Abstract
Despite extensive research, the exact cause of oral lichenoid lesions remains unknown. The chronic inflammatory tissue reaction mediated by T cells is the basis of the etiological process. However, oral lichenoid lesions often occur in the presence of certain drugs. Our aim was to conduct a preliminary retrospective study to assess the correlation between the administration of statins or HMG-inhibitor CoA reductase, which are commonly used for treating hypercholesterolemia, and the sartans or blockers of the angiotensin II receptor, which are used for treating hypertension, in relation to the occurrence of oral lichenoid lesions. This preliminary retrospective study included 2158 patients who attended the Oral Medicine and Maxillofacial Surgery (Mo-Max) Department of Oral Science and Maxillofacial Surgery, Sapienza University of Rome, from 2019 to 2022. A significant association was found between the presence of oral lichenoid lesions and the simultaneous administration of sartans and statins (χ2 = 46.49; p < 0.001). Of the 2158 patients, 118 (5.5%) were diagnosed with oral lichenoid lesions. In the analysis of standardized residues, we found that pathology developed in 16.3% of patients taking statins and 15.9% of those taking sartans. Oral lichenoid lesions only developed in 4.4% of those not taking these drugs.
1. Introduction
1.1. Literature Review
Oral lichenoid lesions (OLLs) are eruptions in the oral cavity that closely resemble oral lichen planus (OLP) both clinically and histologically but are typically associated with identifiable external triggers, unlike OLP, which has an autoimmune origin. These triggers may include dental materials like amalgams, composite resins, cobalt, and gold. Mercury in amalgam fillings has been particularly debated, with some studies reporting minimal sensitization and no improvement after their removal, while others point to mercury sensitivity as a significant cause. Additionally, certain drugs—particularly nonsteroidal anti-inflammatory drugs (NSAIDs) and angiotensin-converting enzyme (ACE) inhibitors—are well-documented triggers for OLLs. Although other medications, such as beta blockers and methyldopa, have also been implicated, the evidence is often limited to isolated cases [1,2,3,4,5,6,7,8].
Clinically, OLLs tend to appear as unilateral or localized lesions near an identifiable trigger, whereas OLP typically presents with a bilateral and symmetrical distribution. Both conditions present with various lesion types, including whitish lesions such as striae, plaques, and papules, as well as reddish alterations indicative of atrophy, erosion/ulceration, or bullae (Figure 1 and Figure 2). These manifestations may occur independently or in various combinations. OLLs may also display more intense erythema and isolated erosions. Histologically, OLLs and OLP share features, such as a lichenoid inflammatory infiltrate and basal cell degeneration. However, OLLs often demonstrate a more diffuse lymphocytic infiltrate and include inflammatory cells like eosinophils and plasma cells, which are less common in OLP [9,10,11,12,13]. These characteristics suggest greater epithelial instability in OLLs, including cellular atypia, hyperplasia, and neovascularization, potentially increasing its risk of malignant transformation. This epithelial instability, combined with chronic inflammation, creates a pro-oncogenic environment characterized by oxidative DNA damage and cytokine-driven immune dysregulation. Recent studies have indicated that the risk of malignant transformation is higher in OLLs than in OLP, which is driven by a combination of factors such as etiology, histopathology, and inflammation. These findings underline the necessity for the vigilant, long-term monitoring of patients with OLLs, particularly those with additional risk factors like tobacco use, alcohol consumption, hepatitis C infection, or the presence of red-type lesions (atrophic, erosive, or bullous forms) [14,15,16].
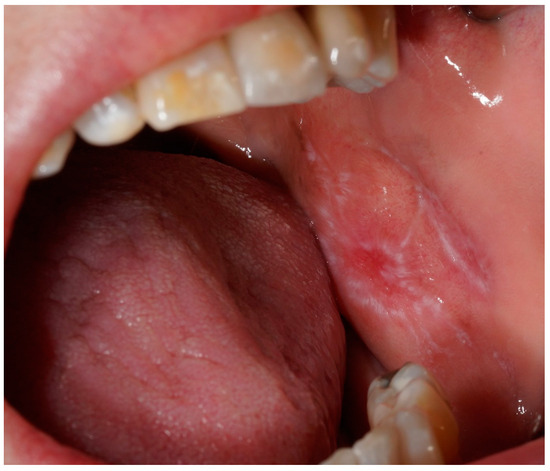
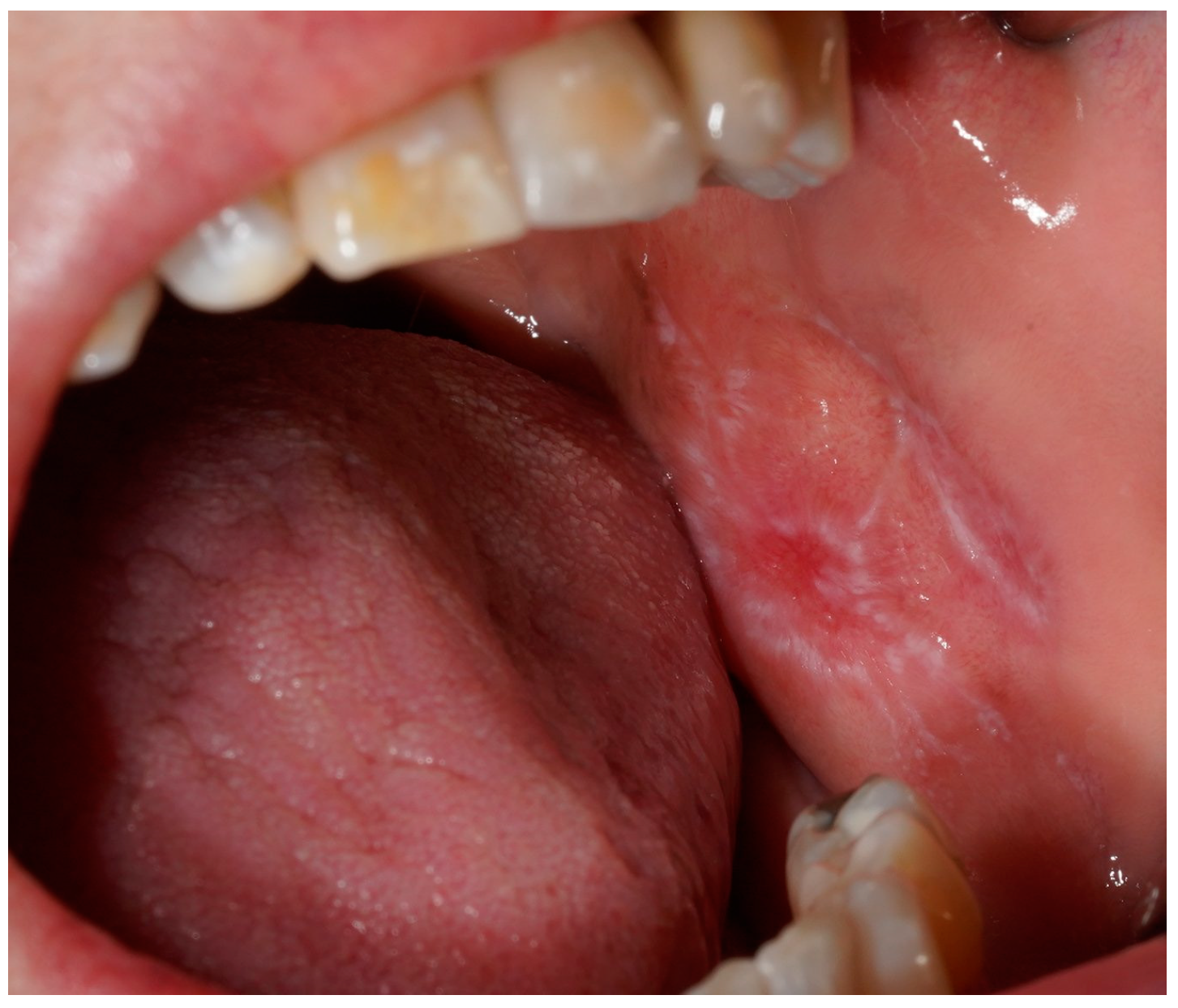
Figure 1.
