Insect Frass as an Agricultural Resource Against Abiotic and Biotic Crop Stresses: Mechanisms of Action and Possible Negative Effects
Abstract
:1. Introduction
2. Abiotic Stresses of Agricultural Importance
3. Agricultural Biotic Stresses: Pests and Pathogens
4. The Insect Farming Industry Around the World
5. Insect Frass as an Agricultural Resource
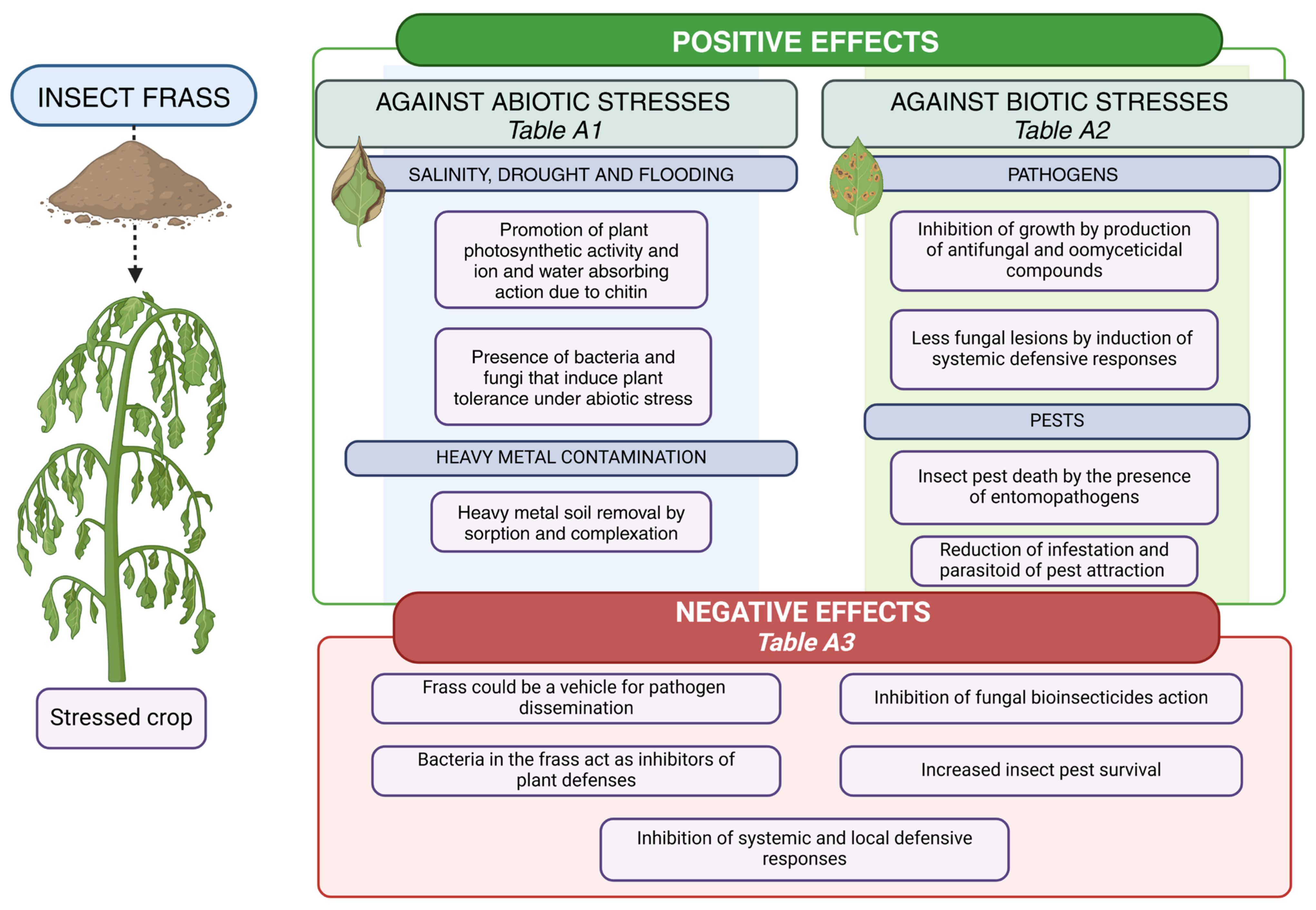
6. Analysis Conducted
7. Insect Frass Against Abiotic Plant Stresses
8. Insect Frass Against Agricultural Pests and Pathogens
8.1. Against Plant Pathogens
8.2. Against Insect Pests
9. Negative Stress-Related Effects of the Use of Insect Frass in Agriculture
10. Conclusions and Future Prospects
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Insects | Crop/Plant | Form of Application and Dosage | Abiotic Stress | Effects | Mechanism of Action | References | |
|---|---|---|---|---|---|---|---|
| Order | Species | ||||||
| Coleoptera | Alphitobius diaperinus | - | Frass: in soil at 2.5% or 5% (w/w) | Heavy metal | Heavy metal soil removal | Sorption and complexation of heavy metals | [77] |
| Tenebrio molitor | Bean | Frass: in soil at 2% (v/v) | Salinity Drought Flooding | Greater plant biomass formation under abiotic stress | Presence of bacteria and fungi that induce plant tolerance under abiotic stress Absorbent action of saline ions and/or water of the chitin/chitosan present in the frass | [61] | |
| - | Biochar from frass (using pyrolysis at 600 °C for 90–120 min) | Heavy metal | Heavy metal soil removal | Sorption of heavy metals | [120] | ||
| - | Frass: in soil at 2.5% or 5% (w/w) | Heavy metal | Heavy metal soil removal | Sorption and complexation of heavy metals | [77] | ||
| - | Frass: in soil at 2% or 4% | Heavy metal | Heavy metal soil removal | Complexation of heavy metals | [93] | ||
| - | Frass | Heavy metal | Heavy metal soil removal | Sorption of heavy metals | [108] | ||
| Diptera | Hermetia illucens | Rice | Frass: in soil at 2–8% (w/w) | Heavy metal | Heavy metal soil removal | Sorption and complexation of heavy metals | [20] |
| - | Frass: in soil at 2.5% or 5% (w/w) | Heavy metal | Heavy metal soil removal | Sorption and complexation of heavy metals | [77] | ||
| Lettuce | Frass: in field at 1.5 t ha−1 | Heavy metal | Less accumulation of Pb in the plant | Increase in microbial enzymatic activity in the soil | [92] | ||
| Barley | Frass: in soil at 10–12.5 g/L | Drought | Increased plant tolerance to drought | Promotion of plant photosynthetic activity | [131] | ||
| Lettuce | Frass: 12.5 g per plant | Drought | Increased plant tolerance to drought | Promotion of plant photosynthetic activity | [109] | ||
| Insects | Crop/Plant | Form of Application and Dosage | Abiotic Stress | Effects | Mechanism of Action | References | |
|---|---|---|---|---|---|---|---|
| Order | Species | ||||||
| Coleoptera | Allomyrina dichotoma | Rice Tomato Wheat Barley Pepper | As microorganisms isolated from frass | Pathogens: Rhizoctonia solani (fungus), Phytophthora capsici (oomycete), Colletotrichum coccodes (fungus), and Fusarium oxysporum (fungus) | Inhibition of fungal and oomycete growth (in vitro) | Production of antifungal and oomyceticidal compounds via Bacillus amyloliquefaciens (bacteria) | [141] |
| Calosoma sayi | - | Frass | Insect pest: Spodoptera frugiperda (Lepidoptera) | Insect pest death | Entomopathogens vehicle (nuclear polyhedrosis virus and Vairimorpha sp.) | [128] | |
| Diaprepes abbreviates | Citrus crops | As frass extracts | Insect pest: Diaprepes abbreviatus (Coleoptera) | Insect pest death | Entomopathogens attraction (Steinernema diaprepesi and Heterorhabditis indica) | [114] | |
| Hylobius abietis | Scots pine (Pinus sylvestris) | As fungal volatiles (extracted from frass) | Insect pest: Hylobius abietis (Coleoptera) | Reduction in infestation | Fungal volatiles cancel out the volatiles from the host plant | [124] | |
| Scots pine (Pinus sylvestris) | As bacteria volatiles (extracted from frass) | Insect pest: H. abietis (Coleoptera) | Reduction in infestation | Bacteria volatiles act as antifeedant compounds | [133] | ||
| Hylotrupes bajulus | - | Frass | Insect pest: Hylotrupes bajulus (Coleoptera) | Not identified | Parasitoid attraction (Sclerodermus cereicollis and S. domesticus) | [132] | |
| Hypothenemus hampei | - | As frass extracts | Insect pest: Hypothenemus hampei (Coleoptera) | Not identified | Parasitoid attraction (Cephalonomia stephanoderis and Prorops nasuta) | [102,112] | |
| Osphranteria coerulescens | Apricot | Frass | Insect pest: Osphranteria coerulescens (Coleoptera) | Insect pest death | Entomopathogens vehicle (Heterorhabditis bacteriophora and Steinernema carpocapsae) | [135] | |
| Phyllophaga vandinei | - | Frass | Insect pest: Phyllophaga vandinei (Coleoptera) | Insect pest death | Entomopathogen vehicle (invertebrate iridescent virus 6) | [130] | |
| Prostephanus truncatus | - | As frass extracts | Insect pest: Prostephanus truncatus (Coleoptera) | Not identified | Predator attraction (Teretrius nigrescens) | [138] | |
| Protaetia brevitarsis | - | As bacteria isolated from frass | Pathogens: Sclerotinia sclerotiorum, S. rolfsii and F. oxysporum (fungi) | Inhibition of fungal growth (in vitro) | Production of antifungal compounds via Bacillus subtilis (bacteria) | [134] | |
| - | As bacteria isolated from frass | Pathogens: Fusarium oxysporum, Pyricularia grisea, Rhizoctonia solani, and Stagonosporopsis cucurbitacearum (fungi) | Inhibition of fungal growth (in vitro) | Production of antifungal compounds via Streptomyces albidoflavus and Nocardiopsis flavescens (bacteria) | [88] | ||
| Rhyzopertha dominica | Wheat (grains) | Frass | Insect pest: Rhyzopertha dominica (Coleoptera) | Not identified | Parasitoid attraction (Theocolax elegans) | [125] | |
| Tenebrio molitor | Arabidopsis thaliana | Frass: in soil at 2% (v/v) | Pathogen: Botrytis cinerea (fungus) | Less fungal lesions | Induction of systemic defensive responses | [111] | |
| Trichoferus holosericeus | - | Frass | Insect pest: Trichoferus holosericeus (Coleoptera) | Not identified | Parasitoid attraction (Sclerodermus cereicollis and S. domesticus) | [132] | |
| Collembola * | Heteromurus nitidus | Annual meadow grass (Poa annua) (in microcosm chambers) White clover (Trifolium repens) (in microcosm chambers) | Frass | Insect pest: Myzus persicae (aphids) | Reduction in aphid reproduction | Not identified | [137] |
| Onychiurus scotarius | Annual meadow grass (P. annua) (in microcosm chambers) White clover (T. repens) (in microcosm chambers) | Frass | Insect pest: M. persicae (aphids) | Reduction in aphid reproduction | Not identified | [137] | |
