Evaluating the Link Between Cardiovascular Risk and Alzheimer’s Disease: A Comprehensive Case-Control Study in Castilla y León, Spain
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Ethical Considerations
2.3. Cohort Study
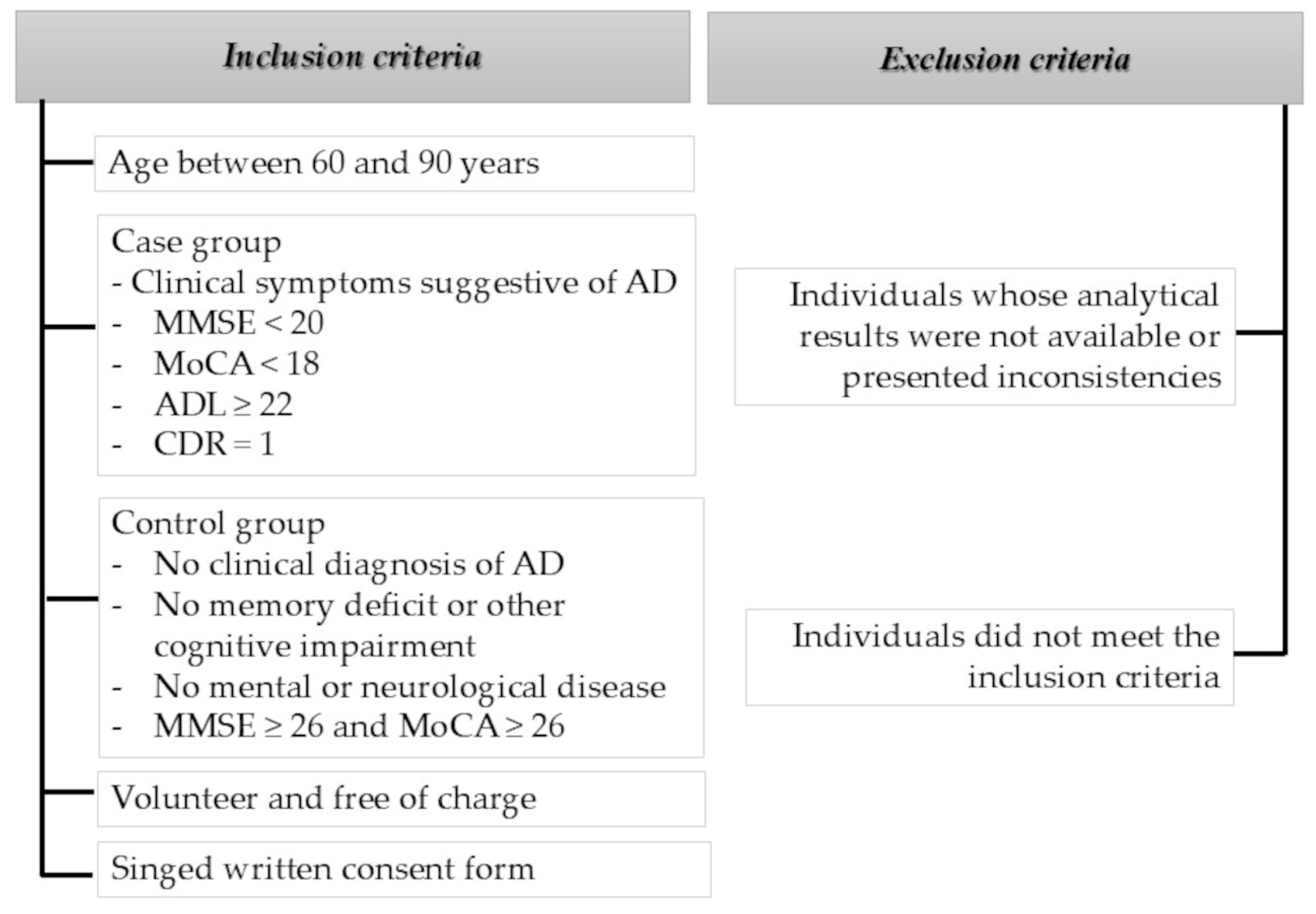
2.4. Inclusion and Exclusion Criteria
2.5. Socio-Demographic Data and Clinical Data
2.6. Statistical Analysis
3. Results
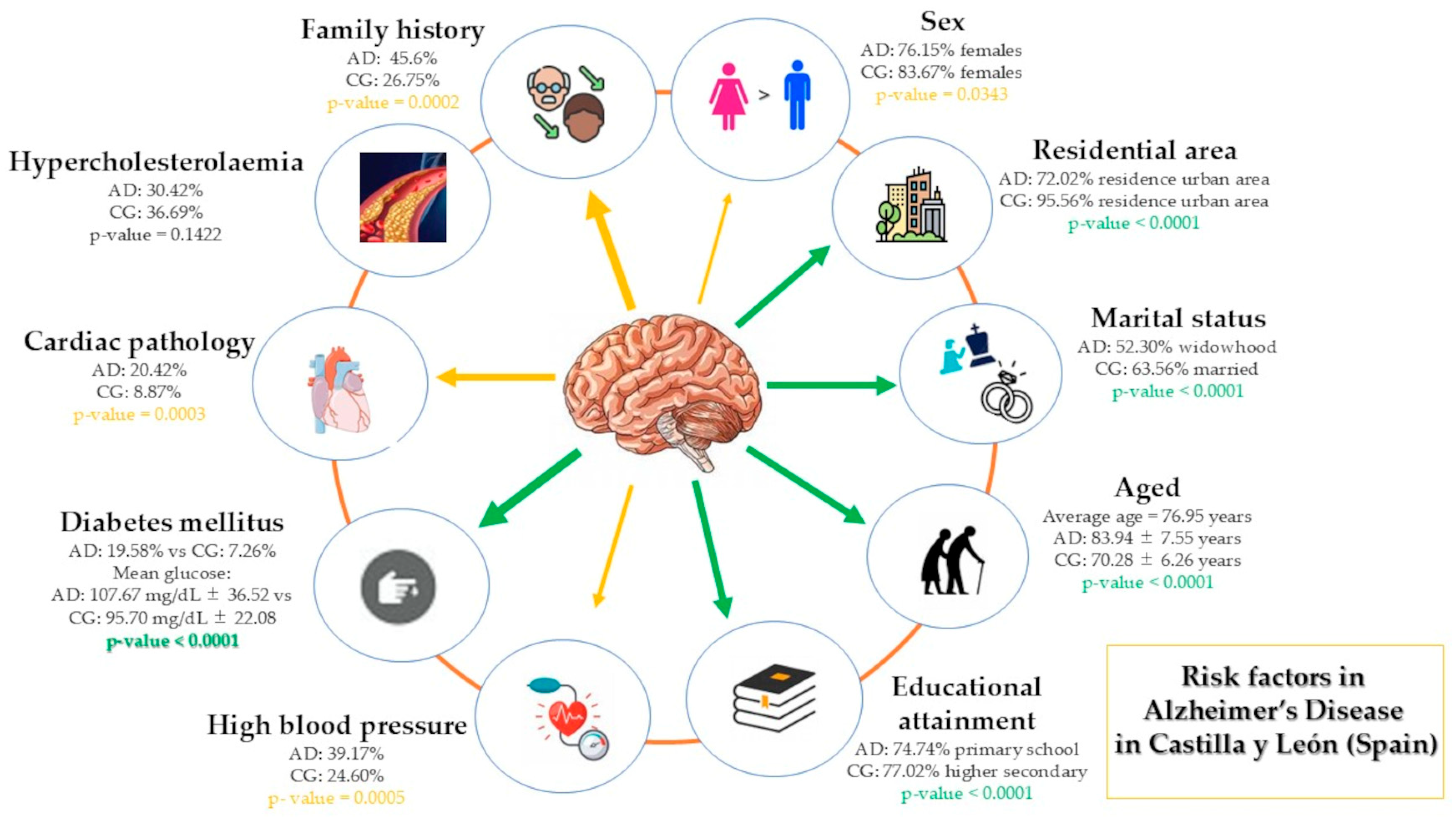
3.1. Sociodemographic Data
3.1.1. Age and Sex
3.1.2. Marital Status
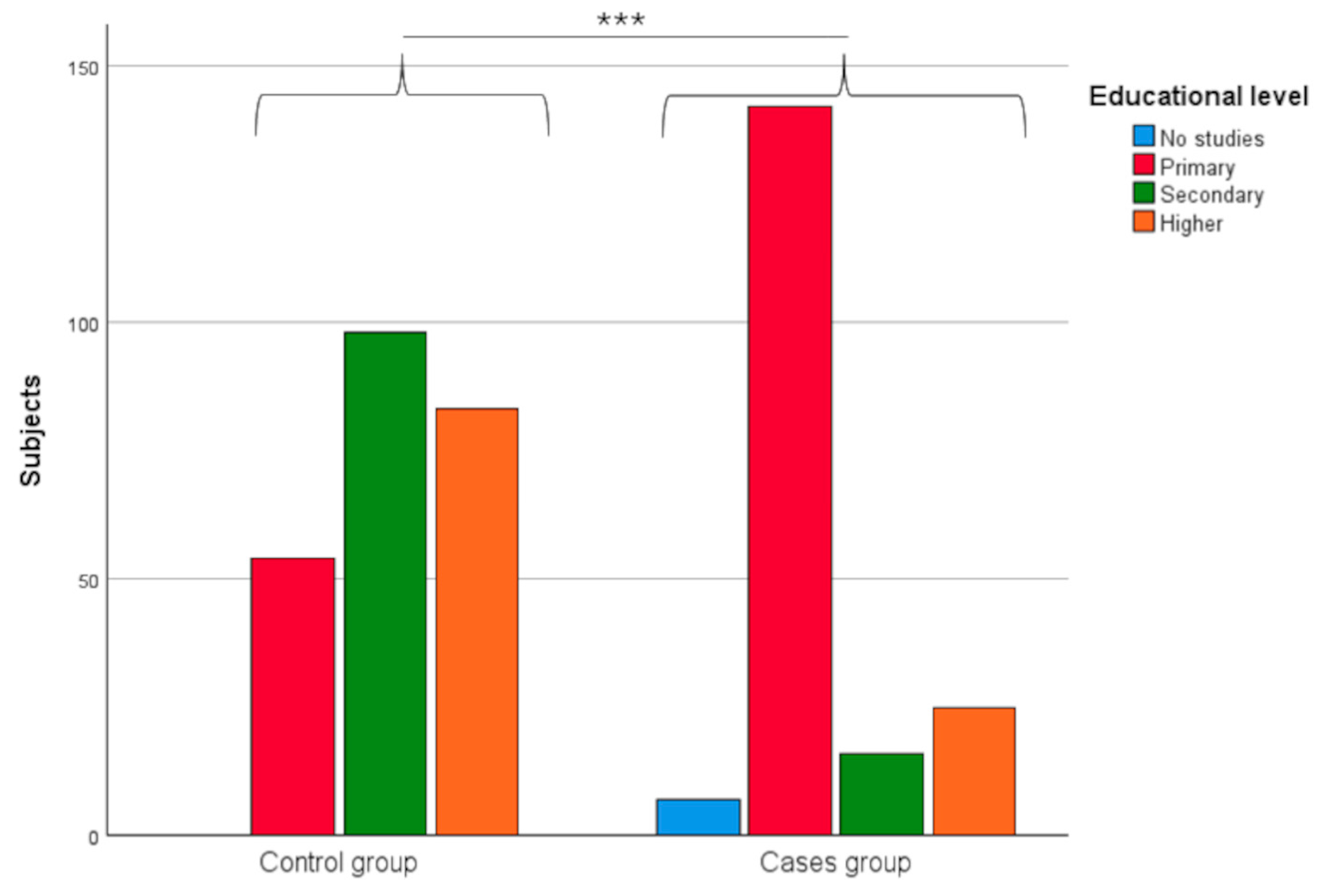
3.1.3. Educational Level
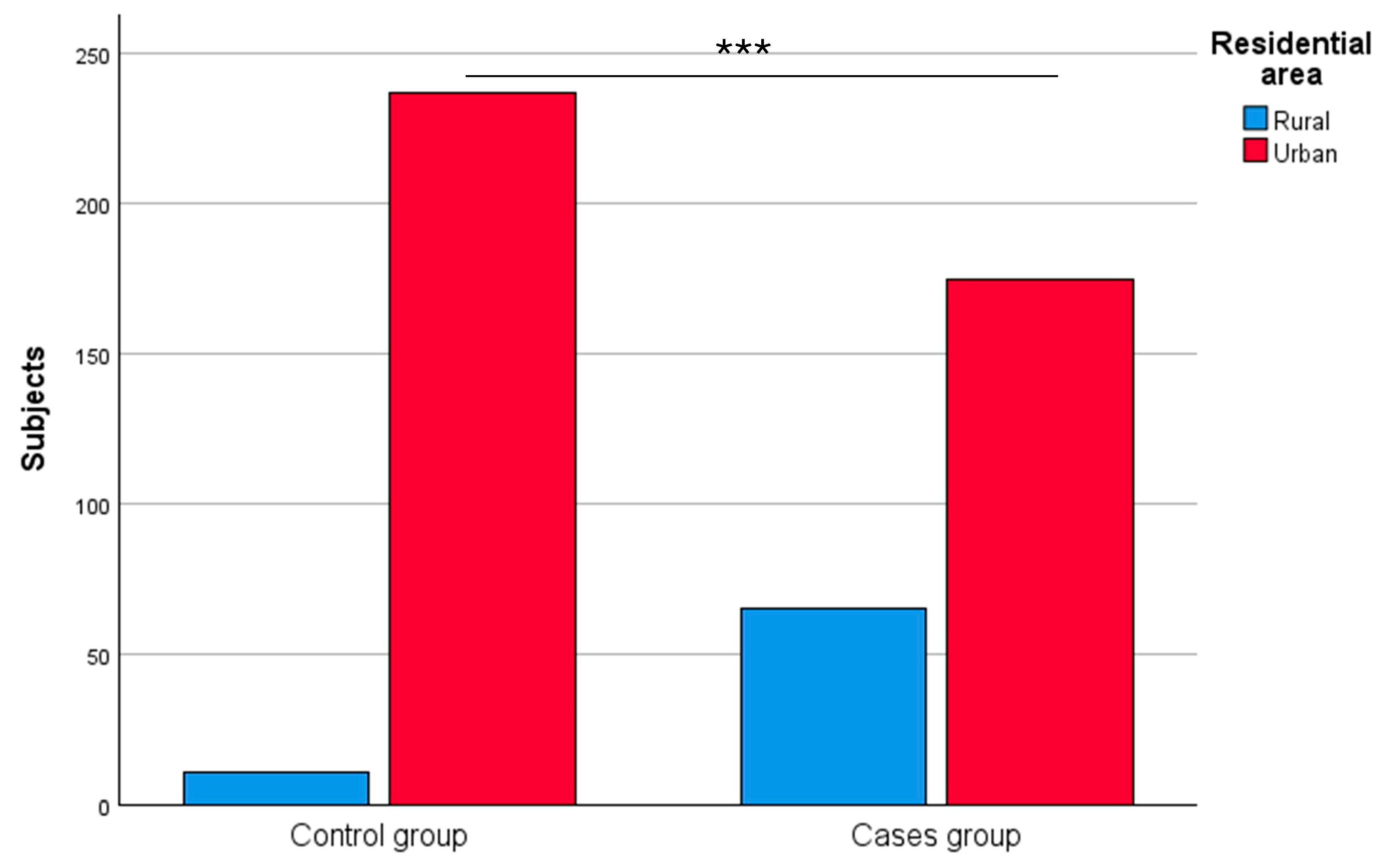
3.1.4. Residential Area
3.1.5. Family History of Probable Alzheimer’s Disease
3.2. Clinical Data
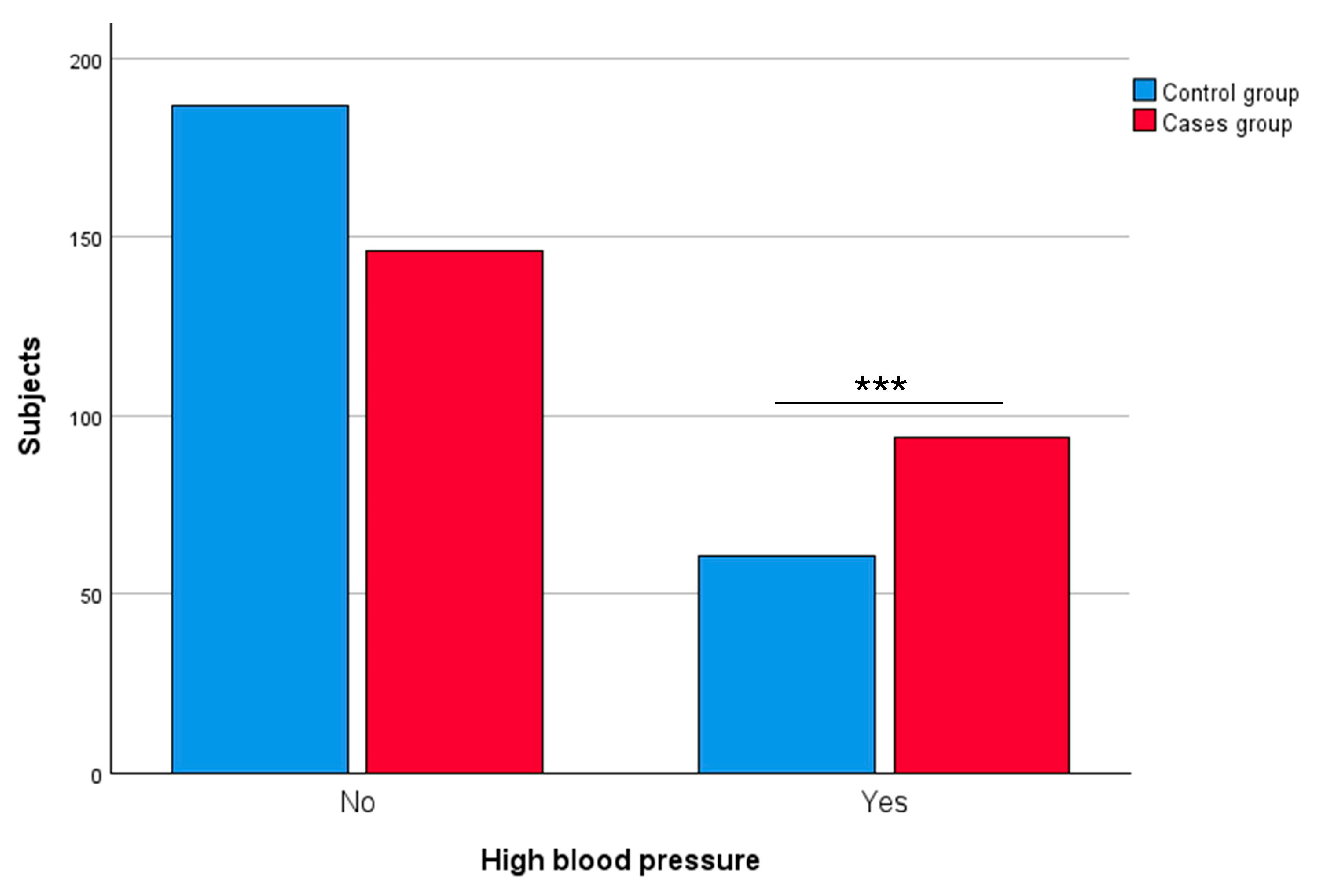
3.2.1. High Blood Pressure
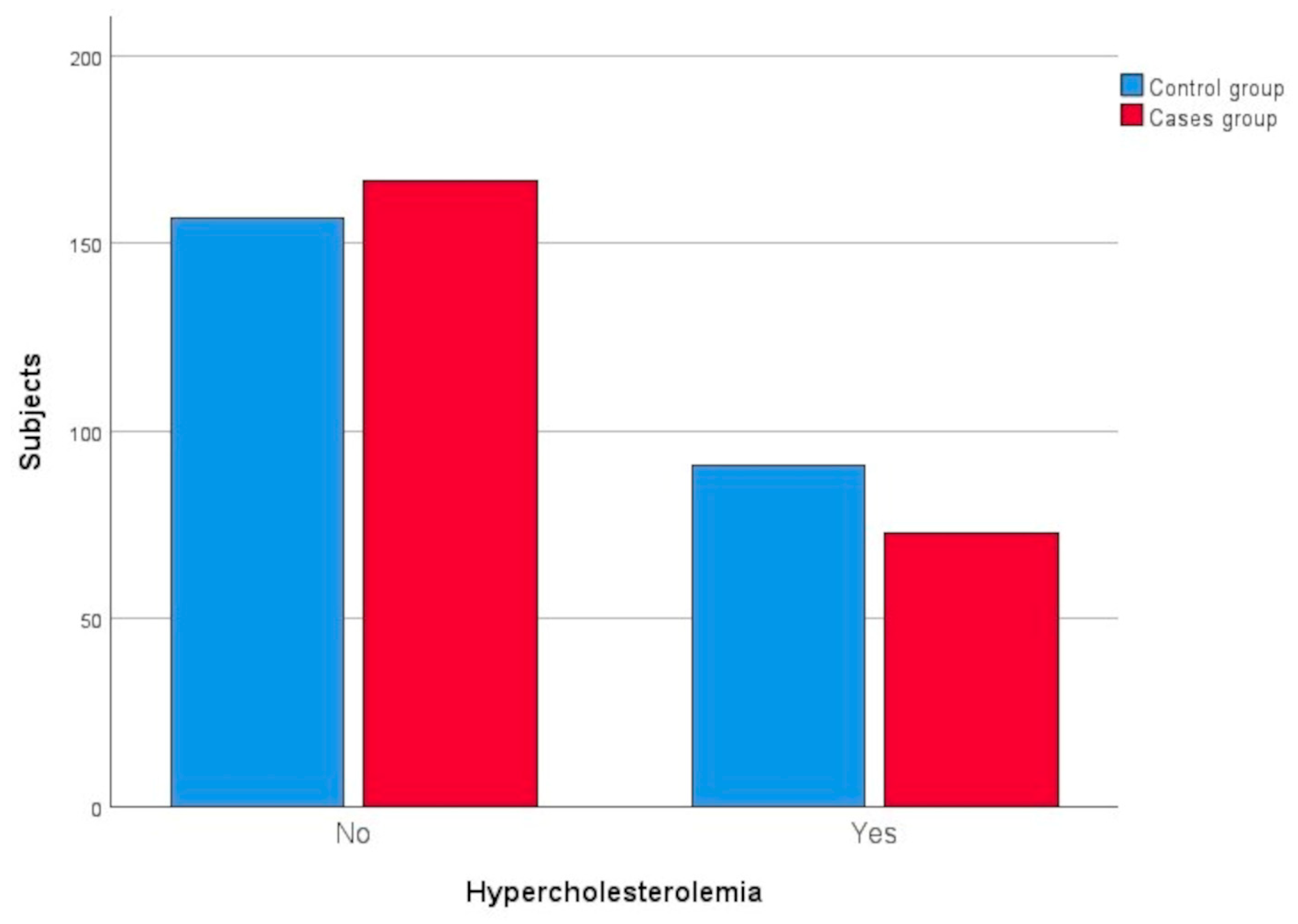
3.2.2. Hypercholesterolaemia
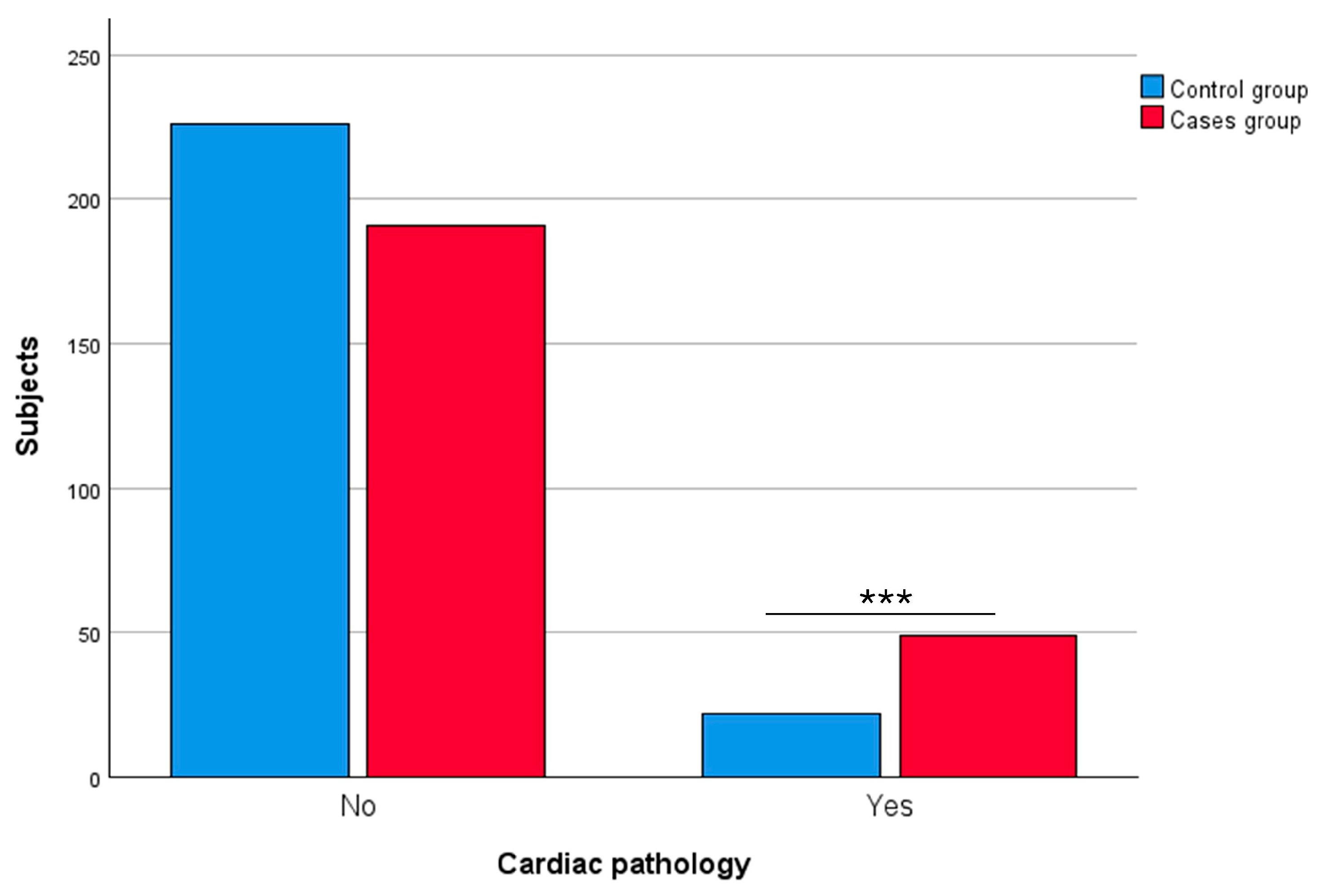
3.2.3. Cardiac Pathology
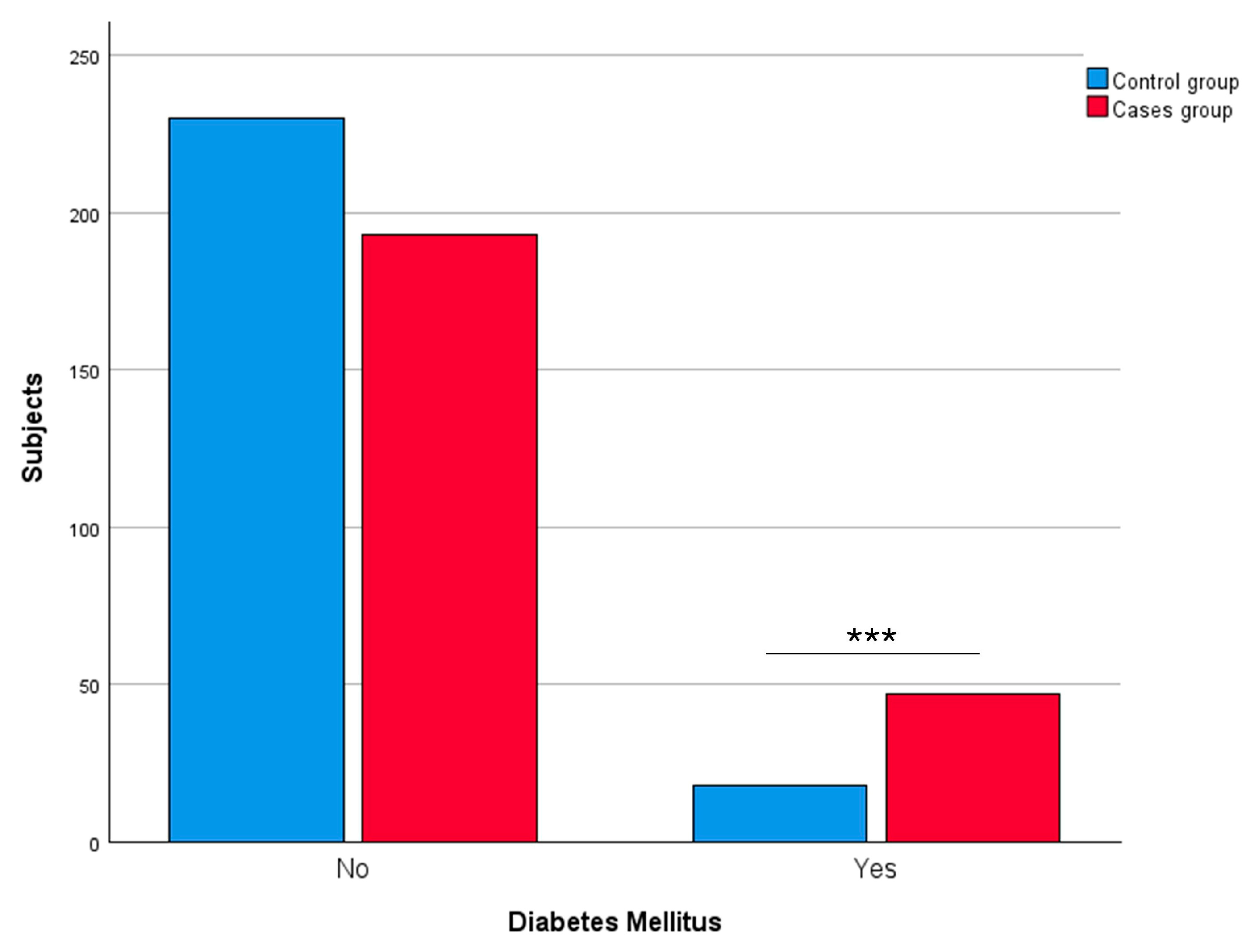
3.2.4. Diabetes Mellitus
4. Discussion
4.1. Socio-Demographic Data of Participants
4.2. Alzheimer’s Disease and Cardiovascular Risk Factors
4.2.1. High Blood Pressure and Alzheimer’s Disease
4.2.2. Hypercholesterolaemia and Alzheimer’s Disease
4.2.3. Cardiac Pathology and Alzheimer’s Disease
4.2.4. Diabetes Mellitus and Alzheimer’s Disease
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olivari, B.S.; French, M.E.; Mcguire, L.C. The Public Health Road Map to Respond to the Growing Dementia Crisis. Innov. Aging 2020, 4, igz043. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, G.; Rátiva, N.; González, J.; Ramos, T.; Miranda, C.; León, T. La historia olvidada del alzhéimer: Recordando a Oskar Fischer. Rev. Chil. Neuropsiquiatr. 2024, 62, 82–88. [Google Scholar] [CrossRef]
- Orgeta, V.; Mukadam, N.; Sommerlad, A.; Livingston, G. The Lancet Commission on Dementia Prevention, Intervention, and Care: A call for action. Ir. J. Psychol. Med. 2019, 36, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Deture, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Lendel, C. Extracellular protein components of amyloid plaques and their roles in Alzheimer’s disease pathology. Mol. Neurodegener. 2021, 16, 59. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Niculescu, A.G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef]
- Migliore, L.; Coppedè, F. Gene–environment interactions in Alzheimer disease: The emerging role of epigenetics. Nat. Rev. Neurol. 2022, 18, 643–660. [Google Scholar] [CrossRef]
- Behl, T.; Kyada, A.; Roopashree, R.; Nathiya, D.; Arya, R.; Kumar, M.R.; Khalid, M.; Gulati, M.; Sachdeva, M.; Fareed, M.; et al. Epigenetic biomarkers in Alzheimer’s disease: Diagnostic and prognostic relevance. Ageing Res. Rev. 2024, 102, 102556. [Google Scholar] [CrossRef]
