Abstract
Plant-based proteins are increasingly recognized for their environmental, ethical, and nutritional benefits. However, their digestibility varies due to factors such as molecular structure, amino acid composition, and processing methods. This review comprehensively analyzes methods used to assess plant protein digestibility, including in vivo, in vitro, and ex vivo approaches. While in vivo studies, particularly those using pigs, are considered the gold standard, in vitro and ex vivo models offer cost-effective and reproducible alternatives for simulating digestion. Additionally, antinutritional factors present in plant proteins can hinder digestibility, necessitating processing strategies such as fermentation, enzymatic hydrolysis, and high-pressure treatments to enhance protein bioavailability. Advances in evaluation techniques, including the Digestible Indispensable Amino Acid Score (DIAAS) and dynamic digestion models, offer more precise assessments of protein quality. By systematically comparing these methods, this review aims to guide food scientists and manufacturers in selecting appropriate evaluation strategies to improve the nutritional quality of plant-based protein products. Understanding the mechanisms influencing plant protein digestibility is essential for optimizing food formulations and supporting the broader adoption of sustainable protein sources in human diets.
1. Introduction
Dietary protein is obtained from animal and plant sources. Still, there are significant distinctions in the type of protein they provide, including their molecular makeup, amino acid composition, technical properties, and digestibility [1]. These differences can impact how bioavailable they are for human nutrition, as well as the taste and texture of foods that contain animal or plant proteins.
While animal protein is in high demand, it is generally deemed to be less environmentally sustainable. As a result, transitioning from animal- to plant-based protein foods may be necessary to promote environmental sustainability, ethical considerations, affordable food, enhanced food safety, meeting consumer demand, and addressing protein-energy malnutrition [2]. By understanding the fundamental differences between animal and plant proteins, food manufacturers can make informed decisions when substituting animal protein with plant protein to develop more sustainable and nutritious food products. Thus, the importance of consuming a diverse range of plant proteins lies in ensuring all the essential amino acids our bodies require. Proteins are crucial elements of the human diet, comprising amino acids bonded by peptide bonds [3]. Since our bodies cannot synthesize protein independently, it is essential to consume protein from dietary sources rich in essential amino acids, such as plants.
The surge in plant protein consumption in recent years, particularly from legumes, has led to many plant-based foods as alternatives to animal-based foods [4,5]. As a result, protein ingredients have become increasingly popular in the food industry due to their nutritional benefits and functional properties in food formulations [6].
Assessing the protein quality of plant-based sources is crucial for developing alternative protein sources and formulating plant-based food products that meet the body’s nutritional requirements. This involves determining a food protein’s ability to meet the body’s metabolic requirements for amino acids and nitrogen, which encompasses the protein’s amino acid composition, bioavailability, and digestibility [7,8]. However, not all plant proteins are created equal, and some are better than others regarding their amino acid composition and digestibility.
Protein digestibility is influenced by factors such as the protein’s structural makeup, the intensity of thermal processing, and the existence of antinutritional factors (ANFs) that can impede protein digestion [3]. Thus, understanding the differences in protein quality among plant-based sources is essential to selecting the best protein sources for developing plant-based food products that are nutritionally complete and bioavailable.
Despite the growing interest in plant-based proteins and their role in sustainable food systems, no comprehensive review currently serves as a decision-making guide for selecting the most appropriate method to evaluate plant protein digestibility. Existing studies focus on specific techniques or protein sources but lack a comparative framework synthesizing available methodologies and their applicability [3,6,9,10,11].
Thus, this comprehensive review, mainly covering the most relevant literature of the last 10 years in the protein digestibility field, addresses this gap by systematically analyzing protein digestibility assessment methods, highlighting their advantages, limitations, and relevance for different plant protein sources. By offering a structured comparison, this work aims to support food scientists and manufacturers in making informed decisions regarding evaluating plant protein digestibility, ultimately contributing to developing nutritionally optimized and functional plant-based food products. Therefore, this knowledge can help food manufacturers make informed decisions when selecting plant protein sources and processing methods to ensure that the final product provides high-quality protein to consumers. This comprehensive analysis provides the main springboard for our present article.
2. Plant Protein Quality
Protein quality plays a crucial role in ensuring optimal nutrition and maintaining good health, as it directly impacts the body’s ability to repair tissues, support immune function, and produce essential enzymes and hormones [3]. It refers to the ability of the dietary protein to offset the nitrogen loss that occurs during metabolic processes in organisms [12]. Several factors, such as age, health status, physiological condition, and energy balance, contribute to the determination of the nutritional value of a food product. Furthermore, the presence and bioavailability of essential amino acids and protein digestibility are significant factors that affect human growth and the maintenance of good health [3,13,14]. Thus, understanding the various factors that influence protein quality is essential for ensuring that dietary protein intake meets the nutritional requirements of individuals.
3. Protein Digestibility
Protein digestion occurs in two phases, namely, the gastric and the intestinal phases, beginning in the stomach and ending in the small intestine. The stomach acts as a reservoir, regulating the release of nutrients into the small intestine, which serves as the primary site of hydrolysis and absorption [15]. In the human stomach, acids and pepsin facilitate the breakdown of proteins, while pancreatic and intestinal enzymes aid in protein digestion in the small intestine [14]. Understanding the sequential events and mechanisms involved in protein digestion is crucial for evaluating protein quality and optimizing nutrient uptake in the human body.
The stomach environment undergoes temporal and spatial variations, which are critical in regulating digestion kinetics. Upon food entry, the stomach’s pH becomes relatively high, and enzyme concentration remains low, with values depending on the buffering capacity [15]. Several complex processes occur in the gastric compartment during digestion, including enzymatic, physical, and chemical processes that are crucial in controlling digestion kinetics [16]. The gastric phase of digestion begins with the secretion of pepsinogen and hydrochloric acid (HCl) from the chief cells and parietal cells, respectively. HCl secretion causes a decrease in pH, leading to the secretion of pepsinogen, the zymogen of pepsin, and gastric lipase [15]. Pepsin, activated by gastric acid, hydrolyses proteins, with a preference for cleaving large aliphatic or aromatic side groups, thus completing around 10–20% of protein digestion [16]. The stomach’s acidic environment serves as a critical barrier against microbial pathogens as it inhibits or eliminates many microorganisms. However, aging is associated with a decline in gastric fluid secretion, leading to a reduction in stomach acidity and a condition known as achlorhydria, which can increase the risk of gastrointestinal infections [17].
The stomach connects the esophagus to the duodenum, the initial segment of the small intestine, and remains relatively stable at both its esophageal and duodenal junctions. Thus, after gastric digestion, the pylorus, which holds the chyme (semifluid mass of partly digested food), acts as a filter, permitting only liquids and small food particles to pass through the pylorus (2–3 mm) to the pyloric sphincter [18]. During gastric emptying, rhythmic mixing waves propel small portions of chyme through the pyloric sphincter into the duodenum. If too much chyme were released at once, it would exceed the small intestine’s capacity to process it efficiently. The remaining chyme is pushed back into the stomach body, where it continues to mix. This cycle repeats as subsequent mixing waves move additional chyme into the duodenum [19]. The precise regulation of the rate of gastric emptying of chyme into the duodenum is critical for further digestion and absorption in the small intestines, which is regulated by feedback from the intestines via a variety of gastrointestinal hormones [18].
Upon entering the intestines, the chyme transitions to the intestinal phase, where pepsin is inactivated at a pH greater than 4.5 to prevent auto-digestion. As chyme empties into the small intestine, pancreatic proteases are secreted along with pancreatic bicarbonate. Key proteases of the small intestine have an optimal pH range of 7.0–9.0 [17]. The contents of the small intestine are thoroughly mixed, and the relatively high enzyme activity leads to the rapid production of hydrolysis products [15]. The resulting products of the gastric and intestinal phases are absorbed by enterocytes in the jejunum and, to a lesser extent, in the ileum, and then metabolized in various body organs [20,21]. Bile, secreted from the gall bladder into the duodenum, is mostly reabsorbed in the distal ileum, with only approximately 5% passing into the colon [15]. The intestinal phase is also crucial in gastric emptying and protein digestion. Delayed gastric emptying in response to nutrients has been attributed to decreased gastric contractions and increased intestinal contractions [18].
In addition, a crucial step in the final hydrolysis of peptides is their breakdown into free amino acids, which occurs either at the intestinal brush border or within the cytoplasm of the intestinal mucosal cells. This process is facilitated by various peptidases, including brush-border enzymes such as aminopeptidases and dipeptidyl peptidases, as well as intracellular peptidases within enterocytes [22]. A key physiological observation is that dietary protein absorption occurs in two forms: as free amino acids and as peptides. Notably, peptide absorption is often considered a more efficient mechanism than the absorption of individual amino acids, as peptides can utilize specific transport systems, such as the PEPT1 transporter [23], which facilitates the uptake of dipeptides and tripeptides. Once inside the enterocytes, these peptides can be further hydrolyzed into free amino acids before being transported into the bloodstream [24]. However, although dipeptides or tripeptides undergo quick hydrolysis by brush-border peptidases, a significant proportion (approximately 30–50%) is absorbed directly in its peptide form without being fully broken down into free amino acids [22].
These residual proteins, peptides, and amino acids continue their transit through the digestive system and reach the colon via the ileocecal valve. Once in the colon, they may undergo microbial fermentation, leading to the production of short-chain fatty acids (SCFAs), ammonia, and other metabolites, which can influence gut microbiota composition, intestinal health, and systemic metabolism [15].
4. Protein Quality Evaluation Methods
The digestion, absorption, and potential health effects of proteins and amino acids in food are influenced by their type and nutritional quality. Dietary protein quality is determined by its amino acid composition and bioavailability, which is directly related to its digestibility [25,26], as defined by the Food and Agriculture Organization (FAO).
Evaluating protein quality involves assessing the extent to which the protein is digested and absorbed and its amino acids are utilized by the human body [9]. Nevertheless, such experiments are often costly, technically challenging, and time-consuming, and they may present ethical concerns. However, in vivo studies (in humans or animals) are considered the gold standard for evaluating the nutritional quality of the protein component of food [27,28].
One factor that distinguishes protein digestibility assessment methods is how they are conducted: in vivo, in vitro, or ex vivo. Moreover, the digestion of proteins can be evaluated through a wide range of techniques falling within these three categories of protein digestibility assessment (Figure 1).
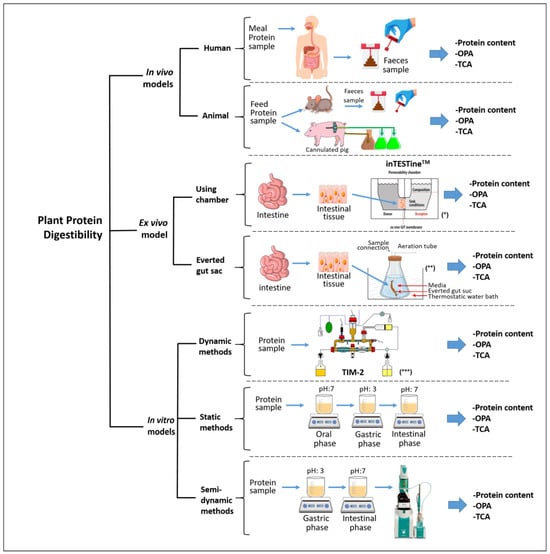
Figure 1.
Schematic representation of the methods for assessing plant protein digestibility. (*) adapted from Sitovs and Mohylyuk [29]; (**) adapted from Antal et al. [30]; (***) adapted from Rehman et al. [31].
4.1. In Vivo Models
In the assessment of protein digestibility, in vivo methods involve feeding trials on animals or humans. Animal models are critical in designing in vivo and ex vivo experimental studies [32]. Controlled feeding experiments with rodents, chickens, or pigs are preferred for obtaining accurate results [33]. Nonetheless, caution must be exercised when extrapolating findings and inferences from animal trials to humans, given the significant differences between the gastrointestinal tracts of the two groups.