| Dermaptera | Forficula auricularia | - | Frass | Pathogens: Staphylococcus aureus (bacteria) and Aspergillus niger (fubgus) | Inhibition of bacterial multiplication and fungal growth | Presence of antimicrobial compounds | [113] |
| Diptera | Ceratitis capitata | Coffee (in field) | As frass extracts | Insect pest: Ceratitis capitata (Diptera) | Reduction in infestation | Pheromone-deterrent effect | [101] |
| Hermetia illucens | Kale Swiss chard | Frass: in field at 10.3 t ha−1 | Insect pest: Aphis spp. (Hemiptera), Plutella xylostella (Lepidoptera), Bemisia tabaci (Hemiptera), and Liriomyza spp. (Diptera) | Reduces pest infestation | Induction of systemic defensive responses | [143] | |
| Brussels sprouts | Frass: in soil at 5 g/kg | Insect pest: Delia radicum (Diptera) | Insect pest death | Unidentified | [87] | ||
| - | As frass extracts | Pathogens: Alternaria solani, Botrytis cinerea, Fusarium oxysporum, Rhizoctonia solani, Sclerotinia sclerotiorum (fungi), and Phytophthora capsici (oomycete) | Inhibition of fungal and oomycete growth | Presence of antifungal and oomyceticidal compounds from microorganisms | [129] | ||
| Mustard (Brassica rapa) | Frass: in soil at 2 g/kg | Insect pest: D. radicum (Diptera) and P. xylostella (Lepidoptera) | Insect pest death | Unidentified | [119] | ||
| Spinach | As frass extracts: 100 mL per plant | Pathogen: Meloidogyne incognita (nematode) | Suppressed nematode egg hatchability J2s paralysis and death Suppression of gall development | Presence of nematicidal compounds | [123] | ||
| Brussels sprouts | Frass: in soil at 5 g/Kg | Insect pest: D. radicum (Diptera) | Insect pest death | Unidentified | [126] | ||
| Lepidoptera | Anticarsia gemmatalis | Soya | Frass | Insect pest: Anticarsia gemmatalis (Lepidoptera) | Insect pest death | Entomopathogen vehicle (Anticarsia gemmatalis multiple nucleopolyhedrovirus) | [100] |
| Busseola fusca | Maize | Frass | Plant pathogen: Fusarium verticillioides (fungus) | Not identified | Biological control agent vehicle: Acremonium zeae (fungus) | [107] | |
| Cydalima perspectalis | - | As frass extracts (volatile compounds) | Insect pest: Cydalima perspectalis (Lepidoptera) | Not identified | Oviposition-repellent effect | [84] | |
| Cydia pomonella | Apple (in vitro) | Frass | Insect pest: Cydia pomonella (Lepidoptera) | Not identified | Parasitoid attraction (Hyssopus pallidus) | [96] | |
| Helicoverpa zea | Tomato | As protein extract from frass | Insect pest: S. frugiperda (Lepidoptera) | Reduction in plant biomass consumption by the insect pest | Induction of systemic defensive responses | [98] | |
| Hyblaea puera | - | Frass | Insect pest: Hyblaea puera (Lepidoptera) | Insect pest death | Entomopathogen vehicle (Hyblaea puera nucleopolyhedrovirus) | [115] | |
| Lymantria dispar | Poplar (Populus nigra) (in vitro) | Frass | Insect pest: Lymntria dispar (Lepidoptera) | Insect pest death | Parasitoid attraction (Glyptapanteles flavicoxis) | [116] | |
| - | Frass | Insect pest: L. dispar (Lepidoptera) | Insect pest death | Entomopathogens vehicle (Nosema lymantriae and Vairimorpha disparis) | [99] | ||
| Ostrinia nubilalis | Maize | As protein extract from frass | Insect pest: Ostrinia nubilalis (Lepidoptera) | Reduction in plant biomass consumption by the insect pest | Induction of systemic defensive responses | [98] | |
| Phthorimaea operculella | - | As frass extracts (volatile compounds) | Insect pest: Phthorimaea operculella (Lepidoptera) | Not identified | Oviposition-repellent Effect | [139] | |
| Spodoptera frugiperda | - | Frass | Insect pest: Spodoptera frugiperda (Lepidoptera) | Not identified | Parasitoid attraction (Cotesia marginiventris) | [117] | |
| Maize | As protein extract from frass | Pathogen: Cochlioblus heterostrophus (fungus) | Reduction in disease severity | Induction of systemic and local defensive responses | [97] | ||
| Maize Rice | As protein extract from frass | Insect pest: S. frugiperda (Lepidoptera) | Reduction in plant biomass consumption by the insect pest | Induction of systemic defensive responses | [98] | ||
| Trichoplusia ni | Cabbage | As protein extract from frass | Insect pest: Trichoplusia ni (Lepidoptera) | Reduction in plant biomass consumption by the insect pest | Induction of systemic defensive responses | [98] | |
| Tuta absoluta | - | Frass | Insect pest: Tuta absoluta (Lepidoptera) | Not identified | Predator attraction (Nesidiocoris tenuis) | [89] | |
| - | Frass | Insect pest: T. absoluta (Lepidoptera) | Not identified | Parasitoid attraction (Dolichogenidea gelechiidivoris) | [86] | ||
| Insects | Crop/Plant | Form of Application and Dosage | Abiotic Stress | Effects | Mechanism of Action | References | |
|---|---|---|---|---|---|---|---|
| Order | Species | ||||||
| Blattodea | Blattella germanica | - | Bacteria isolated from frass | Insect pests: unspecified | Inhibition of fungal bioinsecticides action | Production of antifungal compounds | [88] |
| Coleoptera | Acalymma vittatum | Cucumber | Frass | Pathogen: Erwinia tracheiphila (bacteria) | Pathogen dissemination | Plant pathogen vehicle: Erwinia tracheiphila (bacteria) | [103] |
| Cosmopolites sordidus | Banana | Frass | Pathogen: F. oxysporum f.sp. cubense (fungus) | Pathogen dissemination | Plant pathogen vehicle: F. oxysporum f.sp. cubense (fungus) | [90] | |
| Leptinotarsa decemlineata | Potato | Frass: 20 mg diluted in 20 μL of sterile water per plant | Insect pest: Leptinotarsa decemlineata (Coleoptera) | Inhibition of local plant defense responses | Bacteria in the frass act as inhibitors of plant defenses | [91] | |
| Odontotaenius disjunctus | - | Bacteria isolated from frass | Insect pests: unspecified | Inhibition of fungal bioinsecticides action | Production of antifungal compounds | [118] | |
| Tenebrio molitor | Wheat | Frass | Pathogen: Fusarium proliferatum (fungus) | Pathogen dissemination | Plant pathogen vehicle: Fusarium proliferatum (fungus) | [140] | |
| Mustard (Brassica rapa) | Frass: in soil at 2 g/kg | Insect pest: D. radicum (Diptera) and P. xylostella (Lepidoptera) | Increased insect pest survival | Unidentified | [119] | ||
| Xyleborinus Saxesenii X. affinis X. ferrugineus X. perforans | ʻŌhiʻa lehua (Metrosideros polymorpha) | Frass | Pathogen: Ceratocystis lukuohia and Ceratocystis huliohia (fungi) | Pathogen dissemination | Plant pathogen vehicle: Ceratocystis lukuohia and Ceratocystis huliohia (fungi) | [121] | |
| Dermaptera | Forficula auricularia | - | Frass | Insect pest: Forficula auricularia (Dermaptera) | Increase in pest insect population | Coprophagy | [110] |