- Bello-Corral, L.; Seco-Calvo, J.; Molina Fresno, A.; González, A.I.; Llorente, A.; Fernández-Lázaro, D.; Sánchez-Valdeón, L. Prevalence of ApoE Alleles in a Spanish Population of Patients with a Clinical Diagnosis of Alzheimer’s Disease: An Observational Case-Control Study. Medicina 2024, 60, 1941. [Google Scholar] [CrossRef]
- Loeffler, D.A. Modifiable, Non-Modifiable, and Clinical Factors Associated with Progression of Alzheimer’s Disease. J. Alzheimers Dis. 2021, 80, 1–27. [Google Scholar] [CrossRef]
- Litke, R.; Garcharna, L.C.; Jiwani, S.; Neugroschl, J. Modifiable Risk Factors in Alzheimer Disease and Related Dementias: A Review. Clin. Ther. 2021, 43, 953. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.E.; Yaffe, K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011, 10, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Śliwińska, S.; Jeziorek, M. The role of nutrition in Alzheimer’s disease. Rocz. Panstw. Zakl. Hig. 2021, 72, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, P.L.; Castillo-García, A.; Morales, J.S.; de la Villa, P.; Hampel, H.; Emanuele, E.; Lista, S.; Lucia, A. Exercise benefits on Alzheimer’s disease: State-of-the-science. Ageing Res. Rev. 2020, 62, 101108. [Google Scholar] [CrossRef]
- Wanleenuwat, P.; Iwanowski, P.; Kozubski, W. Alzheimer’s dementia: Pathogenesis and impact of cardiovascular risk factors on cognitive decline. Postgrad. Med. 2019, 131, 415–422. [Google Scholar] [CrossRef]
- Waigi, E.W.; Webb, R.C.; Moss, M.A.; Uline, M.J.; McCarthy, C.G.; Wenceslau, C.F. Soluble and insoluble protein aggregates, endoplasmic reticulum stress, and vascular dysfunction in Alzheimer’s disease and cardiovascular diseases. GeroScience 2023, 45, 1411–1438. [Google Scholar] [CrossRef]
- Leszek, J.; Mikhaylenko, E.V.; Belousov, D.M.; Koutsouraki, E.; Szczechowiak, K.; Kobusiak-Prokopowicz, M.; Mysiak, A.; Diniz, B.S.; Somasundaram, S.G.; Kirkland, C.E.; et al. The Links between Cardiovascular Diseases and Alzheimer’s Disease. Curr. Neuropharmacol. 2021, 19, 152–169. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann. Intern. Med. 2007, 147, 573–577. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Serdar, C.C.; Cihan, M.; Yücel, D.; Serdar, M.A. Sample size, power and effect size revisited: Simplified and practical approaches in pre-clinical, clinical and laboratory studies. Biochem. Medica 2021, 31, 1–27. [Google Scholar] [CrossRef]
- World Medical Association World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [CrossRef] [PubMed]
- Jack, C.R.; Albert, M.S.; Knopman, D.S.; McKhann, G.M.; Sperling, R.A.; Carrillo, M.C.; Thies, B.; Phelps, C.H. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.; Almeida, C.; Ferreira De Sousa, L.; Ii, F.; Moreira, P.; Iii, R.; Marques, B.; Iv, S. International Classification of Diseases—11th revision: From design to implementation. Rev. Saude Publica 2020, 54, 104. [Google Scholar] [CrossRef]
- Zhao, J.; Huai, J. Role of primary aging hallmarks in Alzheimer’s disease. Theranostics 2023, 13, 197–230. [Google Scholar] [CrossRef]