The scientific community has widely employed animal models to investigate the impact of diet quality on health. Dietary intervention studies in animals are essential for understanding the biological functions of specific nutrients before human validation [32]. In the past century, rats were the most common model for biochemical research due to their simple ileal digestibility model. However, their popularity has decreased in the last decades due to limitations in performing reverse genetics in rats [34]. The key methodology used in these studies is the measurement of the digestive losses of nitrogen and amino acids [35]. However, to minimize the impact of colonic microbiota activity, it is important to assess nitrogen and amino acid losses in the ileum content rather than in the faeces, since amino acids are absorbed in the small intestine. Nonetheless, no standardized rat model method is currently available to evaluate ileal protein digestibility [36]. For instance, Wang et al. [37] evaluated the protein digestibility of microalgae species. For this study, the researchers used a rat model and fed rats with three different microalgae species (Chlorella vulgaris, Chlorella sorokiniana, and Acutodesmus obliquus) for 3 days. After that, faeces were collected from individual rats for 5 days, with a daily recording of food intake. The collected faeces were then pooled and stored at −80 °C for further analysis. The results revealed that the true protein digestibility of Chlorella vulgaris was 64.7%, 59.3% for Chlorella sorokiniana, and 37.9% for Acutodesmus obliquus.
In a recent study, Guillin et al. [34] investigated the digestibility of gluten and pea protein using the cecal digestibility technique in rats as an alternative to the ileal digestibility method. The rationale for this alternative is that the caecum of rodents is large and accumulates digesta from the small intestine before it enters the colon [38]. As a result, a considerable amount of dietary nitrogen and amino acid losses could be retained in the caecum after a sufficient digestion period, potentially eliminating the necessity for an indigestible marker [34]. In this study, the rats were first fed with a standard chow diet for six days to adapt to the experimental conditions. Following that, they were trained to consume a calibrated meal of 4 g dry weight containing 16 mg of Cr2O3 (10.9 mg of chromium) in a short time at the beginning of the dark period. After 10 days of habituation to the diets, the rats were given a calibrated meal of their respective diets and euthanized six hours later using gaseous anesthesia and decapitation. The study’s primary finding suggested that true caecal digestibility could be an adequate approximation of true ileal digestibility and eliminate the need for a non-absorbable marker, provided that the nitrogen amount in each digestive tract segment is known.
In an ideal scenario, the true ileal protein digestibility of foods should be determined in humans using techniques such as naso-ileal tube sampling [39]. However, due to practical and ethical limitations, this method is not recommended for routine studies. Alternatively, ileum-fistulated growing pigs are used as animal models, as recommended by Hodgkinson et al. [40]. Pigs are considered superior to rats as they are meal-eating animals and have a gastrointestinal anatomy like that of humans [41].
The use of animal models to estimate the digestibility of crude protein (CP) and amino acids (AA) in human foods is well established, with pigs being recognized as a suitable model for this purpose. This is supported by studies such as Mathai et al. [42] and the FAO, who have stated that the pig is a more appropriate model than the rat for determining the ileal digestibility of AA in food proteins [43].
Thus, the growing pig model is recognized as a reliable in vivo system for studying human Digestible Indispensable Amino Acid Score (DIAAS) values, rather than Protein Digestibility-Corrected Amino Acid Score (PDCAAS) values, utilizing substrates with predicted differences in digestibility. This validation was accomplished as part of the PROTEOS project, a continuous international scientific partnership led by the Global Dairy Platform and supported by a consortium of food industry companies and sectors. The project aims to establish a large data set of AA digestibility for human foods to implement the DIAAS measure [44,45].
4.1.1. Protein Digestibility-Corrected Amino Acid Score (PDCAAS)
For over three decades, the PDCAAS has been the primary system for evaluating the quality of proteins in human foods. The PDCAAS calculates a digestibility value using the overall digestibility of crude protein (CP) measured in rats. This value is then multiplied by the concentration of the primary limiting amino acid (AA), determined by contrasting the AA profile of a protein sample with the estimated AA requirements of young children. However, limitations of PDCAAS have been identified, as the total tract digestibility of CP may not accurately reflect AA digestibility, and PDCAAS assumes equal digestibility for all AAs [42,43].
Therefore, there is a growing need for more accurate methods for evaluating protein quality, which has led to the development of the Digestible Indispensable Amino Acid Score (DIAAS) method. The DIAAS method is recommended by the Food and Agriculture Organization of the United Nations (FAO) as an alternative to PDCAAS, and it overcomes the limitations of the PDCAAS method by considering the true ileal digestibility of AA, rather than total tract digestibility of CP, and using a more accurate estimation of the AA requirements for humans [46,47].
Although PDCAAS was previously considered the “gold standard” for protein quality determination, the DIAAS method has emerged as a more reliable alternative for evaluating protein quality in human foods.
4.1.2. Digestibility Indispensable Amino Acid Score (DIAAS)
In 2013, the Food and Agricultural Organization of the United Nations/World Health Organization (FAO/WHO) proposed the DIAAS as an alternative to the PDCAAS for evaluating protein quality in human foods. Unlike PDCAAS, which is based on the total tract digestibility of crude protein (CP) in rats and assumes equal digestibility for all amino acids (AAs), DIAAS is based on the true ileal digestibility of each indispensable AA. As a result, the DIAAS method provides a more accurate evaluation of protein quality [44]. Since its recommendation by the FAO/WHO, DIAAS has replaced the previous PDCAAS method in evaluating protein quality in human foods. To calculate the DIAAS for protein quality evaluation, it is necessary to determine the true ileal digestibility of individual AAs, which can be accomplished by surgically inserting a T-cannula into the end of the small intestine in pigs. The pig has been recognized as an appropriate model for estimating crude protein (CP) and AA digestibility in foods for humans, and this method has been widely adopted to determine the ileal digestibility of AAs associated with various foods and food ingredients [41,42]. Thus, the surgical insertion of a T-cannula is an effective approach used globally to measure AA digestibility in pigs.
The direct determination of the digestibility of each AA in the small intestine is feasible by collecting digest at its terminal end, which enables the application of specific digestibility to each AA. Conversely, faecal collections used in the PDCAAS method only permit the estimation of crude protein digestibility, which is then uniformly applied to all AAs under the assumption that the digestibility of all AAs is equal. However, this assumption is incorrect [42]. Therefore, the DIAAS method is preferred as it allows for determining the true ileal digestibility of each AA, providing a more accurate assessment of protein quality. Therefore, the DIAAS methodology has emerged as a superior alternative to the PDCAAS method for evaluating protein quality. It considers the individual ileal digestibility of AAs, with pigs being preferred models over rats. Unlike the PDCAAS method, which calculates the digestibility of CP and applies it to all AAs, DIAAS applies unique digestibility values to each AA. Furthermore, reactive digestible lysine, instead of total digestible lysine, is recommended for determining the DIAAS of processed and cooked foods [47]. The DIAAS value determines the quality of the protein, which is classified as <75 (no statement regarding quality), 75–99 (premium protein), and ≥100 (superior-quality protein) [41]. The FAO has revised the age-specific AA reference scoring patterns and recommends classifying the population into three distinct age groups: 0–6 months (infants), 0.5–3 years (children), and individuals older than 3 years (the remaining population) [47].
Table 1 summarizes the protein digestibility of various plant protein sources obtained by in vivo methods.

Table 1.
Protein digestibility of different plant protein sources measured using in vivo models.
4.2. Ex Vivo Models
In vitro absorption models range from basic membrane-based systems to more advanced cell-based models, while ex vivo models utilize intestinal tissues. Still, their viability time is limited to the typical intestinal transit time [15]. To overcome this limitation, ex vivo models employ gastrointestinal fluids from humans as digestive agents rather than using commercially available enzymes derived from animals. The enzymes in juice can differ in activity or specificity from commercial enzymes as they are composed of different isoforms. Human gastric juice (HGJ) contains pepsin and gastric lipase, while human duodenal juice (HDJ) consists of proteolytic and lipolytic enzymes, different inhibitors, and bile salts. To reduce inter-individual variability related to enzymes and other juice components, the use of gastrointestinal juice pools is suggested. Therefore, ex vivo models have the potential to accurately replicate human digestion by incorporating juices that contain various enzymes, inhibitors, and salts.
Ex vivo absorption methods involve using animal or human gut segments, which provide a more accurate representation of the intestinal wall’s morphological and physiological features. This includes the presence of all relevant cell types and architecture and a mucus layer that allows for the simulation of possible further hydrolysis by brush border membrane enzymes. As a result, ex vivo absorption models are a more realistic representation of in vivo absorption compared to in vitro models [80,81].
Ex vivo models of intestinal absorption offer a comprehensive assessment of the morphological and physiological characteristics of the intestinal wall, including the presence of all relevant cell types, the architecture, and the mucus layer, enabling the investigation of nutrient or bioactive compound transport, permeability, absorption, and mucus layer interactions [80]. The approach’s limitation is the tissue’s limited viability, usually between 1 and 3 h, depending on the presence or absence of muscle layers. Intestinal rings or segments are among the simpler techniques, where the segment of interest is immersed in an oxygenated buffer containing the compound. However, the lack of isolation between the apical and basolateral sides results in the accumulation of the compound in enterocytes rather than transport. To separate the two compartments, the everted sac model is employed by suturing the segment on one end and introducing the compound under study before suturing the open end and immersing it in a physiological solution [15]. An alternative approach is to mount the tissue in an Ussing Chamber by cutting the intestinal segment longitudinally and pinning it to a frame that divides the two semi-chambers. Nevertheless, few studies have utilized ex vivo methods to investigate the digestibility of plant proteins [82,83]. While human intervention studies remain the gold standard for evaluating protein digestion and nutritional value, they are time-consuming, costly, and challenging to control. Therefore, in vitro methods are valuable in guiding protein selection for in vivo models and reducing costs in identifying suitable alternative proteins.
4.3. In Vitro Models
In vitro simulations of human digestion have become a widely used alternative to in vivo methods, given their advantages of being less labor-intensive, more cost-effective, faster, and ethically unrestricted [15]. However, differences in operation and experimental parameters among various in vitro methods have resulted in significant variations in measured digestibility, thereby complicating the comparison of results.
Moreover, accurately replicating the complex and dynamic process of human digestion in laboratory experiments presents a challenge, and caution must be taken when interpreting the results of in vitro methods for measuring and assessing plant protein digestibility. The following section summarizes the various techniques used in such experiments.
4.3.1. Dynamic Methods
Beyond biochemical factors, dynamic models also strive to replicate the physiological processes of digestion, including peristalsis, gastric emptying, nutrient absorption, and gastrointestinal transit [84].
Several dynamic models have demonstrated their suitability for simulating the digestion of various food and pharmaceutical products across diverse population groups and research objectives [28]. Mono- and multicompartmental systems have been utilized within these dynamic models to investigate protein digestion. Within these models, the conditions within each compartment are modified over time to accurately simulate the in vivo digestion processes. Dynamic digestion models include specific digestive secretions at designated time points into the respective compartments of the model [33]. The addition of substances can be regulated through various methods in gastrointestinal simulation models. One approach involves maintaining a stable secretion rate, like the simulated gastric juice in the human gastric simulator. Alternatively, a pre-programmed pattern can be employed, allowing the secretion rate to vary over time, as demonstrated in the TIM-1 model [85]. The TIM-1 is one of the main dynamic models designed, and was developed by the Netherlands Organization for Applied Scientific Research (TNO). TIM-1 was designed to replicate the dynamic conditions in the upper gastrointestinal tract. This model accurately mimics the peristaltic mixing of food with digestion fluids that possess physiological relevance, while maintaining controlled pH values and realistic transit times like those observed in the human gastrointestinal system [86]. The TIM-1 consists of four compartments designed to replicate the physiological conditions of the stomach, duodenum, jejunum, and ileum. This model accurately reproduces dynamic processes, including peristalsis, gastric emptying, and nutrient absorption within the jejunum and ileum [87]. Furthermore, the secretion rate can be programmed to respond to other parameters, such as the volume of the model, as observed in the dynamic gastric model proposed by Thuenemann et al. [88]. However, a newer version of this equipment, referred to as the TIM-2 model, is currently available.