| Diptera | Bradysia impatiens | - | Frass | Pathogen: Thielaviopsis basicola (fungus) | Pathogen dissemination | Plant pathogen vehicle: Thielaviopsis basicola (fungus) | [95] |
| Drosophila suzukii | Raspberry | Frass | Pathogens: Cladosporium cladosporioides and C. pseudocladosporioides (fungi) | Pathogen dissemination | Plant pathogen vehicle: Cladosporium cladosporioides and C. pseudocladosporioides (fungi) | [104] | |
| Raspberry | Frass | Pathogens: Cladosporium spp. and Botrytis spp. (fungi) | Pathogen dissemination | Plant pathogen vehicle: Cladosporium spp. and Botrytis spp. (fungi) | [139] | ||
| Psychoda spp. | - | Frass | Pathogen: Thielaviopsis basicola (fungus) | Pathogen dissemination | Plant pathogen vehicle: Thielaviopsis basicola (fungus) | [95] | |
| Scatella stagnalis | - | Frass | Pathogen: T. basicola (fungus) | Pathogen dissemination | Plant pathogen vehicle: T. basicola (fungus) | [105] | |
| - | Frass | Pathogen: T. basicola (fungus) | Pathogen dissemination | Plant pathogen vehicle: T. basicola (fungus) | |||
| Lepidoptera | Busseola fusca | Maize | Frass | Pathogen: Aspergillus spp. and Fusarium spp. (fungi) | Pathogen dissemination | Plant pathogen vehicle: Aspergillus spp. and Fusarium spp. (fungi) | [107] |
| Spodoptera frugiperda | Maize | As protein extract from frass | Insect pest: Spodoptera frugiperda (Lepidoptera) | Increased consumption of plant biomass by the insect pest | Inhibition of systemic and local defensive responses | [97,98] | |
| S. littoralis | Cotton | As bacteria volatiles (extracted from frass) | Insect pest: Spodoptera littoralis (Lepidoptera) | Increased plant infection | Bacterial volatiles act as attractants for insect pest larvae | [85] | |
| Orthoptera | Locusta migratoria | Maize | Frass | Pathogen: Colletotrichum graminicola (fungus) | Pathogen dissemination | Plant pathogen vehicle: Colletotrichum graminicola (fungus) | [83] |
| Melanoplus bivittatus | Alfalfa | Frass (10 fecal pellets per plant) | Pathogen: Verticillium albo-atrum (fungus) | Pathogen dissemination | Plant pathogen vehicle: Verticillium albo-atrum (fungus) | [106] | |
| M. sanguinipes | Alfalfa | Frass (10 fecal pellets per plant) | Pathogen: V. albo-atrum (fungus) | Pathogen dissemination | Plant pathogen vehicle: V. albo-atrum (fungus) | [106,127] | |
| Schistocerca gregaria | Maize | Frass | Pathogen: C. graminicola (fungus) | Pathogen dissemination | Plant pathogen vehicle: C. graminicola (fungus) | [83] | |
References
- Menegat, S.; Ledo, A.; Tirado, R. Greenhouse Gas Emissions from Global Production and Use of Nitrogen Synthetic Fertilisers in Agriculture. Sci. Rep. 2022, 12, 14490. [Google Scholar] [CrossRef]
- United Nations Department of Economic and Social Affairs Population Division. World Population Prospects 2024: Summary of Results; United Nations Publications: New York, NY, USA, 2024. [Google Scholar]
- The Global Economy. Value Added in the Agricultural Sector as a Percentage of GDP. 2023. Available online: https://www.theglobaleconomy.com/rankings/share_of_agriculture/ (accessed on 25 January 2025).
- van Dijk, M.; Morley, T.; Rau, M.L.; Saghai, Y. A Meta-Analysis of Projected Global Food Demand and Population at Risk of Hunger for the Period 2010–2050. Nat. Food 2021, 2, 494–501. [Google Scholar] [CrossRef]
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2023; FAO: Rome, Italy, 2023. [Google Scholar]
- Dhuldhaj, U.P.; Singh, R.; Singh, V.K. Pesticide Contamination in Agro-Ecosystems: Toxicity, Impacts, and Bio-Based Management Strategies. Environ. Sci. Pollut. Res. 2023, 30, 9243–9270. [Google Scholar]
- Anjaria, P.; Vaghela, S. Toxicity of Agrochemicals: Impact on Environment and Human Health. J. Toxicol. 2024, 2024, 250. [Google Scholar] [CrossRef]
- European Commission. Farm to Fork Strategy; European Commission. 2019. Available online: https://ec.europa.eu/food/farm2fork_en (accessed on 2 January 2025).
- Hénault-Ethier, L.; Quinche, M.; Reid, B.; Hotte, N.; Fortin, A.; Normandin, É.; de La Rochelle Renaud, G.; Rasooli Zadeh, A.; Deschamps, M.H.; Vandenberg, G. Opportunities and Challenges in Upcycling Agri-Food Byproducts to Generate Insect Manure (Frass): A Literature Review. Waste Manag. 2024, 176, 169–191. [Google Scholar] [CrossRef]
- Oshunsanya, S.O.; Nwosu, N.J.; Li, Y. Abiotic Stress in Agricultural Crops Under Climatic Conditions. In Sustainable Agriculture, Forest and Environmental Management; Springer: Singapore, 2019; pp. 71–100. [Google Scholar]
- Razzaq, A.; Wani, S.H.; Saleem, F.; Yu, M.; Zhou, M.; Shabala, S. Rewilding Crops for Climate Resilience: Economic Analysis and de Novo Domestication Strategies. J. Exp. Bot. 2021, 72, 6123–6139. [Google Scholar]
- Kopecká, R.; Kameniarová, M.; Černý, M.; Brzobohatý, B.; Novák, J. Abiotic Stress in Crop Production. Int. J. Mol. Sci. 2023, 24, 6603. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Prueger, J.H. Temperature Extremes: Effect on Plant Growth and Development. Weather. Clim. Extrem. 2015, 10, 4–10. [Google Scholar] [CrossRef]
- Breshears, D.D.; Fontaine, J.B.; Ruthrof, K.X.; Field, J.P.; Feng, X.; Burger, J.R.; Law, D.J.; Kala, J.; Hardy, G.E.S.J. Underappreciated Plant Vulnerabilities to Heat Waves. New Phytol. 2021, 231, 32–39. [Google Scholar] [CrossRef]
- Brás, T.A.; Seixas, J.; Carvalhais, N.; Jagermeyr, J. Severity of Drought and Heatwave Crop Losses Tripled over the Last Five Decades in Europe. Environ. Res. Lett. 2021, 16, 065012. [Google Scholar] [CrossRef]
- Hristov, J.; Toreti, A.; Pérez Domínguez, I.; Dentener, F.; Fellmann, T.; Elleby, C.; Ceglar, A.; Fumagalli, D.; Niemeyer, S.; Cerrani, I.; et al. Analysis of Climate Change Impacts on EU Agriculture by 2050 JRC PESETA IV Project-Task 3; Publications Office of the European Union: Luxembourg, 2020. [Google Scholar]
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2024: Financing to End Hunger, Food Insecurity and Malnutrition in All Its Forms; FAO: Rome, Italy, 2024. [Google Scholar]
- Balasubramaniam, T.; Shen, G.; Esmaeili, N.; Zhang, H. Plants’ Response Mechanisms to Salinity Stress. Plants 2023, 12, 2253. [Google Scholar] [CrossRef]
- Angon, P.B.; Islam, M.S.; KC, S.; Das, A.; Anjum, N.; Poudel, A.; Suchi, S.A. Sources, Effects and Present Perspectives of Heavy Metals Contamination: Soil, Plants and Human Food Chain. Heliyon 2024, 10, e28357. [Google Scholar] [CrossRef]
- Wang, X.; Wu, N.; Wu, X.; Geng, W.; Xu, X. Effect of Insect Feces (Hermetia illucens) on Rice Growth and Heavy Metal Migration from Polluted Soil to Rice Plant. Environ. Sci. Pollut. Res. 2022, 29, 14695–14704. [Google Scholar] [CrossRef]
