the vibrational magnetometry conducted earlier. To demonstrate this issue, Figure 8 depicts the materials as-measured values vs. values normalized to each sample’s respective γ-Fe2O3 content. These normalized values represent the values one would achieve if particle surfaces were entirely pure. This could be accomplished by applying post-synthesis treatments (e.g., thermal annealing or wet-chemistry procedures [2,15,57]). Since water, carbonates, and carboxylates are diamagnetic, they do not contribute to magnetic responses in VSM measurements, making the normalization procedure mentioned above plausible for the data.
- (2)
- Moreover, the individual mass fractions of water and carbonates/carboxylates can be determined by differentiating the TGA mass losses according to their respective TGA temperature ranges (below 150 °C for water desorption and above for thermal decomposition of organic species). This temperature threshold can be chosen because it represents a balanced compromise between the water desorption process and the organic species oxidation process (cf. Figure 7). Examining the TGA data this way reveals that the materials exhibit a weight loss of ~14–18 wt.% due to the oxidation of carbonates/carboxylates and of ~2–3 wt.% caused by water desorption (Table 3). Given the fact that these species are located on the particle surface, one can subsequently normalize their relative mass fractions to the samples’ respective SSA, yielding values for the particle surface loading (PSL, unit: mg m−2). This normalization procedure yields PSL values for water and organics, and corresponding values are shown in Table 3. As presented there, organic PSLs are independent of the precursor concentration. However, they depend on the burner type applied: SpraySyn1 samples exhibit organic PSLs at ~0.87 mg m−2, while SpraySyn2 samples attain slightly lower organic PSLs in the range of ~0.69–0.73 mg m−2. On the one hand, this indicates that SpraySyn2 yields particles with fewer carboxylates on the surface. On the other hand, this also indicates that surface-bond carboxylates could have lower average molar masses for SpraySyn2 samples. Both possibilities could coincide with a slightly more complete combustion for this burner type, likely attributable to differences in the burners’ flame activity profile: Recently, the study of Kennedy et al. indicated in experiments on a single SFS burner type that more transient combustion conditions can cause higher carboxylate and carbonate contents, which aligns well with the findings here [18]. Because the above-mentioned calculation of organic PSL values enables the comparison of the purity of SFS samples independently of the particle size, our methodology may be very valuable to denote/compare SFS-made materials in a more systematic way in the future. It would be interesting to prove whether this normalization approach is also transferable on other SFS materials.