- José, M.; Fructuoso, P.; García Revilla, R.; Moure, O.M.; Moure, R.C. Analysis of Aging in Spain: Contemporary Sociological and Demographic Implications. Societies 2025, 15, 46. [Google Scholar] [CrossRef]
- Riedel, B.C.; Thompson, P.M.; Brinton, R.D. Age, APOE and Sex: Triad of Risk of Alzheimer’s Disease. J. Steroid Biochem. Mol. Biol. 2016, 160, 134. [Google Scholar] [CrossRef]
- Xia, X.; Jiang, Q.; McDermott, J.; Han, J.D.J. Aging and Alzheimer’s disease: Comparison and associations from molecular to system level. Aging Cell 2018, 17, e12802. [Google Scholar] [CrossRef]
- Azam, S.; Haque, M.E.; Balakrishnan, R.; Kim, I.S.; Choi, D.K. The Ageing Brain: Molecular and Cellular Basis of Neurodegeneration. Front. Cell Dev. Biol. 2021, 9, 683459. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Garrosa, E.; Seco-Calvo, J.; Garrosa, M. Potential Satellite Cell-Linked Biomarkers in Aging Skeletal Muscle Tissue: Proteomics and Proteogenomics to Monitor Sarcopenia. Proteomes 2022, 10, 29. [Google Scholar] [CrossRef]
- Miramontes, S.; Pereda Serras, C.; Woldemariam, S.R.; Khan, U.; Li, Y.; Tang, A.S.; Tsoy, E.; Oskotsky, T.T.; Sirota, M. Alzheimer’s disease as a women’s health challenge: A call for action on integrative precision medicine approaches. NPJ Women’s Health 2024, 2, 17. [Google Scholar] [CrossRef]
- Cui, S.S.; Jiang, Q.W.; Chen, S. Di Sex difference in biological change and mechanism of Alzheimer’s disease: From macro- to micro-landscape. Ageing Res. Rev. 2023, 87, 101918. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lee, C.; Torres, E.R.S.; Carling, G.; Gan, L. Mechanisms of sex differences in Alzheimer’s disease. Neuron 2024, 112, 1208–1221. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Montagne, A.; Zhao, Z. Alzheimer’s pathogenic mechanisms and underlying sex difference. Cell. Mol. Life Sci. 2021, 78, 4907–4920. [Google Scholar] [CrossRef] [PubMed]
- Calvo, N.; Einstein, G. Steroid hormones: Risk and resilience in women’s Alzheimer disease. Front. Aging Neurosci. 2023, 15, 1159435. [Google Scholar] [CrossRef]
- Subramaniapillai, S.; Rajagopal, S.; Snytte, J.; Otto, A.R.; Einstein, G.; Rajah, M.N. Sex differences in brain aging among adults with family history of Alzheimer’s disease and APOE4 genetic risk. NeuroImage Clin. 2021, 30, 102620. [Google Scholar] [CrossRef]
- Barnes, L.L.; Wilson, R.S.; Bienias, J.L.; Schneider, J.A.; Evans, D.A.; Bennett, D.A. Sex differences in the clinical manifestations of Alzheimer disease pathology. Arch. Gen. Psychiatry 2005, 62, 685–691. [Google Scholar] [CrossRef]
- Buckley, R.F.; Mormino, E.C.; Amariglio, R.E.; Properzi, M.J.; Rabin, J.S.; Lim, Y.Y.; Papp, K.V.; Jacobs, H.I.L.; Burnham, S.; Hanseeuw, B.J.; et al. Sex, amyloid, and APOE ε4 and risk of cognitive decline in preclinical Alzheimer’s disease: Findings from three well-characterized cohorts. Alzheimers Dement. 2018, 14, 1193–1203. [Google Scholar] [CrossRef]