A clinical intraoral image showing a unilateral whitish, reticulated lesion on the buccal mucosa, with areas of mild erythema. The lesion exhibits a slightly irregular but well-defined pattern, characteristic of lichenoid mucosal alterations.
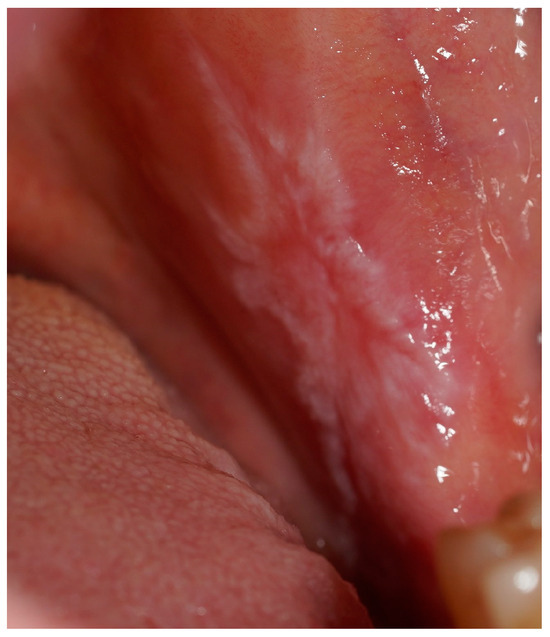
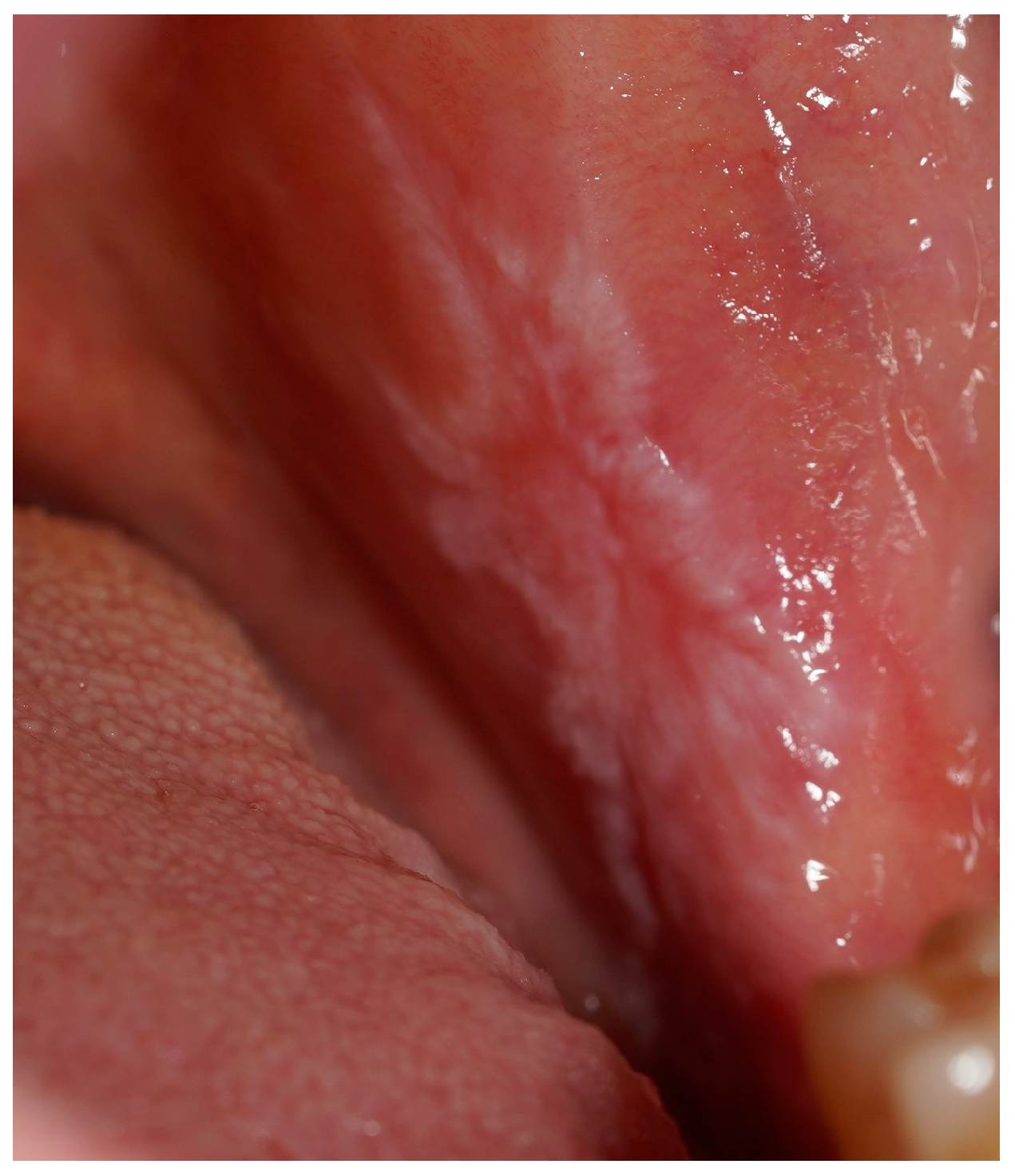
Figure 2.
