Reaction Dynamics of Plant Phenols in Regeneration of Tryptophan from Its Radical Cation Formed via Photosensitized Oxidation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Ultraviolet–Visible (UV-Vis) Absorption Spectroscopy
2.3. Laser Flash Photolysis and Transient Absorption Spectroscopy
3. Results
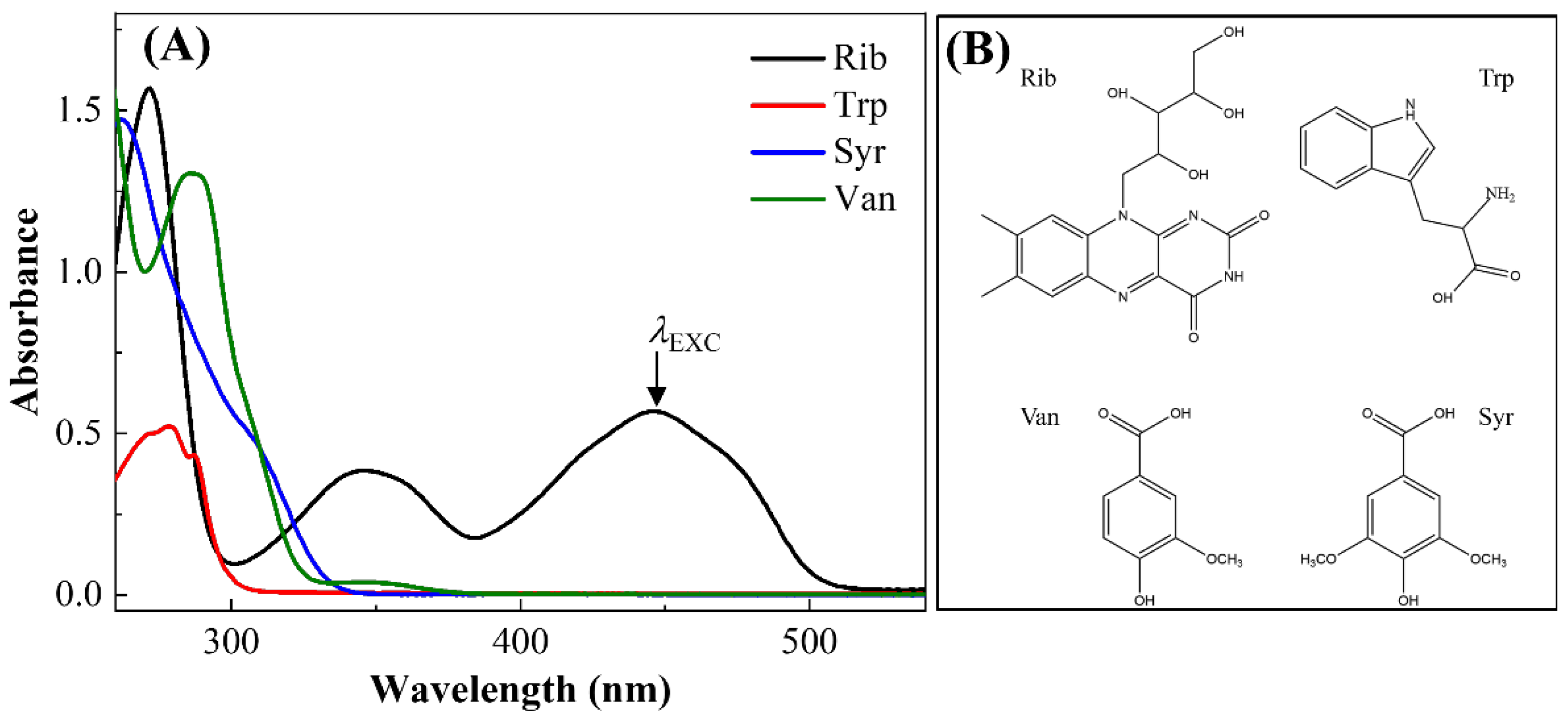
3.1. UV-Vis Steady-State Absorption Characterizations
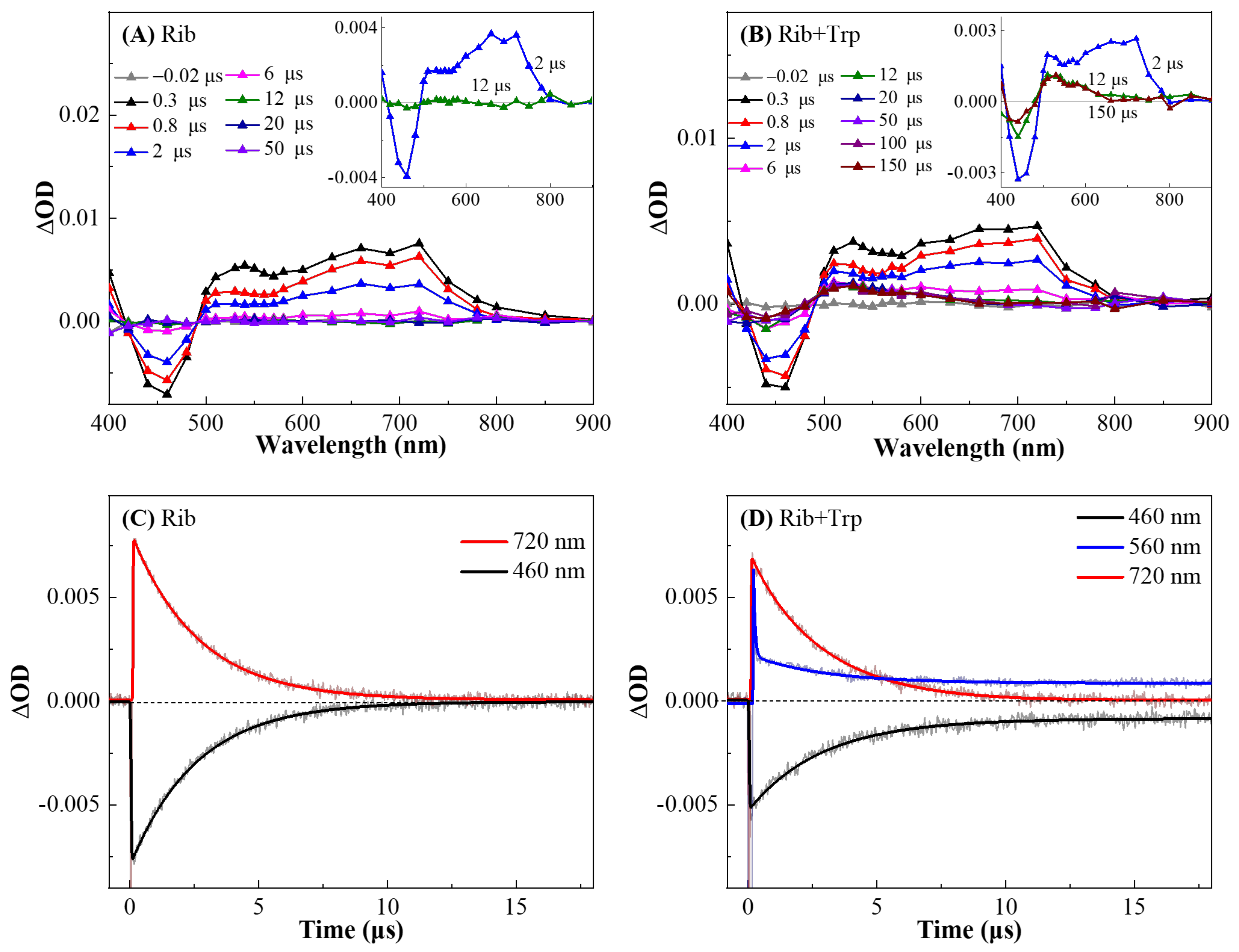
3.2. The Oxidation of Trp by Triplet State of Rib Probed by ns-TA Spectroscopy
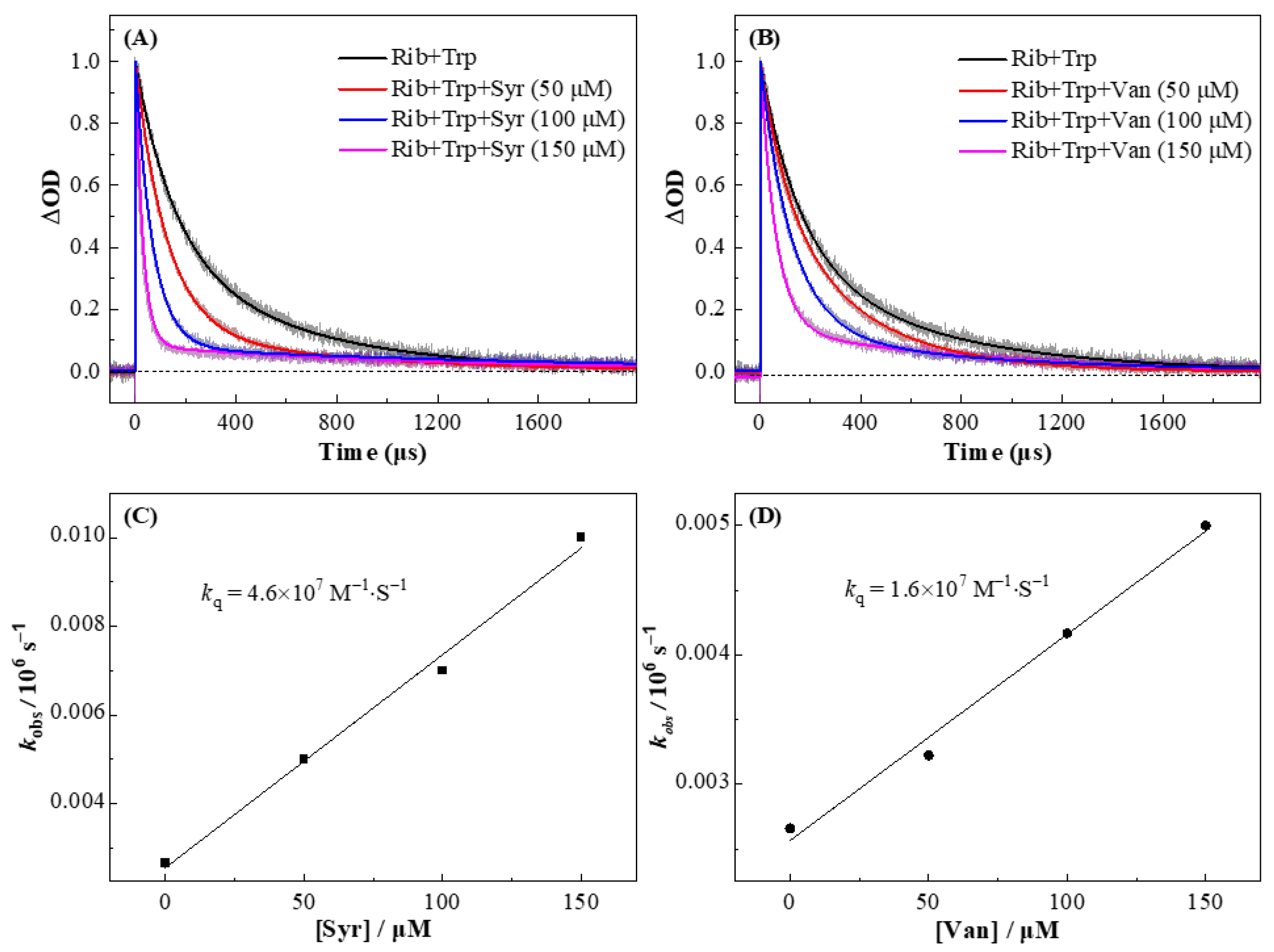
3.3. Concentration Effect of Syr and Van for Regeneration of Trp from the Trp•+
3.4. Higher pH Enhanced Syr and Van for Regeneration of Trp from the Trp•+
3.5. E Dependence on the Regeneration of Trp from Trp•+
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jung, M.Y.; Lee, K.H.; Kim, S.Y. Riboflavin-sensitized photochemical changes in β-lactoglobulin in an aqueous buffer solution as affected by ascorbic acid. J. Agric. Food Chem. 2000, 48, 3847–3850. [Google Scholar] [CrossRef] [PubMed]
- Chapman, K.W.; Whited, L.J.; Boor, K.J. Sensory threshold of light-oxidized flavour defects in milk. J. Food Sci. 2002, 67, 2770–2773. [Google Scholar] [CrossRef]
- Berliner, L.J.; Bradley, D.G.; Meinholtz, D.; Min, D.B.; Ogata, T. The detection of riboflavin photosensitized singlet oxygen formation in milk by electron spin resonance. In Supramolecular Structure and Function; Pifat, G., Ed.; Balaban Publishers: Rehovot, Israel, 1993; pp. 67–77. [Google Scholar]
- Mortensen, G.; Sørensen, J.; Stapelfeldt, H. Light-induced oxidation in semihard cheeses. Evaluation of methods used to determine levels of oxidation. J. Agric. Food Chem. 2002, 50, 4364–4370. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, D.R.; Libardi, S.H.; Skibsted, L.H. Riboflavin as a photosensitizer. Effects on human health and food quality. Food Funct. 2012, 3, 487–502. [Google Scholar]