4.3.2. Static Methods
Static and dynamic models are the two primary types of in vitro digestion methods that are typically utilized. In vitro digestion methods are frequently employed to investigate food behavior under simulated upper gastrointestinal tract physiological conditions, encompassing the oral, gastric, and small intestinal phases [89].
Dynamic models have been demonstrated as effective tools for simulating food and pharmaceutical product digestion across various groups and purposes [28]. However, their complexity and the cost of implementation and maintenance limit access to these models for many food researchers. In contrast, static in vitro digestion models have shown great potential in predicting in vivo digestion outcomes [9,90], making them valuable tools in the field.
Static models have been extensively employed for food, animal feed, and pharmaceutical purposes due to their simplicity. The static in vitro models offer several significant advantages, including excellent outstanding intra- and inter-laboratory repeatability, sturdiness, easiness, low cost, and the ability to easily assess each phase of digestion. Static models employ a fixed ratio of food, enzymes, and electrolytes, as well as a consistent pH corresponding to each stage of digestion. In vitro digestion models have demonstrated significant efficacy in predicting in vivo digestion outcomes [89]. Standardized static models of varying complexity are available to simulate the gastrointestinal performance of pharmaceutical products, as outlined in United States Pharmacopeia protocols [9]. However, various static models exhibit different modifications in variables such as pH, time, the activity and concentration of enzyme, and simulated digestive fluid composition. To address the issue of diverse static models with varying parameters, the international INFOGEST network of multidisciplinary experts was established. Their first goal was to establish a standardized set of digestion parameters for static in vitro simulation of adult digestion, suitable for food. By doing so, INFOGEST aimed to harmonize these methods and facilitate their use in food and nutritional research [91].
For static digestion methods, foods are exposed to three consecutive phases, oral, gastric, and intestinal, with experimental conditions remaining constant throughout each phase. During the oral phase, the food is diluted 1:1 (w/w) with simulated salivary fluid (SSF), with or without the inclusion of salivary amylase. For solids or semisolids, simulated mastication of the food is also performed [92]. If salivary amylase is used, the food is exposed to it for a maximum of 2 min at pH 7. The oral phase is a mandatory component of all simulated digestion procedures, irrespective of the food’s state (liquid or solid), to ensure consistent dilution. This phase plays a vital role in the initial breakdown of food and the hydrolysis of macronutrients, especially carbohydrates, by salivary enzymes, thereby influencing the subsequent phases of protein digestion.
After completion of the oral phase, the resulting bolus is diluted with simulated gastric fluid (SGF) and gastric enzymes, including pepsin and gastric lipase, in a 1:1 (v/v) ratio. The mixture is then subjected to agitation and incubated at pH 3.0 for 2 h to simulate the gastric phase of protein digestion. Subsequently, the gastric chyme is diluted into simulated intestinal fluid (SIF), bile salts, and pancreatic enzymes (pancreatin, based on trypsin activity or each enzyme) in a 1:1 (v/v) ratio, followed by incubation at pH 7 for another 2 h to mimic the intestinal phase of protein digestion [93].
The final step in protein digestion includes the sampling, treatment, storage, and analysis of the digested samples. It is crucial to carefully consider this step before commencing digestion as it may vary depending on the purpose of the experiment, the type of analysis, and the desired endpoints [89]. The proper planning and execution of the sampling and storage procedures are essential to ensure the integrity and reliability of the data obtained from the simulated digestion experiment.
4.3.3. Semi-Dynamic Methods
Static methods have limitations in assessing crucial dynamic processes involved in gastrointestinal digestion, such as pH gradients, gradual enzyme and gastric fluid addition, and continuous gastric emptying [94]. In contrast, dynamic models offer a more physiologically relevant approach by incorporating these factors and others. However, these models are highly complex, requiring significant hardware and software resources, and are generally expensive to implement and not easily accessible [89,95]. Therefore, semi-dynamic models arise as a more accessible option for protein digestibility essays. Semi-dynamic methods combine elements of both in vitro and in vivo methods to measure protein digestibility. Thus, these methods simulate the in vivo digestive process by using enzymes and conditions that mimic the physiological environment of the stomach and small intestine.
Researchers have shown increasing interest in semi-dynamic in vitro models in recent years. These models have gained attention due to their unique capability to enable researchers to strategically select and incorporate specific dynamic factors during different phases of the gastrointestinal tract (GI) [96]. By allowing the deliberate inclusion of chosen dynamic elements, such as pH gradients, enzyme and gastric fluid addition, and gastric emptying, semi-dynamic models offer researchers the ability to create a more targeted and physiologically relevant representation of the digestion process. This approach has emerged as a valuable tool in bridging the gap between static and fully dynamic models, providing a flexible and adaptable platform for investigating GI digestion under controlled laboratory conditions.
A recent development in the field involves the creation of a cost-effective semi-dynamic method. This method utilizes parameters derived from in vivo data on dairy product digestion to establish a more realistic and physiologically relevant representation of the process [95]. By incorporating equivalent in vivo data, this approach enables researchers to capture key dynamic factors involved in the digestion of dairy products, while maintaining a more affordable and accessible experimental setup. However, there are considerations about semi-dynamic methods that should be considered. For instance, these methods do not account for the impact of microbial metabolism, which may affect protein digestibility. Additionally, semi-dynamic methods are unsuitable for measuring protein digestibility in infants, as they have a different digestive physiology than adults. However, there are several considerations related to the food matrix, composition, and human and animal physiology before deciding which model to use for conducting a protein digestibility assay [33].
Table 2 summarizes the protein digestibility of various plant protein sources obtained by in vitro methods.

Table 2.
Protein digestibility of different plant protein sources measured using in vitro models.
5. Antinutritional Factors and Reduction Strategies
A range of factors can affect protein digestibility, including the formation of protein conjugates, such as polysaccharides and polyphenols, which can lead to structural modifications that hinder enzymatic hydrolysis, as well as the presence of antinutritional compounds.
Antinutritional factors (ANFs), also known as anti-nutrients, are naturally occurring compounds found in plant sources that interfere with the absorption or utilization of nutrients in the body. Antinutritional factors present in plant sources have been shown to impede the process of protein digestion and elevate endogenous nitrogen losses in faeces, resulting in reduced protein digestibility and heightened protein requirements [3]. Numerous studies have shown the negative impact of these compounds on protein digestibility, with some indicating their susceptibility to heat degradation, while others suggest their resistance to heat-induced changes [108,109]. The critical challenges in the plant protein field encompass the quest for plant sources that exhibit nutritional profiles comparable to animal-based proteins, and the exploration and refinement of innovative food processing methodologies aimed at augmenting the nutritional attributes of conventional plant protein sources [3]. Hence, it is imperative to mitigate or eradicate these substances to enhance the biological utilization of plant proteins, which typically exhibit lower digestibility (75–80%) in comparison to animal proteins (90–95%) such as meat, poultry, eggs, and milk [3]. Furthermore, it should be noted that plant proteins encounter reduced enzyme accessibility due to the presence of rigid cell walls and seed coats [110].
The major ANFs found in consumable crops involve amylase inhibitors, protease inhibitors, phytic acids, tannins, saponins, lectins, gossypol, and goitrogens [111]. These compounds negatively affect the protein and AA digestibility [112,113] by decreasing bioavailability and disrupting metabolic processes, leading to harmful effects on the gastrointestinal tract’s physiology [114,115,116,117]. Among the ANFs, phytate is one of the major compounds present in many cereals, legumes, and oilseeds [118]. Phytate is a chelating agent and primarily impacts the bioavailability of micronutrients such as calcium, cooper, zinc, and iron [119]. Thus, high phytate intake can lead to mineral deficiencies over time. Nevertheless, phytochemicals, including phenolic compounds, have demonstrated notable antioxidant activity by effectively scavenging reactive oxygen species (ROS), exhibiting strong reducing power, and displaying metal-chelating capabilities towards ferric and ferrous ions [111]. Although not all polyphenols possess chelating properties, certain phytochemicals, such as isoflavones, genistein, and biochanin A, are particularly valued as antioxidants due to their dual attributes as reducing agents and metal ion-chelating agents [120]. Regarding proteins, phytate interferes with protein digestibility by competing for essential mineral cofactors required to activate peptidases. Additionally, phytate directly interacts with the protein, further affecting its digestibility [121].
In addition, protease inhibitors such as trypsin and chymotrypsin are other ANFs found in legumes such as soybeans and kidney beans. These ANFs can impact the globular structure of proteins, hindering digestive enzyme activity in the small intestine [3]. By interfering with the digestion process, protease inhibitors inhibit the enzymes responsible for protein breakdown, potentially resulting in reduced protein digestibility.
Haemagglutinins such as lectins are other ANFs that are proteins that bind to carbohydrates. Lectins are found in various plant sources, including legumes, grains, cereals, and certain vegetables, but are mainly present in legumes. Lectins can cause the agglutination of red blood cells by reversibly binding to specific mono-oligosaccharides and oligosaccharides found in glycoproteins and glycolipids [122]. Lectins in legumes can interfere with the absorption of nutrients and crucial minerals like zinc, calcium, phosphorus, and iron. Therefore, lectins may interact with intestinal epithelial cells, modifying intestinal permeability and causing hypertrophy and hyperplasia [123]. Thus, lectins can cause damage to the lining of the digestive tract, mainly in the small intestine, potentially causing a decrease in protein digestibility.
On the other hand, oxalates are ANFs found in certain foods such as spinach, rhubarb, and beet greens [122]. They exhibit potent acidic properties and can form water-soluble salts by binding with minerals like sodium or potassium and water-insoluble salts by binding to calcium, iron, or zinc [124]. Consequently, in susceptible individuals, this can lead to the formation of kidney stones. In addition, the results of Dogra [125] on buckwheat suggest a negative correlation between protein digestibility and oxalate content, which shows that a higher content of oxalates leads to lower protein digestibility.
Tannins are a polyphenol commonly found in tea, coffee, fruits, and certain vegetables. These compounds are categorized as ANFs because of their capacity to hinder the absorption of essential minerals, particularly iron, and can result in digestive disturbances when consumed in excessive quantities [126]. Furthermore, in conjunction with phytic acid, tannins are widely recognized ANFs for their significant interaction with proteins, reducing their digestibility [127].
It is important to note that many of these ANFs may also exert some beneficial properties [120,128,129]. However, the beneficial effects of ANFs depend on molar ratios between nutrients and antinutrients [11]. For instance, phytates can act as antioxidants and have anticancer properties [130]. In addition, new studies have shown the possible beneficial effects of these compounds on the risk of osteoporosis or age-related cardiovascular calcifications [131,132].
However, the negative effects of antinutritional factors can often be minimized through various processing methods, such as soaking, fermentation, cooking, or heat treatment, which can either reduce their levels or deactivate them.
6. Strategies for Improving Protein Digestibility
Protein digestibility is a crucial factor that determines the bioavailability of amino acids, which are essential for human health. Low digestibility can result from various factors, including anti-nutritional compounds, the structural complexity of proteins, and interactions with other food components. Several strategies have been developed to address these challenges to enhance protein digestibility, each with unique benefits and applications.
6.1. Thermal Processing
Numerous studies have focused on improving plant protein quality through food processing techniques, particularly thermal techniques. These methods aim to enhance plant-based protein sources’ nutritional, functional, and sensory properties. Some of the common thermal processing techniques include cooking [110,116], autoclaving [133], microwave [134], and extrusion [135]. Heat denatures protein structures, making them more accessible to digestive enzymes. Additionally, thermal processing can reduce or eliminate anti-nutritional factors such as trypsin inhibitors and tannins, which otherwise hinder protein digestion. However, excessive heating should be avoided, as it may lead to the formation of undesirable compounds like Maillard reaction products, which can reduce protein quality. For instance, in vitro and in vivo studies have demonstrated that wet sorghum cooking significantly decreases its protein digestibility [136].