- Kumar, S.; Shah, S.H.; Vimala, Y.; Jatav, H.S.; Ahmad, P.; Chen, Y.; Siddique, K.H.M. Abscisic Acid: Metabolism, Transport, Crosstalk with Other Plant Growth Regulators, and Its Role in Heavy Metal Stress Mitigation. Front. Plant Sci. 2022, 13, 972856. [Google Scholar] [CrossRef]
- Singh, A.; Roychoudhury, A. Abscisic Acid in Plants under Abiotic Stress: Crosstalk with Major Phytohormones. Plant Cell Rep. 2023, 42, 961–974. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Li, R.; Ge, Y.; Li, Y.; Li, R. Plants’ Response to Abiotic Stress: Mechanisms and Strategies. Int. J. Mol. Sci. 2023, 24, 10915. [Google Scholar] [CrossRef]
- Marcuello, C.; Foulon, L.; Chabbert, B.; Aguié-Béghin, V.; Molinari, M. Atomic Force Microscopy Reveals How Relative Humidity Impacts the Young’s Modulus of Lignocellulosic Polymers and Their Adhesion with Cellulose Nanocrystals at the Nanoscale. Int. J. Biol. Macromol. 2020, 147, 1064–1075. [Google Scholar] [CrossRef]
- Choi, S.J.; Lee, Z.; Kim, S.; Jeong, E.; Shim, J.S. Modulation of Lignin Biosynthesis for Drought Tolerance in Plants. Front. Plant Sci. 2023, 14, 1116426. [Google Scholar] [CrossRef]
- García-Latorre, C.; Rodrigo, S.; Santamaría, O. Endophytes as Plant Nutrient Uptake-Promoter in Plants. In Endophytes: Mineral Nutrient Management; Maheshwari, D.K., Dheeman, S., Eds.; Springer: Cham, Switzerland, 2021; Volume 3, pp. 247–265. [Google Scholar]
- Poveda, J. Cyanobacteria in Plant Health: Biological Strategy against Abiotic and Biotic Stresses. Crop Prot. 2021, 141, 105450. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.K. Abiotic Stress Responses in Plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef]
- Grover, M.; Ali, S.Z.; Sandhya, V.; Rasul, A.; Venkateswarlu, B. Role of Microorganisms in Adaptation of Agriculture Crops to Abiotic Stresses. World J. Microbiol. Biotechnol. 2011, 27, 1231–1240. [Google Scholar] [CrossRef]
- Chandran, V.; Shaji, H.; Mathew, L. Endophytic Microbial Influence on Plant Stress Responses. In Microbial Endophytes: Functional Biology and Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 161–193. ISBN 9780128196540. [Google Scholar]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The Global Burden of Pathogens and Pests on Major Food Crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef]
- Junaid, M.; Gokce, A. Global agricultural losses and their causes. Bull. Biol. Allied Sci. Res. 2024, 2024, 66. [Google Scholar] [CrossRef]
- Savary, S.; Bregaglio, S.; Willocquet, L.; Gustafson, D.; Mason D’Croz, D.; Sparks, A.; Castilla, N.; Djurle, A.; Allinne, C.; Sharma, M.; et al. Crop Health and Its Global Impacts on the Components of Food Security. Food Secur. 2017, 9, 311–327. [Google Scholar] [CrossRef]
- Gai, Y.; Wang, H. Plant Disease: A Growing Threat to Global Food Security. Agronomy 2024, 14, 1615. [Google Scholar] [CrossRef]
- Ekwomadu, T.I.; Mwanza, M. Fusarium Fungi Pathogens, Identification, Adverse Effects, Disease Management, and Global Food Security: A Review of the Latest Research. Agriculture 2023, 13, 1810. [Google Scholar] [CrossRef]
- Wegulo, S.N.; Baenziger, P.S.; Hernandez Nopsa, J.; Bockus, W.W.; Hallen-Adams, H. Management of Fusarium Head Blight of Wheat and Barley. Crop Prot. 2015, 73, 100–107. [Google Scholar] [CrossRef]
- Overton, K.; Maino, J.L.; Day, R.; Umina, P.A.; Bett, B.; Carnovale, D.; Ekesi, S.; Meagher, R.; Reynolds, O.L. Global Crop Impacts, Yield Losses and Action Thresholds for Fall Armyworm (Spodoptera frugiperda): A Review. Crop Prot. 2021, 145, 105641. [Google Scholar] [CrossRef]
- Tortella, G.; Rubilar, O.; Pieretti, J.C.; Fincheira, P.; de Melo Santana, B.; Fernández-Baldo, M.A.; Benavides-Mendoza, A.; Seabra, A.B. Nanoparticles as a Promising Strategy to Mitigate Biotic Stress in Agriculture. Antibiotics 2023, 12, 338. [Google Scholar] [CrossRef]
- Liang, Y.; Nikolic, M.; Bélanger, R.; Gong, H.; Song, A. Silicon in Agriculture: From Theory to Practice; Springer: Dordrecht, The Netherlands, 2015; ISBN 9789401799782. [Google Scholar]
- Gulzar, N.; Ali, S.; Shah, M.A.; Kamili, A.N. Silicon Supplementation Improves Early Blight Resistance in Lycopersicon esculentum Mill. by Modulating the Expression of Defense-Related Genes and Antioxidant Enzymes. 3 Biotech 2021, 11, 232. [Google Scholar] [CrossRef]
- Verma, K.K.; Song, X.P.; Tian, D.D.; Guo, D.J.; Chen, Z.L.; Zhong, C.S.; Nikpay, A.; Singh, M.; Rajput, V.D.; Singh, R.K.; et al. Influence of Silicon on Biocontrol Strategies to Manage Biotic Stress for Crop Protection, Performance, and Improvement. Plants 2021, 10, 2163. [Google Scholar] [CrossRef] [PubMed]
- González Guzmán, M.; Cellini, F.; Fotopoulos, V.; Balestrini, R.; Arbona, V. New Approaches to Improve Crop Tolerance to Biotic and Abiotic Stresses. Physiol. Plant 2022, 174, e13547. [Google Scholar] [CrossRef]
- Segaran, G.; Shah, P.; Gurumurthy, J.; Sathiavelu, M. Fungal Metabolites as Crop Pathogen Controllers. In Fungal Metabolites for Agricultural Applications: Biostimulation and Crop Protection by Fungal Biotechnology; Poveda, J., Santamaría, O., Martín-García, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2024; pp. 105–136. [Google Scholar]
- Olivadese, M.; Dindo, M.L. Edible Insects: A Historical and Cultural Perspective on Entomophagy with a Focus on Western Societies. Insects 2023, 14, 690. [Google Scholar] [CrossRef] [PubMed]
- van Huis, A.; van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013. [Google Scholar]
- Cadinu, L.A.; Barra, P.; Torre, F.; Delogu, F.; Madau, F.A. Insect Rearing: Potential, Challenges, and Circularity. Sustainability 2020, 12, 4567. [Google Scholar] [CrossRef]
- Sogari, G.; Bellezza Oddon, S.; Gasco, L.; van Huis, A.; Spranghers, T.; Mancini, S. Review: Recent Advances in Insect-Based Feeds: From Animal Farming to the Acceptance of Consumers and Stakeholders. Animal 2023, 17, 100904. [Google Scholar]
- Tuhumury, H.C.D. Edible Insects: Alternative Protein for Sustainable Food and Nutritional Security. In IOP Conference Series: Earth and Environmental Science; IOP Publishing Ltd.: Bristol, UK, 2021; Volume 883. [Google Scholar]
- Lin, X.; Wang, F.; Lu, Y.; Wang, J.; Chen, J.; Yu, Y.; Tao, X.; Xiao, Y.; Peng, Y. A Review on Edible Insects in China: Nutritional Supply, Environmental Benefits, and Potential Applications. Curr. Res. Food Sci. 2023, 7, 100596. [Google Scholar]
- Niyonsaba, H.H.; Hohler, J.; Kooistra, J.; van der Fels-Klerx, H.J.; Meuwissen, M.P.M. Profitability of Insect Farms. J. Insects Food Feed. 2021, 7, 923–934. [Google Scholar] [CrossRef]
- Barragán-Fonseca, K.B.; Gómez, D.; Lalander, C.H.; Dzepe, D.; Chia, S.Y. Review—Insect Farming for Food and Feed in the Global South: Focus on Black Soldier Fly Production. Animal 2024, 101397. [Google Scholar] [CrossRef]
