Density and Viscosity in Biodiesel + Diesel Mixtures from Recycled Feedstocks
Abstract
1. Introduction
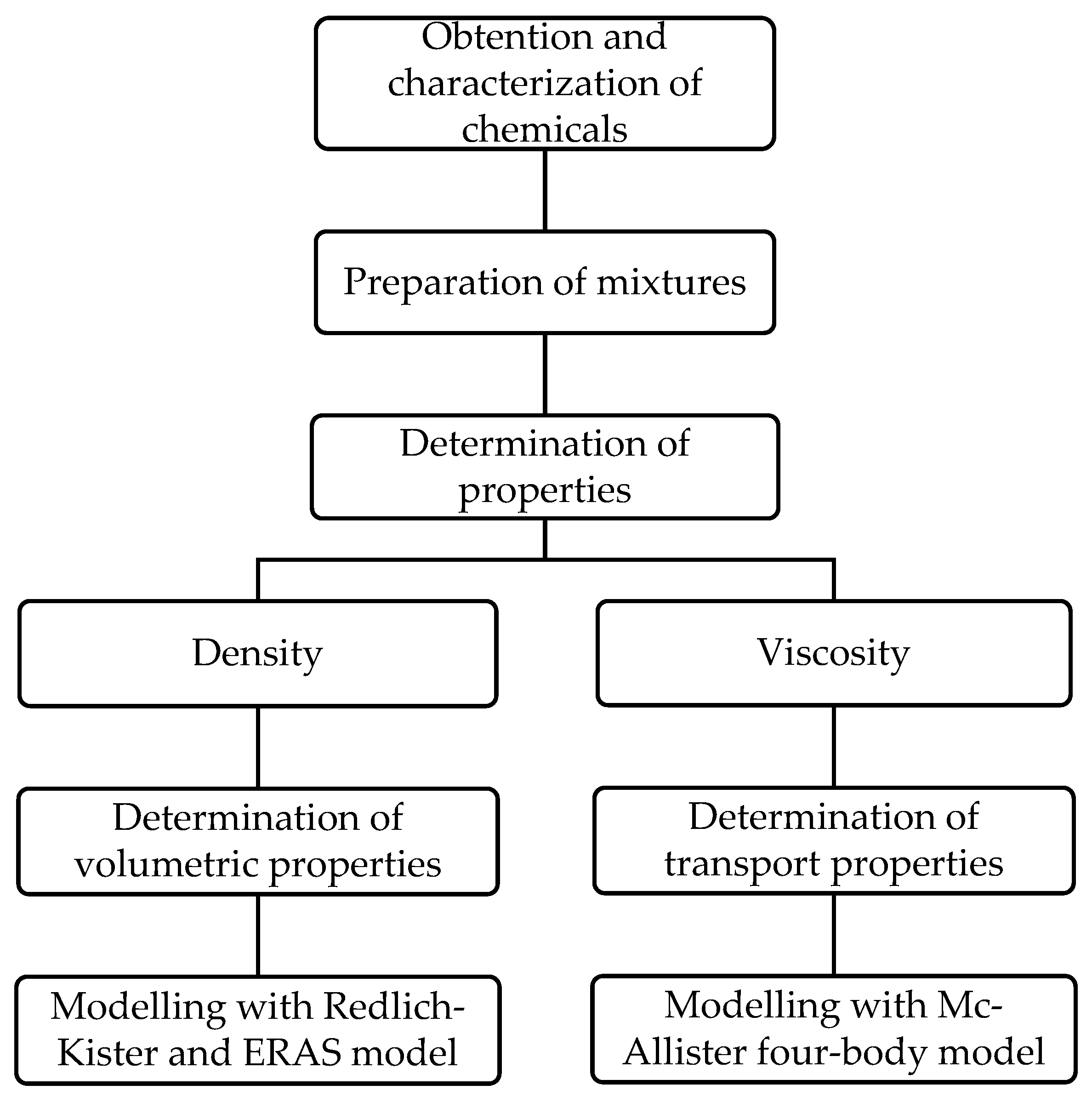
2. Materials and Methods
3. Results and Discussions
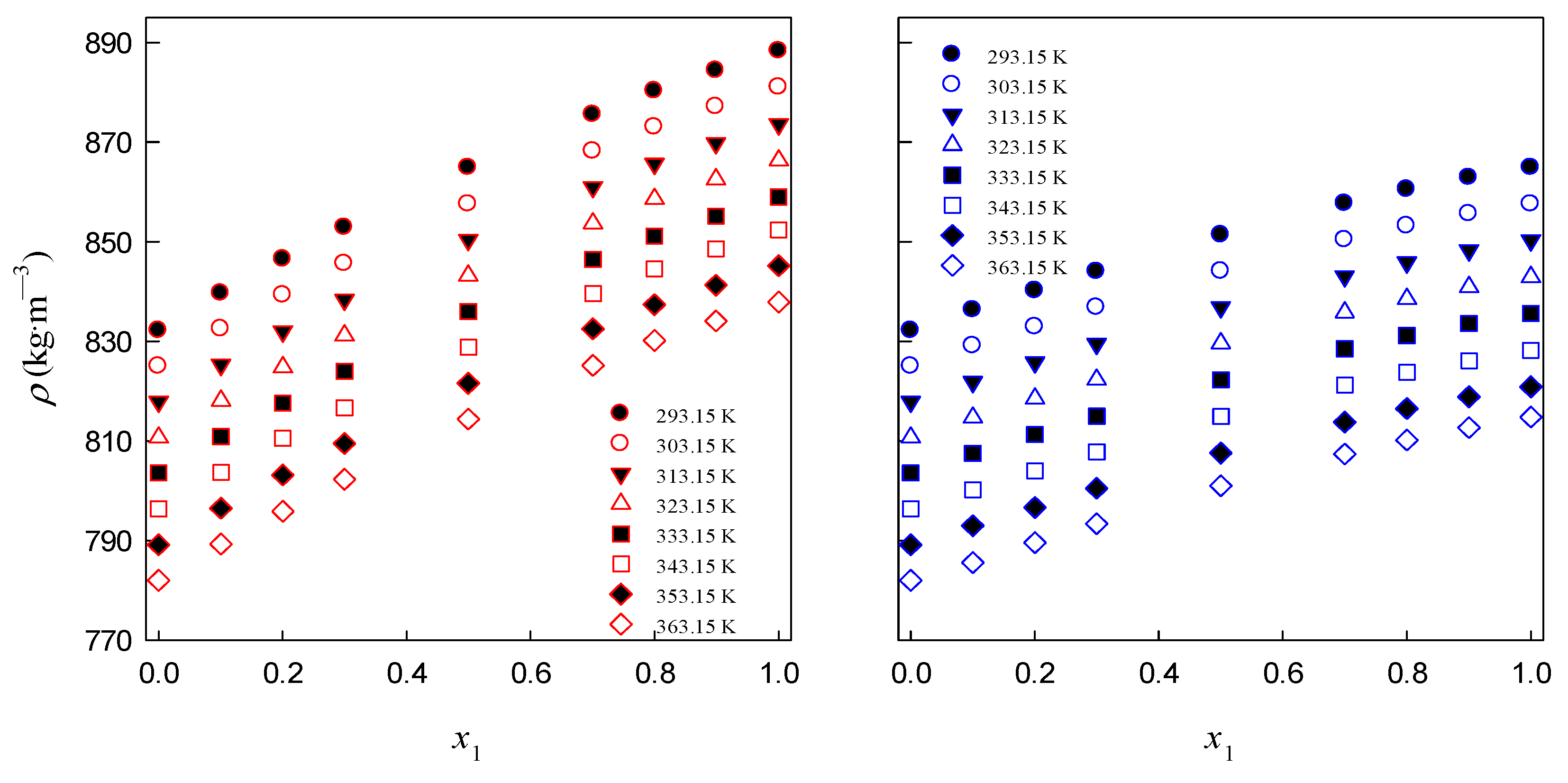
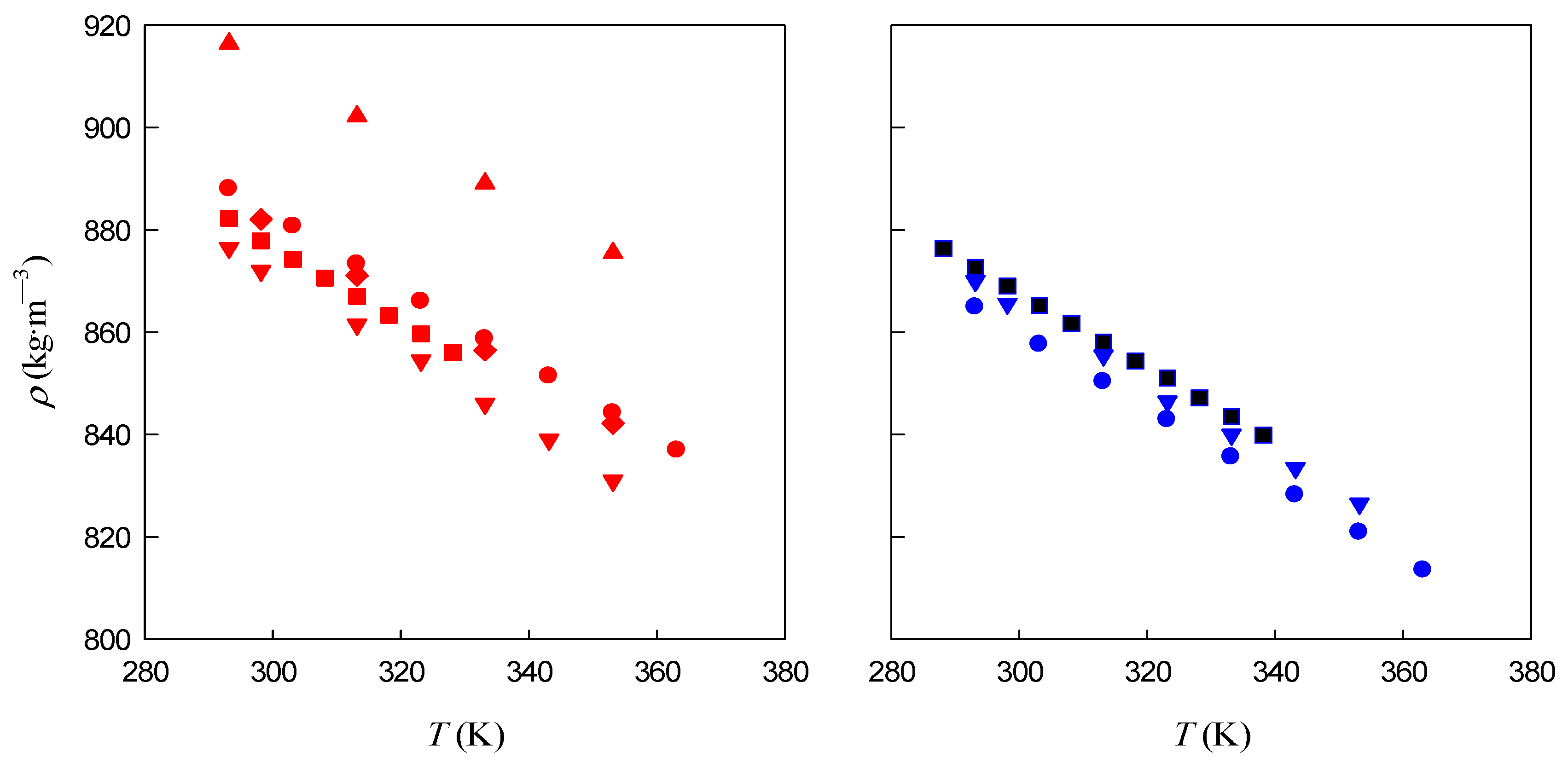
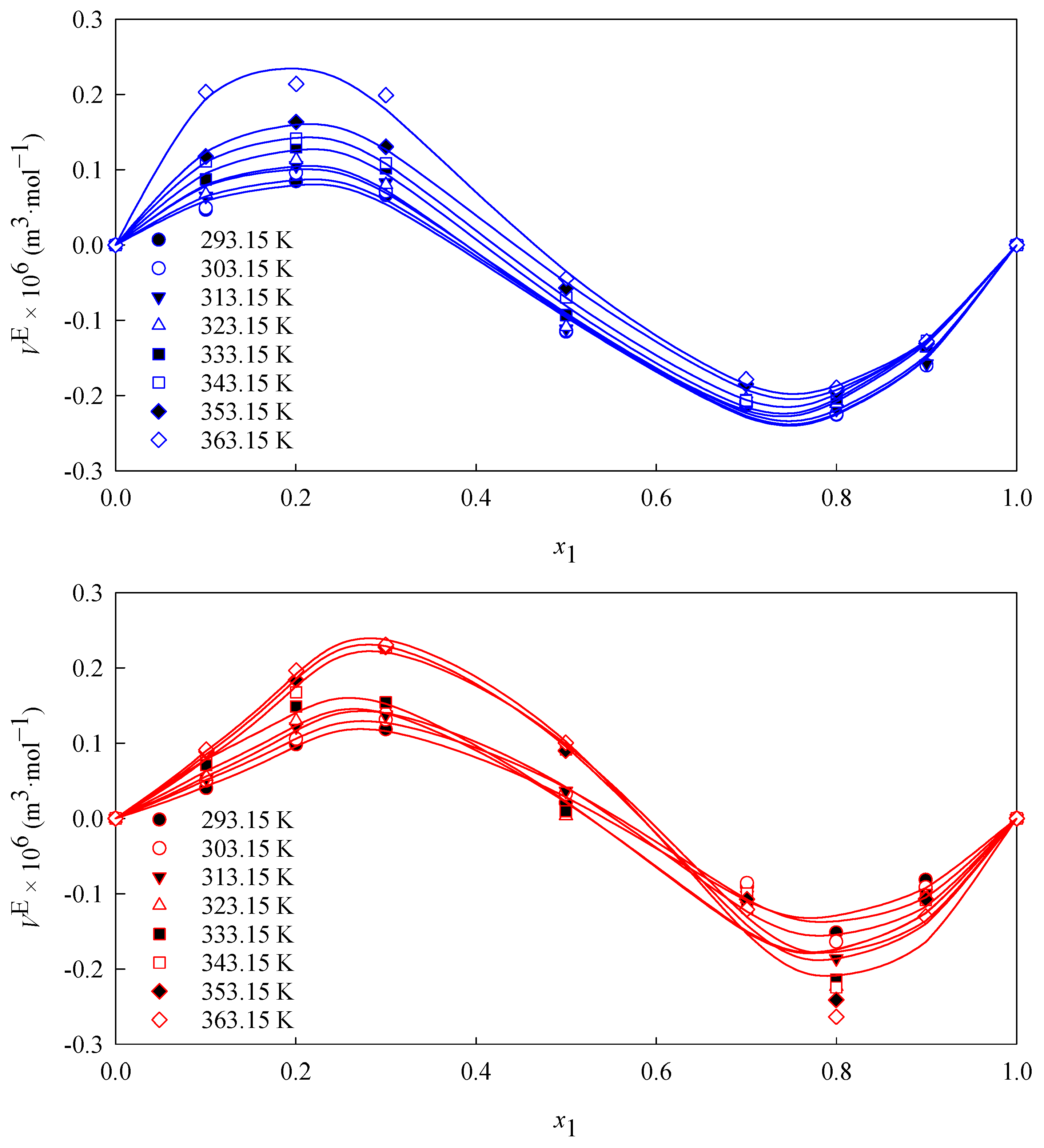
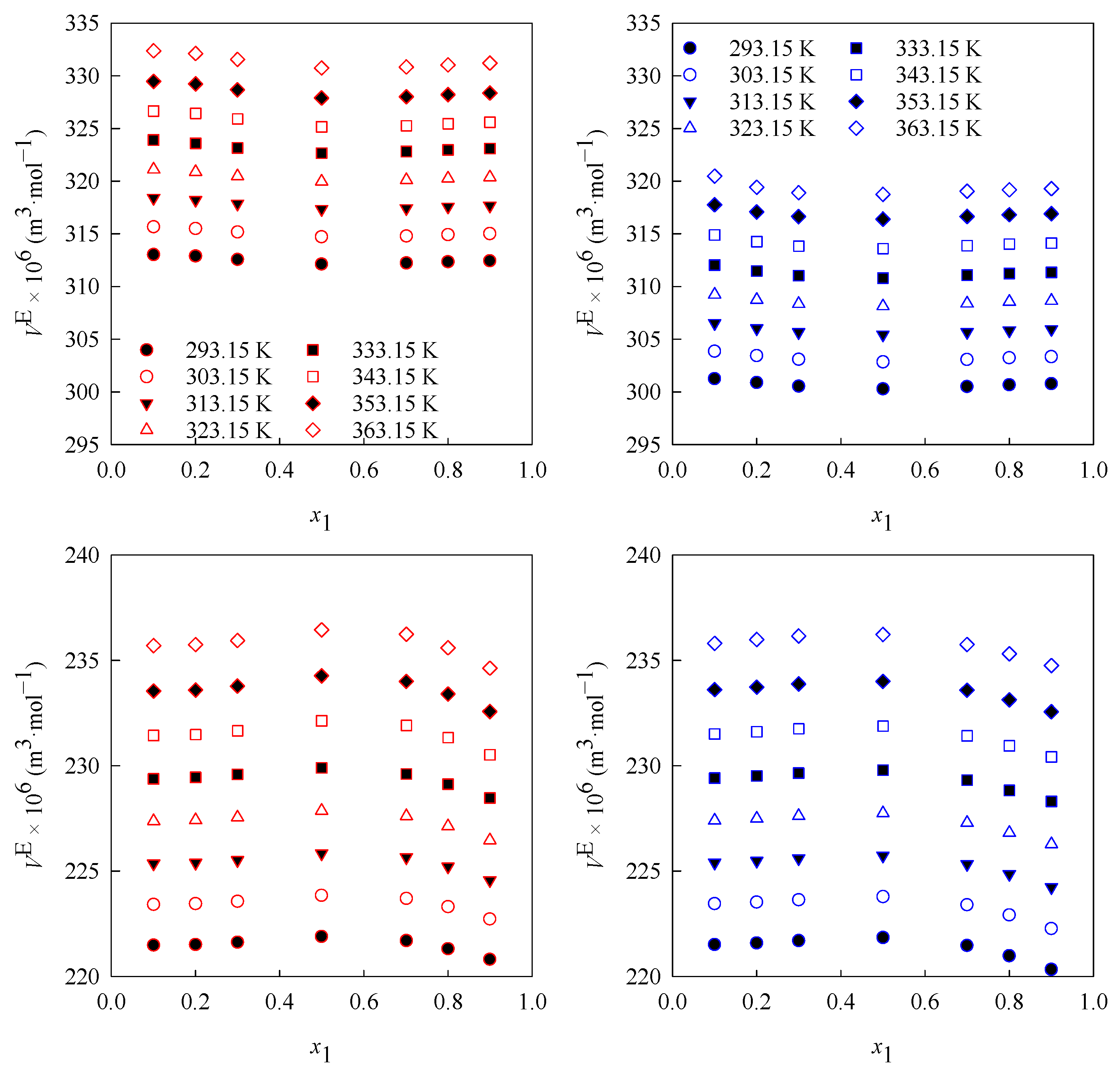
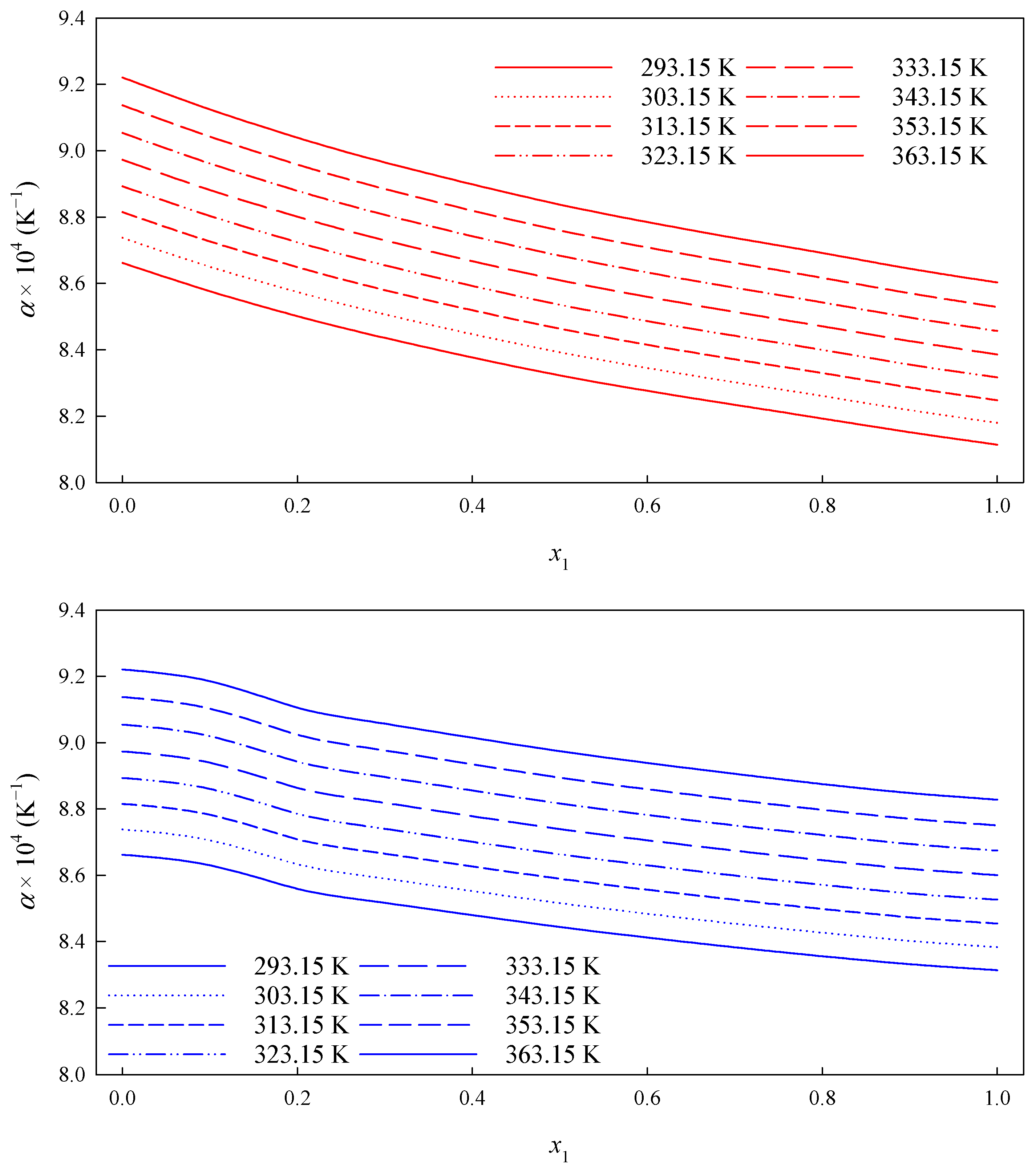
3.1. Density
3.2. Volumetric Properties
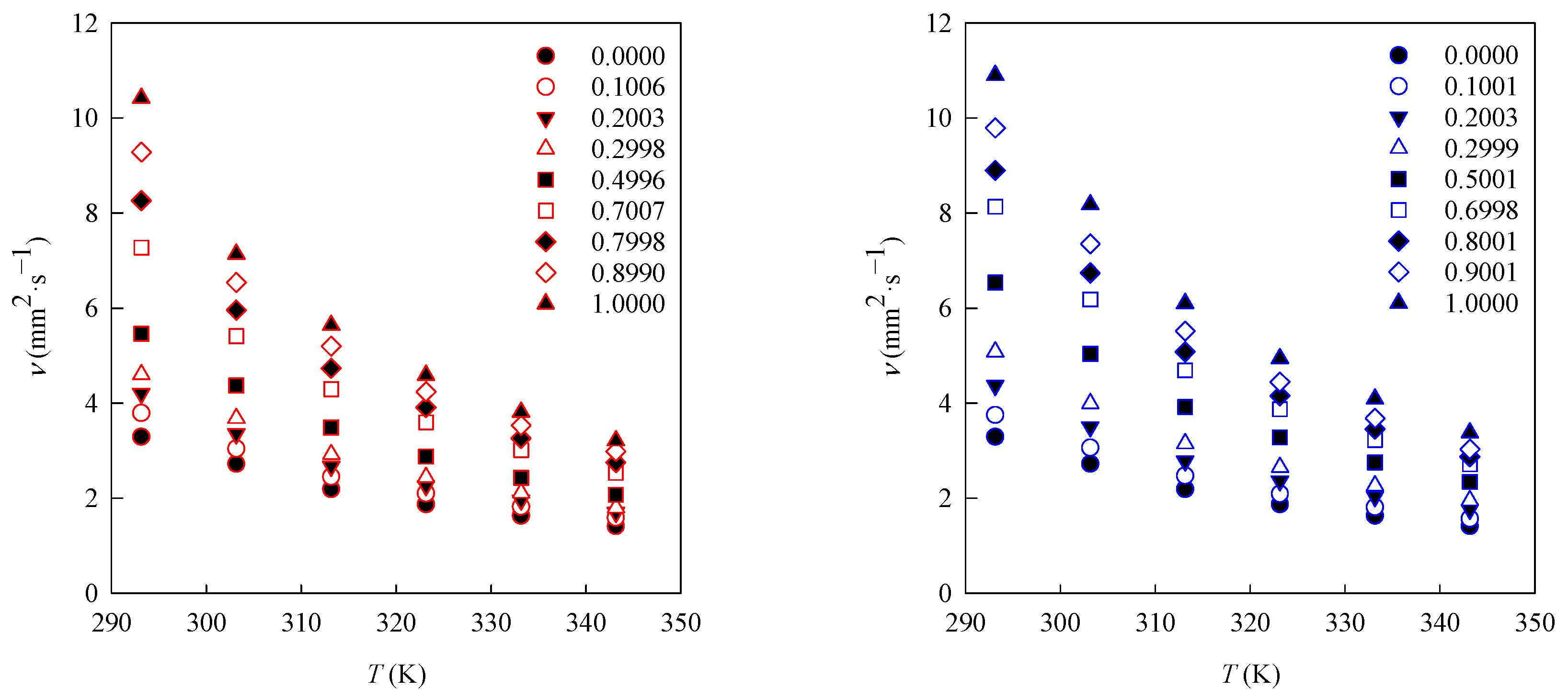
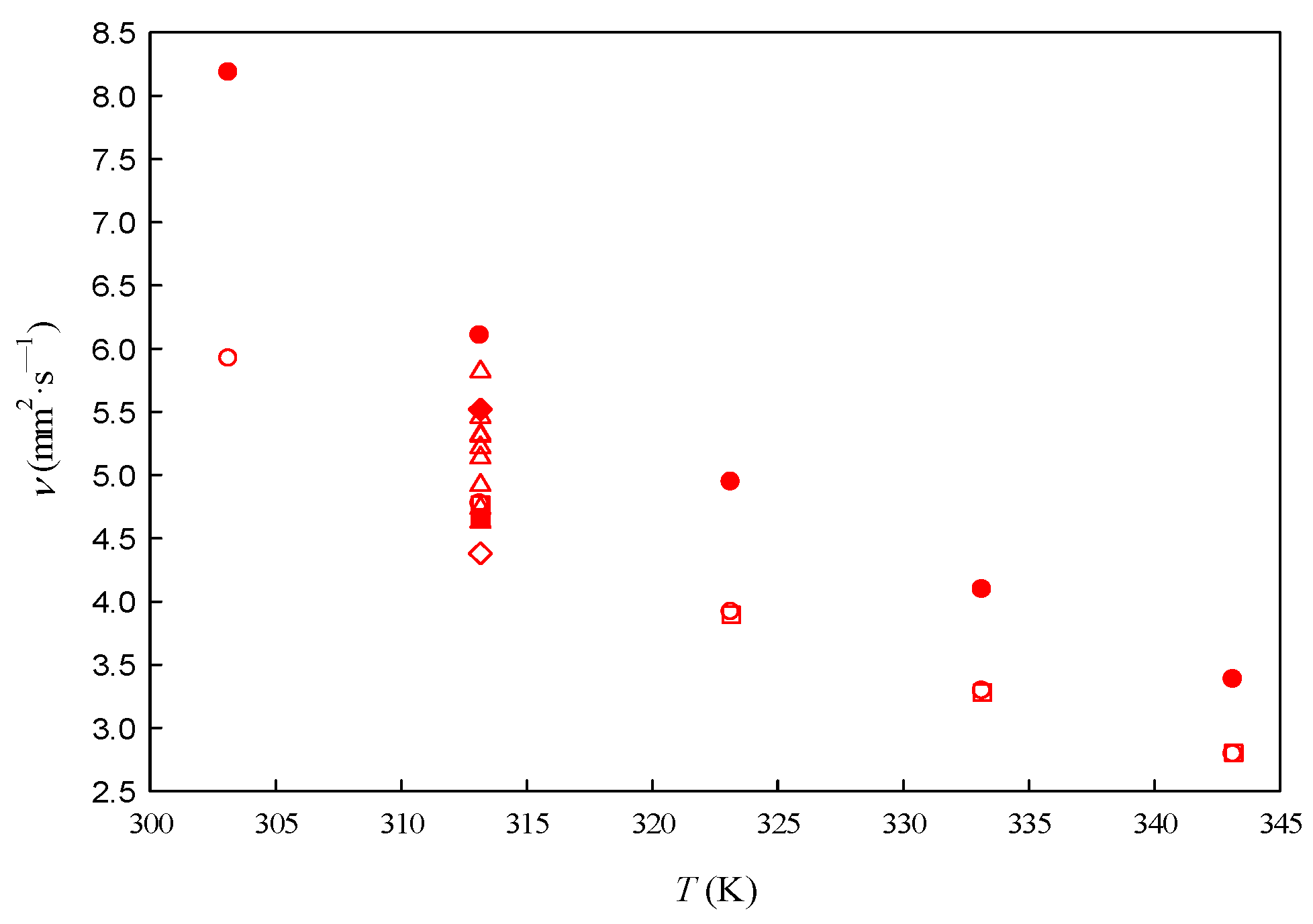
3.3. Viscosity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crippa, M.; Guizzardi, D.; Pagani, F.; Banja, M.; Muntean, M.; Schaaf, E.; Becker, W.; Monforti-Ferrario, F.; Quadrelli, R.; Risquez Martin, A.; et al. GHG Emissions of All World Countries; JRC126363; Publications Office of the European Union: Luxembourg, 2021. [Google Scholar] [CrossRef]
- Encinar, J.M.; González, J.F.; Rodríguez-Reinares, A. Biodiesel from used frying oil. Variables affecting the yields and characteristics of the biodiesel. Ind. Eng. Chem. Res. 2005, 44, 5491–5499. [Google Scholar] [CrossRef]
- Alptekin, E.; Canakci, M. Determination of the density and the viscosities of biodiesel–diesel fuel blends. Renew. Energy 2008, 33, 2623–2630. [Google Scholar] [CrossRef]
- Boehman, A.L. Biodiesel production and processing. Fuel Process. Technol. 2005, 86, 1057–1058. [Google Scholar] [CrossRef]
- Gerpen, J.V. Biodiesel processing and production. Fuel Process. Technol. 2005, 86, 1097–1107. [Google Scholar] [CrossRef]
- Graboski, M.S.; McCormick, R.L. Combustion of fat and vegetable oil derived fuels in diesel engines. Prog. Energy Combust. Sci. 1998, 24, 125–164. [Google Scholar] [CrossRef]