- Valdés, M.T.; Requena, M. The effect of the age at school entry on educational attainment and field of study: An analysis using the Spanish census. High. Educ. 2024, 87, 1061–1083. [Google Scholar] [CrossRef]
- Zhu, W.; Gao, Z.; Li, H.; Huang, Z.; Li, X.; Wang, H.; Wu, X.; Tian, Y.; Zhou, S.; Li, X.; et al. Education reduces cognitive dysfunction in Alzheimer’s disease by changing regional cerebral perfusion: An in-vivo arterial spin labeling study. Neurol. Sci. 2023, 44, 2349–2361. [Google Scholar] [CrossRef]
- Seyedsalehi, A.; Warrier, V.; Bethlehem, R.A.I.; Perry, B.I.; Burgess, S.; Murray, G.K. Educational attainment, structural brain reserve and Alzheimer’s disease: A Mendelian randomization analysis. Brain 2023, 146, 2059–2074. [Google Scholar] [CrossRef]
- Larsson, S.C.; Traylor, M.; Malik, R.; Dichgans, M.; Burgess, S.; Markus, H.S. Modifiable pathways in Alzheimer’s disease: Mendelian randomisation analysis. BMJ 2017, 359, j5375. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, Y.; Zhang, H.; Gao, S.; Wang, L.; Wang, T.; Han, Z.; Sun, B.L.; Liu, G. Cognitive performance protects against Alzheimer’s disease independently of educational attainment and intelligence. Mol. Psychiatry 2022, 27, 4297–4306. [Google Scholar] [CrossRef]
- Lee, W.J.; Liao, Y.C.; Wang, Y.F.; Lin, Y.S.; Wang, S.J.; Fuh, J.L. Summative Effects of Vascular Risk Factors on the Progression of Alzheimer Disease. J. Am. Geriatr. Soc. 2020, 68, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Vemuri, P.; Lesnick, T.G.; Przybelski, S.A.; Knopman, D.S.; Lowe, V.J.; Graff-Radford, J.; Roberts, R.O.; Mielke, M.M.; Machulda, M.M.; Petersen, R.C.; et al. Age, Vascular Health, and Alzheimer’s disease Biomarkers in an Elderly Sample. Ann. Neurol. 2017, 82, 706. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, D.; Mascio, G.; D’Andrea, I.; Fardella, V.; Bell, R.D.; Branchi, I.; Pallante, F.; Zlokovic, B.; Yan, S.S.; Lembo, G. Hypertension induces brain β-amyloid accumulation, cognitive impairment, and memory deterioration through activation of receptor for advanced glycation end products in brain vasculature. Hypertension 2012, 60, 188–197. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, D.; Wen, C.; Zhang, L.; Como, P.; Qiao, Y. Relationship between blood pressure and Alzheimer’s disease in Linxian County, China. Life Sci. 2003, 72, 1125–1133. [Google Scholar] [CrossRef]
- van Arendonk, J.; Neitzel, J.; Steketee, R.M.E.; van Assema, D.M.E.; Vrooman, H.A.; Segbers, M.; Ikram, M.A.; Vernooij, M.W. Diabetes and hypertension are related to amyloid-beta burden in the population-based Rotterdam Study. Brain 2023, 146, 337–348. [Google Scholar] [CrossRef]
- Xu, W.; Tan, L.; Wang, H.F.; Jiang, T.; Tan, M.S.; Tan, L.; Zhao, Q.F.; Li, J.Q.; Wang, J.; Yu, J.T. Meta-analysis of modifiable risk factors for Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2015, 86, 1299–1306. [Google Scholar] [CrossRef]