- Reverberi, M. Edible Insects: Cricket Farming and Processing as an Emerging Market. J. Insects Food Feed. 2020, 6, 211–220. [Google Scholar] [CrossRef]
- Tanga, C.M.; Kababu, M.O. New Insights into the Emerging Edible Insect Industry in Africa. Anim. Front. 2023, 13, 26–40. [Google Scholar] [CrossRef]
- Abril, S.; Pinzón, M.; Hernández-Carrión, M.; Sánchez-Camargo, A.D.P. Edible Insects in Latin America: A Sustainable Alternative for Our Food Security. Front. Nutr. 2022, 9, 904812. [Google Scholar] [CrossRef] [PubMed]
- Madau, F.A.; Arru, B.; Furesi, R.; Pulina, P. Insect Farming for Feed and Food Production from a Circular Business Model Perspective. Sustainability 2020, 12, 5418. [Google Scholar] [CrossRef]
- van Huis, A. Insects as Food and Feed, a New Emerging Agricultural Sector: A Review. J. Insects Food Feed. 2020, 6, 27–44. [Google Scholar] [CrossRef]
- IPIFF. The European Insect Sector Today: Challenges, Opportunities and Regulatory Landscape. In IPIFF Vision Paper on the Future of the Insect Sector Towards 2030; IPIFF: Brussels, Belgium, 2019. [Google Scholar]
- He, L.; Zhang, Y.; Ding, M.Q.; Li, M.X.; Ding, J.; Bai, S.W.; Wu, Q.L.; Zhao, L.; Cao, G.L.; Ren, N.Q.; et al. Sustainable Strategy for Lignocellulosic Crop Wastes Reduction by Tenebrio molitor Linnaeus (Mealworm) and Potential Use of Mealworm Frass as a Fertilizer. J. Clean. Prod. 2021, 325, 129301. [Google Scholar] [CrossRef]
- Lopes, I.G.; Yong, J.W.; Lalander, C. Frass Derived from Black Soldier Fly Larvae Treatment of Biodegradable Wastes. A Critical Review and Future Perspectives. Waste Manag. 2022, 142, 65–76. [Google Scholar] [CrossRef]
- Beesigamukama, D.; Subramanian, S.; Tanga, C.M. Nutrient Quality and Maturity Status of Frass Fertilizer from Nine Edible Insects. Sci. Rep. 2022, 12, 7182. [Google Scholar] [CrossRef]
- Poveda, J.; Jiménez-Gómez, A.; Saati-Santamaría, Z.; Usategui-Martín, R.; Rivas, R.; García-Fraile, P. Mealworm Frass as a Potential Biofertilizer and Abiotic Stress Tolerance-Inductor in Plants. Appl. Soil Ecol. 2019, 142, 110–122. [Google Scholar] [CrossRef]
- Barragán-Fonseca, K.Y.; Nurfikari, A.; van de Zande, E.M.; Wantulla, M.; van Loon, J.J.A.; de Boer, W.; Dicke, M. Insect Frass and Exuviae to Promote Plant Growth and Health. Trends Plant Sci. 2022, 27, 646–654. [Google Scholar] [CrossRef]
- Antoniadis, V.; Molla, A.; Grammenou, A.; Apostolidis, V.; Athanassiou, C.G.; Rumbos, C.I.; Levizou, E. Insect Frass as a Novel Organic Soil Fertilizer for the Cultivation of Spinach (Spinacia oleracea): Effects on Soil Properties, Plant Physiological Parameters, and Nutrient Status. J. Soil Sci. Plant Nutr. 2023, 23, 5935–5944. [Google Scholar] [CrossRef]
- Zunzunegui, I.; Martín-García, J.; Santamaría, Ó.; Poveda, J. Analysis of Yellow Mealworm (Tenebrio molitor) Frass as a Resource for a Sustainable Agriculture in the Current Context of Insect Farming Industry Growth. J. Clean. Prod. 2024, 460, 142608. [Google Scholar] [CrossRef]
- Liu, T.; Klammsteiner, T.; Dregulo, A.M.; Kumar, V.; Zhou, Y.; Zhang, Z.; Awasthi, M.K. Black Soldier Fly Larvae for Organic Manure Recycling and Its Potential for a Circular Bioeconomy: A Review. Sci. Total Environ. 2022, 833, 155122. [Google Scholar] [CrossRef] [PubMed]
- Moruzzo, R.; Riccioli, F.; Espinosa Diaz, S.; Secci, C.; Poli, G.; Mancini, S. Mealworm (Tenebrio molitor): Potential and Challenges to Promote Circular Economy. Animals 2021, 11, 2568. [Google Scholar] [CrossRef] [PubMed]
- Yildirim-Aksoy, M.; Eljack, R.; Schrimsher, C.; Beck, B.H. Use of Dietary Frass from Black Soldier Fly Larvae, Hermetia Illucens, in Hybrid Tilapia (Nile × Mozambique, Oreocromis niloticus × O. mozambique) Diets Improves Growth and Resistance to Bacterial Diseases. Aquac. Rep. 2020, 17, 100373. [Google Scholar] [CrossRef]
- Yildirim-Aksoy, M.; Eljack, R.; Beck, B.H.; Peatman, E. Nutritional Evaluation of Frass from Black Soldier Fly Larvae as Potential Feed Ingredient for Pacific White Shrimp, Litopenaeus vannamei. Aquac. Rep. 2022, 27, 101353. [Google Scholar] [CrossRef]
- Poveda, J. Insect Frass in the Development of Sustainable Agriculture. A Review. Agron. Sustain. Dev. 2021, 41, 5. [Google Scholar] [CrossRef]
- Beesigamukama, D.; Tanga, C.M.; Sevgan, S.; Ekesi, S.; Kelemu, S. Waste to Value: Global Perspective on the Impact of Entomocomposting on Environmental Health, Greenhouse Gas Mitigation and Soil Bioremediation. Sci. Total Environ. 2023, 902, 166067. [Google Scholar]
- Nogalska, A.; Przemieniecki, S.W.; Krzebietke, S.J.; Kosewska, A.; Załuski, D.; Kozera, W.J.; Żarczyński, P.J. Farmed Insect Frass as a Future Organic Fertilizer. Appl. Sci. 2024, 14, 2380. [Google Scholar] [CrossRef]
- Fuertes-Mendizábal, T.; Salcedo, I.; Huérfano, X.; Riga, P.; Estavillo, J.M.; Ávila Blanco, D.; Duñabeitia, M.K. Mealworm Frass as a Potential Organic Fertilizer in Synergy with PGP-Based Biostimulant for Lettuce Plants. Agronomy 2023, 13, 1258. [Google Scholar] [CrossRef]
- Lomonaco, G.; Franco, A.; De Smet, J.; Scieuzo, C.; Salvia, R.; Falabella, P. Larval Frass of Hermetia illucens as Organic Fertilizer: Composition and Beneficial Effects on Different Crops. Insects 2024, 15, 293. [Google Scholar] [CrossRef]
- Gärttling, D.; Schulz, H. Compilation of Black Soldier Fly Frass Analyses. J. Soil. Sci. Plant Nutr. 2022, 22, 937–943. [Google Scholar] [CrossRef]
- Praeg, N.; Klammsteiner, T. Primary Study on Frass Fertilizers from Mass-Reared Insects: Species Variation, Heat Treatment Effects, and Implications for Soil Application at Laboratory Scale. J. Environ. Manag. 2024, 356, 120622. [Google Scholar] [CrossRef] [PubMed]
- Salomon, M.J.; Cavagnaro, T.R.; Burton, R.A. Potential of Black Soldier Fly Larvae Frass (BSFL) as a Novel Fertilizer: Impacts on Tomato Growth, Nutrient Uptake, and Mycorrhizal Formation. Plant Soil. 2025, 1–18. [Google Scholar] [CrossRef]
- Watson, C.; Schlösser, C.; Vögerl, J.; Wichern, F. Excellent Excrement? Frass Impacts on a Soil’s Microbial Community, Processes and Metal Bioavailability. Appl. Soil Ecol. 2021, 168, 104110. [Google Scholar] [CrossRef]
- Hénault-Ethier, L.; Reid, B.; Hotte, N.; Paris, N.; Quinche, M.; Lachance, C.; Fortin, A.; Normandin, É.; Laderrière, V.; Vandenberg, G. Growth Trials on Vegetables, Herbs and Flowers Using Mealworm Frass, Chicken Manure and Municipal Compost. ACS Agric. Sci. Technol. 2023, 3, 249–259. [Google Scholar] [CrossRef]
- Wang, L.; Aerospace, B. Mealworm Frass and Vermicompost Tea as Novel Plant-Ready Nutrients for Indoor Hydroponics Bok Choy Production System. Master’s Thesis, California State Polytechnic University, Pomona, CA, USA, 2022. [Google Scholar]
