Mineral Content of Four Mexican Edible Flowers Growing in Natural Conditions and Backyards from Indigenous Communities
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Sample Preparation and Mineral Content Evaluations
2.3. Statistical Analysis
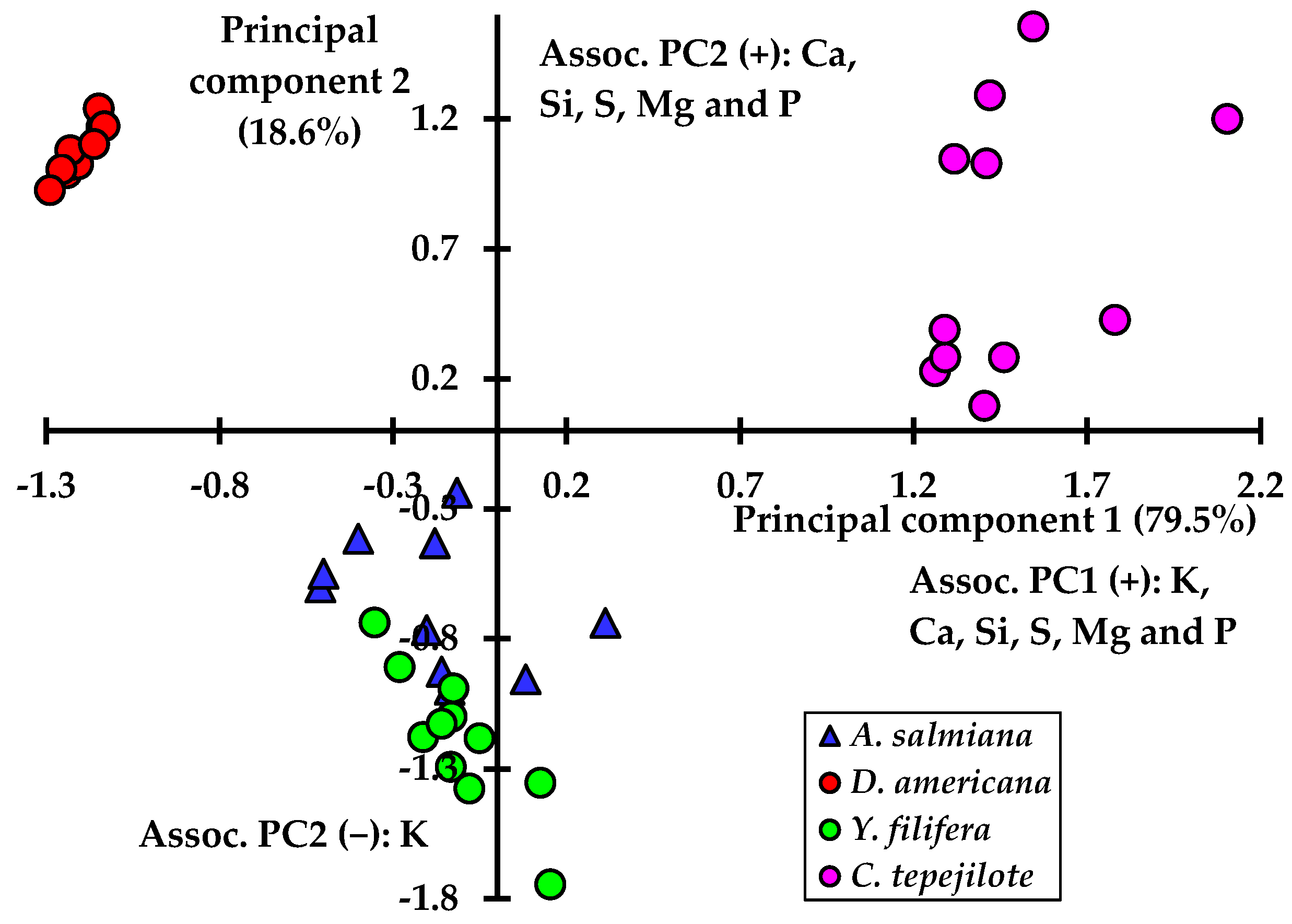
3. Results
3.1. Minerals in Y. filifera Flowers
3.2. Minerals in A. salmiana Flowers
3.3. Minerals in D. americana Flowers
3.4. Minerals in C. tepejilote Flowers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mapes, C.; Basurto, F. Biodiversity and edible plants of Mexico. In Ethnobotany of Mexico, Interactions of People and Plants in Mesoamerica; Lira, R., Casas, A., Blancas, J., Eds.; Springer Natura: New York, NY, USA, 2016; pp. 83–131. [Google Scholar]
- Román-Cortés, N.R.; García-Mateos, M.R.; Castillo-González, A.M.; Sahagún-Castellanos, J.; Jiménez-Arellanes, M.A. Nutritional and nutraceutical characteristics of vegetables of ancestral use in Mexico. Rev. Fitotec. Mex. 2018, 41, 245–253. [Google Scholar]
- Santiago-Saenz, Y.O.; Hernández-Fuentes, A.D.; López-Palestina, C.U.; Garrido-Cauich, J.H.; Alatorre-Cruz, J.M.; Monroy-Torres, R. Importancia nutricional y actividad biológica de los compuestos bioactivos de quelites consumidos en México (Nutritional importance and biological activity of bioactive compounds from quelites consumed in Mexico). Rev. Chil. Nut. 2019, 46, 593–605. [Google Scholar] [CrossRef]
- Cáceres, A.; Lange, K.; Cruz, S.M.; Velásquez, R.; Lima, S.; Menéndez, M.C.; Dardón, R.; Córdova, D.; González, J. Assessment of antioxidant activity of 24 native plants used in Guatemala for their potential application in natural product industry. Acta Hortic. 2012, 964, 85–92. [Google Scholar] [CrossRef]
- Lee, H.W.; Bi, X.; Henry, C.J. Comparative evaluation of minerals content of common green leafy vegetables consumed by the Asian populations in Singapore. J. Food Compos. Anal. 2024, 125, 105787. [Google Scholar] [CrossRef]
- de Lima-Franzen, F.; Rodrigues de Oliveira, M.S.; Lidório, H.F.; Farias-Menegaes, J.; Martins-Fries, L.L. Chemical composition of rose, sunflower and calendula flower petals for human food use. Cienc. Tecnol. Agropec. 2019, 20, 149–158. [Google Scholar]
- Rop, O.; Mlcek, J.; Jurikova, T.; Neugebauerova, J.; Vabkova, J. Edible flowers—A new promising source of mineral elements in human nutrition. Molecules 2012, 17, 6672–6683. [Google Scholar] [CrossRef]
- Cunningham, E. What nutritional contribution do edible flowers make? J. Acad. Nut. Diet. 2015, 115, 856. [Google Scholar] [CrossRef]
- Drava, G.; Lobbi, V.; Govaerts, R.; Minganti, V.; Copetta, A.; Ruffoni, B.; Bisio, A. Trace elements in edible flowers from Italy: Further insights into health benefits and risks to consumers. Molecules 2020, 25, 2891. [Google Scholar] [CrossRef]
- Benvenuti, S.; Mazzoncini, M. The biodiversity of edible flowers: Discovering new teste and new health benefits. Front. Plant Sci. 2021, 11, 569499. [Google Scholar] [CrossRef]
- Pires, E.O.; di Giola, F.; Rouphael, Y.; García-Caparrós, P.; Tzortzakis, N.; Ferreira, I.C.F.R.; Barros, L.; Petropoulos, S.A.; Caleja, C. Edible flowers as an emerging horticultural product: A review on sensorial properties, mineral and aroma profile. Trends Food Sci. Technol. 2023, 137, 31–54. [Google Scholar] [CrossRef]