- García-Morales, R.; Verónico-Sánchez, F.J.; Domenzaín-González, J.; Zúñiga-Moreno, A.; Bouchot, C.; Elizalde-Solis, O. Liquid to solid phase transition detection by using a vibrating tube densimeter along with densities up to 137 MPa of beef tallow fatty acid alkyl esters. J. Chem. Thermodyn. 2024, 192, 107259. [Google Scholar] [CrossRef]
- Knothe, G. Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process. Technol. 2005, 86, 1059–1070. [Google Scholar] [CrossRef]
- Monyem, A.; Canakci, M.; van Gerpen, J.H. Investigation of biodiesel thermal stability under simulated in-use conditions. Appl. Eng. Agric. 2000, 16, 373–378. [Google Scholar] [CrossRef]
- Altiparmak, D.; Keskin, A.; Koca, A.; Gürü, M. Alternative fuel properties of tall oil fatty acid methyl ester-diesel fuel blends. Bioresour. Technol. 2007, 98, 241–246. [Google Scholar] [CrossRef]
- Canakci, M.; van Gerpen, J.H. Comparison of engine performance and emissions for petroleum diesel fuel, yellow grease biodiesel, and soybean oil biodiesel. Trans. ASAE 2003, 46, 937–944. [Google Scholar] [CrossRef]
- Suh, H.K.; Lee, C.S. A review on atomization and exhaust emissions of a biodiesel-fueled compression ignition engine. Renew. Sustain. Energy Rev. 2016, 58, 1601–1620. [Google Scholar] [CrossRef]
- Kinast, J.A. Production of Biodiesels from Multiple Feedstocks and Properties of Biodiesels and Biodiesel-Diesel Blends; National Renewable Energy Laboratory Report; NREL/SR-510–31460; Department of Energy: Des Plaines, IL, USA, 2003. [CrossRef]
- Tat, M.E.; van Gerpen, J.H. The specific gravity of biodiesel and its blends with diesel fuel. J. Am. Oil. Chem. Soc. 2000, 77, 115–119. [Google Scholar] [CrossRef]
- Bahadur, N.P.; Boocock, D.G.B.; Konar, S.K. Liquid hydrocarbons from catalytic pyrolysis of sewage sludge lipid and canola oil: Evaluation of fuel properties. Energy Fuels 1995, 9, 248–256. [Google Scholar] [CrossRef]