- European Commission. Commission Regulation (EU) 2021/1925 of 5 November 2021 Amending Certain Annexes to Regulation (EU) No 142/2011 as Regards the Requirements for Placing on the Market of Certain Insect Products and the Adaptation of a Containment Method; European Commission: Brussels, Belgium, 2021; pp. 4–8. [Google Scholar]
- Verified Market Reports. Global Insect Frass (Biofertilizers) Market by Type (Molitor Larvae, Mealworm), by Application (Seed Treatment, Soil Treatment); Verified Market Reports: Washington, DC, USA, 2025. [Google Scholar]
- Martín-Martín, A.; Thelwall, M.; Orduna-Malea, E.; Delgado López-Cózar, E. OpenCitations’ COCI: A Multidisciplinary Comparison of Coverage via Citations. Scientometrics 2021, 126, 871–906. [Google Scholar] [CrossRef]
- Hasan, S. The Possible Role of Two Species of Orthoptera in the Dissemination of a Plant Pathogenic Fungus. Ann. Appl. Biol. 1982, 101, 205–209. [Google Scholar]
- Molnár, B.P.; Tóth, Z.; Kárpáti, Z. Synthetic Blend of Larval Frass Volatiles Repel Oviposition in the Invasive Box Tree Moth, Cydalima perspectalis. J. Pest Sci. 2017, 90, 873–885. [Google Scholar] [CrossRef]
- Revadi, S.V.; Giannuzzi, V.A.; Vetukuri, R.R.; Walker, W.B.; Becher, P.G. Larval Response to Frass and Guaiacol: Detection of an Attractant Produced by Bacteria from Spodoptera littoralis Frass. J. Pest Sci. 2021, 94, 1105–1118. [Google Scholar] [CrossRef]
- Ayelo, P.M.; Mohamed, S.A.; Chailleux, A.; Yusuf, A.A.; Pirk, C.W.W.; Deletre, E. The Parasitoid Dolichogenidea Gelechiidivoris eavesdrops on Semiochemicals from Its Host Tuta absoluta and Tomato. J. Pest. Sci. 2022, 95, 633–652. [Google Scholar] [CrossRef]
- Wantulla, M.; Dicke, M.; van Loon, J.J.A. Effects of Amending Soil with Black Soldier Fly Frass on Survival and Growth of the Cabbage Root Fly (Delia radicum) Depend on Soil Type. J. Pest. Sci. 2024, 97, 1451–1459. [Google Scholar] [CrossRef]
- Zhang, F.; Sun, X.X.; Zhang, X.C.; Zhang, S.; Lu, J.; Xia, Y.M.; Huang, Y.H.; Wang, X.J. The Interactions between Gut Microbiota and Entomopathogenic Fungi: A Potential Approach for Biological Control of Blattella germanica (L.). Pest Manag. Sci. 2018, 74, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Ayelo, P.M.; Pirk, C.W.W.; Yusuf, A.A.; Chailleux, A.; Mohamed, S.A.; Deletre, E. Exploring the Kairomone-Based Foraging Behaviour of Natural Enemies to Enhance Biological Control: A Review. Front. Ecol. Evol. 2021, 9, 641974. [Google Scholar]
- Guillen Sánchez, C.; Tixier, P.; Tapia Fernández, A.; Conejo Barboza, A.M.; Sandoval Fernández, J.A.; de Lapeyre de Bellaire, L. Can the Banana Weevil Cosmopolites sordidus Be a Vector of Fusarium oxysporum f. sp. cubense Race 1? Unravelling the Internal and External Acquisition of Effective Inoculum. Pest Manag. Sci. 2021, 77, 3002–3012. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Ju, X.; Yang, M.; Xue, R.; Li, Q.; Fu, K.; Guo, W.; Tong, L.; Song, Y.; Zeng, R.; et al. Colorado Potato Beetle Exploits Frass-Associated Bacteria to Suppress Defense Responses in Potato Plants. Pest Manag. Sci. 2022, 78, 3778–3787. [Google Scholar] [CrossRef]
- Cardarelli, M.; El Chami, A.; Iovieno, P.; Rouphael, Y.; Bonini, P.; Colla, G. Organic Fertilizer Sources Distinctively Modulate Productivity, Quality, Mineral Composition, and Soil Enzyme Activity of Greenhouse Lettuce Grown in Degraded Soil. Agronomy 2023, 13, 194. [Google Scholar] [CrossRef]
- Thalassinos, G.; Levizou, E.; Antoniadis, V. Can Soil Improvers (Biochar, Compost, Insect Frass, Lime, and Zeolite) Achieve Phytostabilization of Potentially Toxic Elements in Heavily Contaminated Soil with the Use of Purslane (Portulaca oleracea)? Agronomy 2023, 13, 2827. [Google Scholar] [CrossRef]
- El-Hamalawi, Z.A. Attraction, Acquisition, Retention and Spatiotemporal Distribution of Soilborne Plant Pathogenic Fungi by Shore Flies. Ann. Appl. Biol. 2008, 152, 169–177. [Google Scholar] [CrossRef]
- El-Hamalawi, Z.A. Acquisition, Retention and Dispersal of Soilborne Plant Pathogenic Fungi by Fungus Gnats and Moth Flies. Ann. Appl. Biol. 2008, 153, 195–203. [Google Scholar] [CrossRef]
- Gandolfi, M.; Mattiacci, L.; Dorn, S. Mechanisms of behavioral alterations of parasitoids reared in artificial systems. J. Chem. Ecol. 2003, 29, 1871–1887. [Google Scholar] [CrossRef]
- Ray, S.; Alves, P.C.M.S.; Ahmad, I.; Gaffoor, I.; Acevedo, F.E.; Peiffer, M.; Jin, S.; Han, Y.; Shakee, S.; Felton, G.W.; et al. Turnabout Is Fair Play: Herbivory-Induced Plant Chitinases Excreted in Fall Armyworm Frass Suppress Herbivore Defenses in Maize. Plant Physiol. 2016, 171, 694–706. [Google Scholar] [CrossRef]
- Ray, S.; Basu, S.; Rivera-Vega, L.J.; Acevedo, F.E.; Louis, J.; Felton, G.W.; Luthe, D.S. Lessons from the Far End: Caterpillar FRASS-Induced Defenses in Maize, Rice, Cabbage, and Tomato. J. Chem. Ecol. 2016, 42, 1130–1141. [Google Scholar] [CrossRef] [PubMed]
- Goertz, D.; Hoch, G. Modeling Horizontal Transmission of Microsporidia Infecting Gypsy Moth, Lymantria Dispar. (L.), Larvae. Biol. Control. 2011, 56, 263–270. [Google Scholar] [CrossRef]
- Del-Angel, C.; Lasa, R.; Mercado, G.; Rodríguez-del-Bosque, L.A.; Caballero, P.; Williams, T. Acquisition of Lethal Infection, Hypermobility and Modified Climbing Behavior in Nucleopolyhedrovirus Infected Larvae of Anticarsia gemmatalis. Biol. Control 2018, 125, 90–97. [Google Scholar] [CrossRef]
- Arredondo, J.; Díaz-Fleischer, F. Oviposition Deterrents for the Mediterranean Fruit Fly, Ceratitis capitata (Diptera: Tephritidae) from Fly Faeces Extracts. Bull. Entomol. Res. 2006, 96, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Chiu-Alvarado, P.; Valle-Mora, J.; Rojas, J.C. Chemical Cues from the Coffee Berry Borer Influence the Locomotory Behaviour of Its Bethylid Parasitoids. Bull. Entomol. Res. 2010, 100, 707–714. [Google Scholar] [CrossRef]
- Mitchell, R.F.; Hanks, L.M. Insect Frass as a Pathway for Transmission of Bacterial Wilt of Cucurbits. Environ. Entomol. 2009, 38, 395–403. [Google Scholar]
- Lewis, M.T.; Koivunen, E.E.; Sweet, C.L.; Hamby, K.A. Associations Between Drosophila suzukii (Diptera: Drosophilidae) and Fungi in Raspberries. Environ. Entomol. 2019, 48, 68–79. [Google Scholar]
- Stanghellini, M.E.; Rasmussen, S.L.; Kim, D.H. Ecology and Population Biology Aerial Transmission of Thielaviopsis basicola, a Pathogen of Corn-Salad, by Adult Shore Flies. Phytopathology 1999, 89, 476–479. [Google Scholar] [CrossRef]
- Huang, H.C.; Harper, A.M. Ecology and Epidemiology Survival of Verticillium albo-atrum from Alfalfa in Feces of Leaf-Chewing Insects. Phytopathology 1984, 75, 206–208. [Google Scholar]