- Ou, Y.N.; Tan, C.C.; Shen, X.N.; Xu, W.; Hou, X.H.; Dong, Q.; Tan, L.; Yu, J.T. Blood Pressure and Risks of Cognitive Impairment and Dementia: A Systematic Review and Meta-Analysis of 209 Prospective Studies. Hypertension 2020, 76, 217–225. [Google Scholar] [CrossRef]
- Vicario, A.; Cerezo, G.H.; Coca, A.; Gaseck, D.; Zaninelli, A.; Lovic, D.; Manios, E.; Hering, D.; Sierra, C.; Cunha, P. Efectos del tratamiento y las drogas anti-hipertensivas sobre la función cognitiva: Deterioro cognitivo, demencia y enfermedad de Alzheimer. Rev. Fed. Argentina Cardiol. 2019, 48, 98–106. [Google Scholar]
- Rodrigue, K.M.; Rieck, J.R.; Kennedy, K.M.; Devous, M.D.; Diaz-Arrastia, R.; Park, D.C. Risk factors for β-amyloid deposition in healthy aging: Vascular and genetic effects. JAMA Neurol. 2013, 70, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.; Van Der Graaf, Y.; Visseren, F.L.; Mali, W.P.T.M.; Geerlings, M.I. Hypertension and longitudinal changes in cerebral blood flow: The SMART-MR study. Ann. Neurol. 2012, 71, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Wharton, W.; Goldstein, F.C.; Tansey, M.G.; Brown, A.L.; Tharwani, S.D.; Verble, D.D.; Cintron, A.; Kehoe, P.G. Rationale and Design of the Mechanistic Potential of Antihypertensives in Preclinical Alzheimer’s (HEART) Trial. J. Alzheimers Dis. 2018, 61, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Testa, G.; Giannelli, S.; Staurenghi, E.; Cecci, R.; Floro, L.; Gamba, P.; Sottero, B.; Leonarduzzi, G. The Emerging Role of PCSK9 in the Pathogenesis of Alzheimer’s Disease: A Possible Target for the Disease Treatment. Int. J. Mol. Sci. 2024, 25, 13637. [Google Scholar] [CrossRef]
- Kalmijn, S.; Foley, D.; White, L.; Burchfiel, C.M.; Curb, J.D.; Petrovitch, H.; Ross, G.W.; Havlik, R.J.; Launer, L.J. Metabolic cardiovascular syndrome and risk of dementia in Japanese-American elderly men. The Honolulu-Asia aging study. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2255–2260. [Google Scholar] [CrossRef]
- Notkola, I.L.; Sulkava, R.; Pekkanen, J.; Erkinjuntti, T.; Ehnholm, C.; Kivinen, P.; Tuomilehto, J.; Nissinen, A. Serum total cholesterol, apolipoprotein E epsilon 4 allele, and Alzheimer’s disease. Neuroepidemiology 1998, 17, 14–20. [Google Scholar] [CrossRef]
- Xu, C.; Apostolova, L.G.; Oblak, A.L.; Gao, S. Association of Hypercholesterolemia with Alzheimer’s Disease Pathology and Cerebral Amyloid Angiopathy. J. Alzheimers Dis. 2020, 73, 1305. [Google Scholar] [CrossRef]
- Kivipelto, M.; Helkala, E.L.; Laakso, M.P.; Hänninen, T.; Hallikainen, M.; Alhainen, K.; Iivonen, S.; Mannermaa, A.; Tuomilehto, J.; Nissinen, A.; et al. Apolipoprotein E epsilon4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late-life Alzheimer disease. Ann. Intern. Med. 2002, 137, 149–155. [Google Scholar] [CrossRef]