- Das, M.; Sarkar, M.; Datta, A.; Santra, A.K. Study on viscosity and surface tension properties of biodiesel-diesel blends and their effects on spray parameters for CI engines. Fuel 2018, 220, 769–779. [Google Scholar] [CrossRef]
- Acaroglu, M.; Demirbas, A. Relationships between viscosity and density measurements of biodiesel fuels. Energy Sources, Part A Recover Util. Environ. Eff. 2007, 29, 705–712. [Google Scholar] [CrossRef]
- Amin, A.; Gadallah, A.; El Morsi, A.K.; El-Ibiari, N.N.; El-Diwani, G.I. Experimental and empirical study of diesel and castor biodiesel blending effect, on kinematic viscosity, density and calorific value. Egyptian J. Pet. 2016, 25, 509–514. [Google Scholar] [CrossRef]
- Hoang, A.T. Prediction of the density and viscosity of biodiesel and the influence of biodiesel properties on a diesel engine fuel supply system. J. Mar. Eng. Technol. 2018, 20, 299–311. [Google Scholar] [CrossRef]
- Bukkarapu, K.R.; Rahul, T.S.; Kundla, S.; Vardhan, V. Effects of blending on the properties of diesel and palm biodiesel. IOP Conf. Ser. Mater. Sci. Eng. 2018, 330, 012092. [Google Scholar] [CrossRef]
- Gülüm, M.; Bilgin, A. Measurements and empirical correlations in predicting biodiesel-diesel blends’ viscosity and density. Fuel 2017, 199, 567–577. [Google Scholar] [CrossRef]
- Ramírez-Verduzco, L.F.; Hernández-Sánchez, M.J. Blends of green diesel (synthetized from palm oil) and petroleum diesel: A study on the density and viscosity. Bioenergy Res. 2021, 14, 1002–1013. [Google Scholar] [CrossRef]
- Moradi, G.R.; Karami, B.; Mohadesi, M. Densities and kinematic viscosities in biodiesel−diesel blends at various temperatures. J. Chem. Eng. Data 2013, 58, 99–105. [Google Scholar] [CrossRef]
- Parente, R.C.; Nogueira, C.A.; Carmo, F.R.; Lima, L.P.; Fernandes, F.A.N.; de Santiago-Aguiar, R.S.; de Sant’Ana, H.B. Excess volumes and deviations of viscosities of binary blends of sunflower biodiesel + diesel and fish oil biodiesel + diesel at various temperatures. J. Chem. Eng. Data 2011, 56, 3061–3067. [Google Scholar] [CrossRef]