- Mlcek, J.; Plaskova, A.; Jurikova, T.; Sochor, J.; Baron, M.; Ercisli, S. Chemical, nutritional and sensory characteristics of six ornamental edible flowers species. Foods 2021, 10, 2053. [Google Scholar] [CrossRef]
- Bieżanowska-Kopeć, R.; Ambroszczyk, A.M.; Piątkowska, E.; Leszczyńska, T. Nutritional value and antioxidant activity of fresh pumpkin flowers (Cucurbita sp.) grown in Poland. Appl. Sci. 2022, 12, 6673. [Google Scholar] [CrossRef]
- Marchioni, I.; Gabriele, M.; Carmassi, G.; Ruffoni, B.; Pistelli, L.; Pistelli, L.; Najar, B. Phytochemical, nutritional and mineral content of four edible flowers. Foods 2024, 13, 939. [Google Scholar] [CrossRef]
- Fernandes, L.; Casal, S.; Pereira, J.A.; Saraiva, J.A.; Ramalhosa, E. Edible flowers: A review of the nutritional, antioxidant, antimicrobial properties and effects on human health. J. Food Compos. Anal. 2017, 60, 38–50. [Google Scholar]
- Cormick, G.; Belizán, J.M. Calcium intake and health. Nutrients 2019, 11, 1606. [Google Scholar] [CrossRef]
- Ciosek, Ż.; Kot, K.; Kosik-Bogacka, D.; Łanocha-Arendarczyk, N.; Rotter, I. The effects of calcium, magnesium, phosphorus, fluoride, and lead on bone tissue. Biomolecules 2021, 11, 506. [Google Scholar] [CrossRef]
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The essential metals for humans: A brief overview. J. Inorg. Biochem. 2019, 195, 120–129. [Google Scholar]
- Mezzaroba, L.; Alfieri, D.F.; Simão, A.N.C.; Reiche, E.M.V. The role of zinc, copper, manganese and iron in neurodegenerative diseases. Neurotoxicology 2019, 74, 230–241. [Google Scholar]
- Gombart, A.F.; Pierre, A.; Maggini, S. A review of micronutrients and the immune system–working in harmony to reduce the risk of infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef] [PubMed]
- da Costa, N.D.S.; Lima, L.S.; Galiciolli, M.E.A.; Ribeiro, D.H.F.; Ribeiro, M.M.; Garcia, G.D.P.J.; Ribeiro, M.M.; Garica, G.P.J.; Marçal, I.S.; da Silva, J.F.; et al. Drug-induced osteoporosis and mechanisms of bone tissue regeneration through trace elements. J. Trace Elem. Med. Biol. 2024, 84, 127446. [Google Scholar]
- Centurión-Hidalgo, D.; Alor-Chávez, M.J.; Espinosa-Moreno, J.; Gómez-García, E.; Solano, M.L.; Poot-Matu, J.E. Contenido nutricional de inflorescencias de palmas en la sierra del estado de Tabasco. Univ. Cienc. 2009, 25, 193–199. [Google Scholar]
- Manzanero-Medina, G.I.; Pérez-Herrera, A.; Lustre-Sánchez, H.; Vásquez-Dávila, M.A.; Santos-Sánchez, N.F.; Sánchez-Medina, M.A. Ethnobotanical and Nutritional Study of Quelites Sold in Two Traditional Markets of Oaxaca, Mexico. BioRxiv 2018, 2018, 453225. [Google Scholar] [CrossRef]
- Nabhan, G.P.; Colunga-García-Marín, P.; Zizumbo-Villarreal, D. Reviving the arid American diet in the face of climate change: Assessing its composition, links to the Mesomerican diet and potential to advance indigenous health and diabetes prevention. Int. J. Water Res. Arid Environ. 2022, 11, 81–112. [Google Scholar]
- Castañeda-Rodríguez, R.; Quiles, A.; Llorca, E.; Ozuna, C. How to cook Yucca spp. flowers? An analysis of their chemical composition, microstructure, and bioactive compound bioaccessibility. Appl. Food Res. 2024, 4, 100414. [Google Scholar] [CrossRef]
- Marcos-Gómez, R.; Vera-Guzmán, A.M.; Pérez-Ochoa, M.L.; Martínez-Martínez, L.; Hernández-Delgado, S.; Martínez-Sánchez, D.; Chávez-Servia, J.L. Phenolic compounds and antioxidant activity in edible flower species from Oaxaca. Appl. Sci. 2024, 14, 3136. [Google Scholar] [CrossRef]