- Goldstein, F.C.; Ashley, A.V.; Endeshaw, Y.W.; Hanfelt, J.; Lah, J.J.; Levey, A.I. Effects of hypertension and hypercholesterolemia on cognitive functioning in patients with alzheimer disease. Alzheimer Dis. Assoc. Disord. 2008, 22, 336–342. [Google Scholar] [CrossRef]
- Pappolla, M.A.; Refolo, L.; Sambamurti, K.; Zambon, D.; Duff, K. Hypercholesterolemia and Alzheimer’s Disease: Unraveling the Connection and Assessing the Efficacy of Lipid-Lowering Therapies. J. Alzheimers Dis. 2024, 101, S371–S393. [Google Scholar] [CrossRef]
- O’Connell, E.M.; Lohoff, F.W. Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) in the Brain and Relevance for Neuropsychiatric Disorders. Front. Neurosci. 2020, 14, 548891. [Google Scholar] [CrossRef]
- Cermakova, P.; Eriksdotter, M.; Lund, L.H.; Winblad, B.; Religa, P.; Religa, D. Heart failure and Alzheimer’s disease. J. Intern. Med. 2014, 277, 406. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Sulaiman, G.M.; Mohammed, H.A.; Mohammed, S.G.; Al-Gareeb, A.I.; Albuhadily, A.K.; Dawood, R.A.; Al Ali, A.; Abu-Alghayth, M.H. Amyloid-β and heart failure in Alzheimer’s disease: The new vistas. Front. Med. 2025, 12, 1494101. [Google Scholar] [CrossRef]
- Arega, Y.; Shao, Y. Heart failure and late-onset Alzheimer’s disease: A Mendelian randomization study. Front. Genet. 2022, 13, 1015674. [Google Scholar] [CrossRef]
- Zheng, Y.M.; Zhao, Y.Y.; Zhang, T.; Hou, X.H.; Bi, Y.L.; Ma, Y.H.; Xu, W.; Shen, X.N.; Dong, Q.; Tan, L.; et al. Left Ventricular Ejection Fraction and Cerebrospinal Fluid Biomarkers of Alzheimer’s Disease Pathology in Cognitively Normal Older Adults: The CABLE Study. J. Alzheimers Dis. 2021, 81, 743–750. [Google Scholar] [CrossRef]
- Murphy, J.; Le, T.N.V.; Fedorova, J.; Yang, Y.; Krause-Hauch, M.; Davitt, K.; Zoungrana, L.I.; Fatmi, M.K.; Lesnefsky, E.J.; Li, J.; et al. The Cardiac Dysfunction Caused by Metabolic Alterations in Alzheimer’s Disease. Front. Cardiovasc. Med. 2022, 9, 850538. [Google Scholar] [CrossRef]
- Liang, X.; Huang, Y.; Han, X. Associations between coronary heart disease and risk of cognitive impairment: A meta-analysis. Brain Behav. 2021, 11, e02108. [Google Scholar] [CrossRef]
- Starmans, N.L.P.; Leeuwis, A.E.; Biessels, G.J.; Kappelle, L.J.; van der Flier, W.M.; Tolboom, N. Cerebral amyloid-β deposition in patients with heart disease or carotid occlusive disease: A systematic review and meta-analysis. J. Neurol. Sci. 2023, 445, 120551. [Google Scholar] [CrossRef]
- Papanastasiou, C.A.; Theochari, C.A.; Zareifopoulos, N.; Arfaras-Melainis, A.; Giannakoulas, G.; Karamitsos, T.D.; Palaiodimos, L.; Ntaios, G.; Avgerinos, K.I.; Kapogiannis, D.; et al. Atrial Fibrillation Is Associated with Cognitive Impairment, All-Cause Dementia, Vascular Dementia, and Alzheimer’s Disease: A Systematic Review and Meta-Analysis. J. Gen. Intern. Med. 2021, 36, 3122–3135. [Google Scholar] [CrossRef]