| Burner type | SpraySyn1 | SpraySyn2 | ||
| INN conc. | 0.1 M | 0.2 M | 0.1 M | 0.2 M |
| Total mass loss during TGA in air/% | 19.80 | 17.01 | 21.41 | 18.83 |
| γ-Fe2O3 content/% | 80.20 | 82.99 | 78.59 | 81.17 |
| Water relative mass/% | 2.70 | 2.43 | 3.35 | 2.83 |
| Carbonate/carboxylate relative mass/% | 17.10 | 14.59 | 18.06 | 16.00 |
| PSL by water/mg m−2 | 0.14 | 0.14 | 0.14 | 0.12 |
| PSL by carboxylates/carbonates/mg m−2 | 0.87 | 0.87 | 0.73 | 0.69 |
3.2. Local Particle Characteristics Probed by TS-TEM
3.2.1. TS-TEM Conducted at 15 cm HAB
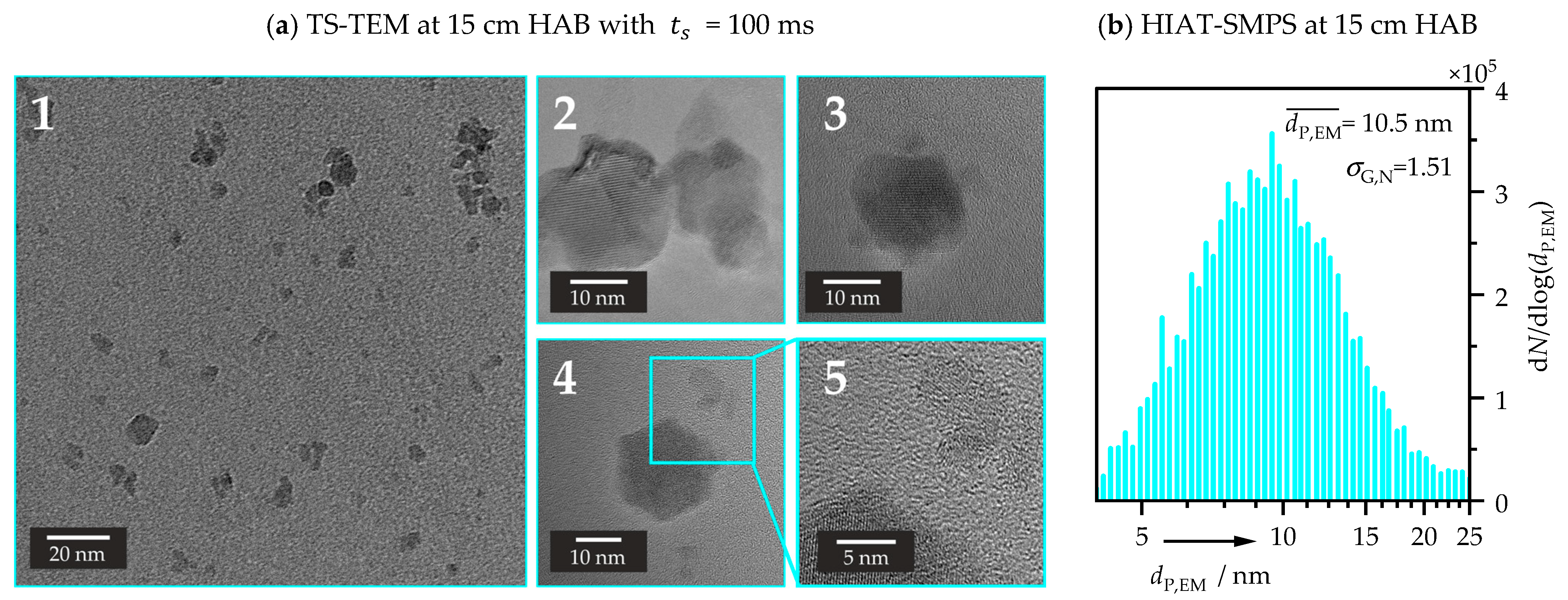
3.2.2. TS-TEM Conducted at 5 cm HAB
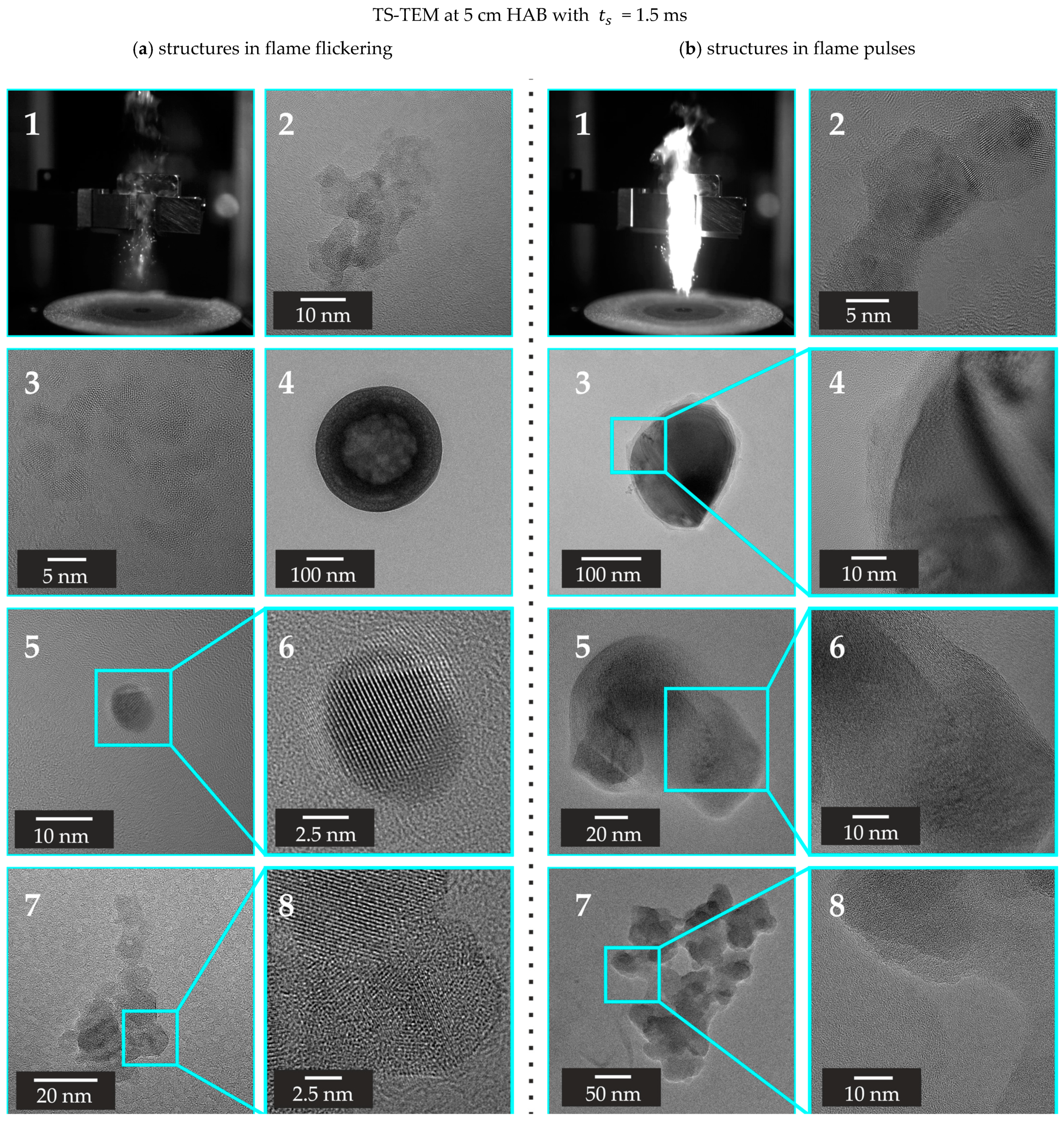
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pankhurst, Q.A.; Conolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. Appl. Phys. B 2003, 36, R167–R181. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef] [PubMed]
- Gul, S.; Khan, S.B.; Rehman, I.U.; Khan, M.A.; Khan, M.I. A Comprehensive Review of Magnetic Nanomaterials Modern Day Theranostics. Front. Mater. 2019, 6, 179. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences, and Uses; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Schwertmann, U.; Cornell, R.M. Iron Oxides in the Laboratory: Preparation and Characterization; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Li, S.; Ren, Y.; Biswas, P.; Tse, S.D. Flame aerosol synthesis of nanostructured materials and functional devices: Processing, modeling, and diagnostics. Prog. Energy Combust. Sci. 2016, 55, 1–59. [Google Scholar] [CrossRef]
- Strobel, R.; Pratsinis, S.E. Flame aerosol synthesis of smart nanostructured materials. J. Mater. Chem. 2007, 17, 4743. [Google Scholar] [CrossRef]
- Teoh, W.Y.; Amal, R.; Mädler, L. Flame spray pyrolysis: An enabling technology for nanoparticles design and fabrication. Nanoscale 2010, 2, 1324–1347. [Google Scholar] [CrossRef]
- Sorvali, M.; Nikka, M.; Juuti, P.; Honkanen, M.; Salminen, T.; Hyvärinen, L.; Mäkelä, J.M. Controlling the phase of iron oxide nanoparticles fabricated from iron (III) nitrate by liquid flame spray. Int. J. Ceram. Eng. Sci. 2019, 1, 194–205. [Google Scholar] [CrossRef]
- Sorvali, M.; Honkanen, M.; Hyvärinen, L.; Kuisma, R.; Larjo, J.; Mäkelä, J.M. Crystallographic phase formation of iron oxide particles produced from iron nitrate by liquid flame spray with a dual oxygen flow. Int. J. Ceram. Eng. Sci. 2021, 3, 227–236. [Google Scholar] [CrossRef]