- Sotelo, A.; López-García, S.; Basurto-Peña, F. Content of nutrient and antinutrient in edible flowers of wild plants in Mexico. Plant Foods Hum. Nutr. 2007, 62, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Medina-Galván, M.I.; Bernardino-Nicanor, A.; Castro-Rosas, J.; Negrete-Rodríguez, M.d.l.L.X.; Conde-Barajas, E.; González-Cruz, L. Antimicrobial and antioxidant activity of flower scape extracts of Agave Salmiana: Effect of the extraction solvent and development stage. Res. J. Biotechnol. 2018, 13, 1–9. [Google Scholar]
- Cáceres, A.; Cruz, S.M. Edible seeds, leaves and flowers as Maya super foods: Function and composition. Int. J. Phytocosmetics Nat. Ingred. 2019, 6, 2. [Google Scholar] [CrossRef]
- Hernández-Castillo, J.B.E.; Bernardino-Nicanor, A.; Vivar-Vera, M.D.L.Á.; Montañez-Soto, J.L.; Teniente-Martínez, G.; Juárez-Goiz, J.M.S.; González-Cruz, L. Modifications of the protein characteristics of pacaya caused by thermal treatment: A spectroscopic, electrophoretic and morphological study. Polymers 2020, 12, 1016. [Google Scholar] [CrossRef]
- Pascual-Mendoza, S.; Saynes-Vásquez, A.; Pérez-Herrera, A.; Meneses, M.E.; Coutiño-Hernández, D.; Sánchez-Medina, M.A. Nutritional composition and bioactive compounds of quelites consumed by indigenous communities in the municipality of Juquila Vijanos, Sierra Norte of Oaxaca, Mexico. Plant Foods Hum. Nut. 2023, 78, 193–200. [Google Scholar] [CrossRef]
- Grzeszczuk, M.; Grzeszczuk, M.; Meller, E.; Stefaniak, A.; Wysocka, G. Mineral composition of some edible flowers. J. Elem. 2018, 23, 151–162. [Google Scholar] [CrossRef]
- Ghosh, P.; Rana, S.S. Physicochemical, nutritional, bioactive compounds and fatty acid profiling of pumpkin flower (Cucurbita maxima), as a potential functional food. SN Appl. Sci. 2021, 3, 216. [Google Scholar]
- Nicknezhad, S.; Hashemabadi, D.; Allahyari, M.S. Nutritional value of some flowers found in green spaces as new food sources. J. Ornam. Plants 2022, 12, 143–155. [Google Scholar]
- Mulík, S.; Ozuna, C. Mexican edible flowers: Cultural background, traditional culinary uses, and potential health benefits. Int. J. Gastron. Food Sci. 2020, 21, 100235. [Google Scholar]
- Mancera-Castro, P.; Bernardino-Nicanor, A.; Juárez-Goiz, J.M.S.; Teniente-Martínez, G.; González-Cruz, L. Effect of the type of thermal treatment on the nutritional and nutraceutical characteristics of pacaya inflorescences (Chamaedorea tepejilote Liebm). Biol. Life Sci. Forum. 2022, 18, 36. [Google Scholar]
- AACC. Approved Methods of the AACC; American Association of Cereal Chemists: St. Paul, MN, USA, 1976. [Google Scholar]
- Martínez-Martínez, R.; Chávez-Servia, J.L.; Vera-Guzmán, A.M.; Aquino-Bolaños, E.N.; Carrillo-Rodríguez, J.C.; Pérez-Herrera, A. Phenotypic variation in grain mineral compositions of pigmented maize conserved in indigenous communities of Mexico. Maydica 2019, 64, 1–13. [Google Scholar]
- Olguín-Hernández, L.; Carrillo-Rodríguez, J.C.; Mayek-Pérez, N.; Aquino-Bolaños, T.; Vera-Guzmán, A.M.; Chávez-Servia, J.L. Patterns and relationships of pesticide use in agricultural crops of Latin America: Review and analysis of statistical data. Agronomy 2024, 14, 2889. [Google Scholar] [CrossRef]
- SAS Institute Inc. Base SAS®9.1.3 Procedures Guide, 2nd ed.; SAS Institute Inc.: Cary, NC, USA, 2006. [Google Scholar]