A clinical intraoral image showing a unilateral whitish, reticulated lesion on the buccal mucosa, accompanied by areas of erythema. The lesion presents a slightly irregular but well-defined pattern, consistent with lichenoid mucosal alterations.
Despite overlapping clinical presentations, OLLs and OLP require different management approaches. In OLLs, addressing the underlying trigger, such as replacing dental materials or discontinuing drugs, can resolve the condition. Conversely, OLP often necessitates long-term treatment, with topical corticosteroids being the first-line therapy. Emerging treatments such as photobiomodulation (PBM) have shown promise in cases unresponsive to corticosteroids, offering anti-inflammatory and biostimulatory benefits [17,18,19,20].
In conclusion, while OLLs and OLP share certain similarities, they are distinct entities with differing etiologies and treatment protocols. Proper differentiation is essential for effective management and improved patient outcomes.
1.2. Theories
OLLs are increasingly recognized as adverse reactions to a wide variety of medications, including antihypertensives, lipid-lowering agents, antimalarials, diuretics, nonsteroidal anti-inflammatory drugs (NSAIDs), and antibiotics. Among antihypertensives, sartans (angiotensin receptor blockers, ARBs) and ACE inhibitors are frequently implicated due to their potential to modulate inflammatory pathways and trigger immune dysregulation. Beta blockers, calcium channel blockers (e.g., amlodipine), and diuretics (e.g., hydrochlorothiazide) have also been associated with OLLs, primarily through mechanisms involving T cell activation, oxidative stress, and delayed hypersensitivity reactions [21,22,23].
Statins, widely used for lipid management, are also notable for their pleiotropic effects on inflammation and T cell modulation, which may contribute to OLL development in predisposed individuals. Elevated cholesterol levels modulate the immune responses of T cells because cholesterol is a key component of the cell membrane. Lymphocyte responses to external signals are coordinated by many molecules that accumulate in the cholesterol-rich regions of the cell membrane called lipid rafts. These lipid rafts serve as platforms for immune cell activation and the inhibition of cholesterol synthesis. Statins interrupt these lipid rafts, affecting the functioning of lymphocytes [24,25,26,27].
OLP, which shares histopathological features with OLLs, is caused by a change in the immune response, in which the T helper 1 lymphocytes no longer intervene. Instead, T helper 2 cells trigger the reactivity of B cells, leading to the production of pathogenic self-antibodies. Although the exact mechanisms linking statins to OLLs remain unclear, their immunomodulatory properties suggest a possible role in triggering or exacerbating these lesions. Histopathological findings in statin-induced OLLs often reveal lymphocytic infiltrates along the basal membrane, apoptotic keratinocytes, and eosinophilic infiltrates, indicating an immune-mediated cytotoxic reaction similar to that observed in drug-induced lichenoid eruptions [28,29].
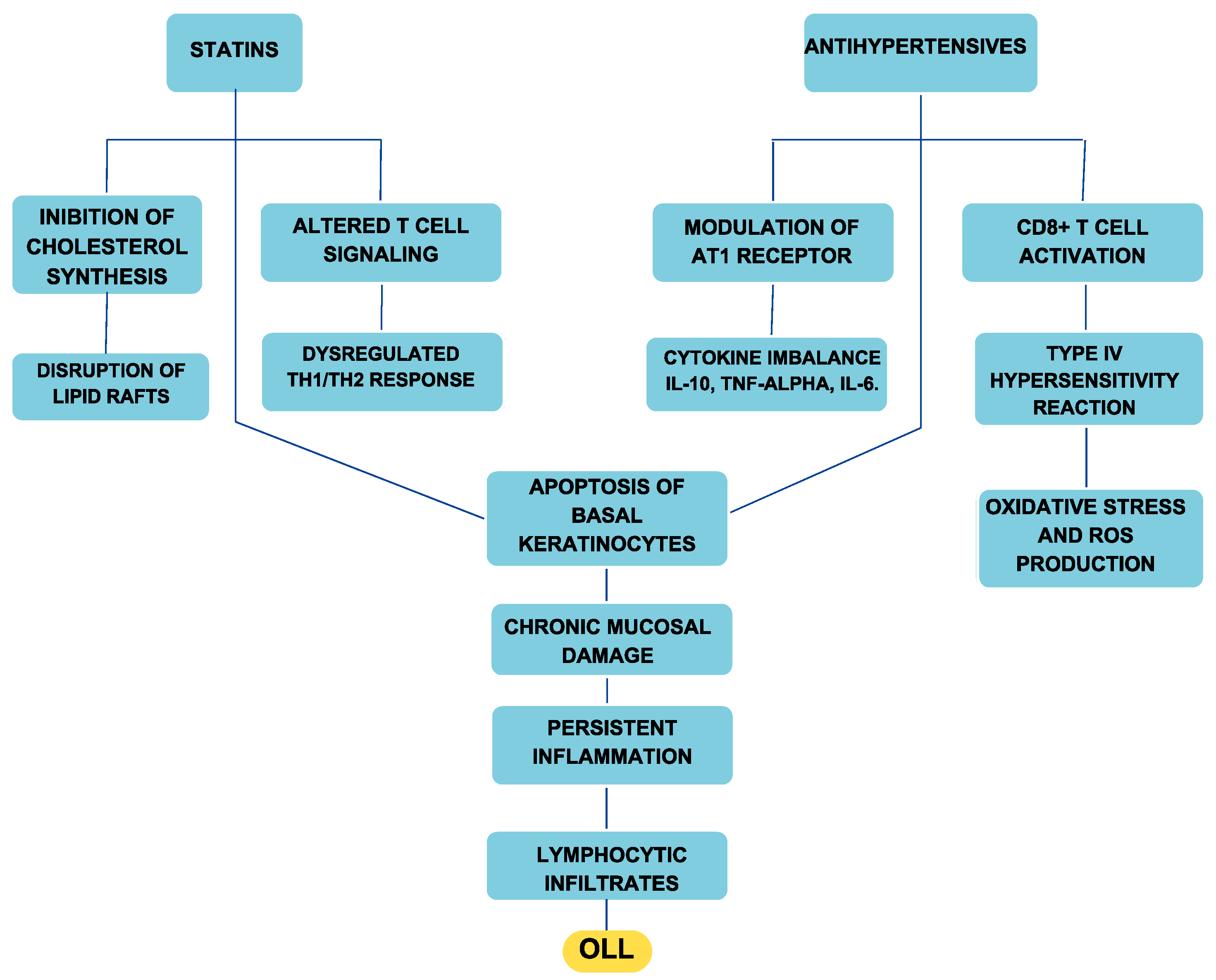
Antihypertensives contribute to OLLs through multiple mechanisms. Type IV hypersensitivity reactions play a crucial role, in which drugs such as ACE inhibitors, sartans, beta blockers, and calcium channel blockers alter antigen presentation and activate CD8+ T lymphocytes, leading to basal keratinocyte apoptosis and chronic mucosal inflammation. Oxidative stress is another significant factor, particularly with beta blockers and calcium antagonists, which can increase reactive oxygen species (ROS), disrupt epithelial integrity, and amplify inflammatory responses. Furthermore, the immunomodulatory effects of antihypertensives, especially sartans, can interfere with cytokine signaling and induce an autoimmune-like response, further exacerbating oral mucosal damage (Figure 3) [30].