- Tischendorf, R.; Simmler, M.; Weinberger, C.; Bieber, M.; Reddemann, M.; Fröde, F.; Lindner, J.; Pitsch, H.; Kneer, R.; Tiemann, M.; et al. Examination of the evolution of iron oxide nanoparticles in flame spray pyrolysis by tailored in situ particle sampling techniques. J. Aerosol Sci. 2021, 154, 105722. [Google Scholar] [CrossRef]
- Hammad, M.; Hardt, S.; Mues, B.; Salamon, S.; Landers, J.; Slabu, I.; Wende, H.; Schulz, C.; Wiggers, H. Gas-phase synthesis of iron oxide nanoparticles for improved magnetic hyperthermia performance. J. Alloys Compd. 2020, 824, 153814. [Google Scholar] [CrossRef]
- Carvajal, L.; Buitrago-Sierra, R.; Santamaría, A.; Angel, S.; Wiggers, H.; Gallego, J. Effect of Spray Parameters in a Spray Flame Reactor During FexOy Nanoparticles Synthesis. J. Therm. Spray Technol. 2020, 29, 368–383. [Google Scholar] [CrossRef]
- Meierhofer, F.; Li, H.; Gockeln, M.; Kun, R.; Grieb, T.; Rosenauer, A.; Fritsching, U.; Kiefer, J.; Birkenstock, J.; Mädler, L.; et al. Screening Precursor-Solvent Combinations for Li4Ti5O12 Energy Storage Material Using Flame Spray Pyrolysis. ACS Appl. Mater. Interfaces 2017, 9, 37760–37777. [Google Scholar] [CrossRef] [PubMed]
- Alkan, B.; Medina, D.; Landers, J.; Heidelmann, M.; Hagemann, U.; Salamon, S.; Andronescu, C.; Wende, H.; Schulz, C.; Schuhmann, W.; et al. Spray-Flame-Prepared LaCo 1– xFexO3 Perovskite Nanoparticles as Active OER Catalysts: Influence of Fe Content and Low-Temperature Heating. ChemElectroChem 2020, 7, 2564–2574. [Google Scholar] [CrossRef]
- Grimm, S.; Stelzner, T.; Leuthäußer, J.; Barth, S.; Heide, K. Particle size effects on the thermal behaviour of maghemite synthesised by flame pyrolysis. Thermochim. Acta 1997, 300, 141–148. [Google Scholar] [CrossRef]
- Grimm, S.; Schulz, M.; Barth, S.; Müller, R. Flame pyrolysis—A preparation route for ultrafine pure gamma-Fe2O3 powders and the control of their particle size and properties. J. Mater. Sci. 1997, 32, 1083–1092. [Google Scholar] [CrossRef]
- Kennedy, C.M.; Zhang, Y.; Zou, S.; Dunn, M.J.; Masri, A.R. Influence of flame stability on iron oxide nanoparticle growth during FSP. J. Aerosol Sci. 2025, 183, 106475. [Google Scholar] [CrossRef]
- Strobel, R.; Pratsinis, S.E. Direct synthesis of maghemite, magnetite and wustite nanoparticles by flame spray pyrolysis. Adv. Powder Technol. 2009, 20, 190–194. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Y.; Huang, G.; Li, C. Metallic iron nanoparticles: Flame synthesis, characterization and magnetic properties. Particuology 2013, 11, 460–467. [Google Scholar] [CrossRef]
- Herrmann, I.K.; Grass, R.N.; Mazunin, D.; Stark, W.J. Synthesis and Covalent Surface Functionalization of Nonoxidic Iron Core−Shell Nanomagnets. Chem. Mater. 2009, 21, 3275–3281. [Google Scholar] [CrossRef]
- Ren, Y.; Ran, K.; Kruse, S.; Mayer, J.; Pitsch, H. Flame synthesis of carbon metal-oxide nanocomposites in a counterflow burner. Proc. Combust. Inst. 2021, 38, 1269–1277. [Google Scholar] [CrossRef]