- dos Santos, I.C.; Novaes-Reis, S. Edible flowers: Traditional and current use. Ornam. Hortic. 2021, 27, 438–445. [Google Scholar]
- Mulík, S.; Hernández-Carrión, M.; Pacheco-Pantoja, S.E.; Ozuna, C. Endemic edible flowers in the Mexican diet: Understanding people’s knowledge, consumption, and experience. Future Foods 2024, 9, 100374. [Google Scholar]
- Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; Otten, J.J., Hellwig, J.P., Meyers, L.D., Eds.; Institute of Medicine of the National Academies, National Academy of Sciences: Washington, DC, USA, 2006; ISBN 0-309-65646-X. [Google Scholar]
- Morales de León, J.C.; Bourges Rodríguez, H.; Camacho Parra, M.E. Tablas de Composición de Alimentos y Productos Alimenticios Mexicanos (Versión Condesada 2015); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán: México City, México, 2016; pp. 55–589. [Google Scholar]
- Jiménez-Aguilar, D.M.; Grusak, M.A. Minerals, vitamin C, phenolics, flavonoids and antioxidant activity of Amaranthus leafy vegetables. J. Food Compos. Anal. 2017, 58, 33–39. [Google Scholar]
- Pinedo-Espinoza, J.M.; Gutiérrez-Tlahque, J.; Santiago-Saenz, Y.O.; Aguirre-Mancilla, C.L.; Reyes-Fuentes, M.; López-Palestina, C.U. Nutritional composition, bioactive compounds and antioxidant activity of wild edible flowers consumed in semiarid regions of Mexico. Plant Foods Hum. Nutr. 2020, 75, 413–419. [Google Scholar] [PubMed]
- Gálvez, A.; Peña, C. Revalorización de la dieta tradicional mexicana: Una visión interdisciplinaria. Rev. Dig. Univ. 2015, 16, 1–117. [Google Scholar]
- Shukla, S.; Bhargava, A.; Chatterjee, A.; Srivastava, J.; Singh, N.; Singh, S.P. Mineral profile and variability in vegetable amaranth (Amaranthus tricolor). Plant Foods Hum. Nutr. 2006, 61, 21–26. [Google Scholar]


| Y. filifera (Izote): | ||||||
| Sources of Variation | Macroelements | |||||
| P | Mg | K | Na | S | Ca | |
| Origin locations | 41,482 ** | 7864.8 ** | 247,688 ** | 0.34 ns | 1580 ** | 40,262 ** |
| Error | 29.5 | 4.7 | 1665.5 | 0.501 | 7.65 | 93.6 |
| Coeff. of variation (%) | 0.8 | 0.9 | 1.6 | 12.8 | 1.7 | 1.8 |
| Sources of Variation | Microelements | |||||
| Cu | Mn | Mo | Si | Zn | Fe | |
| Origin locations | 14.11 ns | 7.08 ** | 0.14 ** | 14.9 ** | 468.1 ** | 365.6 ** |
| Error | 6.15 | 0.11 | 0.002 | 1.84 | 0.61 | 7.00 |
| Coeff. of variation (%) | 24.2 | 3.1 | 9.7 | 13.0 | 1.5 | 7.1 |
| A. salmiana (Maguey Pulquero): | ||||||
| Sources of Variation | Macroelements | |||||
| P | Mg | K | Na | S | Ca | |
| Origin locations | 38,899 ** | 11,697 ** | 328,080 ** | 0.41 ns | 685.3 ** | 310,324 ** |
| Error | 16.6 | 3.86 | 2350.4 | 0.43 | 12.3 | 102.2 |
| Coeff. of variation (%) | 0.7 | 0.6 | 2.2 | 22.9 | 3.6 | 1.2 |
| Sources of Variation | Microelements | |||||
| Cu | Mn | Mo | Si | Zn | Fe | |
| Origin locations | 6.9 ** | 91.2 ** | 0.34 ** | 498.7 ns | 217.0 ** | 49.2 ** |
| Error | 0.103 | 0.068 | 0.018 | 226.3 | 1.71 | 3.81 |
| Coeff. of variation (%) | 4.4 | 1.6 | 2.5 | 26.5 | 2.92 | 6.3 |
| D. americana (Cuachepil): | ||||||
| Sources of Variation | Macroelements | |||||
| P | Mg | K | Na | S | Ca | |
| Origin locations | 10,702 ** | 1595.5 ** | 1157.4 ** | 5.39 ns | 2086.7 ** | 44,200 ** |
| Error | 42.3 | 6.32 | 15.6 | 3.04 | 14.9 | 49.2 |
| Coeff. of variation (%) | 1.1 | 0.8 | 1.7 | 21.3 | 2.4 | 1.6 |
| Sources of Variation | Microelements | |||||
| Cu | Mn | Mo | Si | Zn | Fe | |