In contrast, this effect is not observed in maize, where cooking has only a minimal impact on protein digestibility [137]. The reduced digestibility of sorghum protein is primarily attributed to the formation of enzymatically resistant protein polymers during cooking, caused by disulfide bonding between β and γ-kafirins and matrix proteins [136]. These disulfide crosslinks limit the access of proteolytic enzymes to the more abundant and digestible α-kafirin found at the core of the protein body, which may explain the observed reduction in digestibility [138].
Autoclaving improves the protein digestibility of beans by reducing antinutritional factors and modifying the structural properties of the seeds [139]. The process significantly decreases the content of trypsin inhibitors, which are known to interfere with protein digestion, thus enhancing the accessibility of proteins to digestive enzymes. In addition, autoclaving enhances the protein digestibility of soybeans by effectively inactivating antinutritional factors such as trypsin inhibitors, which interfere with enzymatic protein breakdown [107]. The high temperature and pressure conditions during autoclaving denature the proteins, making their structure more accessible to digestive enzymes. This process also reduces the presence of other compounds, like lectins, that impair nutrient absorption, thereby increasing protein bioavailability. As a result, autoclaving transforms soybeans into a more digestible and nutritionally valuable food ingredient, suitable for direct consumption and use in various food formulations.
Microwave treatment can enhance protein digestibility because it modifies enzyme function, leading to improved protein–protease interactions. Microwaves influence enzyme activity through four key mechanisms [134]. Microwave treatment enhances protein digestibility through several mechanisms: (1) It denatures thermally unstable enzymes, rendering them inactive; (2) it induces molecular rearrangement and protein unfolding, making specific regions of the protein more accessible to enzymatic hydrolysis; (3) it reduces the particle size of treated protein, increasing surface area and exposing more cleavage sites for protease activity; and (4) it promotes the development of proteins with a high zeta potential, stabilizing the protein suspension, preventing aggregation in water, and increasing the availability of sites for protein–protease interaction [140,141].
A study on the effects of extrusion demonstrated that this processing method can significantly improve protein digestibility by altering both the secondary and tertiary structures of plant proteins [142]. Extrusion reduces the α-helix/β-sheet ratio in the protein’s secondary structure, decreasing disulfide bonds and hydrophobic interactions in the tertiary structure. These structural modifications make the proteins more accessible to digestive enzymes, promoting their digestion and absorption. De la Rosa-Millán et al. [143] reported that low-moisture (LM) extrusion increased the in vitro digestibility of soybean protein by modifying its secondary structure. Similarly, Lin et al. [144] showed that LM extrusion enhanced the soluble nitrogen content of wheat protein digestion by affecting its chemical bonds, highlighting extrusion’s ability to improve protein bioavailability.
6.2. Enzymatic Hydrolysis
Enzymatic hydrolysis involves breaking down proteins into smaller peptides and amino acids using proteolytic enzymes. This approach improves digestibility and enhances the bio-functional properties of proteins, such as antioxidant or antihypertensive activities. Enzymatic hydrolysis is commonly employed in producing protein hydrolysates for specialized applications, such as infant formulas, medical nutrition, and sports supplements.
Enzymatic hydrolysis enhances protein digestibility by breaking down high-molecular-weight proteins into smaller peptides and amino acids, making them more accessible to digestive enzymes. A study demonstrated that limited hydrolysis using papain significantly reduced the molecular weight of a soy protein isolate and corn zein mixture while increasing the proportion of random coil structures [145]. These structural modifications facilitated protein breakdown, resulting in a remarkable improvement in in vitro digestibility. Kamiloglu et al. [146] indicated that enzymatic hydrolysis significantly improves the digestibility of plant proteins by breaking down large, complex protein structures into smaller peptides and free amino acids, making them more accessible to digestive enzymes in the gastrointestinal tract. This process also eliminates antinutritional factors, such as phytate, that form insoluble complexes with proteins and minerals, otherwise hindering enzymatic degradation and nutrient absorption [3]. By degrading cell walls and disrupting protein–phytate complexes, hydrolysis enhances the bioavailability of vital nutrients like calcium and improves the overall nutritional value of plant proteins [147]. In addition, Beaubier et al. [148] showed in a study on rapeseed that enzymatic hydrolysis significantly enhances protein digestibility by breaking down complex protein structures into smaller peptides and amino acids, making them more accessible to digestive enzymes. The study demonstrated that proteolysis improves digestibility and enhances functional properties. As a result, hydrolyzed plant proteins offer better digestibility, addressing one of the primary limitations of plant-based diets.
6.3. Fermentation
Fermentation is a natural process that uses microorganisms to modify the structure and properties of proteins [108]. Microbial enzymes break down complex proteins and reduce the levels of anti-nutritional factors, improving the digestibility of pulses by lowering the concentration of non-nutritive compounds that interfere with digestive enzymes, such as trypsin and chymotrypsin inhibitors [149]. Additionally, it reduces the presence of compounds like phenolics and tannins, which contribute to protein crosslinking. Furthermore, fermentation promotes the production of microbial proteases, which partially break down proteins and release them from the matrix [150]. For instance, a study on the fermentation of soybeans showed that this process significantly enhances protein digestibility by reducing antinutritional factors and breaking down structural barriers that hinder enzymatic activity [151]. As digestion progressed, fermented soybeans exhibited the highest protein digestibility, suggesting that microbial activity facilitated protein breakdown and improved bioaccessibility. The biochemical changes induced by fermentation likely promoted better interaction between digestive enzymes and protein substrates, leading to a more efficient digestion process. In addition, the fermentation of pea flour showed that this process significantly enhances protein digestibility while also improving the bioaccessibility of essential minerals such as manganese (Mn) and iron (Fe) [152].
6.4. Physical Treatments
Emerging physical methods, such as high-pressure processing (HPP), ultrasonication, irradiation, and pulsed electric field (PEF), have gained attention for their ability to improve protein digestibility. These techniques disrupt protein structures without using high temperatures, making them ideal for heat-sensitive proteins. This method can unfold proteins and increase enzyme accessibility [153], while ultrasonication breaks protein aggregates into smaller, more digestible fragments. For instance, the high-pressure processing (HPP) of pea protein concentrate (PPC) showed that this treatment enhances gastric proteolysis by inducing structural modifications in proteins [154]. HPP-treated PPC underwent greater proteolysis and exhibited distinct peptide patterns compared to untreated and heat-treated samples. In addition, a study on lupin proteins demonstrated that this treatment could enhance their in vitro digestibility, particularly at higher pressures [155].
High-intensity ultrasound processing significantly enhances the digestibility of plant proteins by inducing physical and chemical modifications. The fluctuating pressure waves generated during ultrasonication disrupt protein aggregates, alter structural configurations, and expose hydrolytic sites, making proteins more accessible to digestive enzymes [156]. This technology can modify proteins’ primary, secondary, tertiary, and quaternary structures, facilitating their enzymatic breakdown [157]. Additionally, ultrasonication helps release proteins from plant matrices and disrupts antinutritional factors that inhibit digestion [158]. By improving the extraction, functionality, and bioavailability of plant proteins, ultrasound processing boosts their digestibility and enhances their overall nutritional profile [157], making it a promising tool for developing plant-based food products.
Irradiation is a modification technique that uses ionizing radiation, such as gamma rays, X-rays, or accelerated electrons, to alter the properties of food ingredients. Irradiation can enhance the digestibility of plant-based proteins by addressing some of their inherent limitations, such as poor aqueous solubility and the presence of anti-nutritional factors like hemicellulose, lignin, and other poorly digestible polysaccharides [159]. By applying ionizing radiation, proteins can be denatured [160], altering their structure to improve their accessibility to digestive enzymes, thus increasing their bioavailability. This process can also reduce the presence of antinutrients, such as lectins and protease inhibitors, which hinder protein digestion and absorption [161]. Additionally, irradiation can enhance the solubility of plant proteins, making them more easily dispersible in aqueous environments, which is crucial for improving their functionality and digestibility [159].
Pulsed electric field (PEF) processing is a non-thermal technology that applies short bursts of high voltage to food products, leading to structural modifications in proteins. In soy proteins, PEF treatment can cause the unfolding of protein molecules and the dissociation of subunits, exposing hydrophobic and thiol groups previously buried within the protein structure [162]. These structural changes can enhance the accessibility of proteolytic enzymes during digestion, thereby improving protein digestibility.
6.5. Soaking and Germination
Soaking and germination are traditional methods often used for legumes and grains. These processes activate endogenous enzymes that degrade antinutritional factors and partially hydrolyze proteins. Germination also increases the bioavailability of essential nutrients, including amino acids, while enhancing the overall digestibility of proteins. Some studies have shown that soaking increases nutritional properties like protein digestibility and decreases antinutrients such as trypsin inhibitors, oligosaccharides, and phytic acid [163]. Gu et al. [164] indicated that soaking is a food processing method that improves legumes’ protein digestibility by reducing antinutritional factors, such as phytates, which inhibit protein breakdown. By hydrating the legumes, soaking helps soften cell walls, making proteins more accessible to digestive enzymes and, thereby, enhancing bioavailability [165]. While soaking generally enhances protein digestibility, the effectiveness can vary depending on the legume type and other processing methods.
Germination improves legume protein digestibility by reducing antinutritional factors like protease inhibitors, activating proteases, and breaking down storage proteins, including allergens [166]. This process increases free amino acids, particularly asparagine. The extent of digestibility improvement varies by species and strain, and further research is needed to identify the key factors. In addition, Di et al. [167] indicated that germination can improve sesame protein’s structural and functional properties. Therefore, germination is an effective approach to improve the protein digestibility of plant seed protein.
6.6. Combination Approaches
Combining multiple strategies, such as enzymatic hydrolysis with fermentation or thermal processing with soaking, can further enhance protein digestibility. These synergies can effectively address the limitations of individual techniques, leading to improved protein quality and functionality. Bera et al. [166] indicated that combining germination with other treatments, such as heating or fermentation, could further enhance digestibility. Also, soaking following cooking treatments significantly decreased phytic acid, tannins, and lectin activity, which significantly increased the protein digestibility of raw bitter and sweet lupin seeds [168].
A study demonstrated that enzymatic hydrolysis combined with short-time fermentation effectively improves the protein digestibility of soybean meal [169]. Using alcalase after fermentation with microorganisms such as Lactobacillus plantarum, Bacillus subtilis, and Aspergillus oryzae significantly increased peptides with molecular weights below 10 kDa, indicative of enhanced proteolysis.
Therefore, improving protein digestibility requires a multifaceted approach tailored to the specific protein source and application. By leveraging advanced technologies and traditional methods, it is possible to enhance proteins’ nutritional value and functional properties, meeting the demands of diverse consumer needs.
7. Final Considerations
The digestibility and quality of plant-based proteins are fundamental for developing nutritious and sustainable foods. However, plant proteins face significant challenges, such as reduced digestibility, incomplete essential amino acid profiles, and the presence of antinutritional factors. To overcome these limitations, innovative and multidisciplinary strategies have been developed, including advanced evaluation methods and the application of emerging food processing technologies.
This review highlights that while traditional methods like PDCAAS have been valuable in the past, more accurate approaches such as DIAAS allow for a better assessment of protein quality by considering the true ileal digestibility of each essential amino acid. Additionally, dynamic and semi-dynamic models achieve a balance between complexity and physiological relevance, positioning them as key tools for future research on protein digestibility.
Finally, implementing thermal treatments, fermentation, enzymatic hydrolysis, and physical technologies such as high-pressure processing have proven effective in improving amino acid bioavailability and reducing antinutritional factors. These strategies, combined with advancements in evaluation models and a deeper understanding of proteins’ technological and nutritional properties, pave the way for designing plant protein ingredients capable of meeting both nutritional requirements and consumers’ sustainability expectations.