- Karaminejad, S.; Dupont, S.M.; Bieber, M.; Reddemann, M.A.; Kneer, R.; Dreier, T.; Endres, T.; Schulz, C. Characterization of spray parameters and flame stability in two modified nozzle configurations of the SpraySyn burner. Proc. Combust. Inst. 2023, 39, 2673–2682. [Google Scholar] [CrossRef]
- Bieber, M.; Al-Khatib, M.; Fröde, F.; Pitsch, H.; Reddemann, M.A.; Schmid, H.J.; Tischendorf, R.; Kneer, R. Influence of angled dispersion gas on coaxial atomization, spray and flame formation in the context of spray-flame synthesis of nanoparticles. Exp. Fluids 2021, 62, 98. [Google Scholar] [CrossRef]
- Kumar, A.; Kirchmann, J.; Beyrau, F.; Kronenburg, A. Jet flapping and its effect on flame oscillations in the SPP1980 SpraySyn burner. Exp. Therm. Fluid Sci. 2023, 142, 110826. [Google Scholar] [CrossRef]
- Schneider, F.; Suleiman, S.; Menser, J.; Borukhovich, E.; Wlokas, I.; Kempf, A.; Wiggers, H.; Schulz, C. SpraySyn—A standardized burner configuration for nanoparticle synthesis in spray flames. Rev. Sci. Instrum. 2019, 90, 85108. [Google Scholar] [CrossRef]
- Fröde, F.; Grenga, T.; Dupont, S.M.; Kneer, R.; Tischendorf, R.; Massopa, O.; Schmid, H.J.; Pitsch, H. Large Eddy Simulation of Iron Oxide Formation in a Laboratory Spray Flame. Appl. Energy Combust. Sci. 2023, 16, 100191. [Google Scholar] [CrossRef]
- Skenderović, I.; Kruis, F.E. Investigation and simulation of droplet breakup and iron oxide nanoparticle formation in spray-flame synthesis. J. Aerosol Sci. 2025, 185, 106535. [Google Scholar] [CrossRef]
- Abdelsamie, A.; Guan, W.; Nanjaiah, M.; Wlokas, I.; Wiggers, H.; Thévenin, D. Investigating the impact of dispersion gas composition on the flame structure in the SpraySyn burner using DNS. Proc. Combust. Inst. 2024, 40, 105398. [Google Scholar] [CrossRef]
- Lang, P.; Kücükmeric, E.; Huber, F.J.T.; Will, S. Investigation of iron oxide nanoparticle formation in a spray-flame synthesis process using laser-induced incandescence. Appl. Phys. B 2024, 130, 199. [Google Scholar] [CrossRef]
- Klukas, S.; Giglmaier, M.; Underberg, M.; Schnurre, S.M.; Prenting, M.M.; Endres, T.; Wiggers, H.; Schulz, C.; Sieber, M.; Schimek, S.; et al. Iron oxide nanoparticle synthesis: Simulation-based comparison of laboratory- and pilot plant-scale spray-flame synthesis. Appl. Energy Combust. Sci. 2024, 18, 100263. [Google Scholar] [CrossRef]
- Kirstein, T.; Aßmann, S.; Furat, O.; Will, S.; Schmidt, V. Determination of droplet size from wide-angle light scattering image data using convolutional neural networks. Mach. Learn. Sci. Technol. 2024, 5, 15049. [Google Scholar] [CrossRef]