| Origin locations | 26.6 ** | 48.7 ** | 18.7 ** | 29.9 ** | 100.3 ** | 152.8 ** |
| Error | 3.03 | 0.05 | 0.019 | 1.09 | 0.54 | 1.76 |
| Coeff. of variation (%) | 14.0 | 1.2 | 3.2 | 11.2 | 1.6 | 2.6 |
| C. tepejilote (Tepejilote): | ||||||
| Sources of Variation | Macroelements | |||||
| P | Mg | K | Na | S | Ca | |
| Origin locations | 20,002 ** | 112,073 ** | 282,853 ** | 1.41 ns | 11,710 ** | 389,290 ** |
| Error | 34.6 | 52.3 | 3695.4 | 1.02 | 58.9 | 965.4 |
| Coeff. of variation (%) | 0.7 | 0.9 | 1.9 | 15.8 | 0.7 | 1.2 |
| Sources of Variation | Microelements | |||||
| Cu | Mn | Mo | Si | Zn | Fe | |
| Origin locations | 1.09 ns | 1511.5 ** | ND | 2652.2 ** | 136.9 ** | 702.5 ** |
| Error | 0.603 | 1.51 | ND | 24.1 | 4.4 | 15.1 |
| Coeff. of variation (%) | 18.6 | 1.5 | ND | 3.6 | 2.5 | 4.0 |
| Communitarian Origins of Samples (ID) | Macroelements (mg 100 g−1) | |||||
| P | Mg | K | Na | S | Ca | |
| Magdalena Jaltepec-010 | 538.9 ± 3.4 g 1 | 212.4 ± 3.5 e | 2237.5 ± 5.8 f | 11.8 ± 6.6 a | 144.5 ± 0.9 g | 500.3 ± 15.9 e |
| Magdalena Jaltepec-01a | 549.9 ± 2.1 fg | 164.5 ± 0.7 h | 2449.7 ± 12.5 e | 8.6 ± 1.3 a | 126.0 ± 2.2 h | 467.9 ± 8.6 f |
| Magdalena Jaltepec-01b | 588.9 ± 11.5 e | 185.0 ± 3.1 g | 2435.7± 42.4 e | 4.8 ± 3.3 a | 129.8 ± 0.1 h | 678.3 ± 11.9 b |
| Magdalena Jaltepec-01c | 465.7 ± 2.3 h | 205.9 ± 2.9 f | 2494.9 ± 12.2 de | 12.4 ± 8.6 a | 149.6 ± 1.2 fg | 566.0 ± 3.8 d |
| Magdalena Jaltepec-02a | 679.3 ± 3.1 d | 184.0 ± 0.4 g | 2614.2 ± 3.0 c | 9.8 ± 1.6 a | 160.7 ± 3.3 de | 636.4 ± 4.2c |
| Magdalena Jaltepec-02b | 736.9 ± 6.3 c | 238.4 ± 1.2 d | 3129.7 ± 40.6 a | 12.4 ± 0.4 a | 181.2 ± 4.0 b | 521.8 ± 5.6 e |
| Magdalena Jaltepec-02c | 869.0 ± 4.0 a | 249.7 ± 1.7 c | 2879.9 ± 48.6 b | 19.6 ± 13.4 a | 206.1 ± 1.6 a | 730.5 ± 3.1 a |
| Santo Tomas Tamazulapan | 671.5 ± 4.2 d | 288.1 ± 2.1 b | 2488.0 ± 44.3 de | 9.6 ± 0.2 a | 170.5 ± 3.0 c | 394.2 ± 6.0 g |
| Santa Maria Sola | 764.9 ± 2.6 b | 289.6 ± 1.2 b | 2594.8 ± 64.9 cd | 20.5 ± 20.5 a | 157.2 ± 3.9 ef | 388.6 ± 7.9 g |
| Santa Ana Miahuatlan | 559.1 ± 5.5 f | 302.1 ± 1.6 a | 2075.8 ± 72.1 g | 5.5 ± 2.8 a | 148.7 ± 2.0 g | 496.0 ± 16.9 ef |
| Ejutla de Crespo | 666.5 ± 6.4 d | 301.0 ± 3.5 a | 2699.4 ± 28.6 c | 13.0 ± 6.4 a | 167.5 ± 3.6 cd | 407.0 ± 8.4 g |
| Communitarian Origins of Samples (ID) | Microelements (mg 100 g−1) | |||||
| Cu | Mn | Si | Zn | Fe | Mo | |
| Magdalena Jaltepec-010 | 0.86 ± 0.24 a 1 | 1.15 ± 0.06 bc | 9.41 ± 3.48 bc | 3.94 ± 0.04 g | 2.89 ± 0.32 de | 0.05 ± 0.0 cd |
| Magdalena Jaltepec-01a | 1.00 ± 0.55 a | 0.92 ± 0.03 d | 5.50 ± 0.77 c | 3.75 ± 0.05 g | 2.41 ± 0.34 e | 0.04 ± 0.0 c–e |
| Magdalena Jaltepec-01b | 0.74 ± 0.13 a | 0.81 ± 0.02 e | 9.38 ± 3.24 bc | 3.73 ± 0.07 g | 2.72 ± 0.13 de | 0.06 ± 0.0 bc |
| Magdalena Jaltepec-01c | 0.77 ± 0.12 a | 1.16 ± 0.06 a–c | 14.01 ± 4.65 b | 4.67 ± 0.16 e | 3.30 ± 0.62 b–d | 0.05 ± 0.0 c |
| Magdalena Jaltepec-02a | 1.39 ± 0.34 a | 0.81 ± 0.01 e | 9.73 ± 1.44 bc | 4.33 ± 0.12 f | 3.18 ± 0.04 cd | 0.03 ± 0.0 f |
| Magdalena Jaltepec-02b | 1.24 ± 0.25 a | 1.13 ± 0.01 bc | 11.59 ± 2.80 bc | 5.15 ± 0.04 d | 3.94 ± 0.05 bc | 0.03 ± 0.0 d–f |
| Magdalena Jaltepec-02c | 1.13 ± 0.06 a | 1.21 ± 0.02 ab | 7.45 ± 2.77 bc | 7.07 ± 0.04 a | 4.04 ± 0.14 b | 0.03 ± 0.0 ef |
| Santo Tomas Tamazulapan | 1.20 ± 0.32 a | 1.25 ± 0.00 a | 24.38 ± 1.95 a | 5.59 ± 0.04 c | ND | 0.07 ± 0.0 b |