Author Contributions
Conceptualization, M.O.-N., C.B.-D. and C.B.-R.; investigation, M.O.-N., C.B.-D., C.B.-R. and I.G.-P.; writing—original draft preparation, M.O.-N., C.B.-D., C.B.-R., I.G.-P. and M.C.-F.; writing—review and editing, M.O.-N., M.C.-F., J.E.R. and L.M.; visualization, C.B.-R. and M.C.-F.; supervision, M.O.-N. and C.B.-D.; funding acquisition, M.O.-N. and C.B.-D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by ANID (Agencia Nacional de Investigación y Desarrollo, Chile) through FONDECYT-INICIACIOÓN project N° 11230612, FONDECYT-INICIACIÓN project N° 11240860, and FONDECYT-REGULAR project N° 1240824.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to legal reasons.
Acknowledgments
Authors (M.O.N., C.B.D., C.B.R., and I.G.F.) are members of the AlProSos network (sustainable regional protein-rich foods), funded by CYTED (ref. 125RT0165).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Day, L.; Cakebread, J.A.; Loveday, S.M. Food proteins from animals and plants: Differences in the nutritional and functional properties. Trends Food Sci. Technol. 2022, 119, 428–442. [Google Scholar] [CrossRef]
- Langyan, S.; Yadava, P.; Khan, F.N.; Dar, Z.A.; Singh, R.; Kumar, A. Sustaining protein nutrition through plant-based foods. Front. Nutr. 2022, 8, 772573. [Google Scholar]
- Sá, A.G.A.; Moreno, Y.M.F.; Carciofi, B.A.M. Food processing for the improvement of plant proteins digestibility. Crit. Rev. Food Sci. Nutr. 2020, 60, 3367–3386. [Google Scholar] [PubMed]
- Ma, K.K.; Greis, M.; Lu, J.; Nolden, A.A.; McClements, D.J.; Kinchla, A. Functional performance of plant proteins. Foods 2022, 11, 594. [Google Scholar] [CrossRef]
- Burgos-Díaz, C.; Opazo-Navarrete, M.; Wandersleben, T.; Soto-Añual, M.; Barahona, T.; Bustamante, M. Chemical and nutritional evaluation of protein-rich ingredients obtained through a technological process from yellow lupin seeds (Lupinus luteus). Plant Foods Hum. Nutr. 2019, 74, 508–517. [Google Scholar]
- Akhrume, F.U.; Aluko, R.E.; Adedeji, A.A. Modification of plant proteins for improved functionality: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 198–224. [Google Scholar] [CrossRef] [PubMed]
- Pencharz, P.B.; Elango, R.; Wolfe, R.R. Recent developments in understanding protein needs—How much and what kind should we eat? Appl. Physiol. Nutr. Metab. 2016, 41, 577–580. [Google Scholar] [PubMed]
- Han, S.; Chee, K.; Cho, S. Nutritional quality of rice bran protein in comparison to animal and vegetable protein. Food Chem. 2015, 172, 766–769. [Google Scholar]
- Bohn, T.; Carriere, F.; Day, L.; Deglaire, A.; Egger, L.; Freitas, D.; Golding, M.; Le Feunteun, S.; Macierzanka, A.; Menard, O.; et al. Correlation between in vitro and in vivo data on food digestion. What can we predict with static in vitro digestion models? Crit. Rev. Food Sci. Nutr. 2018, 58, 2239–2261. [Google Scholar] [CrossRef]
- Dhull, S.B.; Kinabo, J.; Uebersax, M.A. Nutrient profile and effect of processing methods on the composition and functional properties of lentils (Lens culinaris Medik): A review. Legume Sci. 2022, 5, e156. [Google Scholar] [CrossRef]
- Nath, H.; Samtiya, M.; Dhewa, T. Beneficial attributes and adverse effects of major plant-based foods anti-nutrients on health: A review. Nutr. Metab. 2022, 28, 200147. [Google Scholar]
- Aguilar, E.G.; Albarracín, G.J.; Uñates, M.A.; Piola, H.D.; Camiña, J.M.; Escudero, N.L. Evaluation of the nutritional quality of the grain protein of new amaranths varieties. Plant Foods Hum. Nutr. 2015, 70, 21–26. [Google Scholar] [PubMed]
- Arribas, C.; Cabellos, B.; Sánchez, C.; Cuadrado, C.; Guillamón, E.; Pedrosa, M. The impact of extrusion on the nutritional composition, dietary fiber and in vitro digestibility of gluten-free snacks based on rice, pea and carob flour blends. Food Funct. 2017, 8, 3654–3663. [Google Scholar] [CrossRef]
- Avila Ruiz, G.; Opazo-Navarrete, M.; Meurs, M.; Minor, M.; Sala, G.; van Boekel, M.; Stieger, M.; Janssen, A.E.M. Denaturation and in vitro gastric digestion of heat-treated quinoa protein isolates obtained at various extraction pH. Food Biophys. 2019, 11, 184–197. [Google Scholar]
- Mackie, A.; Mulet-Cabero, A.-I.; Torcello-Gómez, A. Simulating human digestion: Developing our knowledge to create healthier and more sustainable foods. Food Funct. 2020, 11, 9397–9431. [Google Scholar]
- Mulet-Cabero, A.-I.; Mackie, A.R.; Brodkorb, A.; Wilde, P.J. Dairy structures and physiological responses: A matter of gastric digestion. Crit. Rev. Food Sci. Nutr. 2022, 60, 3737–3752. [Google Scholar]
- Wang, C.; Zhao, F.; Bai, Y.; Li, C.; Xu, X.; Kristiansen, K.; Zhou, G. Effect of gastrointestinal alterations mimicking elderly conditions on in vitro digestion of meat and soy proteins. Food Chem. 2022, 383, 132465. [Google Scholar]
- Goyal, R.K.; Guo, Y.; Mashimo, H. Advances in the physiology of gastric emptying. Neurogastroenterol. Motil. 2018, 31, e135446. [Google Scholar]
- McQuilken, S.A. The mouth, stomach and intestines. Anaesth. Intensive Care 2023, 22, 330–335. [Google Scholar]
- Verhoeckx, K.; Cotter, P.; López-Espósito, I.; Kleiveland, C.; Lea, T.; Mackie, A.; Requena, T.; Swiatecka, D.; Wichers, H. Dynamic gastric model (DGM). In The Impact of Food Bioactives on Health In Vitro and Ex Vivo Models; Verhoeckx, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; p. 338. [Google Scholar]
- Fan, P.; Li, L.; Rezaei, A.; Eslamfam, S.; Che, D.; Ma, X. Metabolites of dietary protein and peptides by intestinal microbes and their impacts on gut. Curr. Protein Pept. Sci. 2015, 16, 646–654. [Google Scholar] [CrossRef]
- Bhutta, Z.A.; Sadiq, K. Protein digestion and bioavailability. Encycl. Hum. Nutr. 2013, 4, 116–122. [Google Scholar]
- Khavinson, V.; Linkova, N.; Kozhevnikova, E.; Dyatlova, A.; Petukhov, M. Transport of biologically active ultrashort peptides using POT and LAT carriers. Int. J. Mol. Sci. 2022, 23, 7733. [Google Scholar] [CrossRef]
- Lee, V.H.; Dodda-Kashi, S.; Grass, G.M.; Rubas, W. Oral route of peptide and protein drug delivery. In Peptide and Protein Drug Delivery; CRC Press: Boca Raton, FL, USA, 2024; pp. 691–738. [Google Scholar]
- De Bhowmick, G.; Hayes, M. In vitro digestibility of selected seaweeds. Foods 2022, 11, 289. [Google Scholar] [CrossRef]
- Bleakley, S.; Hayes, M. Algal proteins: Extraction, application, and challenges concerning production. Foods 2017, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Sousa, R.; Portmann, R.; Dubois, S.; Recio, I.; Egger, L. Protein digestion of different protein sources using the INFOGEST static digestion model. Int. Food Res. 2020, 130, 108996. [Google Scholar] [CrossRef]
- Dupont, D.; Alric, M.; Blanquet-Diot, S.; Bornhorst, G.; Cueva, C.; Deglaire, A.; Denis, S.; Ferrua, M.; Havenaar, R.; Lelieveld, J.; et al. Can dynamic in vitro digestion system mimic the physiological reality? Crit. Rev. Food Sci. Nutr. 2019, 59, 1546–1562. [Google Scholar] [CrossRef]
- Sitovs, A.; Mohylyuk, V. Ex vivo permeability study of poorly soluble drugs across gastrointestinal membranes: Acceptor compartment media composition. Drug Discov. Today 2024, 29, 104214. [Google Scholar] [CrossRef] [PubMed]
- Antal, O.; Dalmadi, I.; Takács, K. Upgrading in vitro digestion protocols with absorption models. Appl. Sci. 2024, 14, 8320. [Google Scholar] [CrossRef]
- Rehman, A.; Heinsen, F.-A.; Koenen, M.E.; Venema, K.; Knecht, H.; Hellmig, S.; Schreiber, S.; Ott, S.J. Effects of probiotics and antibiotics on the intestinal homeostasis in a computer controlled model of the large intestine. BMC Microbiol. 2012, 12, 47. [Google Scholar] [CrossRef]
- Chalvon-Demersay, T.; Blachier, F.; Tomé, D.; Blais, A. Animal models for the study of the relationships between diet and obesity: A focus on dietary protein and strogen deficiency. Front. Nutr. 2017, 4, 5. [Google Scholar] [CrossRef]
- Giromini, C.; Cheli, C.; Rebucci, R.; Baldi, A. Dairy proteins and bioactive peptides: Modeling digestion and the intestinal barrier. JDS 2019, 102, 929–942. [Google Scholar]
- Guillin, F.M.; Gaudichon, C.; Guérin-Deremaux, L.; Lefranc-Millot, C.; Khodorova, N.; Besançon, S.; Calvez, J. Caecal digestibility as an approximation of ileal protein digestibility evaluation in rats. J. Nutr. Sci. 2023, 12, e18. [Google Scholar] [CrossRef] [PubMed]
- Teng, P.-Y.; Yadav, S.; Shi, H.; Kim, W.K. Evaluating endogenous loss and standard ileal digestibility of amino acids in response to the graded severity levels of E. maxima infection. Poult. Sci. 2021, 100, 101426. [Google Scholar] [PubMed]
- Gaudichon, C.; Calvez, J. Determinants of amino acid bioavailability from ingested protein in relation to gut health. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 55–61. [Google Scholar] [CrossRef]
- Wang, Y.; Tibbetts, S.M.; Berrue, F.; McGinn, P.J.; MacQuarrie, S.P.; Puttaswamy, A.; Patelakis, S.; Schmidt, D.; Melanson, R.; MacKenzie, S.E. A rat study to evaluate the protein quality of three green microalgal species and the impact of mechanical cell wall disruption. Foods 2020, 9, 1531. [Google Scholar] [CrossRef] [PubMed]
- Bartolí, R.; Boix, J.; Ódena, G.; De la Ossa, N.D.; Moreno, V.; Lorenzo-Zúñiga, V. Colonoscopy in rats: An endoscopic, histological and tomography study. World J. Gastrointest. Endosc. 2013, 5, 226–230. [Google Scholar]
- Moughan, P.J.; Wolfe, R.R. Determination of dietary amino acid digestibility in humans. J. Nutr. 2019, 149, 2101–2109. [Google Scholar] [CrossRef]
- Hodgkinson, S.M.; Stein, H.H.; de Vries, S.; Hendriks, W.H.; Moughan, P.J. Determination of true ileal amino acid digestibility in the growing pig for calculation of digestible indispensable amino acid score (DIAAS). J. Nutr. 2020, 150, 2621–2623. [Google Scholar]
- Bailey, H.M.; Stein, H.H. Can the digestible indispensable amino acid score methodology decrease protein malnutrition. Anim. Front. 2019, 9, 18–23. [Google Scholar]