- Mesquita, F.M.R.; Feitosa, F.X.; de Santiago-Aguiar, R.S.; de Sant’Ana, H.B. Experimental density data and excess molar volumes of coconut biodiesel + n-hexadecane and coconut biodiesel + diesel at different temperatures. Braz. J. Chem. Eng. 2014, 31, 543–551. [Google Scholar] [CrossRef]
- Mesquita, F.M.R.; Feitosa, F.X.; Santiago, R.S.; de Sant’Ana, H.B. Density, excess volumes, and partial volumes of binary mixtures of soybean biodiesel + diesel and soybean biodiesel + n-hexadecane at different temperatures and atmospheric pressure. J. Chem. Eng. Data 2011, 56, 153–157. [Google Scholar] [CrossRef]
- Yue, H.; Liu, Z. Densities and surface tensions of binary mixtures of biodiesel, diesel, and n-butanol. Korean J. Chem. Eng. 2016, 33, 1692–1697. [Google Scholar] [CrossRef]
- Santos, S.M.; Maciel, M.R.W.; Fregolente, L.V. Kinematic Viscosity Prediction Guide: Reviewing and Evaluating Empirical Models for Diesel Fractions, and Biodiesel–Diesel Blends According to the Temperature and Feedstock. Int. J. Therm. 2021, 42, 121. [Google Scholar] [CrossRef]
- García-Morales, R.; Verónico-Sánchez, F.J.; Zúñiga-Moreno, A.; González-Vargas, O.A.; Ramírez-Jiménez, E.; Elizalde-Solis, O. Fatty acid alkyl ester production by one-step supercritical transesterification of beef tallow by using ethanol, iso-butanol, and 1-butanol. Processes 2023, 11, 742. [Google Scholar] [CrossRef]
- García-Morales, R.; Zúñiga-Moreno, A.; Verónico-Sánchez, F.J.; Domenzain-González, J.; Pérez-López, H.I.; Bouchot, C.; Elizalde-Solis, O. Fatty acid methyl esters from waste beef tallow using supercritical methanol tranesterification. Fuel 2022, 313, 122706. [Google Scholar] [CrossRef]