- Eitner, A.; Al-Kamal, A.K.; Ali, M.Y.; Sheikh, M.-A.; Schulz, C.; Wiggers, H. Spray-flame synthesis of Nb-doped TiO2 nanoparticles and their electrochemical performance in sodium-ion batteries. Appl. Energy Combust. Sci. 2024, 17, 100252. [Google Scholar] [CrossRef]
- Baik, S.-J.; Martins, F.J.W.A.; Beyrau, F.; Kempf, A. Synthetic Inlet Conditions for Inhomogeneous Flows from Filters, Packed Beds, or Sinter Plates. Flow Turbul. Combust. 2024, 112, 483–489. [Google Scholar] [CrossRef]
- Aßmann, S.; Huber, F.J.; Will, S. In situ characterization of particle formation in spray flame synthesis using wide-angle light scattering. Particuology 2024, 86, 304–312. [Google Scholar] [CrossRef]
- Al-Kamal, A.K.; Hammad, M.; Yusuf Ali, M.; Angel, S.; Segets, D.; Schulz, C.; Wiggers, H. Titania/graphene nanocomposites from scalable gas-phase synthesis for high-capacity and high-stability sodium-ion battery anodes. Nanotechnology 2024, 35, 225602. [Google Scholar] [CrossRef]
- Lang, P.; Schneider, N.E.; Huber, F.J.; Will, S. Characterization of the SpraySyn 2.0 burner: Droplet diameters, flame stability and particle sizes. Appl. Energy Combust. Sci. 2025, 22, 100324. [Google Scholar] [CrossRef]
- Jüngst, N.; Ersoy, V.; Smallwood, G.J.; Kaiser, S.A. Neural networks for classification and segmentation of thermally-induced droplet breakup in spray-flame synthesis. J. Aerosol Sci. 2024, 176, 106314. [Google Scholar] [CrossRef]
- Kirchmann, J.; Kronenburg, A.; Prenting, M.M.; Karaminejad, S.; Dreier, T.; Endres, T.; Patil, S.; Beyrau, F. Characterizing the SpraySyn burners with MMC-LES. Appl. Energy Combust. Sci. 2023, 15, 100182. [Google Scholar] [CrossRef]
- Tischendorf, R.; Massopo, O.; Prymak, O.; Dupont, S.; Fröde, F.; Pitsch, H.; Kneer, R.; Schmid, H.-J. Maghemite nanoparticles synthesis via spray flame synthesis and particle characterization by hole in a tube sampling and scanning mobility particle sizing (HIAT-SMPS). Appl. Energy Combust. Sci. 2024, 17, 100235. [Google Scholar] [CrossRef]
- Hanesch, M. Raman spectroscopy of iron oxides and (oxy)hydroxides at low laser power and possible applications in environmental magnetic studies. Geophys. J. Int. 2009, 177, 941–948. [Google Scholar] [CrossRef]
- Altenhoff, M.; Teige, C.; Storch, M.; Will, S. Novel electric thermophoretic sampling device with highly repeatable characteristics. Rev. Sci. Instrum. 2016, 87, 125108. [Google Scholar] [CrossRef]
- Kunze, F.; Kuns, S.; Spree, M.; Hülser, T.; Schulz, C.; Wiggers, H.; Schnurre, S.M. Synthesis of silicon nanoparticles in a pilot-plant-scale microwave plasma reactor: Impact of flow rates and precursor concentration on the nanoparticle size and aggregation. Powder Technol. 2019, 342, 880–886. [Google Scholar] [CrossRef]