- Ashton, N.J.; Moseby-Knappe, M.; Benedet, A.L.; Grötschel, L.; Lantero-Rodriguez, J.; Karikari, T.K.; Hassager, C.; Wise, M.P.; Stammet, P.; Kjaergaard, J.; et al. Alzheimer Disease Blood Biomarkers in Patients With Out-of-Hospital Cardiac Arrest. JAMA Neurol. 2023, 80, 388–396. [Google Scholar] [CrossRef]
- Agrawal, S.; Schneider, J.A. Vascular pathology and pathogenesis of cognitive impairment and dementia in older adults. Cereb. Circ.-Cogn. Behav. 2022, 3, 100148. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.; Lopez, O.; Cohen, A.; Reis, S.E. Cardiovascular Disease and Alzheimer’s Disease: The Heart–Brain Axis. J. Am. Heart Assoc. 2023, 12, 30780. [Google Scholar] [CrossRef]
- Ott, A.; Stolk, R.P.; Hofman, A.; Van Harskamp, F.; Grobbee, D.E.; Breteler, M.M.B. Association of diabetes mellitus and dementia: The Rotterdam Study. Diabetologia 1996, 39, 1392–1397. [Google Scholar] [CrossRef] [PubMed]
- Ott, A.; Stolk, R.P.; Van Harskamp, F.; Pols, H.A.P.; Hofman, A.; Breteler, M.M.B. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology 1999, 53, 1937–1942. [Google Scholar] [CrossRef]
- Mejía-Arango, S.; Zúñiga-Gil, C. Diabetes mellitus as a risk factor for dementia in the Mexican elder population. Rev. Neurol. 2011, 53, 397–405. [Google Scholar] [CrossRef]
- Plastino, M.; Fava, A.; Pirritano, D.; Cotronei, P.; Sacco, N.; Sperlì, T.; Spanò, A.; Gallo, D.; Mungari, P.; Consoli, D.; et al. Effects of insulinic therapy on cognitive impairment in patients with Alzheimer disease and diabetes mellitus type-2. J. Neurol. Sci. 2010, 288, 112–116. [Google Scholar] [CrossRef]
- Singh, D.D.; Shati, A.A.; Alfaifi, M.Y.; Elbehairi, S.E.I.; Han, I.; Choi, E.-H.; Yadav, D.K.; Singh, D.D.; Shati, A.A.; Alfaifi, M.Y.; et al. Development of Dementia in Type 2 Diabetes Patients: Mechanisms of Insulin Resistance and Antidiabetic Drug Development. Cells 2022, 11, 3767. [Google Scholar] [CrossRef]
- Kong, Y.; Wang, F.; Wang, J.; Liu, C.; Zhou, Y.; Xu, Z.; Zhang, C.; Sun, B.; Guan, Y. Pathological Mechanisms Linking Diabetes Mellitus and Alzheimer’s Disease: The Receptor for Advanced Glycation End Products (RAGE). Front. Aging Neurosci. 2020, 12, 217. [Google Scholar] [CrossRef]
- Kciuk, M.; Kruczkowska, W.; Gałęziewska, J.; Wanke, K.; Kałuzińska-Kołat, Ż.; Aleksandrowicz, M.; Kontek, R. Alzheimer’s Disease as Type 3 Diabetes: Understanding the Link and Implications. Int. J. Mol. Sci. 2024, 25, 11955. [Google Scholar] [CrossRef]




| Characteristics | pAD Case Group | Control Group | p-Value | |||
|---|---|---|---|---|---|---|
| Frequency | % | Frequency | % | |||
| Gender | Men | 62 | 23.85 | 41 | 16.33 | 0.0343 * |
| Women | 198 | 76.15 | 210 | 83.67 | ||
| Civil status | Single | 12 | 5.02 | 22 | 8.91 | <0.0001 *** |
| Married | 100 | 41.84 | 157 | 63.56 | ||
| Widowed | 125 | 52.30 | 50 | 20.24 | ||