- Mathai, J.K.; Liu, Y.; Stein, H.H. Values for digestible indispensable amino acid scores (DIAAS) for some dairy and plant proteins may better describe protein quality than values calculated using the concept for protein digestibility-corrected amino acid scores (PDCAAS). Br. J. Nutr. 2017, 117, 490–499. [Google Scholar] [CrossRef]
- Abelilla, J.J.; Liu, Y.; Stein, H.H. Digestible indispensable amino acid score (DIAAS) and protein digestibility corrected amino acid score (PDCAAS) in oat protein concentrate measured in 20- to 30-kilogram pigs. J. Sci. Food Agric. 2017, 98, 410–414. [Google Scholar] [CrossRef]
- Sousa, R.; Recio, I.; Heimo, D.; Dubois, S.; Moughan, P.J.; Hodgkinson, S.M.; Portmann, R.; Egger, L. In vitro digestibility of dietary proteins and in vitro DIAAS analytical workflow based on the INFOGEST static protocol and its validation with in vivo data. Food Chem. 2023, 404, 134720. [Google Scholar] [PubMed]
- Hodgkinson, S.M.; Stroebinger, N.; van der Wielen, N.; Mensink, M.; Montoya, C.; Hendriks, W.H.; de Vries, S.; Stein, H.H.; Moughan, P.J. Comparison of true ileal amino acid digestibility between adult humans and growing pigs. J. Nutr. 2022, 152, 1635–1646. [Google Scholar] [CrossRef]
- Loveday, S.M. Food Proteins: Technological, nutritional, and sustainability attributes of traditional and emerging proteins. Annu. Rev. Food Sci. 2019, 10, 311–339. [Google Scholar] [CrossRef]
- Herreman, L.; Nommensen, P.; Pennings, B.; Laus, M.C. Comprehensive overview of the quality of plant- And animal sourced proteins based on the digestible indispensable amino acid score. Food Sci. Nutr. 2020, 8, 5379–5391. [Google Scholar] [CrossRef]
- Speak, J.W.; Van Wesemael, D. CVB Feed Table 2021. Chemical Composition and Nutritional Values of Feedstuffs. Central Bureau for Livestock Feeding (CVB): Lelystad, The Netherlands, 2018. [Google Scholar]
- Jaeger, A.; Zanini, E.; Sahin, A.W.; Arendt, E.K. Barley protein properties, extraction and applications, with a focus on brewers’ spent grain protein. Foods 2021, 10, 1389. [Google Scholar] [CrossRef]
- Walter, S.; Zehring, J.; Mink, K.; Quendt, U.; Zocher, K.; Rohn, S. Protein content of peas (Pisum sativum) and beans (Vicia faba)—Influence of cultivation conditions. J. Food Compos. Anal. 2022, 105, 104257. [Google Scholar] [CrossRef]
- Punzalan, J.K.M.; Rosentrater, K.A. Copra meal: A review of its production, properties, and prospects. Animals 2024, 14, 1689. [Google Scholar] [CrossRef] [PubMed]
- Rangel, A.; Saraiva, K.; Schwengber, P.; Narciso, M.S.; Domont, G.B.; Ferreira, S.T.; Pedrosa, C. Biological evaluation of a protein isolate from cowpea (Vigna unguiculata) seeds. Food Chem. 2004, 87, 491–499. [Google Scholar] [CrossRef]
- Abebe, B.K.; Alemayehu, M.T. A review of the nutritional use of cowpea (Vigna unguiculata L. Walp) for human and animal diets. J. Agric. Food Res. 2022, 10, 100383. [Google Scholar] [CrossRef]
- Bletner, J.K.; Chalhoub, N.E.; Goff, O.E. The horsebean (Vicia faba L.) as a vegetable protein concentrate in chick diets. Poult. Sci. 1963, 42, 562–568. [Google Scholar]
- Guterres, L.L.; Pinton, M.B.; dos Santos, B.A.; Correa, L.P.; Cordeiro, M.W.S.; Wagner, R.; Cichoski, A.J.; Lorenzo, J.M.; Campagnol, P.C.B. Hydrogelled emulsion from linseed oil and pea protein as a strategy to produce healthier pork burgers with high technological and sensory quality. Meat Sci. 2023, 195, 109028. [Google Scholar] [CrossRef]
- Lima, L.S.; Palin, M.F.; Santos, G.T.; Benchaar, C.; Lima, L.C.R.; Chouinard, P.Y.; Petit, H.B. Effect of flax meal on the production performance and oxidative status of dairy cows infused with flax oil in the abomasum. Livest. Sci. 2014, 170, 53–62. [Google Scholar]
- Panasiewicz, K. Chemical composition of lupin (Lupinus spp.) as influenced by variety and tillage system. Agriculture 2022, 12, 263. [Google Scholar] [CrossRef]
- Ochieng’, I.O.; Gitari, H.I.; Mochoge, B.; Rezaei-Chiyaneh, E.; Gweyi-Onyango, J. Optimizing maize yield, nitrogen efficacy and grain protein content under different N forms and rates. J. Soil Sci. Plant Nutr. 2021, 21, 1867–1880. [Google Scholar] [CrossRef]
- Speak, J.W.; Blok, M.C. CVB Feed Table 2018. Chemical Composition and Nutritional Values of Feedstuff. Central Bureau for Livestock Feeding (CVB): Lelystad, The Netherlands, 2018. [Google Scholar]
- Zhang, Y.; He, H.; Xu, M.; Zhang, X.; Cao, S.; Hu, Y.; Luan, G. Physicochemical properties and protein structure of extruded corn gluten meal: Implication of temperature. Food Chem. 2023, 399, 133985. [Google Scholar]
- Hou, S.; Men, Y.; Wei, M.; Zhang, Y.; Li, H.; Sun, Z.; Han, Y. Total protein content, amino acid composition and eating-quality evaluation of foxtail millet (Setaria italica (L.) P. Beauv. Foods 2023, 12, 31. [Google Scholar] [CrossRef]
- Byadagi, S.S.; Geetha, K. Nutritional profile of selected Niger seed varieties. Mysore J. Agric. Sci. 2013, 47, 603–608. [Google Scholar]
- Kumar, L.; Sehrawat, R.; Kong, Y. Oat properties: A perspective on functional properties. LWT—Food Sci. Technol. 2021, 152, 112307. [Google Scholar]
- Bhutto, R.A.; Bhutto, N.H.; Khanal, S.; Wang, M.; Iqbal, S.; Fan, Y.; Yi, J. Potato protein as an emerging high-quality: Source, extraction, purification, properties (functional, nutritional, physicochemical, and processing), applications, and challenges using potato protein. Food Hydrocoll. 2024, 157, 110415. [Google Scholar] [CrossRef]
- Rajkovic, D.; Jeromela, A.M.; Pezo, L.; Loncar, B.; Zanetti, F.; Monti, A.; Spika, A.K. Yield and quality prediction of winter rapeseed—Artificial neural network and random forest models. Agronomy 2022, 12, 58. [Google Scholar]
- Jia, W.; Rodriguez-Alonso, E.; Bianeis, M.; Keppler, J.K.; van de Goot, A.J. Assessing functional properties of rapeseed protein concentrate versus isolate for food applications. Innov. Food Sci. Emerg. Technol. 2021, 68, 102636. [Google Scholar] [CrossRef]
- Siaw, M.; Wang, Y.-J.; McClung, A.M.; Mauromoustakos, A. Effect of protein denaturation and lipid removal on rice physicochemical properties. LWT—Food Sci. Technol. 2021, 150, 112015. [Google Scholar] [CrossRef]
- Ma, C.; Ren, Z.; Zhang, Z.; Du, J.; Jin, C.; Yin, X. Development of simplified models for nondestructive testing of rice (with husk) protein content using hyperspectral imaging technology. Vib. Spectrosc. 2021, 114, 103230. [Google Scholar] [CrossRef]
- Xhaferaj, M.; Muskovics, G.; Schall, E.; Bugyi, Z.; Tömösközi, S.; Scherf, K.A. Characterization of rye flours and their potential as reference material for gluten analysis. Food Chem. 2023, 408, 135148. [Google Scholar] [CrossRef]
- Li, X.; Yi, J.; Wu, T.; Wang, J.; Li, L.; Liu, P. Steam explosion modification on phytate, protein, and lignan in sesame cake. Ind. Crops Prod. 2023, 206, 117697. [Google Scholar] [CrossRef]
- Zarei, M.; Amirkolaei, A.K.; Trushenski, J.T.; Sealey, W.M.; Schwarz, M.H.; Ovissipour, R. Sorghum as a potential valuable aquafeed ingredient: Nutritional quality and digestibility. Agriculture 2022, 12, 669. [Google Scholar] [CrossRef]
- Xiao, R.; Lou, H.; Hu, R.; Li, S.; Zheng, Y.; Wang, D.; Xu, Y.; Xu, Y.; Li, Y. Enzymatic production and physicochemical and functional properties of sorghum protein isolates. Int. J. Biol. Macromol. 2024, 283, 137421. [Google Scholar] [CrossRef]
- Janocha, A.; Milczarek, A.; Pietrusiak, D.; Laski, K.; Saleh, M. Efficiency of soybean products in broiler chicken nutrition. Animals 2022, 12, 294. [Google Scholar] [CrossRef]
- Liu, S.X.; Chen, D.; Plumier, B.; Berhow, M.; Xu, J.; Byars, J.A. Impact of particle size fractions on composition, antioxidant activities, and functional properties of soybean hulls. J. Food Meas. Charact. 2021, 15, 1547–1562. [Google Scholar] [CrossRef]
- Guo, B.; Sun, L.; Jiang, S.; Ren, H.; Sun, R.; Wei, Z.; Hong, H.; Luan, X.; Wang, J.; Xu, D.; et al. Soybean genetic resources contributing to sustainable protein production. Theor. Appl. Genet. 2022, 135, 4095–4121. [Google Scholar]
- Banjac, V.; Vukmirovic, D.; Pezo, L.; Draganovic, V.; Duragic, O.; Coloviv, R. Impact of variability in protein content of sunflower meal on the extrusion process and physical quality of the extruded salmonid feed. J. Food Process Eng. 2021, 44, e13640. [Google Scholar]
- Camerlengo, F.; Kiszonas, A.M. Genetic factors influencing triticale quality for food. J. Cereal Sci. 2023, 11, 103744. [Google Scholar]
- Schopf, M.; Wehrli, M.C.; Becker, T.; Jekle, M.; Scherf, K.A. Fundamental characterization of wheat gluten. Eur. Food Res. Technol. 2021, 247, 985–997. [Google Scholar] [CrossRef]
- Jimenez-Pulido, I.J.; Rico, D.; Perez, J.; Martínez-Villaluenga, C.; De Luis, D.; Diana, A.B.M. Impact of protein content on the antioxidants, anti-inflammatory properties and glycemic index of wheat and wheat bran. Foods 2022, 11, 2049. [Google Scholar] [CrossRef]
- Gunnes, N.; Michiels, J.; De Smet, S.; Vanhaecke, L.; Kravchuk, O.; Gidley, M. Blood lipids—Soluble dietary fibres: Study of bile salts diffusion across intestinal mucosa using the Ussing chamber system. J. Nutr. Intermed. Metab. 2016, 4, 35. [Google Scholar]
- Kalungwana, N.; Marshall, L.; Mackie, A.; Boesch, C. An ex vivo intestinal absorption model is more effective than an in vitro cell model to characterise absorption of dietary carotenoids following simulated gastrointestinal digestion. Food Res. Int. 2023, 166, 112558. [Google Scholar] [PubMed]
- Nitride, C.; Vegarud, G.E.; Comi, I.; Devold, T.G.; Roset, A.; Marti, A.; Iametti, S.; Mamone, G.; Picariello, G.; Alfieri, F.; et al. Effect of sprouting on the proteome of chickpea flour and on its digestibility by ex vivo gastroduodenal digestion complemented with jejunal brush border membrane enzymes. Int. Food Res. 2022, 154, 111012. [Google Scholar]
- Assad-Bustillos, M.; Palier, J.; Rabesona, H.; Choiset, Y.; Valle, G.D.; Feron, G. Role of the bolus degree of structure on the protein digestibility during in vitro digestion of a pea protein-fortified sponge cake chewed by elderly. J. Texture Stud. 2020, 51, 134–143. [Google Scholar]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzynski, M.; Nowakowska, D.; Matwijczuk, A. Influence of in vitro digestion on composition, bioaccessibility and antioxidant activity of food polyphenols—A non-systematic review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef]