- ASTM D86; Standard Test Method for Distillation of Petroleum Products and Liquid Fuels at Atmospheric Pressure. ASTM International: West Conshohocken, PA, USA, 2018.
- ASTM D93; Standard Test Methods for Flash Point by Pensky-Martens Closed Cup Tester. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D7039; Standard Test Method for Sulfur in Gasoline, Diesel Fuel, Jet Fuel, Kerosine, Biodiesel, Biodiesel Blends, and Gasoline-Ethanol Blends by Monochromatic Wavelength Dispersive X-Ray Fluorescence Spectrometry. ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM D4737; Standard Test Method for Calculated Cetane Index by Four Variable Equation. ASTM: West Conshohocken, PA, USA, 2023.
- Elizalde-Solis, O.; Zúñiga-Moreno, A.; Camacho-Camacho, L.E.; García-Morales, R.; González-Arias, S.; Verónico-Sánchez, F.J. Densities and excess molar volumes of the binary systems of the ionic liquid trihexyl(tetradecyl) phosphonium bromide mixed with acetonitrile or tetrahydrofuran at temperatures from 293.15 to 313.15 K. Front. Phys. 2023, 11, 1208382. [Google Scholar] [CrossRef]
- Picard, A.; Davis, R.S.; Gläser, M.; Fujii, K. Revised formula for the density of moist air (CIPM-2007). Metrologia 2008, 45, 149–155. [Google Scholar] [CrossRef]
- Wagner, W.; Pruß, A. The IAPWS formulation 1995 for the thermodynamic properties of ordinary water substance for general and scientific use. J. Phys. Chem. Ref. Data 2002, 31, 387–535. [Google Scholar] [CrossRef]
- Joint Committee for Guides in Metrology. Evaluation of Measurement Data—Guide to the Expression of Uncertainty in Measurement, 1st ed.; JCGM 100:2008; 2008. Available online: https://www.bipm.org/documents/20126/2071204/JCGM_100_2008_E.pdf (accessed on 18 October 2024).
- ASTM D445; Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids (and Calculation of Dynamic Viscosity). American Society for Testing and Materials: West Conshohocken, PA, USA, 2021.