- Li, T.L.; King, J.M.; Min, D.B. Quenching mechanisms and kinetics of carotenoids in riboflavin photosensitized singlet oxygen oxidation of vitamin D2. J. Food Biochem. 2000, 24, 477–492. [Google Scholar] [CrossRef]
- Yoshimura, A.; Ohno, T. Lumiflavin-sensitized photooxygenation of indole. Photochem. Photobiol. 1988, 48, 561–565. [Google Scholar] [CrossRef]
- Kristensen, D.; Orlien, V.; Mortensen, G.; Brockhoff, P.; Skibsted, L.H. Light-induced oxidation in sliced Havarti cheese packaged in modified atmosphere. Int. Dairy J. 2000, 10, 95–103. [Google Scholar] [CrossRef]
- Cardoso, D.R.; Franco, D.W.; Olsen, K.; Andersen, M.L.; Skibsted, L.H. Reactivity of Bovine Whey Proteins, Peptides, and Amino Acids toward Triplet Riboflavin as Studied by Laser Flash Photolysis. J. Agric. Food Chem. 2004, 52, 6602–6606. [Google Scholar] [CrossRef]
- Harriman, A. Further Comments on the Redox Potentials of Tryptophan and Tyrosine. J. Phys. Chem. 1987, 91, 6104–6106. [Google Scholar] [CrossRef]
- Bellmaine, S.; Schnellbaecher, A.; Zimmer, A. Reactivity and degradation products of tryptophan in solution and proteins. Free Radic. Biol. Med. 2020, 160, 696–718. [Google Scholar] [CrossRef]
- Bent, D.V.; Hayon, E. Excited State Chemistry of Aromatic Amino Acids and Related Peptides. III. Tryptophan. J. Am. Chem. Soc. 1975, 97, 2612–2619. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Han, R.M.; Lyu, M.K.; Zhang, J.P.; Skibsted, L.H. Regeneration of β-Carotene from the Radical Cation by Tyrosine and Tryptophan. J. Phys. Chem. B 2015, 119, 6603–6610. [Google Scholar] [CrossRef] [PubMed]