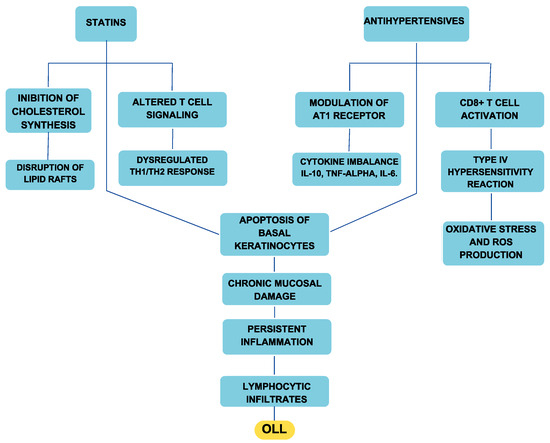
Figure 3.
Diagram illustrating the etiopathogenetic mechanism linking antihypertensives and statins to the onset of OLLs.
A key concern in patients undergoing polydrug therapy is the risk of drug–drug interactions (DDIs), which can amplify adverse effects. DDIs occur when the pharmacokinetics or pharmacodynamics of a drug are altered by the concomitant administration of another medication, potentially intensifying the risk of lichenoid reactions. Sartans, which already exhibit immunomodulatory properties, may further contribute to this risk when combined with statins. A well-known example of drug-induced lichenoid reactions is Grinspan syndrome, in which the concurrent use of antihypertensive and antidiabetic medications has been associated with OLL onset [31].
Diagnosing drug-induced OLLs requires a meticulous evaluation of the patient’s medication history, particularly focusing on recent changes or the long-term use of high-risk drugs. The growing prevalence of OLLs underscores the need for further research into the pharmacological mechanisms underlying these lesions and the development of strategies for their prevention and management.
1.3. Hypotheses
This study aimed to retrospectively share evidence of the correlation between the administration of statins, which are commonly used for the treatment of hypercholesterolemia, and sartans, which are used for the treatment of hypertension, and the occurrence of oral lichenoid lesions (OLLs).
Given their known immunomodulatory effects, we hypothesize that these medications are associated with an increased risk of developing OLLs.
Given that OLLs are included in the World Health Organization’s list of potentially malignant oral disorders, it is crucial to recognize and understand the underlying causes of these lesions. Expanding the current literature through studies such as this one is essential to identify the medications associated with OLLs and improve early detection, management, and prevention strategies, ultimately reducing the risk of malignant transformation [15,16].
2. Materials and Methods
2.1. Selection of Participants
This preliminary retrospective study included 2158 patients who attended the Mo-Max (Oral Medicine and Maxillofacial Surgery) Department of Oral Science and Maxillofacial Surgery, Sapienza University of Rome, from 2019 to 2022. The Mo-Max Department is a task force comprising specialists in different medical disciplines such as oral medicine, prosthetics, maxillofacial surgery, oncology, radiotherapy, and histopathology, who collaborate to provide multidisciplinary group care to patients. The inclusion criteria encompassed adult patients (≥18 years old) with complete medical records documenting their diagnosis, pharmacological treatments, and relevant clinical history. Patients were eligible for this study if they had a confirmed diagnosis of oral lichenoid lesions (OLLs) or were undergoing treatment with statins or sartans, regardless of the presence of OLLs. Only individuals with documented follow-up data over a minimum period of 6 months were considered. Exclusion criteria included patients with incomplete medical records, such as missing information on medication use, diagnosis, or clinical history.
A total of 2158 patient records were analyzed. Of these, 1966 patients (91.1%) were excluded, as they did not meet the inclusion criteria, because they were not undergoing any drug therapy relevant to this study (i.e., neither statins nor sartans) (Table 1 and Table 2). The remaining 192 patients (8.9%) were receiving pharmacological treatment, with 123 (5.7%) on statins and 69 (3.2%) on sartans (Table 2). Among the 2158 patients, 118 (5.5%) were diagnosed with oral lichenoid lesions (OLLs) (Table 3). Of these 118 OLL cases, 20 patients (16.9%) were on statin therapy, 11 (9.3%) were on sartan therapy, and the remaining 87 patients (73.7%) were not undergoing any relevant pharmacological treatment (Table 4). Among these 118 OLL cases, subjects were predominantly female, with 75 women (63.4%) and 43 men, in line with literature reports.

Table 1.
An explanatory table of the cases comparing whether patients were taking drugs.

Table 2.
An explanatory table of cases comparing the total treatment with sartans, statins, or other drugs.

Table 3.
A comparison of the total number of people with oral lichenoid lesions (OLLs) or not diagnosed with OLLs.

Table 4.
An explanatory table of the cases comparing the total drugs (statins, sartans, or other drugs) taken by patients with a proven diagnosis of lichen planus.
2.2. Data Analysis
The data were analyzed with SPSS version 24.0 with the help of GraphPad Prism 10.4.1. The chi-squared test and Fisher’s exact test were used to evaluate the associations between the presence of oral lichenoid lesions (OLLs) and the use of specific drugs (statin and sartans), which allowed for the evaluation of the prevalence of OLLs among patients who were undergoing drug therapy using different categories of drugs.
The results were considered significant at a p-value of <0.05.