- Grozdanić, N.; Radović, I.; Knežević-Stevanović, A.; Kijevčanin, M. Volumetric properties of binary mixtures of tetrahydrofuran, dimethyl adipate, 1-butanol and 2-butanol from (288.15 to 323.15) K and modeling by Prigogine-Flory-Patterson (PFP) and Extended Real Association Solution (ERAS) models. J. Mol. Liq. 2021, 340, 117313. [Google Scholar] [CrossRef]
- Heintz, A. A new theoretical approach for predicting excess properties of alkanol/alkane mixtures. Ber. Bunsenges. Phys. Chem. 1985, 89, 172–181. [Google Scholar] [CrossRef]
- NguyenThi, T.X.; Bazile, J.P.; Bessières, D. Density measurements of waste cooking oil biodiesel and diesel blends over extended pressure and temperature ranges. Energies 2018, 11, 1212. [Google Scholar] [CrossRef]
- Radović, I.R.; Grozdanić, N.D.; Djordjević, B.D.; Šerbanović, S.P.; Kijevčanin, M.L. Prediction of excess molar volumes of binary mixtures by Prigogine-Flory-Patterson (PFP) and extended real association solution (ERAS) models. J. Serb. Chem. Soc. 2017, 82, 1379–1390. [Google Scholar] [CrossRef]
- McAllister, R.A. The viscosity of liquid mixtures. AIChE J. 1960, 6, 427–431. [Google Scholar] [CrossRef]
- Pratas, M.J.; Freitas, S.; Oliveira, M.B.; Monteiro, S.C.; Lima, A.S.; Coutinho, J.A.P. Densities and viscosities of fatty acid methyl and ethyl esters. J. Chem. Eng. Data 2010, 55, 3983–3990. [Google Scholar] [CrossRef]
- Pratas, M.J.; Freitas, S.; Oliveira, M.B.; Monteiro, S.C.; Lima, A.S.; Coutinho, J.A.P. Densities and viscosities of minority fatty acid methyl and ethyl esters present in biodiesel. J. Chem. Eng. Data 2011, 56, 2175–2180. [Google Scholar] [CrossRef]
- Cano-Gómez, J.J.; Iglesias-Silva, G.A.; Rivas, P.; Díaz-Ovalle, C.O.; Cerino-Córdova, F.J. Densities and viscosities for binary liquid mixtures of biodiesel + 1-butanol, + isobutyl alcohol, or + 2-butanol from 293.15 to 333.15 K at 0.1 MPa. J. Chem. Eng. Data 2017, 62, 3391–3400. [Google Scholar] [CrossRef]
- Aitbelale, R.; Abala, I.; Alaoui, F.E.M.; Sahibed-dine, A.; Rujas, N.M.; Aguilar, F. Characterization and determination of thermodynamic properties of waste cooking oil biodiesel: Experimental, correlation and modeling density over a wide temperature range up to 393.15 and pressure up to 140 MPa. Fluid Phase Equilib. 2019, 497, 87–96. [Google Scholar] [CrossRef]
- Vargas-Ibáñez, L.T.; Iglesias-Silva, G.A.; Cano-Gómez, J.J.; Escamilla-Alvarado, C.; Berrones-Eguiluz, M.A. Densities and viscosities for binary liquid mixtures of biodiesel + 1-pentanol, 2-pentanol, or 2-methyl-1-butanol from (288.15 to 338.15) K at 0.1 MPa. J. Chem. Eng. Data 2018, 63, 2438–2450. [Google Scholar] [CrossRef]
- Ramírez-Verduzco, L.F.; Rodríguez-Rodríguez, J.E.; Jaramillo-Jacob, A.R. Predicting cetane number, kinematic viscosity, density and higher heating value of biodiesel from its fatty acid methyl ester composition. Fuel 2012, 91, 102–111. [Google Scholar] [CrossRef]
- Shin, H.-Y.; Lim, S.-M.; Bae, S.-Y.; Oh, S.C. Thermal decomposition and stability of fatty acid methyl esters in supercritical methanol. J. Anal. Appl. Pyrol. 2011, 92, 332–338. [Google Scholar] [CrossRef]
- Vargas-Ibáñez, L.T.; Cano-Gómez, J.J.; Iglesias-Silva, G.A.; Santos-López, I.A.; Rivas-García, P.; Alcalá-Rodríguez, M.M.; Zwolinski, P. Thermophysical and excess properties of diesel + biodiesel with octanol isomers at different temperatures. J. Mol. Liq. 2022, 363, 119779. [Google Scholar] [CrossRef]
- Feyzi, M.; Shahbazi, Z.; Norouzi, L. Excess molar volumes of binary and ternary mixture of sunflower biodiesel, diesel and 2-propanol at 293.15–353.15 K and ambient pressure. J. Mol. Liq. 2018, 249, 1271–1278. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Chen, J. Experimental investigations of density and dynamic viscosity of n-hexadecane with three fatty acid methyl esters. Fuel 2016, 166, 553–559. [Google Scholar] [CrossRef]
- Li, D.; Zheng, Y.; Wang, J.; Pang, Y.; Liu, M. Volumetric properties and viscosity for the ternary system of (1-pentanol + ethylcyclohexane + methyl myristate) and corresponding binary systems at T = 293.15–323.15 K. J. Chem. Thermodyn. 2022, 165, 106660. [Google Scholar] [CrossRef]
- Du, W.; Wang, X. Density and viscosity for binary mixtures of methyl decanoate with 1-propanol, 1-butanol, and 1-pentanol. J. Mol. Liq. 2019, 294, 111647. [Google Scholar] [CrossRef]
- Yang, F.; Wang, X.; Tan, H.; He, S.; Liu, Z. Experimental investigations on the thermophysical properties of methyl myristate in alcoholic solutions. Fuel 2018, 215, 187–195. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, Z.; Xue, S.; Hou, K.; Liu, H.; Liu, X.; He, M. Association effect on the density, viscosity and excess properties of fatty acid ester + alcohol mixtures: Experiment and modeling. Fuel 2022, 316, 123425. [Google Scholar] [CrossRef]
- Mahajan, A.R.; Mirgane, S.R. Excess molar volumes and viscosities for the binary mixtures of n-octane, n-decane, n-dodecane, and n-tetradecane with octan-2-ol at 298.15 K. J. Thermodyn. 2013, 2013, 571918. [Google Scholar] [CrossRef]
- Vargas-Ibáñez, L.T.; Cano-Gómez, J.J.; Iglesias-Silva, G.A.; Santos-López, I.A.; Benavides-Moran, O.E.; Zwolinski, P. Physical properties of biodiesel blended with hexanol isomers at different temperatures: Surface tension, density, viscosity, and refractive index. J. Chem. Eng. Data 2020, 65, 3706–3727. [Google Scholar] [CrossRef]