- Skibsted, L.H. Carotenoids in antioxidant networks. Colorants or radical scavengers. J. Agric. Food Chem. 2012, 60, 2409–2417. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Chen, C.H.; Han, R.M.; Zhang, J.P.; Skibsted, L.H. Thermodynamic versus kinetic control of antioxidant synergism between β-carotene and (iso)flavonoids and their glycosides in liposomes. J. Agric. Food Chem. 2010, 58, 9221–9227. [Google Scholar] [CrossRef]
- Heelis, P.F.; Parsons, B.J.; Phillips, G.O. The pH dependence of the reactions of flavin triplet states with amino acids. Biochim. Biophys. Acta (BBA) Gen. Subj. 1979, 587, 455–462. [Google Scholar] [CrossRef]
- Jung, M.Y.; Kim, S.K.; Kim, S.Y. Riboflavin-sensitized photooxidation of ascorbic acid: Kinetics and amino acid effects. Food Chem. 1995, 53, 397–403. [Google Scholar] [CrossRef]
- Lu, C.; Lin, W.; Wang, W.; Han, Z.; Yao, S.; Lin, N. Riboflavin (VB2) photosensitized oxidation of 2′-deoxyguanosine-5′-mono phosphate (dGMP) in aqueous solution: A transient intermediates study. Phys. Chem. Chem. Phys. 2000, 2, 329–334. [Google Scholar] [CrossRef]
- Skibsted, L.H. Light-induced changes in dairy products. Bull. Int. Dairy Fed. 2000, 346, 4–9. [Google Scholar]
- Simic, A.; Manojlovic, D.; Segan, D.; Todorovic, M. Electrochemical behavior and antioxidant and prooxidant activity of natural phenolics. Molecules 2007, 12, 2327–2340. [Google Scholar] [CrossRef]
- Sarkar, B.; Das, U.; Bhattacharyya, S.; Bose, S.K. Studies on the aerobic photooxidation of cysteine using riboflavin as sensitizer: Evidence for the photogeneration of a superoxide anion and hydrogen peroxide. Biol. Pharm. Bull. 1997, 20, 910–912. [Google Scholar] [CrossRef]
- Toyosaki, T.; Mineshita, T. Mechanism of milk riboflavin photolysis in model system. Milchwissenschaft 1989, 44, 292–295. [Google Scholar]