3. Results
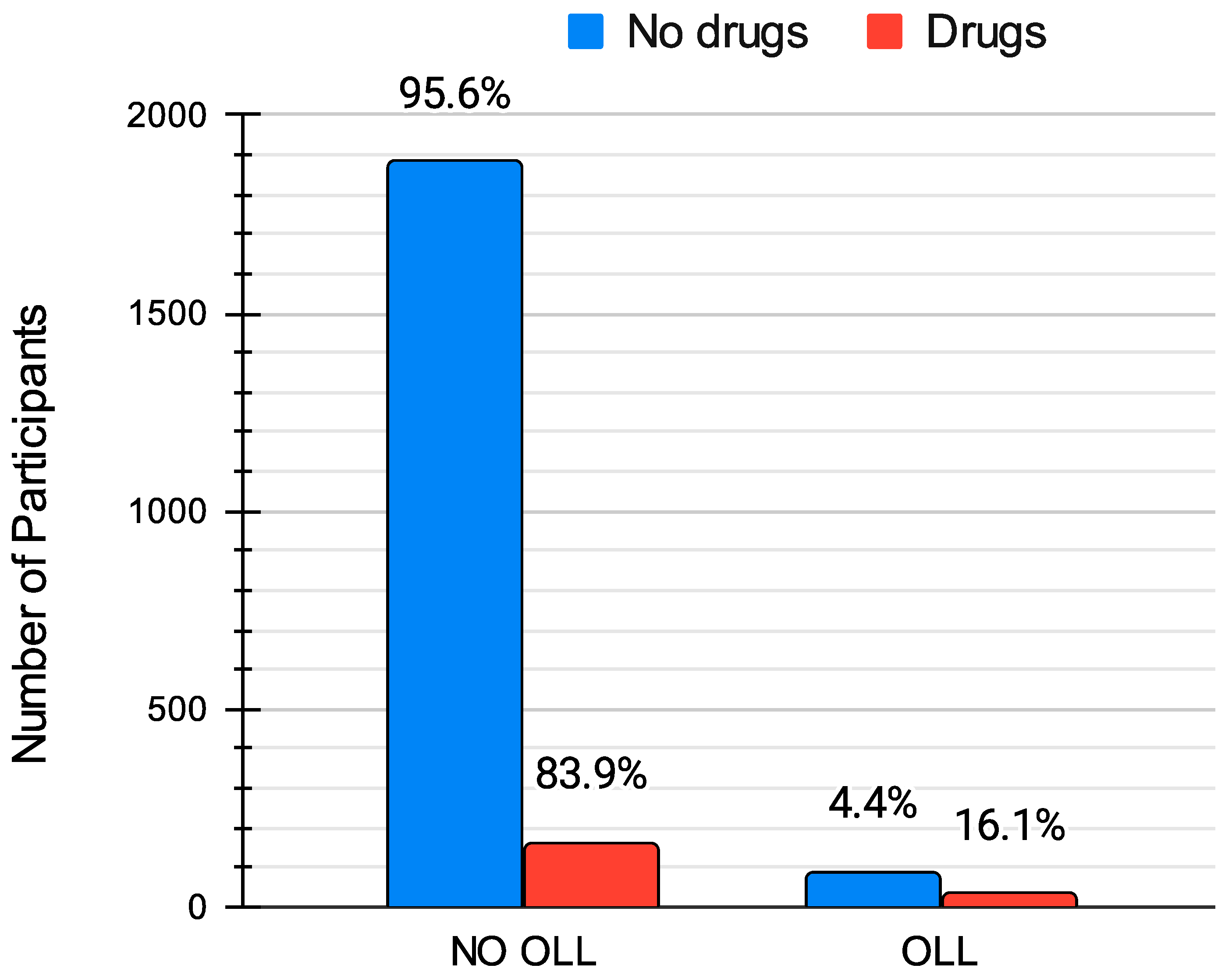
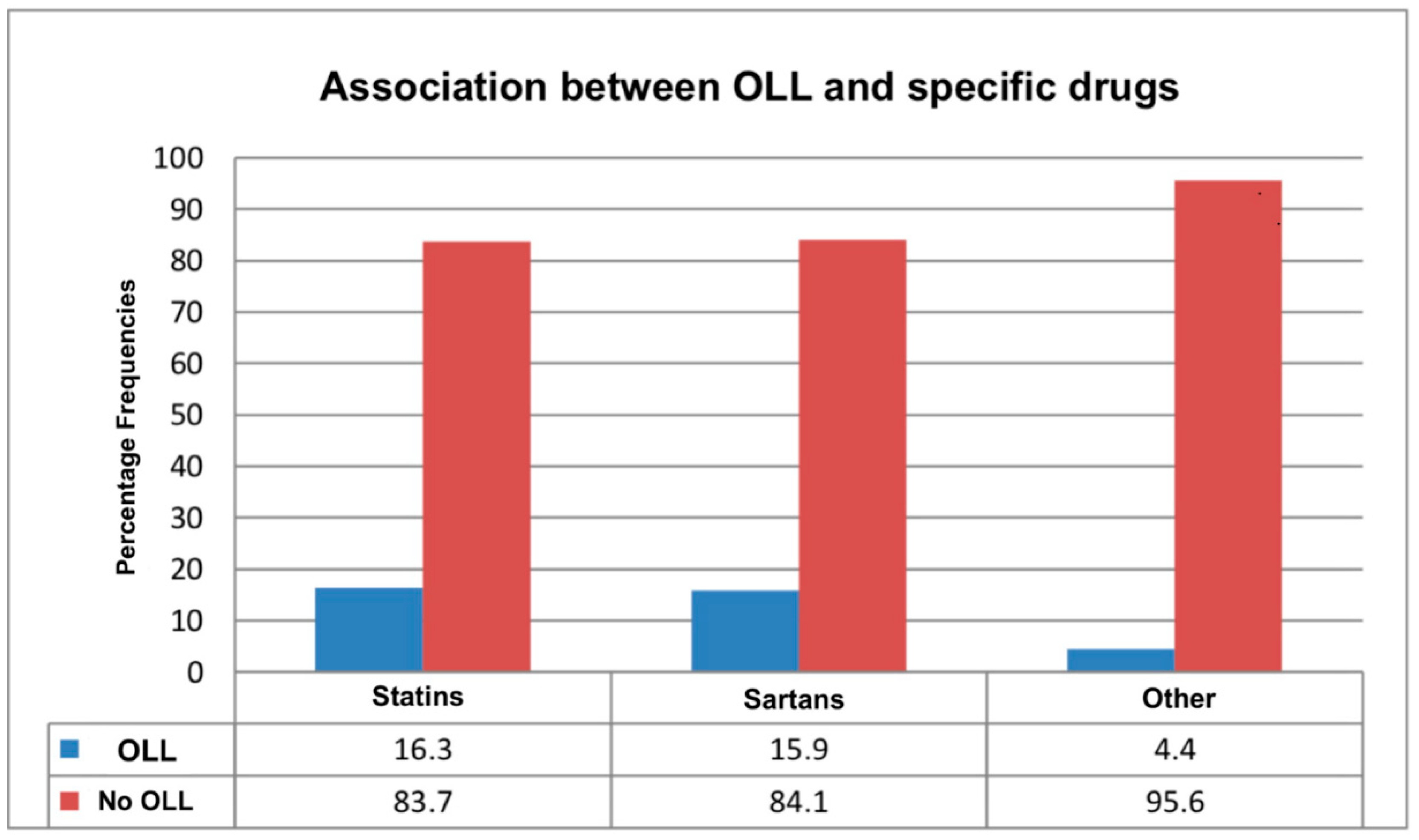
The data analysis revealed a significant association between the presence of oral lichenoid lesions and undergoing pharmacological therapy with statins or sartans (χ2 = 46.49; p < 0.001). Specifically, standardized residuals analysis showed that 16.1% of patients on these medications developed the condition compared with only 4.4% of those not taking such drugs (Table 5, Figure 4). This difference between patients taking statins or sartans and those not on these medications was statistically significant. Furthermore, the analysis highlighted a significant association between OLLs and the specific type of medication used (χ2 = 46.49; p < 0.001). Based on the results of our study, standardized residual analysis indicated that 16.3% of patients taking statins and 15.9% of those on sartans developed the condition. These percentages are notably higher compared with the 4.4% of cases observed among patients not on similar medications (Table 6, Figure 5). However, the analysis did not show a statistically significant difference in the risk of developing OLLs between patients taking statins and those taking sartans.