- Oliveira, F.D.; Cunha, R.L. Soy protein-based delivery systems as carriers of trans-resveratrol: Bioaccessibility using different in vitro digestion models. Food Res. Int. 2022, 161, 111837. [Google Scholar] [CrossRef]
- Havenaar, R.; Maathuis, A.; de Jong, A.; Mancinelli, D.; Berger, A.; Bellmann, S. Herring roe protein has a high digestible indispensable amino acid score (DIAAS) using a dynamic in vitro gastrointestinal model. Nutr. Res. 2016, 36, 798–807. [Google Scholar] [CrossRef]
- Souza Simões, L.; Madalena, D.A.; Pinheiro, A.C.; Teixeira, J.A.; Vicente, A.A.; Ramos, O.L. Micro- and nano bio-based delivery systems for food applications: In vitro behavior. Adv. Colloid Interface Sci. 2017, 243, 23–45. [Google Scholar]
- Thuenemann, E.C.; Mandalari, G.; Rich, G.T.; Faulks, R.M. Dynamic gastric model (DGM). In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Springer: Cham, Switzerland, 2015; pp. 47–59. [Google Scholar]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Balance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [PubMed]
- Sanchón, J.; Fernández-Tomé, S.; Miralles, B.; Hernández-Ledesma, B.; Tomé, D.; Gaudichon, C.; Recio, I. Protein degradation and peptide release from milk proteins in human jejunum. Comparison with in vitro gastrointestinal simulation. Food Chem. 2018, 239, 486–494. [Google Scholar] [PubMed]
- Egger, L.; Ménard, O.; Delgado-Andrade, C.; Alvito, P.; Assunc¸ao, R.; Balance, S.; Barberá, R.; Brodkorb, A.; Cattenoz, T.; Clemente, A.; et al. The harmonized INFOGEST in vitro digestion method: From knowledge to action. Food Res. Int. 2015, 88, 217–225. [Google Scholar]
- Catalayud, M.; Xiong, C.; Laing, G.D.; Raber, G.; Francesconi, K.; van de Wiele, T. Salivary and gut microbiomes play a significant role in in vitro oral bioaccessibility, biotransformation, and intestinal absorption of arsenic from food. Environ. Sci. Technol. 2018, 52, 14422–14435. [Google Scholar]
- Calero, V.; Rodrigues, P.M.; Dias, T.; Ainla, A.; Villaca, A.; Pastrana, L.; Xavier, M.; Goncalves, C. A miniaturised semi-dynamic in-vitro model for human digestion. Sci. Rep. 2024, 14, 11923. [Google Scholar] [CrossRef]
- Alegria, A.; García-Llatas, G.; Cilla, A.; Verhoeckx, K.; Cotter, P.; López-Expósito, I.; Kleiveland, C.; Lea, T.; Mackie, A.; Requena, T.; et al. (Eds.) The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Springer: Cham, Switzerland, 2015; Chapter 1. [Google Scholar]
- Mulet-Cabero, A.-I.; Torcello-Gómez, A.; Saha, S.; Mackie, A.R.; Wilde, P.J.; Brodkorb, A. Impact of caseins and whey proteins ratio and lipid content on in vitro digestion and ex vivo absorption. Food Chem. 2020, 319, 126514. [Google Scholar] [CrossRef] [PubMed]
- Verkempinck, S.H.E.; Duijsens, D.; Michels, D.; Guevara-Zambrano, J.M.; Infantes-García, M.R.; Pälchen, K.; Grauwet, T. Studying semi-dynamic digestion kinetics of food: Establishing a computer-controlled multireactor approach. Int. Food Res. 2022, 156, 111301. [Google Scholar] [CrossRef]
- Delfino, R.A.; Canniatti-Brazaca, S.G. Interação de polifenóis e proteínas e o efeito na digestibilidade proteica de feijão comum (Phaseolus vulgaris L.) cultivar Pérola. Ciênc. Tecnol. 2010, 30, 8–12. [Google Scholar]
- Mechi, R.; Caniatti-Brazaca, S.G.; Arthur, V. Avaliação química, nutricional e fatores antinutricionais do feijão preto (Phaseolus vulgaris L.) irradiado. Ciênc. Tecnol. Aliment. 2005, 25, 109–114. [Google Scholar] [CrossRef]
- Shimelis, E.A.; Rakshit, S. Effect of microwave heating on solubility and digestibility of proteins and reduction of antinutrients of selected common bean (Phaseolus vulgaris L.) varieties grown in Ethiopia. Ital. J. Food Sci. 2005, 17, 407–418. [Google Scholar]
- Kalpanadevi, V.; Mohan, V.R. Effect of processing on antinutrients and in vitro protein digestibility of the underutilized legume, Vigna unguiculata (L.) Walp subsp. Unguiculata. LWT—Food Sci. Technol. 2013, 51, 455–461. [Google Scholar]
- Khttatab, R.Y.; Arntfield, S.D.; Nyachoti, C.M. Nutritional quality of legume seeds as affected by some physical treatments, Part 1: Protein quality evaluation. LWT—Food Sci Technol. 2009, 42, 1107–1112. [Google Scholar] [CrossRef]
- Ravindran, G. Seed protein of millets: Amino acid composition, proteinase inhibitors and in-vitro protein digestibility. Food Chem. 1992, 44, 13–17. [Google Scholar] [CrossRef]
- Abioye, V.F.; Babarinde, G.O.; Ogunlakin, G.O.; Adejuyitan, J.A.; Olatunde, S.J.; Abioye, A.O. Varietal and processing influence on nutritional and phytochemical properties of finger millet: A review. Heliyon 2022, 8, e12310. [Google Scholar] [CrossRef]
- Oghbaei, M.; Prakash, J. Bioaccessible nutrients and bioactive components from fortified products prepared using finger millet (Eleusine coracana). J. Sci. Food Agric. 2011, 92, 2281–2290. [Google Scholar]
- Shevkani, K.; Kaur, R.; Singh, N.; Hkanze, D.P. Colour, composition, digestibility, functionality and pasting properties of diverse kidney beans (Phaseolus vulgaris) flours. Curr. Res. Food Sci. 2022, 5, 619–628. [Google Scholar] [CrossRef]
- Berno, L.I.; Guimarães-Lopes, T.G.; Canniatti-Brazaca, S.G. Avaliação da composição centesimal, digestibilidade e atividade inibitoria de tripsina em produtos derivados de soja (Glycine max). Alim. Nutriç. 2007, 18, 277–282. [Google Scholar]
- Lee, J.Y.; Cho, Y.Y.; Ohh, S.J. Effect of gamma irradiation and autoclaving on sterilization and amino acids digestibility of diets for specific pathogen free mini-pigs containing either soybean meal or whey protein. Livest. Sci. 2012, 149, 201–207. [Google Scholar]
- Espinoza-Páez, E.; Alanis-Guzmán, M.G.; Hernández-Luna, C.E.; Báez-González, J.G.; Amaya-Guerra, C.A.; Andrés-Grau, A.M. Increasing antioxidant activity and protein digestibility in Phaseolus vulgaris and Avena sativa by fermentation with the Pleurotus ostreatus fungus. Molecules 2017, 22, 2275. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, G.; Ying, D.; Sanguansri, L.; Augustin, M.A. Effect of extrusion conditions on the physico-chemical properties and in vitro protein digestibility of canola meal. Int. Food Res. 2017, 100, 658–664. [Google Scholar] [CrossRef]
- Annor, G.A.; Tyl, C.; Marcone, M.; Ragaee, S.; Marti, S. Why do millets have slower starch and protein digestibility than other cereals? Trends Food Sci. Technol. 2017, 66, 73–83. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhewa, T. Plant food anti-nutritional factors and their reduction strategies: An overview. Food Prod. Process. Nutr. 2020, 2, 6. [Google Scholar] [CrossRef]
- Anaya, K.; Cruz, A.C.B.; Cunha, D.C.S.; Monteiro, S.M.N.; Santos, E.A. Growth impairment caused by raw linseed consumption: Can trypsin inhibitors be harmful for health? Plant Foods Hum. Nutr. 2015, 70, 338–343. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, Y.; Lu, Z.; Wang, Y. Solid-state fermentation of corn-soybean meal mixed feed with Bacillus subtilis and Enterococcus faecium for degrading antinutritional factors and enhancing nutritional value. J. Anim. Sci. Biotechnol. 2017, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Adenekan, M.K.; Fadimu, G.J.; Odunmbaku, L.A.; Oke, E.K. Effect of isolation techniques on the characteristics of pigeon pea (Cajanus cajan) protein isolates. Food Sci. Nutr. 2018, 6, 146–152. [Google Scholar] [CrossRef]
- Tusnio, A.; Taciak, M.; Barszcz, M.; Swiceh, E.; Bachanek, I.; Skomiał, J. Effect of replacing soybean meal by raw or extruded pea seeds on growth performance and selected physiological parameters of the ileum and distal colon of pigs. PLoS ONE 2017, 12, e0169467-16. [Google Scholar] [CrossRef] [PubMed]
- Kamela, A.L.S.; Mouokeu, R.S.; Ashish, R.; Tazoho, G.M. Influence of processing methods on proximate composition and dieting of two amaranthus species from west Cameroon. Int. J. Food Sci. 2016, 2016, 6707313. [Google Scholar]
- Boye, J.; Wijesinha-Bettoni, R.; Burlingame, B. Protein quality evaluation twenty years after the introduction of the protein digestibility corrected amino acid score method. Br. J. Nutr. 2012, 108, S183–S211. [Google Scholar] [CrossRef] [PubMed]
- Foster, M.; Samman, S. Implications of a plant-based diet on zinc requirements and nutritional status. In Vegetarian and Plant-Based Diets in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2017; pp. 683–713. [Google Scholar]
- Kaushik, G.; Singhal, P.; Chaturvedi, S. Food processing for increasing consumption: The case of legumes. In Food Processing for Increased Quality and Consumption; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–28. [Google Scholar]
- de Camargo, A.C.; da Silva Lima, R. A perspective on phenolic compounds, their potential health benefits, and international regulations: The revised Brazilian normative on food supplements. J. Food Bioact. 2019, 7, 7–17. [Google Scholar]
- Joye, I. Protein digestibility of cereal products. Foods 2019, 8, 199. [Google Scholar] [CrossRef] [PubMed]
- López-Moreno, M.; Garcés-Rimón, M.; Miguel, M. Antinutrients: Lectins, goitrogens, phytates and oxalates, friends or foe? J. Funct. Foods 2022, 89, 104938. [Google Scholar]
- Muramoto, K. Lectins as bioactive proteins in foods and feeds. Food Sci. Technol. Res. 2017, 23, 487–494. [Google Scholar]
- Lo, D.; Wang, H.-I.; Wu, W.-J.; Yang, R.-Y. Anti-nutrient components and their concentrations in edible parts in vegetable families. CAB Rev. 2018, 13, 15. [Google Scholar]
- Dogra, D. Common buckwheat: Nutritional profiling of grains. Asian J. Dairy Food Res. 2019, 38, 333–340. [Google Scholar] [CrossRef]
- Delimont, N.M.; Rosenkranz, S.K.; Haub, M.D.; Lindshield, B.L. Salivary proline-rich protein may reduce tannin-iron chelation: A systematic narrative review. Nutr. Metab. 2017, 14, 47. [Google Scholar]