- Huie, R.E.; Clifton, C.L.; Neta, P. Electro-transfer reaction-rates and equilibria of the carbonate and sulfate radical-anions. Int. J. Radiant. Appl. Instrum. Part C Radiat. Phys. Chem. 1991, 38, 477–481. [Google Scholar] [CrossRef]
- Cheng, H.; Han, R.M.; Zhang, J.P.; Skibsted, L.H. Electron Transfer from Plant Phenolates to Carotenoid Radical Cations. Antioxidant Interaction Entering the Marcus Theory Inverted Region. J. Agric. Food Chem. 2014, 62, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. (Ed.) Principle of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006; pp. 278–283. [Google Scholar]
- Li, W.B.; Gandra, N.; Courtney, S.N.; Gao, R.M. Singlet Oxygen Production upon Two-Photon Excitation of TiO2 in Chloroform. Chemphyschem 2009, 10, 1789–1793. [Google Scholar] [CrossRef] [PubMed]
- Marcus, R.A. Electron transfer reactions in chemistry. Theory and experiment (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 1993, 32, 1111–1121. [Google Scholar] [CrossRef]
- Turro, C.M.; Zaleski, J.M.; Karabatsos, Y.G.; Nocera, D. Bimolecular electron transfer in the Marcus inverted region. J. Am. Chem. Soc. 1996, 118, 6060–6067. [Google Scholar] [CrossRef]
- Thanasekaran, P.; Rajendran, T.; Rajagopal, S.; Srinivasan, C.; Ramaraj, R.; Ramamurthy, P.; Venkatachalapathy, B. Marcus inverted region in the photoinduced electron transfer reactions of ruthenium (II)–polypyridine complexes with phenolate ions. J. Phys. Chem. A 1997, 101, 8195–8199. [Google Scholar] [CrossRef]