Table 5.
An explanatory table of the occurrence of oral lichenoid lesions (OLLs) in the presence or absence of statins or sartans.
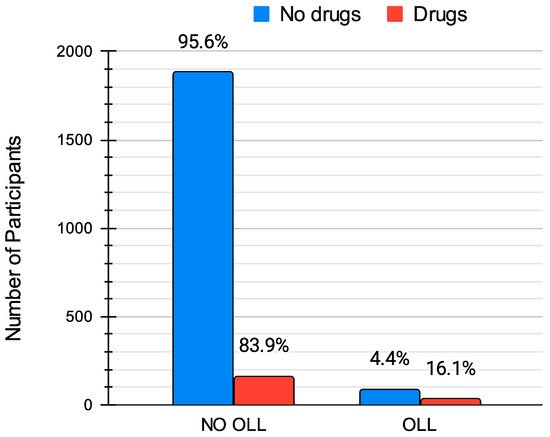
Figure 4.
Comparison of the prevalence of oral lichenoid lesions (OLLs) between patients taking statins or sartans (“Drugs”) and those not on these medications (“No drugs”). The percentages indicate the proportion of patients in each category, showing a significantly higher prevalence of OLLs among those on medication (16.1%) compared to those not taking these drugs (4.4%).

Table 6.
An explanatory table of the occurrence of oral lichenoid lesions (OLLs), with different percentages indicated between the intake of statins and sartans compared with no medication taken.
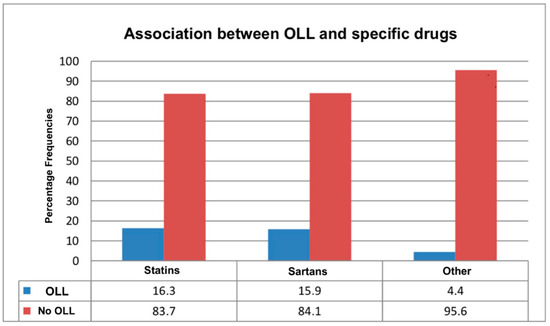
Figure 5.
The proportion of patients who developed oral lichenoid lesions (OLLs) versus those who did not across different drug classes. The blue segments indicate the percentage of patients who developed OLLs, while the red segments show those without OLLs within each drug category.
4. Discussion
Oral lichenoid lesions (OLLs) are a clinical reality that must be properly recognized and managed. Their diagnosis can be challenging, as they often mimic other oral conditions, making it crucial for clinicians to be aware of their characteristics and potential triggers. Among these, drug-induced OLLs represent a significant concern, requiring a thorough understanding of the medications that may be responsible. In our study, the analysis of the standardized residues showed that among those who took statins, 16.3% developed OLLs; among those who took sartans, 15.9% developed OLLs. However, only 4.4% of cases developed OLLs when they did not take similar drugs (Table 6, Figure 1). These findings support prior research implicating antihypertensive agents and lipid-lowering drugs in the onset of OLLs [32,33]. Specifically, ACE inhibitors such as captopril have been linked to lichenoid lesions, often manifesting within months of treatment initiation [32]. Similarly, sartans, including valsartan and losartan, have been associated with lichenoid reactions, with some cases requiring drug discontinuation for lesion resolution [34].
A recent study reported a case of a 60-year-old Chinese man who developed oral and cutaneous lichenoid lesions following amlodipine treatment for hypertension. The lesions resolved gradually after discontinuation of the drug, reinforcing the role of calcium channel blockers in OLL development [35,36].
Building on this, a study involving 465 hypertensive patients aged 20–80 years examined the oral conditions associated with antihypertensive therapy. Among the findings, lichenoid drug lesions were identified in 4.5% of patients and were characterized by bilateral, white, linear striations predominantly on the posterior buccal mucosa. These lesions were strongly linked to the use of ACE inhibitors, particularly captopril [32].
In a related study, the correlation between antihypertensive drugs and OLLs was further explored. Among 450 patients undergoing antihypertensive therapy, 3.3% developed OLLs, with lesions primarily affecting the buccal mucosa, labial mucosa, and retromolar regions. Women were disproportionately affected (66.7% vs. 33.3% of men). OLLs typically appear within an average of 3 months after starting antihypertensive therapy and show regression within weeks to months after discontinuing the suspected drug. The most commonly implicated medications included hydrochlorothiazide, methyldopa, metoprolol, and propranolol. Higher dosages, such as 47.5 mg of metoprolol and 160 mg of valsartan, were particularly associated with an increased risk of OLLs. Conversely, drugs like losartan and bisoprolol demonstrated lower associations with OLLs and did not show significant differences in usage between affected and unaffected patients [33].
Moreover, another study reported a case of OLLs induced by valsartan. A 46-year-old woman developed an itchy rash on her trunk 2 months after starting valsartan. The lesions, appearing within weeks, were erythematous or purplish flat-topped papules. The patient was previously diagnosed with LP and intermittently treated with corticosteroid ointments for 8 months, with little success. As a result, valsartan was changed to another antihypertensive drug, and the lesions were treated with a clobetasol propionate-based ointment. The lesions improved within 2 months [34].