- Carbone, F.; Moslih, S.; Gomez, A. Probing gas-to-particle transition in a moderately sooting atmospheric pressure ethylene/air laminar premixed flame. Part II: Molecular clusters and nascent soot particle size distributions. Combust. Flame 2017, 181, 329–341. [Google Scholar] [CrossRef]
- Vargas, A.M.; Gülder, Ö.L. A multi-probe thermophoretic soot sampling system for high-pressure diffusion flames. Rev. Sci. Instrum. 2016, 87, 55101. [Google Scholar] [CrossRef] [PubMed]
- Leschowski, M.; Dreier, T.; Schulz, C. An automated thermophoretic soot sampling device for laboratory-scale high-pressure flames. Rev. Sci. Instrum. 2014, 85, 45103. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Altman, I.; Choi, M. Design of thermophoretic probe for precise particle sampling. J. Aerosol Sci. 2008, 39, 418–431. [Google Scholar] [CrossRef]
- Talbot, L.; Cheng, R.K.; Schefer, R.W.; Willis, D.R. Thermophoresis of particles in a heated boundary layer. J. Fluid Mech. 1980, 101, 737–758. [Google Scholar] [CrossRef]
- De Faria, D.L.A.; Silva, S.V.; de Oliveira, M.T. Raman Microspectroscopy of Some Iron Oxides and Oxyhydroxides. J. Raman Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- Saito, R.; Hofmann, M.; Dresselhaus, G.; Jorio, A.; Dresselhaus, M.S. Raman spectroscopy of graphene and carbon nanotubes. Adv. Phys. 2011, 60, 413–550. [Google Scholar] [CrossRef]
- Li, D.; Teoh, W.Y.; Selomulya, C.; Woodward, R.C.; Munroe, P.; Amal, R. Insight into microstructural and magnetic properties of flame-made γ-Fe2O3 nanoparticles. J. Mater. Chem. 2007, 17, 4876. [Google Scholar] [CrossRef]
- Zhu, H.J.; Hill, R.H. The photochemical metal organic deposition of manganese oxide films from films of manganese (II) 2-ethylhexanoate: A mechanistic study. J. Non Cryst. Solids 2002, 311, 174–184. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Jun, K.-W.; Park, J.-Y.; Potdar, H.S.; Chikate, R.C. A simple chemical route for the synthesis of γ-Fe2O3 nano-particles dispersed in organic solvents via an iron–hydroxy oleate precursor. J. Ind. Eng. Chem. 2008, 14, 38–44. [Google Scholar] [CrossRef]
- Meierhofer, F.; Mädler, L.; Fritsching, U. Nanoparticle evolution in flame spray pyrolysis—Process design via experimental and computational analysis. AIChE J. 2020, 66, e16885. [Google Scholar] [CrossRef]
- Bargar, J.R.; Kubicki, J.D.; Reitmeyer, R.; Davis, J.A. ATR-FTIR spectroscopic characterization of coexisting carbonate surface complexes on hematite. Geochim. Cosmochim. Acta 2005, 69, 1527–1542. [Google Scholar] [CrossRef]
- Deshpande, K.; Mukasyan, A.; Varma, A. Direct Synthesis of Iron Oxide Nanopowders by the Combustion Approach: Reaction Mechanism and Properties. Chem. Mater. 2004, 16, 4896–4904. [Google Scholar] [CrossRef]
- Angel, S.; Tapia, J.D.; Gallego, J.; Hagemann, U.; Wiggers, H. Spray-Flame Synthesis of LaMnO 3+δ Nanoparticles for Selective CO Oxidation (SELOX). Energy Fuels 2021, 35, 4367–4376. [Google Scholar] [CrossRef]