- Kaspchak, E.; Mafra, L.I.; Mafra, M.R. Effect of heating and ionic strength on the interaction of bovine serum albumin and the antinutrients tannic and phytic acids, and its influence on in vitro protein digestibility. Food Chem. 2018, 252, 1–8. [Google Scholar] [PubMed]
- Thakur, A.; Sharma, V.; Thakur, A. An overview of anti-nutritional factors in food. Int. J. Chem. Stud. 2019, 7, 2472–2479. [Google Scholar]
- Li, L.; Tsao, R. UF-LC-DAD-MSn for discovering enzyme inhibitors for nutraceuticals and functional foods. J. Food Bioact. 2019, 7, 27–35. [Google Scholar] [CrossRef]
- Abdulwaliyu, I.; Arekemase, S.O.; Adudu, J.A.; Batari, M.L.; Egbule, M.N.; Okoduwa, S.I.R. Investigation of the medicinal significance of phytic acid as an indispensable anti-nutrient in diseases. Clin. Nutr. Exp. 2019, 28, 42–61. [Google Scholar] [CrossRef]
- Gonzalez, A.A.L.; Grases, F.; Mari, B.; Tomas-Salva, M.; Rodriguez, A. Urinary phytate concentration and risk of fracture determined by the FRAX index in a group of postmenopausal women. Turk. J. Med. Sci. 2019, 49, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Palomeque, C.; Grau, A.; Perelló, J.; Sanchis, P.; Isern, B.; Prieto, R.M.; CostaBauzá, A.; Caldés, O.J.; Bonnin, O.; Garcia-Raja, A.; et al. Relationship between Urinary Level of Phytate and Valvular Calcification in an Elderly Population: A Cross-Sectional Study. PLoS ONE 2015, 10, e0136560. [Google Scholar] [CrossRef] [PubMed]
- Mukhar, K.; Nabi, B.G.; Ahmed, W.; Suleman, R.; Aadil, R.M. Effect of thermal processing on the digestion of plant proteins. Process. Technol. Food Protein Dig. 2023, 16, 407–428. [Google Scholar]
- Deng, X.; Huang, H.; Huang, S.; Yang, M.; Wu, J.; Ci, Z.; He, Y.; Wu, Z.; Han, L.; Zhang, D. Insight into the incredible effects of microwave heating: Driving changes in the structure, properties and functions of macromolecular nutrients in novel food. Front. Nutr. 2022, 9, 941527. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Liu, Y.; Wang, L.; Li, D.; Mao, Z. Effects of extrusion parameters on physicochemical properties of flaxseed snack and process optimization. Int. J. Agric. Biol. Eng. 2015, 8, 121–131. [Google Scholar]
- Fombang, E.N.; Taylor, J.R.N.; Mbofung, C.M.F.; Minnnar, A. Use of γ-irradiation to alleviate the poor protein digestibility of sorghum porridge. Food Chem. 2005, 91, 695–703. [Google Scholar] [CrossRef]
- Duodu, K.G.; Nunes, A.; Delgadillo, I.; Parker, M.L.; Mills, E.N.S.; Belton, P.S.; Taylor, J.R.N. Effect of grain structure and cooking on sorghum and maize in vitro protein digestibility. J. Cereal Sci. 2002, 35, 161–174. [Google Scholar] [CrossRef]
- Labuschagne, M.T. A review of cereal grain proteomics and its potential for sorghum improvement. J. Cereal Sci. 2018, 84, 151–158. [Google Scholar] [CrossRef]
- Batista, K.A.; Pereira, W.J.; Moreira, B.R.; Silva, C.N.S.; Fernandes, K.F. Effect of autoclaving on the nutritional quality of hard-to-cook common beans (Phaseolus vulgaris). Int. J. Environ. Agric. Biotechnol. 2020, 5, 22–30. [Google Scholar] [CrossRef][Green Version]
- Sun, X.; Ohanenye, I.C.; Ahmed, T.; Udenigwe, C.C. Microwave treatment increased protein digestibility of pigeon pea (Cajanus cajan) flour: Elucidation of underlying mechanisms. Food Chem. 2020, 329, 127196. [Google Scholar] [PubMed]
- Habinshuti, I.; Mu, T.-H.; Zhang, M. Ultrasound microwave-assisted enzymatic production and characterisation of antioxidant peptides from sweet potato protein. Ultrason. Sonochem. 2020, 69, 105262. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Z.; Zhang, C.; Wu, C.; Tan, C.-P.; Liu, Y. Comparative structural, digestion and absorption characterization of three common extruded plant proteins. Food Res. Int. 2024, 177, 113852. [Google Scholar] [PubMed]
- de la Rosa-Millán, J.; Heredia-Olea, E.; Perez-Carrillo, E.; Guajardo-Flores, D.; Serna-Saldívar, S.R.O. Effect of decortication, germination and extrusion on physicochemical and in vitro protein and starch digestion characteristics of black beans (Phaseolus vulgaris L.). LWT—Food Sci. Technol. 2019, 102, 330–337. [Google Scholar] [CrossRef]
- Lin, Q.; Pan, L.; Deng, N.; Sang, M.; Cai, K.; Chen, C.; Han, J.; Ye, A. Protein digestibility of textured-wheat-protein (TWP)-based meat analogues: (I) Effects of fibrous structure. Food Hydrocoll. 2022, 130, 107694. [Google Scholar]
- Wu, D.; Wu, W.; Zhang, N.; Soladoye, O.P.; Aluko, R.E.; Zhang, Y.; Fu, Y. Tailoring soy protein/corn zein mixture by limited enzymatic hydrolysis to improve digestibility and functionality. Food Chem. 2024, 23, 101550. [Google Scholar]
- Kamiloglu, S.; Tomas, M.; Ozkan, G.; Ozdal, T.; Capanoglu, E. In vitro digestibility of plant proteins: Strategies for improvement and health implications. Curr. Opin. Food Sci. 2024, 57, 101148. [Google Scholar]
- Amat, T.; Assifaoui, A.; Schmitt, C.; Saurel, R. Importance of binary and ternary complex formation on the functional and nutritional properties of legume proteins in presence of phytic acid and calcium. Crit. Rev. Food Sci. Nutr. 2022, 63, 12036–12058. [Google Scholar]
- Beaubier, S.; Pineda-Vadillo, C.; Mesieres, O.; Framboisier, X.; Galet, O.; Kapel, R. Improving the in vitro digestibility of rapeseed albumins resistant to gastrointestinal proteolysis while preserving the functional properties using enzymatic hydrolysis. Food Chem. 2023, 407, 135132. [Google Scholar]
- Çabuk, B.; Nosworthy, M.G.; Stone, A.K.; Korber, D.R.; Tanaka, T.; House, J.D.; Nickerson, M.T. Effect of fermentation on the protein digestibility and levels of non-nutritive compounds of pea protein concentrate. Food Technol. Biotechnol. 2018, 56, 257–264. [Google Scholar] [PubMed]
- Chandra-Hioe, M.V.; Wong, C.H.M.; Arcot, J. The potential use of fermented chickpea and faba bean flour as food ingredients. Plant Foods Hum. Nutr. 2016, 7, 90–95. [Google Scholar]
- Ketnawa, S.; Ogawa, Y. In vitro protein digestibility and biochemical characteristics of soaked, boiled and fermented soybeans. Sci. Rep. 2011, 11, 14257. [Google Scholar]
- Skalickova, S.; Ridoskova, A.; Slama, P.; Skladanka, J.; Skarpa, P.; Smykalova, I.; Horacek, J.; Dostalova, R.; Horky, P. Effect of lactic fermentation and cooking on nutrient and mineral digestibility of peas. Front. Nutr. 2022, 9, 838963. [Google Scholar]
- Wang, Y.; Ma, C.-M.; Yang, Y.; Wang, B.; Liu, X.-F.; Wang, Y.; Bian, X.; Chang, G.; Zhang, N. Effect of high hydrostatic pressure treatment on food composition and applications in food industry: A review. Food Res. Int. 2024, 195, 114991. [Google Scholar]
- Hall, A.E.; Moraru, C.I. Comparative effects of high pressure processing and heat treatment on in vitro digestibility of pea protein and starch. NPJ Sci. Food 2022, 6, 2. [Google Scholar] [PubMed]
- De Lamballerie-Anton, M.; Délepine, S.; Chapleau, N. High pressure effect on meat and lupin protein digestibility. High Press. Res. 2002, 22, 649–652. [Google Scholar]
- Aghababaei, F.; McClements, D.J.; Hadidi, M. Ultrasound processing for enhanced digestibility of plant proteins. Food Hydrocoll. 2024, 155, 110188. [Google Scholar]
- Pandita, G.; Sharma, S.; Oommen, I.E.; Madaan, N.; Bhosale, Y.; Nagy, V.; Shaikh, A.M.; Kovács, B. Comprehensive review on the potential of ultrasound for blue food protein extraction, modification and impact on bioactive properties. Ultrason. Sonochem. 2024, 111, 107087. [Google Scholar]
- Rojas, M.; Kubo, M.T.K.; Pastor, A.C.M.; Augusto, P.E.D. Ultrasound processing to enhance the functionality of plant-based beverages and proteins. Curr. Opin. Food Sci. 2022, 48, 100939. [Google Scholar]
- Nasrabadi, M.N.; Doost, A.S.; Mezzenga, R. Modification approaches of plant-based proteins to improve their technofunctionality and use in food products. Food Hydrocoll. 2021, 118, 106789. [Google Scholar]
- Kim, T.-K.; Hwang, K.-E.; Ham, Y.-K.; Kim, H.-W.; Paik, H.-D.; Kim, Y.-B.; Choi, Y.-S. Interactions between raw meat irradiated by various kinds of ionizing radiation and transglutaminase treatment in meat emulsion systems. Radiat. Phys. Chem. 2022, 166, 108452. [Google Scholar]
- Faizal, F.I.; Ahmad, N.H.; Yaacob, J.S.; Abdul Halim-Lim, S.; Abd Rahim, M.H. Food processing to reduce antinutrients in plant-based foods. Int. Food Res. J. 2023, 30, 25–45. [Google Scholar]
- Kohli, V.; Singha, S. Protein digestibility of soybean: How processing affects seed structure, protein and non-protein components. Discov. Food 2024, 4, 7. [Google Scholar]
- Ertas, N.; Bilgicli, N. Effect of different debittering processes on mineral and phytic acid content of lupin (Lupinus albus L.) seeds. J. Food Sci. Technol. 2012, 51, 3348–3354. [Google Scholar] [PubMed]
- Gu, J.; Bk, A.; Wu, H.; Lu, P.; Nawaz, M.A.; Barrow, C.J.; Suleria, H.A.R. Impact of processing and storage on protein digestibility and bioavailability of legumes. Food Rev. Int. 2023, 39, 4697–4724. [Google Scholar]
- Ohanenye, I.C.; Ekezie, F.-G.C.; Sarteshnizi, R.A.; Boachie, R.T.; Emenike, C.U.; Sun, X.; Nwachukwu, I.D.; Udenigwe, C.C. Legume seed protein digestibility as influenced by traditional and emerging physical processing technologies. Foods 2022, 11, 2299. [Google Scholar] [CrossRef]
- Bera, I.; O’Sullivan, M.; Flynn, D.; Shileds, D.C. Relationship between protein digestibility and the proteolysis of legume proteins during seed germination. Molecules 2023, 28, 3204. [Google Scholar] [CrossRef]
- Di, Y.; Chang, X.; Gu, R.; Duan, X.; Liu, F.; Liu, X.; Wang, Y. Impact of germination on structural, functional properties and in vitro protein digestibility of sesame (Sesamum indicum L.) protein. LWT—Food Sci. Technol. 2022, 154, 112651. [Google Scholar]
- Embaby, H. Effect of soaking, dehulling, and cooking methods on certain antinutrients and in vitro protein digestibility of bitter and sweet lupin seeds. Food Sci. Biotechnol. 2010, 19, 1055–1062. [Google Scholar]
- Yang, H.; Qu, Y.; Li, J.; Liu, X.; Wu, R.; Wu, J. Improvement of the protein quality and degradation of allergens in soybean meal by combination fermentation and enzymatic hydrolysis. LWT—Food Sci. Technol. 2020, 128, 109442. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).