- Ncube, E.; Truter, M.; Flett, B.C.; Van Den Berg, J.; Erasmus, A.; Viljoen, A. Fungal Mycoflora Associated with Busseola fusca Frass in Maize Plants. Afr. Entomol. 2020, 28, 394–405. [Google Scholar] [CrossRef]
- Kim, H.B.; Lee, J.H.; Lee, Y.J.; Rho, J.S.; Lee, J.M.; Kim, S.H.; Park, J.H.; Seo, D.C. Adsorption Characteristics and Mechanism of Cd by Mealworm Frass. Appl. Biol. Chem. 2025, 68, 5. [Google Scholar] [CrossRef]
- Sawinska, Z.; Radzikowska-Kujawska, D.; Kowalczewski, P.Ł.; Grzanka, M.; Sobiech, Ł.; Skrzypczak, G.; Drożdżyńska, A.; Ślachciński, M.; Świtek, S. Hermetia illucens Frass Fertilization: A Novel Approach for Enhancing Lettuce Resilience and Photosynthetic Efficiency under Drought Stress Conditions. Appl. Sci. 2024, 14, 2386. [Google Scholar] [CrossRef]
- Körner, M.; Diehl, J.M.C.; Meunier, J. Growing up with Feces: Benefits of Allo-Coprophagy in Families of the European Earwig. Behav. Ecol. 2016, 27, arw113. [Google Scholar] [CrossRef]
- Blakstad, J.I.; Strimbeck, R.; Poveda, J.; Bones, A.M.; Kissen, R. Frass from Yellow Mealworm (Tenebrio molitor) as Plant Fertilizer and Defense Priming Agent. Biocatal. Agric. Biotechnol. 2023, 53, 102862. [Google Scholar] [CrossRef]
- Chiu-Alvarado, P.; Rojas, J.C. Behavioural Responses of Bethylid Parasitoid Species of the Coffee Berry Borer to Chemicals Cues from Host and Non-Host Dust/Frass. BioControl 2011, 56, 45–53. [Google Scholar] [CrossRef]
- Diehl, J.M.C.; Körner, M.; Pietsch, M.; Meunier, J. Feces Production as a Form of Social Immunity in an Insect with Facultative Maternal Care. BMC Evol. Biol. 2015, 15, 40. [Google Scholar] [CrossRef]
- Rivera, M.J.; Martini, X.; Khrimian, A.; Stelinski, L. A Weevil Sex Pheromone Serves as an Attractant for Its Entomopathogenic Nematode Predators. Chemoecology 2017, 27, 199–206. [Google Scholar] [CrossRef]
- Bindu, T.N.; Balakrishnan, P.; Sajeev, T.V.; Sudheendrakumar, V.V. Role of Soil and Larval Excreta in the Horizontal Transmission of the Baculovirus HpNPV and Its Implications in the Management of Teak Defoliator Hyblaea Puera. Curr. Sci. 2022, 122, 00113891. [Google Scholar] [CrossRef]
- Havill, N.P.; Raffa, K.F. Compound Effects of Induced Plant Responses on Insect Herbivores and Parasitoids: Implications for Tritrophic Interactions. Ecol. Entomol. 2000, 25, 171–179. [Google Scholar] [CrossRef]
- Desneux, N.; Ramírez-Romero, R.; Bokonon-Ganta, A.H.; Bernal, J.S. Attraction of the Parasitoid Cotesia marginiventris to Host (Spodoptera frugiperda) Frass Is Affected by Transgenic Maize. Ecotoxicology 2010, 19, 1183–1192. [Google Scholar] [CrossRef]
- Pessotti, R.C.; Hansen, B.L.; Reaso, J.N.; Ceja-Navarro, J.A.; El-Hifnawi, L.; Brodie, E.L.; Traxler, M.F. Multiple Lineages of Streptomyces Produce Antimicrobials within Passalid Beetle Galleries across Eastern North America. eLife 2021, 10, e65091. [Google Scholar] [CrossRef] [PubMed]
- Chia, S.Y.; van Loon, J.J.A.; Dicke, M. Effects of Frass from Larvae of Black Soldier Fly (Hermetia illucens) and Yellow Mealworm (Tenebrio molitor) on Growth and Insect Resistance in Field Mustard (Brassica rapa): Differences between Insect Species and Frass Treatments. Entomol. Exp. Appl. 2024, 172, 394–408. [Google Scholar] [CrossRef]
- Yang, S.S.; Chen, Y.; Zhang, Y.; Zhou, H.M.; Ji, X.Y.; He, L.; Xing, D.F.; Ren, N.Q.; Ho, S.H.; Wu, W.M. A Novel Clean Production Approach to Utilize Crop Waste Residues as Co-Diet for Mealworm (Tenebrio molitor) Biomass Production with Biochar as Byproduct for Heavy Metal Removal. Environ. Pollut. 2019, 252, 1142–1153. [Google Scholar] [CrossRef] [PubMed]
- Roy, K.; Jaenecke, K.A.; Dunkle, E.J.; Mikros, D.; Peck, R.W. Ambrosia Beetles (Coleoptera: Curculionidae) Can Directly Transmit the Fungal Pathogens Responsible for Rapid ʻŌhiʻa Death. For. Pathol. 2023, 53, e12812. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, T.; Geng, L.; Zhang, C.; Xiang, W.; Zhang, J.; Wang, X.; Shu, C. Characterization and Evaluation of Actinomycete from the Protaetia brevitarsis Larva Frass. Front. Microbiol. 2024, 15, 1385734. [Google Scholar] [CrossRef]
- Kisaakye, J.; Beesigamukama, D.; Haukeland, S.; Subramanian, S.; Thiongo, P.K.; Kelemu, S.; Tanga, C.M. Chitin-Enriched Insect Frass Fertilizer as a Biorational Alternative for Root-Knot Nematode (Meloidogyne incognita) Management. Front. Plant Sci. 2024, 15, 1361739. [Google Scholar] [CrossRef]
- Azeem, M.; Rajarao, G.K.; Terenius, O.; Nordlander, G.; Nordenhem, H.; Kazuhiro, N.; Norin, E.; Borg-Karlson, A.-K. A Fungal Metabolite Masks the Host Plant Odor for the Pine Weevil (Hylobius abietis). Fungal Ecol. 2015, 13, 103–111. [Google Scholar] [CrossRef]
- Giunti, G.; Campolo, O.; Caccamo, P.; Laudani, F.; Palmeri, V. Volatile Infochemicals from Rhyzopertha dominica Larvae and Larval Feces Involved in Theocolax elegans Host Habitat Location. Insects 2021, 12, 142. [Google Scholar] [CrossRef]
- Wantulla, M.; van Zadelhoff, K.; van Loon, J.J.A.; Dicke, M. The Potential of Soil Amendment with Insect Exuviae and Frass to Control the Cabbage Root Fly. J. Appl. Entomol. 2023, 147, 181–191. [Google Scholar] [CrossRef]
- Harper, A.M.; Huang, H.C.; Kozub, G.C. Survival of Verticillium albo-atrum on Insect Bodies and in Insect Feces at Various Temperatures. J. Econ. Entomol. 1988, 81, 1799–1802. [Google Scholar] [CrossRef]
- Young, O.P.; Hamm, J.J. Compatibility of two fall armyworm pathogens with the predaceous beetle, Calosoma sayi (Coleoptera: Carabidae). J. Entomol. Sci. 1985, 20, 212–218. [Google Scholar] [CrossRef]
- Arabzadeh, G.; Delisle-Houde, M.; Dorais, M.; Deschamps, M.H.; Derome, N.; Vandenberg, G.W.; Tweddell, R.J. Evaluation of the Antagonistic Activity of Black Soldier Fly Frass Extracts against Plant Pathogens Using Single- and Double-Layer Agar Bioassays. J. Insects Food Feed. 2024, 2, 1–10. [Google Scholar] [CrossRef]
- Jenkins, D.A.; Hunter, W.B.; Goenaga, R. Effects of Invertebrate Iridescent Virus 6 in Phyllophaga vandinei and Its Potential as a Biocontrol Delivery System. J. Insect Sci. 2011, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Grzanka, M.; Sobiech, Ł.; Radzikowska-Kujawska, D.; Sawinska, Z.; Kowalczewski, P.Ł.; Świtek, S.; Skrzypczak, G.; Kardasz, P. The Influence of Hermetia illucens L. Frass on the Health, Stress, and Development of Barley. J. Plant Prot. Res. 2024, 64, 394–401. [Google Scholar] [CrossRef]
- Masini, P.; Austeri, L.; Rebora, M.; Piersanti, S.; de Francesco, F.; Salerno, G. Olfactory Cues in the Host-Location of the European Ecto-Parasitoids Sclerodermus cereicollis and Sclerodermus domesticus (Hymenoptera: Bethylidae). J. Stored Prod. Res. 2024, 109, 102441. [Google Scholar] [CrossRef]