- Rodriguez-Fernandez, H.; Dasappa, S.; Sabado, K.D.; Camacho, J. Production of Carbon Black in Turbulent Spray Flames of Coal Tar Distillates. Appl. Sci. 2021, 11, 10001. [Google Scholar] [CrossRef]
- Frei, M.; Kruis, F.E. Image-based size analysis of agglomerated and partially sintered particles via convolutional neural networks. Powder Technol. 2020, 360, 324–336. [Google Scholar] [CrossRef]
- Frei, M.; Kruis, F.E. Fully automated primary particle size analysis of agglomerates on transmission electron microscopy images via artificial neural networks. Powder Technol. 2018, 332, 120–130. [Google Scholar] [CrossRef]
- Mahr, C.; Stahl, J.; Gerken, B.; Baric, V.; Frei, M.; Krause, F.F.; Grieb, T.; Schowalter, M.; Mehrtens, T.; Kruis, E.; et al. Characterization of mixing in nanoparticle hetero-aggregates by convolutional neural networks. Nano Sel. 2024, 5, 2300128. [Google Scholar] [CrossRef]
- Sipkens, T.A.; Frei, M.; Baldelli, A.; Kirchen, P.; Kruis, F.E.; Rogak, S.N. Characterizing soot in TEM images using a convolutional neural network. Powder Technol. 2021, 387, 313–324. [Google Scholar] [CrossRef]
| INN conc. | 0.1 M | 0.2 M | 0.1 M | 0.2 M |
| Burner type | SpraySyn1 | SpraySyn2 | ||
| H/kJ mol−1 | 3.11 | 3.10 | 3.11 | 3.10 |
| ϕ/- | 0.2624 | 0.2622 | 0.2624 | 0.2622 |
| INN conc. | 0.1 M | 0.2 M | 0.1 M | 0.2 M |
| Burner type | SpraySyn1 | SpraySyn2 | ||
| /nm | 6.2 | 7.2 | 5.0 | 5.3 |
| /emu g−1 | 16.4 | 20.8 | 9.7 | 11.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tischendorf, R.; Duschik, K.; Fröde, F.; Reddemann, M.; Kneer, R.; Pitsch, H.; Schaper, M.; Schmid, H.-J. On the Formation of Carbonaceous By-Product Species in Spray Flame Synthesis of Maghemite Nanoparticles. Appl. Sci. 2025, 15, 3294. https://doi.org/10.3390/app15063294
Tischendorf R, Duschik K, Fröde F, Reddemann M, Kneer R, Pitsch H, Schaper M, Schmid H-J. On the Formation of Carbonaceous By-Product Species in Spray Flame Synthesis of Maghemite Nanoparticles. Applied Sciences. 2025; 15(6):3294. https://doi.org/10.3390/app15063294
Chicago/Turabian StyleTischendorf, Ricardo, Kristina Duschik, Fabian Fröde, Manuel Reddemann, Reinhold Kneer, Heinz Pitsch, Mirko Schaper, and Hans-Joachim Schmid. 2025. "On the Formation of Carbonaceous By-Product Species in Spray Flame Synthesis of Maghemite Nanoparticles" Applied Sciences 15, no. 6: 3294. https://doi.org/10.3390/app15063294
APA StyleTischendorf, R., Duschik, K., Fröde, F., Reddemann, M., Kneer, R., Pitsch, H., Schaper, M., & Schmid, H.-J. (2025). On the Formation of Carbonaceous By-Product Species in Spray Flame Synthesis of Maghemite Nanoparticles. Applied Sciences, 15(6), 3294. https://doi.org/10.3390/app15063294