- Weng, Y.X.; Chan, K.C.; Tzeng, B.C.; Che, C.M. Effect of laser intensity on the determination of intermolecular electron transfer rate constants observation of Marcus inverted region in photo induced back electron transfer reactions. J. Chem. Phys. 1998, 109, 5948–5956. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Ohkubo, K.; Imahori, H.M.; Guldi, D. Driving force dependence of intermolecular electron-transfer reactions of fullerenes. Chem. A Eur. J. 2003, 9, 1585–1593. [Google Scholar] [CrossRef]
- Hong, J.; Kharenko, O.A.; Fan, J.; Xie, F.; Petros, A.K.; Gibney, B.R.; Ogawa, M.Y. Evidence that a miniature CuI metal loprotein undergoes collisional electron transfer in the inverted Marcus region. Angew. Chem. Int. Ed. 2006, 45, 6137–6140. [Google Scholar] [CrossRef]
- Imahori, H.; Yamada, H.; Guldi, D.M.; Endo, Y.; Shimomura, A.; Kundu, S.; Yamada, K.; Okada, T.; Sakata, Y.; Fukuzumi, S. Comparison of reorganization energies for intra- and intermolecular electron transfer. Angew. Chem. Int. Ed. Engl. 2002, 41, 2344–2347. [Google Scholar] [PubMed]
- Castelli, F.; Uccella, N.; Trombetta, D.; Saija, A. Differences between coumaric and cinnamic acids in membrane permeation as evidenced by time-dependent calorimetry. J. Agric. Food Chem. 1999, 47, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Pedulli, G.F.; Cabrini, L.; Zambonin, L.; Landi, L. Solvent and pH effects on the antioxidant activity of caffeic and other phenolic acids. J. Agric. Food Chem. 2006, 54, 2932–2937. [Google Scholar] [CrossRef]
- Han, R.M.; Chen, C.H.; Tian, Y.X.; Zhang, J.P.; Skibsted, L.H. Fast Regeneration of Carotenoids from Radical Cations by Isoflavonoid Dianions: Importance of the Carotenoid Keto Group for Electron Transfer. J. Phys. Chem. A 2010, 114, 126–132. [Google Scholar] [CrossRef] [PubMed]


| [Syr] | [Van] | |||||
|---|---|---|---|---|---|---|
| 50 μM | 100 μM | 150 μM | 50 μM | 100 μM | 150 μM | |