- Jesudason, C.G.; Koon Yau, C. Relevance of the thermodynamic ERAS model for liquid mixtures with components exhibiting a small degree of self-association. J. Mol. Liq. 2017, 234, 1–8. [Google Scholar] [CrossRef]
- Lisar, F.S.; Iloukhani, H.; Khanlarzadeh, K. Application of ERAS-model and PFP theory on excess and deviation properties for binary systems of ionic liquid + amine derivatives. J. Mol. Liq. 2024, 393, 123544. [Google Scholar] [CrossRef]
- Rodríguez-Antón, L.M.; Aparicio, C.; Guignon, B.; Sanz, P.D. Volumetric properties at high pressure of waste oil methyl ester compared with diesel oil. Fuel 2008, 87, 1934–1940. [Google Scholar] [CrossRef]
- Eryilmaz, T.; Yeşilyurt, M.K. investigation of the effect of blending ratio and temperature on the kinematic viscosity and specific gravity of waste cooking oil biodiesel. Fresenius Environmental Bull. Fresenius Environ. Bull. 2015, 24, 1523–1529. [Google Scholar]
- Kassem, Y.; Çamur, H. A laboratory study of the effects of wide range temperature on the properties of biodiesel produced from various waste vegetable oils. Waste Biomass Valor. 2017, 8, 1995–2007. [Google Scholar] [CrossRef][Green Version]
- Li, H.-P.; Zhao, H. Preparing biodiesel from high-acidity waste cooking oil catalyzed by sodium methoxide. Energy Sources Part A Recovery Util. Environ. Eff. 2015, 37, 334–340. [Google Scholar] [CrossRef]
- Azcan, N.; Yilmaz, O. Microwave assisted transesterification of waste frying oil and concentrate methyl ester content of biodiesel by molecular distillation. Fuel 2013, 104, 614–619. [Google Scholar] [CrossRef]
- Sabudak, T.; Yildiz, M. Biodiesel production from waste frying oils and its quality control. Waste Manag. 2010, 30, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Duncan, A.M.; Ahosseini, A.; McHenry, R.; Depcik, C.D.; Stagg-Williams, S.M.; Scurto, A.M. High-Pressure Viscosity of Biodiesel from Soybean, Canola, and Coconut Oils. Energ. Fuel. 2010, 24, 5708–5716. [Google Scholar] [CrossRef]
- Mesquita, F.M.R.; Feitosa, F.X.; do Carmo, F.R.; de Santiago-Aguiar, R.S.; de Sant’Ana, H.B. Viscosities and viscosity deviations of binary mixtures of biodiesel + petrodiesel (or n-hexadecane) at different temperatures. Braz. J. Chem. Eng. 2012, 29, 653–664. [Google Scholar] [CrossRef]
- Dubey, G.P.; Sharma, M.; Dubey, N. Study of densities, viscosities, and speeds of sound of binary liquid mixtures of butan-1-ol with n-alkanes (C6, C8, and C10) at T = (298.15, 303.15, and 308.15) K. J. Chem. Thermodyn. 2008, 40, 309–320. [Google Scholar] [CrossRef]
- Ortega, J.; Espiau, F.; Toledo, F.J.; Dieppa, R. Thermodynamic properties of (an ester + an alkane). XVII. Experimental HEm and VEm values for (an alkyl propanoate + an alkane) at 318.15 K. J. Chem. Thermodyn. 2005, 37, 967–983. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Lang, H. Measurement and correlation of density and viscosity of n-hexadecane with three fatty acid ethyl esters. J. Chem. Thermodyn. 2016, 97, 127–134. [Google Scholar] [CrossRef]
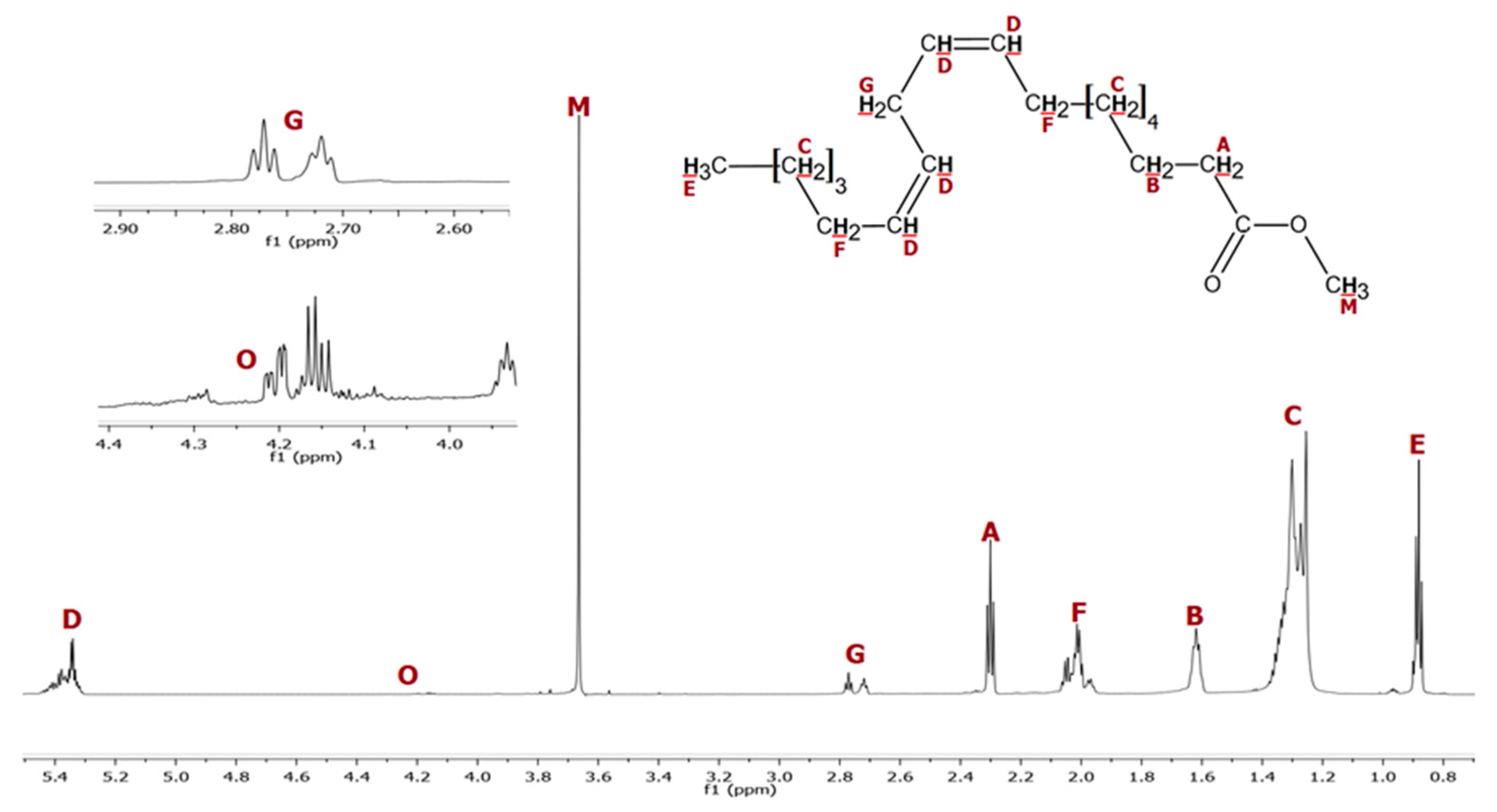

| Cooking Oil Methyl Ester Biodiesel Molar Mass: 277.57 g·mol−1 | Beef Tallow Butyl Ester Biodiesel [29] Molar Mass: 260.18 g·mol−1 |
|---|---|
| Ester profile (mol%) 1 | Ester profile (area%) 1 |
| (C15H30O2, methyl tetradecanoate) 1.063 | (C18H36O2, butyl tetradecanoate) 1.904 |
| (C17H34O2, methyl hexadecanoate) 8.763 | (C20H40O2, butyl hexadecanoate) 25.549 |
| (C19H38O2, methyl octadecanoate) 4.116 | (C22H44O2, butyl octadecanoate) 50.282 |
| (C19H36O2, methyl 9-octadecenoate) 12.025 | (C23H44O2, methyl tert-butyl 9-octadecenoate) 22.264 |
| (C19H34O2, methyl 9,12-octadecadienoate) 74.033 | |
| Yield (%) 2 | |
| 99.46 | 87.7 |
| Alcohol (%) 3 | |
| 0.15 | 0.20 |
| Water content (wt%) 4 | |
| 0.125 | 0.121 |
| Iodine value (g·100 g−1) 5 | |
| 80.57 | 76.01 |
| Saponification number (mgKOH·gFAEE−1) 5 | |
| 191.81 | 167.56 |
| Diesel Molar Mass: 184.33 g·mol−1 | |
|---|---|
| Distillation curve (K) 1 | |
| (Initial) | 427.85 |
| (10%) | 456.95 |
| (50%) | 516.25 |
| (90%) | 592.85 |
| (Final) | 615.85 |
| Flash (K) 2 | 325.88 |
| Sulfur (ppm) 3 | 11.52 |
| Cetane index 4 | 50 |
| Property | Value |
|---|---|
| ρ at 308.15 K (kg·m−3) | 936.7 |
| Molar mass (g·mol−1) | 790.76 |
| High heating value (J·g−1) | 39,562.42 |
| Water content (ppm) | 265 |
| Saponification index | 211.8 |
| %free fatty acid (hexadecanoic acid) | 3.98 |
| %free fatty acid (octadecanoic acid) | 4.19 |
| %free fatty acid (9-octadecenoic acid) | 4.35 |
| Model | Accuracy | Standard Uncertainty | |
|---|---|---|---|
| Synthesis of mixtures by successive loadings method | |||
| Balance | Precisa model EP 520A | ±0.1 mg | 0.0004 in mole fraction |
| Density measurement | |||
| Vibrating tube densimeter | Anton Paar DMA 4500M | 0.01 kg·m−3 | 0.2 kg·m−3 |
| Thermometer | 100-Ω platinum thermometer | 0.01 K | 0.02 K |
| Viscosity measurement | |||
| Capillarity viscometer | Cannon-Fenske | 0.01 mm2·s−1 | 0.14 mm2·s−1 |
| Thermometer | Polyscience, model PD07R | 0.02 K | 0.04 K |