| Divorced | 1 | 0.42 | 13 | 5.26 | ||
| Separated | 1 | 0.42 | 5 | 2.02 | ||
| Not available | 21 | – | 4 | – | ||
| Educational level | Without studies | 7 | 3.68 | 0 | 0 | <0.0001 *** |
| Primary | 142 | 74.74 | 54 | 22.98 | ||
| Secondary | 16 | 8.42 | 98 | 41.70 | ||
| Higher | 25 | 13.16 | 83 | 35.32 | ||
| Not available | 70 | – | 16 | – | ||
| Residential area | Rural | 65 | 27.58 | 11 | 4.44 | <0.0001 *** |
| Urban | 175 | 72.92 | 237 | 95.56 | ||
| Not available | 20 | – | 3 | – | ||
| Family history of AD | Yes | 88 | 45.60 | 65 | 26.75 | 0.0002 *** |
| No | 101 | 52.30 | 174 | 71.60 | ||
| Unknown | 4 | 2.10 | 4 | 1.65 | ||
| Not available | 8 | – | 8 | – | ||
| Frequency | Mean (SD) | Frequency | Mean (SD) | |||
| Age | 238 | 83.94 (7.55) | 249 | 70.28 (6.26) | <0.0001 *** | |
| Medical Diseases and Conditions | pAD Group | Control Group | p-Value | |||
|---|---|---|---|---|---|---|
| Frequency | % | Frequency | % | |||
| High blood pressure | Yes | 94 | 39.17 | 61 | 24.60 | 0.0005 *** |
| No | 146 | 60.83 | 187 | 75.40 | ||
| Not available | 20 | – | 3 | – | ||
| Hypercholesterolemia | Yes | 73 | 30.42 | 91 | 36.69 | 0.1422 |
| No | 167 | 69.58 | 157 | 63.31 | ||
| Not available | 20 | – | 3 | – | ||
| Cardiac pathology | Yes | 49 | 20.42 | 22 | 8.87 | 0.0003 *** |
| No | 191 | 79.58 | 226 | 91.13 | ||
| Not available | 20 | – | 3 | – | ||
| Diabetes mellitus | Yes | 47 | 19.58 | 18 | 7.26 | <0.0001 *** |
| No | 193 | 80.42 | 230 | 92.74 | ||
| Not available | 20 | – | 3 | – | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bello-Corral, L.; Seco-Calvo, J.; Celorrio San Miguel, M.; Garrosa, E.; Fernández-Lázaro, D.; Sánchez-Valdeón, L. Evaluating the Link Between Cardiovascular Risk and Alzheimer’s Disease: A Comprehensive Case-Control Study in Castilla y León, Spain. Appl. Sci. 2025, 15, 3409. https://doi.org/10.3390/app15063409
Bello-Corral L, Seco-Calvo J, Celorrio San Miguel M, Garrosa E, Fernández-Lázaro D, Sánchez-Valdeón L. Evaluating the Link Between Cardiovascular Risk and Alzheimer’s Disease: A Comprehensive Case-Control Study in Castilla y León, Spain. Applied Sciences. 2025; 15(6):3409. https://doi.org/10.3390/app15063409
Chicago/Turabian StyleBello-Corral, Laura, Jesús Seco-Calvo, Marta Celorrio San Miguel, Evelina Garrosa, Diego Fernández-Lázaro, and Leticia Sánchez-Valdeón. 2025. "Evaluating the Link Between Cardiovascular Risk and Alzheimer’s Disease: A Comprehensive Case-Control Study in Castilla y León, Spain" Applied Sciences 15, no. 6: 3409. https://doi.org/10.3390/app15063409
APA StyleBello-Corral, L., Seco-Calvo, J., Celorrio San Miguel, M., Garrosa, E., Fernández-Lázaro, D., & Sánchez-Valdeón, L. (2025). Evaluating the Link Between Cardiovascular Risk and Alzheimer’s Disease: A Comprehensive Case-Control Study in Castilla y León, Spain. Applied Sciences, 15(6), 3409. https://doi.org/10.3390/app15063409