| kobs/s−1 | 5.0 × 103 | 7.0 × 103 | 1.0 × 104 | 3.2 × 103 | 4.2 × 103 | 5.0 × 103 |
| kq/M−1·s−1 | 4.7 × 107 | 4.3 × 107 | 4.9 × 107 | 1.1 × 107 | 1.5 × 107 | 1.6 × 107 |
| kq (average) | 4.6 × 107 | 1.4 × 107 | ||||
| pH | 2 | 5.6 | 7.2 | 9 | 10 |
|---|---|---|---|---|---|
| φ-OH | 98.84% (99.31%) | 2.1% (3.47%) | 0.05% (0.09%) | 0.00% (0.00%) | 0.00% (0.00%) |
| φ-O− | 1.16% (0.69%) | 97.88% (96.53%) | 99.5% (99.79%) | 78.02% (93.18%) | 26.20% (57.74%) |
| φ-O2− | 0.00% (0.00%) | 0.01% (0.00%) | 0.44% (0.12%) | 21.98% (6.82%) | 73.80% (42.26%) |
| Sal | m-hyd | Van | Syr | Caf | Cat | |
|---|---|---|---|---|---|---|
| ∆E | −0.13 | −0.02 | 0.08 | 0.32 | 0.36 | 0.62 |
| k2/M−1·s−1 | 2.20 × 107 | 2.18 × 107 | 5.03 × 107 | 8.73 × 107 | 1.26 × 108 | 7.50 × 107 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhou, Y.; Li, D.; Zhang, J. Reaction Dynamics of Plant Phenols in Regeneration of Tryptophan from Its Radical Cation Formed via Photosensitized Oxidation. Appl. Sci. 2025, 15, 3524. https://doi.org/10.3390/app15073524
Li Y, Zhou Y, Li D, Zhang J. Reaction Dynamics of Plant Phenols in Regeneration of Tryptophan from Its Radical Cation Formed via Photosensitized Oxidation. Applied Sciences. 2025; 15(7):3524. https://doi.org/10.3390/app15073524
Chicago/Turabian StyleLi, Yuqian, Yiming Zhou, Danhong Li, and Jianping Zhang. 2025. "Reaction Dynamics of Plant Phenols in Regeneration of Tryptophan from Its Radical Cation Formed via Photosensitized Oxidation" Applied Sciences 15, no. 7: 3524. https://doi.org/10.3390/app15073524
APA StyleLi, Y., Zhou, Y., Li, D., & Zhang, J. (2025). Reaction Dynamics of Plant Phenols in Regeneration of Tryptophan from Its Radical Cation Formed via Photosensitized Oxidation. Applied Sciences, 15(7), 3524. https://doi.org/10.3390/app15073524