| Santa Maria Sola | 1.16 ± 0.02 a | 1.09 ± 0.00 c | 13.11 ± 3.08 bc | 6.24 ± 0.01 b | 5.60 ± 1.10 a | 0.10 ± 0.0 a |
| Santa Ana Miahuatlan | 0.80 ± 0.03 a | 0.97 ± 0.03 d | 7.17 ± 0.77 bc | 5.61 ± 0.00 c | 3.74 ± 0.16 bc | 0.03 ± 0.2 ef |
| Ejutla de Crespo | 0.98 ± 0.03 a | 1.08 ± 0.03 c | 14.07 ± 3.77 b | 7.14 ± 0.07 a | 5.57 ± 0.09 a | 0.04 ± 0.0 d–f |
| Communitarian Origins of Samples (ID) | Macroelements (mg 100 g−1) | |||||
| P | Mg | K | Na | S | Ca | |
| San Esteban Atatlahuca | 462.2 ± 2.0 h 1 | 303.3 ± 1.7 f | 2075.7 ± 16.5 d | 4.7 ± 2.2 a | 92.2 ± 3.4 c | 989.4 ± 7.4 c |
| San Pedro Molinos | 608.5 ± 0.5 c | 324.0 ± 1.9 d | 2237.6 ± 6.7 c | 1.6 ± 0.01 a | 88.5 ± 6.6 cd | 723.0 ± 7.8 d |
| San Jose Monte Verde | 530.4 ± 4.0 e | 345.7 ± 1.1 c | 2358.3 ± 111.5 bc | 44.7 ± 0.01 a | 113.4 ± 3.4 b | 647.7 ± 8.2 ef |
| Santa Cruz Nundaco | 689.6 ± 1.5 b | 363.5 ± 2.6 b | 2430.6 ± 22.7 b | 6.1 ± 3.1 a | 91.2 ± 3.4 c | 633.1 ± 9.4 f |
| Chalcatongo de Hidalgo | 745.9 ± 3.3 a | 433.6 ± 2.8 a | 2731.0 ± 20.8 a | 10.6 ± 2.3 a | 104.9 ± 3.2 b | 1416.3 ± 18.8 a |
| Santo Tomas Ocotepec | 354.8 ± 6.9 i | 233.1 ± 2.0 h | 1844.5 ± 28.3 e | 6.3 ± 1.8 a | 90.5 ± 2.8 c | 419.6 ± 6.2 h |
| San Juan Teposcolula | 481.6 ± 6.3 g | 221.7 ± 2.4 i | 1815.7 ± 84.6 e | 19.4 ± 22.4 a | 79.7 ± 2.1 de | 472.1 ± 11.4 g |
| San Pedro Tidaa | 599.4 ± 0.8 cd | 268.2 ± 1.4 g | 2623.6 ± 11.9 a | 1.9 ± 0.01 a | 123.9 ± 2.6 a | 1006.4 ± 8.1 c |
| Santa Lucia Monte Verde | 590.4 ± 4.3 d | 310.8 ± 1.9 e | 2030.1 ± 40.8 d | 5.2 ± 0.5 a | 107.0 ± 3.3 b | 1185.6 ± 5.9 b |
| Santa Cruz Itundujia | 516.6 ± 4.6 f | 308.1 ± 0.7 ef | 1846.8 ± 5.5 e | 10.5 ± 3.5 a | 76.2 ± 2.0 e | 668.5 ± 11.2 e |
| Communitarian Origins of Samples (ID) | Microelements (mg 100 g−1) | |||||
| Cu | Mn | Mo | Si | Zn | Fe | |
| San Esteban Atatlahuca | 0.64 ± 0.03 ef 1 | 2.25 ± 0.01 b | 0.052 ± 0.01 d | 4.45 ± 1.56 a | 3.65 ± 0.06 e | 2.83 ± 0.22 c |
| San Pedro Molinos | 0.57 ± 0.0 fg | 2.20 ± 0.03 b | 0.052 ± 0.01 d | 4.67 ± 0.63 a | 4.20 ± 0.03 cd | 2.75 ± 0.13 c |
| San Jose Monte Verde | 0.87 ± 0.0 b | 1.25 ± 0.03 f | 0.103 ± 0.01 b | 6.17 ± 0.97 a | 4.40 ± 0.28 bc | 2.80 ± 0.03 c |
| Santa Cruz Nundaco | 0.57 ± 0.0 fg | 1.82 ± 0.05 c | 0.052 ± 0.01 d | 6.95 ± 1.35 a | 5.64 ± 0.08 a | 3.40 ± 0.16 b |
| Chalcatongo de Hidalgo | 0.99 ± 0.06 a | 2.54 ± 0.01 a | 0.053 ± 0.01 c | 5.11 ± 0.58 a | 4.39 ± 0.10 bc | 3.16 ± 0.24 bc |
| Santo Tomas Ocotepec | 0.81 ± 0.06 bc | 1.09 ± 0.01 g | 0.052 ± 0.01 d | 7.81 ± 3.91 a | 3.08 ± 0.12 f | 3.27 ± 0.17 bc |
| San Juan Teposcolula | 0.52 ± 0.01 g | 1.09 ± 0.01 g | 0.052 ± 0.01 d | 5.55 ± 0.92 a | 3.86 ± 0.17 de | 2.88 ± 0.26 bc |
| San Pedro Tidaa | 0.83 ± 0.01 bc | 1.51 ± 0.01 d | 0.100 ± 0.01 a | 4.52 ± 0.50 a | 4.63 ± 0.05 b | 2.81 ± 0.14 c |
| Santa Lucia Monte Verde | 0.71 ± 0.03 de | 1.04 ± 0.01 g | 0.052 ± 0.01 d | 4.29 ± 0.37 a | 5.65 ± 0.11 a | 2.99 ± 0.12 bc |
| Santa Cruz Itundujia | 0.75 ± 0.03 cd | 1.36 ± 0.05 e | ND | 7.23 ± 0.35 a | 5.30 ± 0.11 a | 4.06 ± 0.32 a |
| Community Origins of Samples (ID) | Macroelements (mg 100 g−1) | |||||
| P | Mg | K | Na | S | Ca | |
| San Andres Paxtlan | 505.6 ± 0.9 g 1 | 331.5 ± 0.8 b | 246.1 ± 1.0 b–d | 7.7 ± 4.5 a | 152.6 ± 1.3 c | 576.7 ± 3.1 b |
| San Felipe Zapotitlan | 521.9 ± 2.2 g | 259.4 ± 0.6 g | 199.1 ± 0.5 h | 1.3 ± 0.01a | 148.7 ± 3.0 c | 608.8 ± 1.7a |
| Santa Cruz Xitla-1 | 702.1 ± 12.1 a | 346.2 ± 5.8 a | 235.4 ± 4.6 d–f | 8.2 ± 1.7 a | 169.4 ± 8.5 b | 354.1 ± 1.9 f |