The role of statins in OLL pathogenesis is particularly relevant, given their effects on lipid metabolism and immune modulation. Statins disrupt cholesterol-rich lipid rafts, key structures in T cell signaling, potentially contributing to immune dysregulation and autoimmunity. Previous case reports highlight instances where statins triggered lichenoid eruptions, with symptom resolution upon drug withdrawal. For example, a 63-year-old man developed an itchy and bullous lichenoid eruption after 2 months of simvastatin therapy. The histological and direct immunofluorescence characteristics were consistent with a diagnosis of lichen planus pemphigoid. Withdrawal of the drug led to lesion resolution [28].
As further evidence of the increasing prevalence of OLLs induced by drugs, another study reported two cases of OLLs due to HMG-CoA reductase inhibitors. The subjects experienced lichenoid lesions induced by fluvastatin 4 weeks after the start of therapy; the rash disappeared after the interruption of fluvastatin. After a period of almost 5 weeks without any medical treatment for hypercholesterolemia, the lesions disappeared, but within 2 weeks of starting therapy with lovastatin, a lichenoid rash appeared that was similar to the initial rash, suggesting a class effect among statins. The rash appeared again and disappeared after the interruption of therapy [37].
Similarly, a 55-year-old woman developed pruritic erythematous-gray telangiectatic patches on her right thigh 1 week after starting rosuvastatin. A skin biopsy suggested a lichenoid drug eruption or lupus erythematosus. The lesions resolved after discontinuing rosuvastatin but recurred with simvastatin, spreading to the back, flank, and oral mucosa. This case highlights possible cross-reactivity between rosuvastatin and simvastatin and is the first report of a lichenoid drug eruption associated with rosuvastatin. Recognizing this reaction is crucial for clinicians when prescribing statins in patients with previous adverse cutaneous events [38].
5. Conclusions
This study highlights a significant association between the administration of statins and sartans and the development of oral lichenoid lesions (OLLs), with a notably higher prevalence among patients using these medications than those not exposed to them.
However, as a preliminary retrospective study, it presents inherent limitations. Data analysis alone is insufficient in drawing definitive conclusions, and experimental studies are required to elucidate the molecular pathways through which statins and sartans may contribute to disease development. These constraints highlight the necessity for further research to clarify the mechanisms underlying this reaction, identify high-risk patient subgroups, and determine whether OLL persistence after drug discontinuation is influenced by individual susceptibility or co-existing conditions. Longitudinal studies with larger cohorts could provide valuable insights.
The clinical implications are significant: both physicians and dentists must be vigilant in recognizing OLLs in patients treated with statins or sartans. Prompt identification and timely interventions, such as medication adjustments, can contribute to improving patients’ overall well-being. Given the potential malignant transformation of OLLs, early detection and monitoring in patients on long-term statin or sartan therapy are essential. Clinicians should be aware of this association to ensure timely intervention.
Author Contributions
Conceptualization, D.P. and G.P.; methodology, D.P. and F.B.; software, M.M.; validation, D.P and G.P.; formal analysis, M.M.; investigation, D.P. and F.B.; resources, G.P.; data curation, D.P. and F.B.; writing—original draft preparation, D.P.; writing—review and editing, F.M.G.; visualization, F.M.G.; supervision, G.P., G.T. and U.R.; project administration, G.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding
Institutional Review Board Statement
This study was conducted in accordance with the principles of the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy reasons.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Regezi, J.A. Oral Pathology: Clinical Pathologic Correlations: First South Asia Edition; Elsevier: New Delhi, India, 2016. [Google Scholar]
- Ismail, S.B.; Kumar, S.K.; Zain, R.B. Oral lichen planus and lichenoid reactions: Etiopathogenesis, diagnosis, management and malignant transformation. J. Oral Sci. 2007, 49, 89–106. [Google Scholar] [CrossRef] [PubMed]
- Yiannias, J.A.; El Azhary, R.A.; Hand, J.H.; Pakzad, S.Y.; Rogers, R.S., III. Relevant contact sensitivities in patients with the diagnosis of oral lichen planus. J. Am. Acad. Dermatol. 2000, 42, 177–178. [Google Scholar] [PubMed]
- Hietanen, J.; Pihlman, K.; Forstrom, L.; Linder, E.; Reunala, T. No evidence of hypersensitivity to dental restorative metals in oral lichen planus. Scand. J. Dent. Res. 1987, 95, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Koch, P.; Bahmer, F.A. Oral lesions and symptoms related to metals used in dental restorations: A clinical, allergological, and histologic study. J. Am. Acad. Dermatol. 1999, 41, 422–430. [Google Scholar] [CrossRef]
- Thompson, D.F.; Skaehill, P.A. Drug-induced lichen planus. Pharmacotherapy 1994, 14, 561–571. [Google Scholar] [PubMed]
- McCartan, B.E.; McCreary, C.E. Oral lichenoid drug eruptions. Oral Dis. 1997, 3, 58–63. [Google Scholar] [CrossRef]
- Park, W.-B.; Moon, J.; Shin, S.; Hong, J.-Y. Oral Lichenoid Lesion following Dental Implant Placement and Successful Management with Free Gingival Graft: A Case Report with 10-Year Follow-Up. Medicina 2023, 59, 2188. [Google Scholar] [CrossRef]
- Roopashree, M.R.; Gondhalekar, R.V.; Shashikanth, M.C.; George, J.; Thippeswamy, S.H.; Shukla, A. Pathogenesis of oral lichen planus—A review. J. Oral Pathol. Med. 2010, 39, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Burkhart, N.W.; Burkes, E.J.; Burker, E.J. Meeting the educational needs of patients with oral lichen planus. Gen. Dent. 1997, 45, 126–132. [Google Scholar] [PubMed]