| Parameter | A0 1 | A1 1 | A2 1 | A3 1 | 1 | AAD (%) |
|---|---|---|---|---|---|---|
| T (K) | waste cooking oil methyl ester biodiesel (1) + diesel (2) | |||||
| 293.15 | 0.1123 | −1.4763 | −0.6015 | 0.8471 | 0.0188 | 13.94 |
| 303.15 | 0.1618 | −1.5012 | −0.7173 | 0.6607 | 0.0222 | 12.69 |
| 313.15 | 0.1653 | −1.6925 | −0.7872 | 0.7788 | 0.0249 | 11.60 |
| 323.15 | 0.0841 | −1.8618 | −0.6734 | 0.8722 | 0.0420 | 94.60 |
| 333.15 | 0.0892 | −1.9400 | −0.5437 | 0.8304 | 0.0332 | 31.50 |
| 343.15 | 0.3930 | −2.2957 | −1.1090 | 1.2698 | 0.0372 | 14.37 |
| 353.15 | 0.3798 | −2.4213 | −1.0682 | 1.3524 | 0.0420 | 13.72 |
| 363.15 | 0.4071 | −2.5143 | −1.2786 | 1.1875 | 0.0420 | 11.75 |
| T (K) | waste beef tallow butyl ester biodiesel (1) + diesel (2) | |||||
| 293.15 | −0.3844 | −1.7615 | −0.1971 | 0.4791 | 0.0183 | 11.39 |
| 303.15 | −0.3726 | −1.7793 | −0.1586 | 0.4493 | 0.0207 | 12.81 |
| 313.15 | −0.3623 | −1.8012 | −0.0256 | 0.3727 | 0.0199 | 10.80 |
| 323.15 | −0.3722 | −1.8347 | 0.1367 | 0.5746 | 0.0163 | 8.98 |
| 333.15 | −0.3235 | −1.9433 | 0.2209 | 0.6131 | 0.0116 | 5.96 |
| 343.15 | −0.2798 | −1.9425 | 0.2941 | 0.4672 | 0.0016 | 0.68 |
| 353.15 | −0.1983 | −1.9498 | 0.2679 | 0.3144 | 0.0078 | 4.30 |
| 363.15 | −0.1390 | −2.1558 | 0.7994 | −0.1146 | 0.0182 | 7.25 |
| 293.15 K | 313.15 K | 333.15 K | = 353.15 K | |
|---|---|---|---|---|
| waste cooking oil methyl ester biodiesel (1) + diesel (2) | ||||
| (–) | −0.4139 | −0.4246 | −0.4066 | −0.4293 |
| (m3·mol−1) | 39.8175 | 44.9050 | 44.4452 | 50.5017 |
| (MPa) | 219.5644 | 237.7053 | 200.1880 | 225.9977 |
| (%) | 13.81 | 10.03 | 27.19 | 13.72 |
| (m3·mol−1) | 0.0333 | 0.0441 | 0.0573 | 0.0751 |
| waste beef tallow butyl ester biodiesel (1) + diesel (2) | ||||
| (–) | 0.1500 | 0.2630 | 0.3182 | 0.3198 |
| (m3·mol−1) | −103.4619 | −61.3002 | −47.3863 | −46.4457 |
| (MPa) | 112.2140 | 101.1186 | 84.3279 | 77.4472 |
| (%) | 10.67 | 10.19 | 7.35 | 4.27 |
| (m3·mol−1) | 0.0293 | 0.0324 | 0.0242 | 0.0131 |
| T (K) | 293.15 | 303.15 | 313.15 | 323.15 | 333.15 | 343.15 |
|---|---|---|---|---|---|---|
| x1 | η × 103 (Pa·s) waste cooking oil methyl ester biodiesel (1) + diesel (2) | |||||
| 0.0000 | 2.74 | 2.24 | 1.79 | 1.51 | 1.31 | 1.12 |
| 0.1006 | 3.18 | 2.53 | 2.02 | 1.72 | 1.47 | 1.27 |
| 0.2003 | 3.56 | 2.81 | 2.23 | 1.86 | 1.60 | 1.38 |
| 0.2998 | 3.92 | 3.11 | 2.45 | 2.03 | 1.74 | 1.47 |
| 0.4996 | 4.72 | 3.75 | 2.97 | 2.42 | 2.04 | 1.71 |
| 0.7007 | 6.36 | 4.69 | 3.69 | 3.06 | 2.54 | 2.13 |
| 0.7998 | 7.27 | 5.20 | 4.10 | 3.35 | 2.77 | 2.32 |
| 0.8990 | 8.21 | 5.74 | 4.52 | 3.66 | 3.02 | 2.52 |
| 1.0000 | 9.25 | 6.29 | 4.93 | 3.98 | 3.27 | 2.74 |
| x1 | η × 103 (Pa·s) waste beef tallow butyl ester biodiesel (1) + diesel (2) | |||||
| 0.0000 | 2.74 | 2.24 | 1.79 | 1.51 | 1.31 | 1.12 |
| 0.1001 | 3.14 | 2.54 | 2.03 | 1.70 | 1.46 | 1.26 |
| 0.2003 | 3.67 | 2.91 | 2.30 | 1.93 | 1.65 | 1.41 |
| 0.2999 | 4.28 | 3.33 | 2.61 | 2.18 | 1.84 | 1.57 |
| 0.5001 | 5.57 | 4.25 | 3.28 | 2.72 | 2.26 | 1.90 |
| 0.6998 | 6.97 | 5.26 | 3.96 | 3.23 | 2.67 | 2.22 |
| 0.8001 | 7.66 | 5.75 | 4.30 | 3.48 | 2.87 | 2.36 |
| 0.9001 | 8.45 | 6.29 | 4.68 | 3.74 | 3.07 | 2.50 |
| 1.0000 | 9.43 | 7.02 | 5.19 | 4.16 | 3.42 | 2.80 |
| Parameter | η1112 1 | η1122 1 | η222 1 | 1 | AAD (%) |
|---|---|---|---|---|---|
| T (K) | waste beef tallow butyl ester biodiesel (1) + diesel (2) | ||||
| 293.15 | 1.62 | 2.64 | 3.83 | 0.02 | 2.93 |
| 303.15 | 1.49 | 2.32 | 3.01 | 0.02 | 3.72 |
| 313.15 | 1.32 | 2.06 | 2.41 | 0.01 | 2.48 |
| 323.15 | 1.06 | 2.05 | 1.97 | 0.01 | 6.17 |
| 333.15 | 0.99 | 1.87 | 1.68 | 0.01 | 7.34 |
| 343.15 | 0.90 | 1.75 | 1.45 | 0.02 | 15.51 |
| T (K) | waste cooking oil methyl ester biodiesel (1) + diesel (2) | ||||
| 293.15 | 4.58 | 1.40 | 4.34 | 0.08 | 7.61 |
| 303.15 | 2.56 | 1.61 | 3.16 | 0.02 | 4.33 |
| 313.15 | 2.35 | 1.46 | 2.50 | 0.01 | 4.25 |
| 323.15 | 2.40 | 1.29 | 2.12 | 0.03 | 21.12 |
| 333.15 | 2.17 | 1.21 | 1.82 | 0.03 | 16.82 |
| 343.15 | 2.29 | 1.04 | 1.62 | 0.02 | 25.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Rodríguez, G.; Domenzaín-González, J.; Verónico-Sánchez, F.J.; Pérez-López, H.I.; Zúñiga-Moreno, A.; Elizalde-Solis, O. Density and Viscosity in Biodiesel + Diesel Mixtures from Recycled Feedstocks. Appl. Sci. 2025, 15, 3812. https://doi.org/10.3390/app15073812
Sánchez-Rodríguez G, Domenzaín-González J, Verónico-Sánchez FJ, Pérez-López HI, Zúñiga-Moreno A, Elizalde-Solis O. Density and Viscosity in Biodiesel + Diesel Mixtures from Recycled Feedstocks. Applied Sciences. 2025; 15(7):3812. https://doi.org/10.3390/app15073812
Chicago/Turabian StyleSánchez-Rodríguez, Gabriela, José Domenzaín-González, Francisco Javier Verónico-Sánchez, Hugo Isidro Pérez-López, Abel Zúñiga-Moreno, and Octavio Elizalde-Solis. 2025. "Density and Viscosity in Biodiesel + Diesel Mixtures from Recycled Feedstocks" Applied Sciences 15, no. 7: 3812. https://doi.org/10.3390/app15073812
APA StyleSánchez-Rodríguez, G., Domenzaín-González, J., Verónico-Sánchez, F. J., Pérez-López, H. I., Zúñiga-Moreno, A., & Elizalde-Solis, O. (2025). Density and Viscosity in Biodiesel + Diesel Mixtures from Recycled Feedstocks. Applied Sciences, 15(7), 3812. https://doi.org/10.3390/app15073812