| Santa Cruz Xitla-2 | 633.9 ± 14.4 b | 317.3 ± 2.1 c | 250.2 ± 9.6 a–c | 7.0 ± 4.9 a | 142.9 ± 6.7 c | 581.2 ± 3.8 b |
| Santa Cruz Xitla-3 | 623.3 ± 6.7 bc | 306.5 ± 3.2 d | 256.1 ± 3.6 ab | 3.8 ± 3.3 a | 168.1 ± 1.3 b | 407.8 ± 5.2 e |
| Agua Fria Sola de Vega-1 | 597.6 ± 4.6 de | 298.9 ± 2.4 ef | 204.4 ± 0.3 h | 5.0 ± 0.3 a | 144.0 ± 1.2 c | 396.9 ± 2.9 e |
| Agua Fria Sola de Vega-2 | 551.4 ± 2.4 f | 325.4 ± 1.2 b | 230.0 ± 1.9 fg | 6.1 ± 2.4 a | 130.3 ± 1.3 d | 243.6 ± 14.3 h |
| Reyes Sola de Vega-1 | 605.3 ± 4.0 cd | 305.2 ± 1.8 de | 221.0 ± 2.4 g | 5.8 ± 5.4 a | 203.1 ± 4.0 a | 305.0 ± 13.9 g |
| Reyes Sola de Vega-2 | 605.4 ± 3.2 cd | 326.5 ± 2.0 b | 258.9 ± 1.7 a | 5.6 ± 1.2 a | 177.0 ± 1.9 b | 509.3 ± 4.8 c |
| San Pedro El Alto | 584.6 ± 1.6 e | 295.1 ± 2.1 f | 242.0 ± 5.2 c–e | 5.4 ± 1.3 a | 204.6 ± 1.7 a | 346.4 ± 5.5 f |
| Santa Lucia Miahuatlan | 509.6 ± 2.9 g | 303.9 ± 1.0 de | 234.1 ± 2.0 ef | 3.4 ± 0.4 a | 127.4 ± 2.9 d | 439.8 ± 4.5 d |
| Community Origins of Samples (ID) | Microelements (mg 100 g−1) | |||||
| Cu | Mn | Mo | Si | Zn | Fe | |
| San Andres Paxtlan | 1.02 ± 0.08 bc 1 | 2.22 ± 0.01 c | 0.55 ± 0.03 d | 11.53 ± 2.18 b | 4.52 ± 0.03 cd | 5.44 ± 0.13 b |
| San Felipe Zapotitlan | 1.24 ± 0.11 ab | 2.04 ± 0.01 d | ND | 15.30 ± 2.28 a | 3.77 ± 0.06 g | 4.65 ± 0.03 e |
| Santa Cruz Xitla-1 | 0.57 ± 0.23 c | 1.66 ± 0.03 f | 0.37 ± 0.01 f | 7.35 ± 0.09 cd | 4.41 ± 0.10 c–e | 5.23 ± 0.20 bc |
| Santa Cruz Xitla-2 | 1.59 ± 0.27 a | 2.03 ± 0.03 d | 0.65 ± 0.03 c | 12.40 ± 0.20 ab | 5.07 ± 0.14 b | 6.31 ± 0.25 a |
| Santa Cruz Xitla-3 | 1.08 ± 0.03 bc | 2.50 ± 0.05 a | 0.313± 0.01 g | 6.99 ± 0.23 de | 5.44 ± 0.03 a | 3.96 ± 0.13 f |
| Agua Fria Sola de Vega-1 | 1.32 ± 0.03 ab | 1.57 ± 0.0 g | 0.052 ± 0.01 h | 11.41 ± 0.43 b | 4.07 ± 0.06 f | 5.41 ± 0.14 bc |
| Agua Fria Sola de Vega-2 | 1.53 ± 0.16 ab | 2.34 ± 0.03 b | 0.051 ± 0.01 h | 7.05 ± 0.12 de | 4.57 ± 0.06 c | 4.57 ± 0.10 e |
| Reyes Sola de Vega-1 | 1.48 ± 0.11 ab | 1.31 ± 0.03 i | 0.692 ± 0.01 b | 8.13 ± 1.03 cd | 5.31 ± 0.11 a | 5.04 ± 0.03 cd |
| Reyes Sola de Vega-2 | 1.53 ± 0.37 ab | 2.36 ± 0.01 b | 0.421 ± 0.01 e | 7.60 ± 0.61 cd | 5.45 ± 0.04 a | 4.75 ± 0.04 de |
| San Pedro El Alto | 1.08 ± 0.06 a–c | 1.78 ± 0.01 e | 0.366 ± 0.01 f | 4.27 ± 0.13 e | 4.31 ± 0.03 de | 4.78 ± 0.03 de |
| Santa Lucia Miahuatlan | 1.24 ± 0.08 ab | 1.45 ± 0.01 h | 0.780 ± 0.01 a | 10.34 ± 0.53 bc | 4.25 ± 0.02 ef | 6.29 ± 0.13 a |
| Communitarian Origins of Samples (ID) | Macroelements (mg 100 g−1) | |||||
| P | Mg | K | Na | S | Ca | |
| Sn. Juan Bta. Valle Nacional | 775.3 ± 5.8 g 1 | 842.7 ± 3.0 b | 2853.3 ± 42.3 f | 15.47 ± 9.2 a | 1094.4 ± 12.8 bc | 2586.1 ± 24.0 e |
| Sn. Fpe. Jalapa de Diaz-1 | 941.7 ± 4.3 a | 852.2 ± 2.5 b | 2802.6 ± 20.4 f | 20.57 ± 4.9 a | 1108.0 ± 9.3 b | 3088.6 ± 17.7 b |
| Sn. Fpe. Jalapa de Diaz-2 | 909.3 ± 2.9 b | 801.8 ± 5.3 c | 2813.4 ± 9.3 f | 17.00 ± 8.9 a | 1112.2 ± 5.0 b | 2915.2 ± 13.3 c |
| Sn. Fpe. Jalapa de Diaz-3 | 882.8 ± 2.2 c | 771.7 ± 11.1 d | 2940.6 ± 36.9 f | 22.50 ± 9.4 a | 1142.9 ± 8.8 a | 2812.2 ± 52.2 d |
| Ayotzintepec-1 | 808.2 ± 9.2 e | 811.3 ± 12.2 c | 3431.5 ± 167.5 bc | 43.67 ± 41.7 a | 959.4 ± 1.0 f | 2387.1 ± 35.7 fg |
| Ayotzintepec-2 | 781.4 ± 7.9 fg | 730.8 ± 9.0 e | 3221.9 ± 40.2 de | 28.17 ± 4.1 a | 1066.1 ± 9.6 de | 2263.1 ± 41.9 h |