- Scully, C.; Beyli, M.; Ferreiro, M.C.; Ficarra, G.; Gill, Y.; Griffiths, M.; Holmstrup, P.; Mutlu, S.; Porter, S.; Wray, D. Update on oral lichen planus: Etiopathogenesis and management. Crit. Rev. Oral Biol. Med. 1998, 9, 86–122. [Google Scholar] [CrossRef] [PubMed]
- Pergolini, D.; Palaia, G.; Stendardo, V.; Del Vecchio, A.; Tenore, G.; Rocchetti, F.; Rosaria, C.; Di Gioia, T.; Romeo, U. Whitish lesions of the oral mucosa. Dent. Cadmos 2023, 90, 5–6. Available online: https://www.dentalcadmos.com/lesioni-biancastre-della-mucosa-orale/ (accessed on 1 March 2023). [CrossRef]
- Eisen, D.; Carrozzo, M.; Bagan Sebastian, J.V.; Thongprasom, K. Oral lichen planus: Clinical features and management. Oral Dis. 2005, 11, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.B.; Wan, L.S.; Gorsky, M.; Zhang, L. Oral lichen planus: Progress in understanding its malignant potential and the implications for clinical management. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003, 96, 32–37. [Google Scholar] [CrossRef] [PubMed]
- González-Moles, M.Á.; Ramos-García, P. An Evidence-Based Update on the Potential for Malignancy of Oral Lichen Planus and Related Conditions: A Systematic Review and Meta-Analysis. Cancers 2024, 16, 608. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-W.; Li, K.Y.; Chan, B.W.A.; McGrath, C.P.; Zheng, L.-W. Rate of Malignant Transformation Differs Based on Diagnostic Criteria for Oral Lichenoid Conditions: A Systematic Review and Meta-Analysis of 24,277 Patients. Cancers 2023, 15, 2537. [Google Scholar] [CrossRef]
- Del Vecchio, A.; Palaia, G.; Grassotti, B.; Tenore, G.; Ciolfi, C.; Podda, G.; Impellizzeri, A.; Mohsen, A.; Galluccio, G.; Romeo, U. Effects of laser photobiomodulation in the management of oral lichen planus: A literature review. Clin. Ter. 2021, 172, 467–483. [Google Scholar]
- Del Vecchio, A.; Tenore, G.; Luzi, M.C.; Palaia, G.; Mohsen, A.; Pergolini, D.; Romeo, U. Laser photobiomodulation (PBM)—A possible new frontier for the treatment of oral cancer: A review of in vitro and in vivo studies. Healthcare 2021, 9, 134. [Google Scholar] [CrossRef]
- Farhi, D.; Dupin, N. Pathophysiology, etiologic factors, and clinical management of oral lichen planus, part I: Facts and controversies. Clin. Dermatol. 2010, 28, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Scully, C.; Carrozzo, M. Oral mucosal disease: Lichen planus. Br. J. Oral Maxillofac. Surg. 2008, 46, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Potts, A.J.; Hamburger, J.; Scully, C. The medication of patients with oral lichen planus and the association of nonsteroidal anti-inflammatory drugs with erosive lesions. Oral Surg. Oral Med. Oral Pathol. 1987, 64, 541–543. [Google Scholar]
- Robertson, W.D.; Wray, D. Ingestion of medication among patients with oral keratoses including lichen planus. Oral Surg. Oral Med. Oral Pathol. 1992, 74, 183–185. [Google Scholar] [PubMed]
- Fischoff, D.K.; Sternbach, S.; Gomez, J.; Shah, S.S. Medications Associated with Oral Lichenoid Lesions: A Single-Site Retrospective Cohort Study. N. Y. State Dent. J. 2022, 88, 16–21. [Google Scholar]
- Weitz-Schmidt, G.; Welzenbach, K.; Brinkmann, V.; Kamata, T.; Kallen, J.; Bruns, C.; Cottens, S.; Takada, Y.; Hommel, U. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat. Med. 2001, 7, 687. [Google Scholar]
- Fehr, T.; Kahlert, C.; Fierz, W.; Joller-Jemelka, H.I.; Riesen, W.F.; Rickli, H.; Wüthrich, R.P.; Ammann, P. Statin-Induced Immunomodulatory Effects on Human T Cells in Vivo. Atherosclerosis 2004, 175, 83–90. [Google Scholar] [CrossRef]
- Bellosta, S.; Corsini, A. Statins: Drug Interactions and Related Adverse Reactions. Expert Opin. Drug Saf. 2012, 11, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Pergolini, D.; Botticelli, A.; Fascetti, R.; Rocchetti, F.; Cirillo, A.; Tenore, G.; Palaia, G.; Polimeni, A.; Romeo, U. Oral Immune-Related Adverse Events Associated with PD-1 Inhibitor Treatment: A Case Series. Appl. Sci. 2022, 12, 12994. [Google Scholar] [CrossRef]
- Stoebner, P.E.; Michot, C.; Ligeron, C.; Durand, L.; Meynadier, J.; Meunier, L. Simvastatin-Induced Lichen Planus Pemphigoides. Ann. Dermatol. Venereol. 2003, 130, 187–190. [Google Scholar] [PubMed]
- Salem, C.B.; Chenguel, L.; Ghariani, N.; Denguezli, M.; Hmouda, H.; Bouraoui, K. Lichen Planus Pemphigoides Indotto da Captopril. Pharmacoepidemiol. Drug Saf. 2008, 17, 722–724. [Google Scholar] [PubMed]
- Dudhia, B.B.; Dudhia, S.B.; Patel, P.S.; Jani, Y.V. Oral Lichen Planus to Oral Lichenoid Lesions: Evolution or Revolution. J. Oral Med. Radiol. 2015, 19, 364. [Google Scholar] [CrossRef]
- Akram, Z.; Anwar, M.A.; Iqrar, S.; Noor, M.; Manji, S.N.; Arham, H. Oral Lichen Planus: Manifestation of Grinspan’s Syndrome or a Lichenoid Reaction to Medications. Pak. J. Med. Health Sci. 2023, 17, 523. [Google Scholar] [CrossRef]
- Kumar, P.; Mastan, K.M.K.; Chowdhary, R.; Shanmugam, K. Oral Manifestations in Hypertensive Patients: A Clinical Study. J. Oral Maxillofac. Pathol. 2012, 16, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Sarafan, N.; Alipour, A.; Shariati, A. Drug-Induced Oral Lichenoid Reactions in Patients Consuming Antihypertensive Drugs. Cardiovasc. Biomed. J. 2022, 2, 3–10. [Google Scholar] [CrossRef]
- Gencoglan, G.; Ceylan, C.; Kazandi, A.C. Linear Lichenoid Drug Eruption Induced by Valsartan. Clin. Exp. Dermatol. 2009, 34, e334–e335. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health Malaysia. Malaysian Statistics on Medicines 2015–2016; Pharmaceutical Services Programme, Ministry of Health Malaysia: Kuala Lumpur, Malaysia, 2020.
- How, O.J.; Muhamad, R.; Muthusamy, R.; Rahman, F.A.; Zulkifli, M.M. Amlodipine-induced Lichenoid Drug Eruption: A Case Report. Bangladesh J. Med. Sci. 2023, 22, 229–233. [Google Scholar] [CrossRef]
- Sebök, B.; Tóth, M.; Anga, B.; Harangi, F.; Schneider, I. Lichenoid Drug Eruption with HMG-CoA Reductase Inhibitors (Fluvastatin and Lovastatin). Acta Derm. Venereol. 2004, 84, 229–230. [Google Scholar] [CrossRef]
- Wong, I.T.Y.; Huang, Y.; Zhou, Y. Drug Eruption to Rosuvastatin with Recurrence on Simvastatin: A Case Report. J. Cutan. Med. Surg. 2018, 22, 359–361. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).