- Axelsson, K.; Konstanzer, V.; Rajarao, G.K.; Terenius, O.; Seriot, L.; Nordenhem, H.; Nordlander, G.; Borg-Karlson, A.K. Antifeedants Produced by Bacteria Associated with the Gut of the Pine Weevil Hylobius abietis. Microb. Ecol. 2017, 74, 177–184. [Google Scholar] [CrossRef]
- Xuan, H.; Gao, P.; Du, B.; Geng, L.; Wang, K.; Huang, K.; Zhang, J.; Huang, T.; Shu, C. Characterization of Microorganisms from Protaetia brevitarsis Larva Frass. Microorganisms 2022, 10, 311. [Google Scholar] [CrossRef]
- Sharifi, S.; Karimi, J.; Hosseini, M.; Rezapanah, M. Efficacy of Two Entomopathogenic Nematode Species as Potential Biocontrol Agents against the Rosaceae Longhorned Beetle, Osphranteria coerulescens, under Laboratory Conditions. Nematology 2014, 16, 729–737. [Google Scholar] [CrossRef]
- Zhang, X.G.; Li, X.; Gao, Y.L.; Liu, Y.; Dong, W.X.; Xiao, C. Oviposition Deterrents in Larval Frass of Potato Tuberworm Moth, Phthorimaea operculella (Lepidoptera: Gelechiidae). Neotrop. Entomol. 2019, 48, 496–502. [Google Scholar] [CrossRef]
- Scheu, S.; Theenhaus, A.; Jones, T.H. Links between the Detritivore and the Herbivore System: Effects of Earthworms and Collembola on Plant Growth and Aphid Development. Oecologia 1999, 119, 541–551. [Google Scholar] [CrossRef]
- Stewart-Jones, A.; Hodges, R.J.; Farman, D.I.; Hall, D.R. Solvent Extraction of Cues in the Dust and Frass of Prostephanus truncatus and Analysis of Behavioural Mechanisms Leading to Arrestment of the Predator Teretrius nigrescens. Physiol. Entomol. 2006, 31, 63–72. [Google Scholar] [CrossRef]
- Swett, C.L.; Hamby, K.A.; Hellman, E.M.; Carignan, C.; Bourret, T.B.; Koivunen, E.E. Characterizing Members of the Cladosporium cladosporioides Species Complex as Fruit Rot Pathogens of Red Raspberries in the Mid-Atlantic and Co-Occurrence with Drosophila suzukii (Spotted Wing Drosophila). Phytoparasitica 2019, 47, 415–428. [Google Scholar] [CrossRef]
- Guo, Z.; Pfohl, K.; Karlovsky, P.; Dehne, H.W.; Altincicek, B. Dissemination of Fusarium Proliferatum by Mealworm Beetle Tenebrio molitor. PLoS ONE 2018, 13, e0204602. [Google Scholar] [CrossRef]
- Nam, H.S.; Yang, H.J.; Oh, B.J.; Anderson, A.J.; Kim, Y.C. Biological Control Potential of Bacillus amyloliquefaciens KB3 Isolated from the Feces of Allomyrina dichotoma Larvae. Plant Pathol. J. 2016, 32, 273–280. [Google Scholar] [CrossRef]
- Ray, S.; Gaffor, I.; Acevedo, F.E.; Helms, A.; Chuang, W.P.; Tooker, J.; Felton, G.W.; Luthe, D.S. Maize Plants Recognize Herbivore-Associated Cues from Caterpillar Frass. J. Chem. Ecol. 2015, 41, 781–792. [Google Scholar] [CrossRef]
- Abiya, A.A.; Kupesa, D.M.; Beesigamukama, D.; Kassie, M.; Mureithi, D.; Thairu, D.; Wesonga, J.; Tanga, C.M.; Niassy, S. Agronomic Performance of Kale (Brassica oleracea) and Swiss Chard (Beta vulgaris) Grown on Soil Amended with Black Soldier Fly Frass Fertilizer under Wonder Multistorey Gardening System. Agronomy 2022, 12, 2211. [Google Scholar] [CrossRef]
- Noble, R.; Roberts, S.J. Eradication of Plant Pathogens and Nematodes during Composting: A Review. Plant Pathol. 2004, 53, 548–568. [Google Scholar] [CrossRef]

| Journal | Number of Papers | Paper References |
|---|---|---|
| Journal of Pest Science | 4 | [84,85,86,87] |
| Pest Management Science | 4 | [88,89,90,91] |
| Agronomy | 3 | [92,93] |
| Annals of Applied Biology | 3 | [83,94,95] |
| Journal of Chemical Ecology | 3 | [96,97,98] |
| Applied Soil Ecology | 2 | [61,77] |
| Biological Control | 2 | [99,100] |
| Bulletin of Entomological Research | 2 | [101,102] |
| Environmental Entomology | 2 | [103,104] |
| Phytopathology | 2 | [105,106] |
| African Entomology | 1 | [107] |
| Applied Biological Chemistry | 1 | [108] |
| Applied Sciences | 1 | [109] |
| Behavioral Ecology | 1 | [110] |
| Biocatalysis and Agricultural Biotechnology | 1 | [111] |
| BioControl | 1 | [112] |
| BMC Evolutionary Biology | 1 | [113] |
| Chemoecology | 1 | [114] |
| Current Science | 1 | [115] |
| Ecological Entomology | 1 | [116] |
| Ecotoxicology | 1 | [117] |
| Elife | 1 | [118] |
| Entomologia Experimentalis et Applicata | 1 | [119] |
| Environmental Pollution | 1 | [120] |
| Environmental Science and Pollution Research | 1 | [20] |
| Forest Pathology | 1 | [121] |
| Frontiers in Microbiology | 1 | [122] |
| Frontiers in Plant Science | 1 | [123] |
| Fungal Ecology | 1 | [124] |
| Insects | 1 | [125] |
| Journal of Applied Entomology | 1 | [126] |
| Journal of Economic Entomology | 1 | [127] |
| Journal of Entomological Science | 1 | [128] |
| Journal of Insects as Food and Feed | 1 | [129] |
| Journal of Insect Science | 1 | [130] |
| Journal of Plant Protection Research | 1 | [131] |
| Journal of Stored Products Research | 1 | [132] |
| Microbial Ecology | 1 | [133] |
| Microorganisms | 1 | [134] |
| Nematology | 1 | [135] |
| Neotropical Entomology | 1 | [136] |
| Oecologia | 1 | [137] |
| Physiological Entomology | 1 | [138] |
| Phytoparasitica | 1 | [139] |
| Plant Physiology | 1 | [97] |
| PLoS ONE | 1 | [140] |
| The Plant Pathology Journal | 1 | [141] |
| Reference | Journal | WoS Citations | Scopus Citations |
|---|---|---|---|
| [137] | Oecologia | - | 148 |
| [61] | Applied Soil Ecology | 98 | 109 |
| [116] | Ecological Entomology | 98 | - |
| [120] | Environmental Pollution | 69 | - |
| [97] | Plant Physiology | 56 | - |
| [142] | Journal of Chemical Ecology | 49 | - |
| [96] | Journal of Chemical Ecology | 44 | - |
| [103] | Environmental Entomology | 43 | - |
| [77] | Applied Soil Ecology | 37 | - |
| [98] | Journal of Chemical Ecology | 32 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zunzunegui, I.; Martín-García, J.; Santamaría, Ó.; Poveda, J. Insect Frass as an Agricultural Resource Against Abiotic and Biotic Crop Stresses: Mechanisms of Action and Possible Negative Effects. Appl. Sci. 2025, 15, 3606. https://doi.org/10.3390/app15073606
Zunzunegui I, Martín-García J, Santamaría Ó, Poveda J. Insect Frass as an Agricultural Resource Against Abiotic and Biotic Crop Stresses: Mechanisms of Action and Possible Negative Effects. Applied Sciences. 2025; 15(7):3606. https://doi.org/10.3390/app15073606
Chicago/Turabian StyleZunzunegui, Irene, Jorge Martín-García, Óscar Santamaría, and Jorge Poveda. 2025. "Insect Frass as an Agricultural Resource Against Abiotic and Biotic Crop Stresses: Mechanisms of Action and Possible Negative Effects" Applied Sciences 15, no. 7: 3606. https://doi.org/10.3390/app15073606
APA StyleZunzunegui, I., Martín-García, J., Santamaría, Ó., & Poveda, J. (2025). Insect Frass as an Agricultural Resource Against Abiotic and Biotic Crop Stresses: Mechanisms of Action and Possible Negative Effects. Applied Sciences, 15(7), 3606. https://doi.org/10.3390/app15073606