| Ayotzintepec-3 | 842.6 ± 7.7 d | 738.1 ± 3.3 e | 3226.0 ± 41.9 de | 20.47 ± 13.2 a | 1075.6 ± 5.4 cd | 2303.3 ± 15.7 gh |
| Santa Maria Jacatepec-1 | 752.0 ± 0.7 h | 533.1 ± 5.9 h | 3167.6 ± 13.6 e | 9.95 ± 1.2 a | 1045.2 ± 4.6 e | 2358.7 ± 22.4 g |
| Santa Maria Jacatepec-2 | 796.6 ± 7.0 ef | 595.3 ± 10.6 g | 3398.6 ± 23.7 cd | 13.60 ± 11.4 a | 1054.3 ± 8.2 de | 2474.2 ± 46.6 f |
| Santa Maria Jacatepec-3 | 643.8 ± 1.4 i | 1281.0 ± 3.7 a | 3588.5 ± 54.3 ab | 20.87 ± 9.0 a | 945.7 ± 3.7 f | 3383.7 ± 23.9 a |
| Santiago Jocotepec | 808.2 ± 7.5 e | 667.0 ± 0.8 f | 3650.3 ± 43.3 a | 30.40 ± 3.7 a | 1110.0 ± 8.3 b | 2771.8 ± 18.0 d |
| Communitarian Origins of Samples (ID) | Microelements (mg 100 g−1) | |||||
| Cu | Mn | Si | Zn | Fe | ||
| San Juan Bta. Valle Nacional | 1.40 ± 0.01 a 1 | 13.65 ± 0.18 a | 141.3 ± 8.9 c | 8.83 ± 0.30 a | 8.32 ± 0.63 ef | |
| San Fpe. Jalapa de Diaz-1 | 1.43 ± 0.09 a | 8.67 ± 0.09 d | 161.4 ± 4.8 b | 9.20 ± 0.34 a | 9.24 ± 0.07 c–f | |
| San Fpe. Jalapa de Diaz-2 | 1.43 ± 0.09 a | 9.21 ± 0.12 c | 136.2 ± 3.1 cd | 8.95 ± 0.14 a | 9.28 ± 0.20 c–e | |
| San Fpe. Jalapa de Diaz-3 | 3.09 ± 2.48 a | 6.25 ± 0.05 hi | 122.7 ± 4.8 de | 8.11 ± 0.24 b | 9.46 ± 0.39 b–d | |
| Ayotzintepec-1 | 2.34 ± 0.94 a | 6.52 ± 0.03 gh | 104.1 ± 5.4 f | 8.11 ± 0.13 b | 9.83 ± 0.52 bc | |
| Ayotzintepec-2 | 2.03 ± 0.96 a | 6.63 ± 0.18 g | 102.7 ± 1.7 f | 7.71 ± 0.23 bc | 8.50 ± 0.25 d–f | |
| Ayotzintepec-3 | 2.42 ± 0.08 a | 7.36 ± 0.18 f | 106.8 ± 3.1 f | 9.11 ± 0.17 a | 10.51 ± 0.31 b | |
| Santa Maria Jacatepec-1 | 1.76 ± 0.29 a | 6.50 ± 0.12 gh | 121.7 ± 2.4 e | 7.27 ± 0.17 c | 8.12 ± 0.32 f | |
| Santa Maria Jacatepec-2 | 1.48 ± 0.09 a | 8.13 ± 0.11 e | 126.5 ± 7.3 de | 7.94 ± 0.13 b | 9.96 ± 0.60 bc | |
| Santa Maria Jacatepec-3 | 1.10 ± 0.09 a | 9.79 ± 0.11 b | 201.0 ± 1.4 a | 8.84 ± 0.18 a | 13.67 ± 0.05 a | |
| Santiago Jocotepec | 1.52 ± 0.05 a | 6.11 ± 0.04 i | 158.5 ± 4.0 b | 9.23 ± 0.18 a | 8.84 ± 0.41 c–f | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcos-Gómez, R.; Vera-Guzmán, A.M.; Pérez-Ochoa, M.L.; Martínez-Martínez, L.; Hernández-Delgado, S.; Martínez-Sánchez, D.; Chávez-Servia, J.L. Mineral Content of Four Mexican Edible Flowers Growing in Natural Conditions and Backyards from Indigenous Communities. Appl. Sci. 2025, 15, 3432. https://doi.org/10.3390/app15073432
Marcos-Gómez R, Vera-Guzmán AM, Pérez-Ochoa ML, Martínez-Martínez L, Hernández-Delgado S, Martínez-Sánchez D, Chávez-Servia JL. Mineral Content of Four Mexican Edible Flowers Growing in Natural Conditions and Backyards from Indigenous Communities. Applied Sciences. 2025; 15(7):3432. https://doi.org/10.3390/app15073432
Chicago/Turabian StyleMarcos-Gómez, Rubí, Araceli M. Vera-Guzmán, Mónica L. Pérez-Ochoa, Laura Martínez-Martínez, Sanjuana Hernández-Delgado, David Martínez-Sánchez, and José L. Chávez-Servia. 2025. "Mineral Content of Four Mexican Edible Flowers Growing in Natural Conditions and Backyards from Indigenous Communities" Applied Sciences 15, no. 7: 3432. https://doi.org/10.3390/app15073432
APA StyleMarcos-Gómez, R., Vera-Guzmán, A. M., Pérez-Ochoa, M. L., Martínez-Martínez, L., Hernández-Delgado, S., Martínez-Sánchez, D., & Chávez-Servia, J. L. (2025). Mineral Content of Four Mexican Edible Flowers Growing in Natural Conditions and Backyards from Indigenous Communities. Applied Sciences, 15(7), 3432. https://doi.org/10.3390/app15073432








