Long-Term Anti-Corrosion Performance of Ultra-High Content Inhibitor Loaded Gel-Epoxy Solid Inhibitor with Temperature-Responisve Effect
Abstract
1. Introduction
2. Experiment Details
2.1. Sample Preparation and Corrosion Testing Procedures
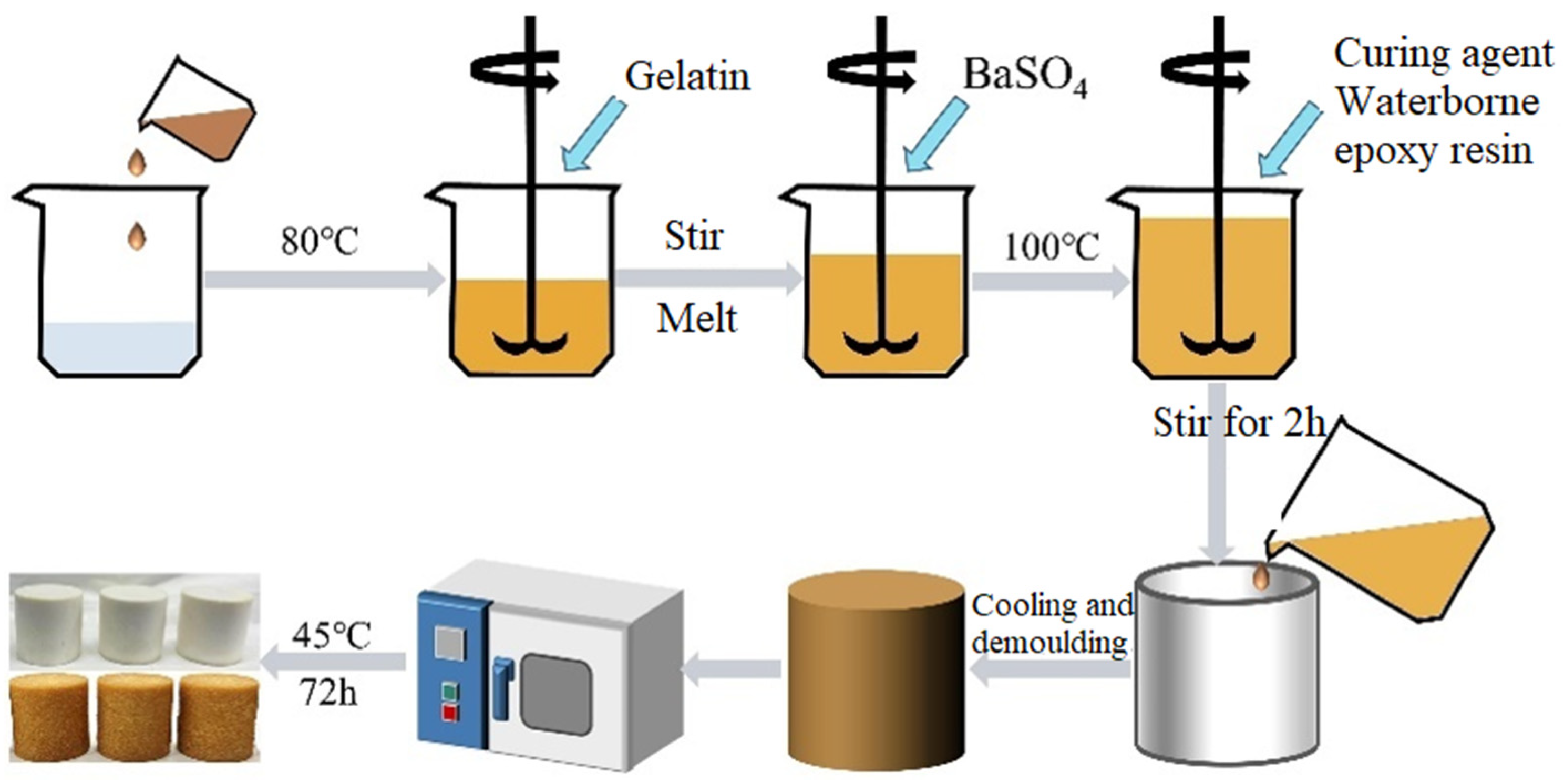
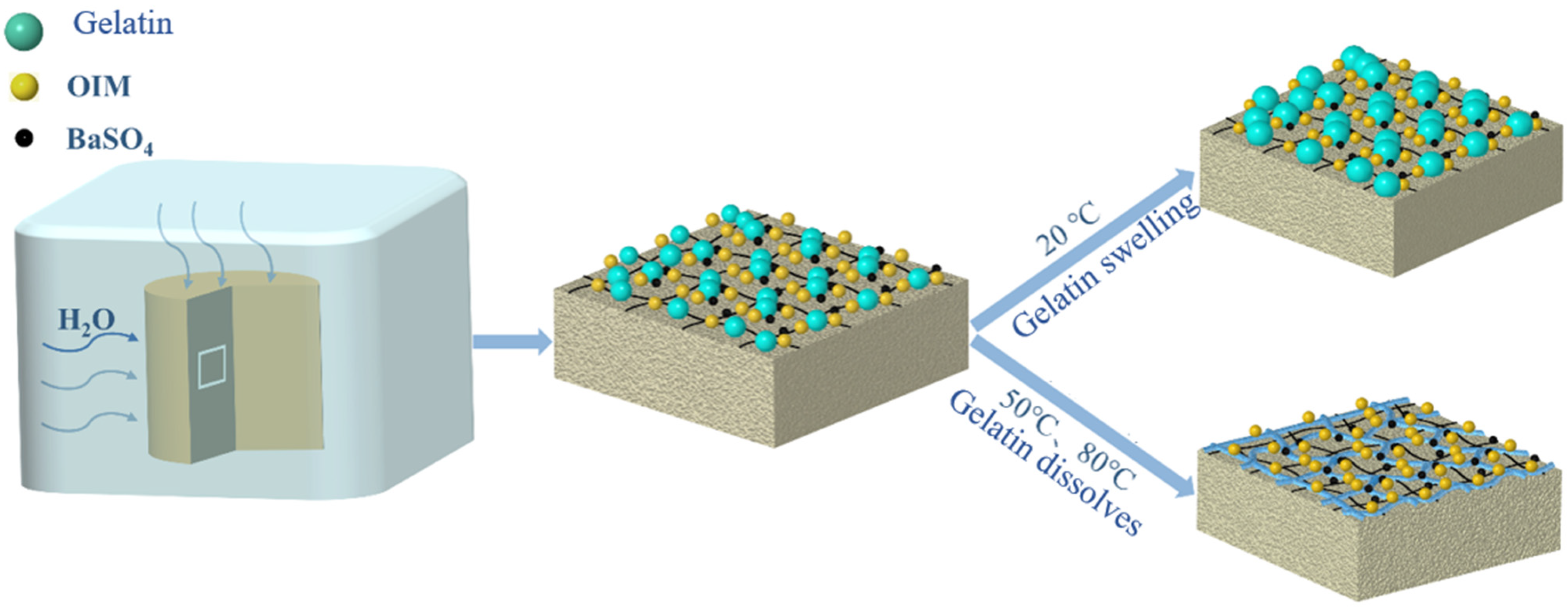
2.2. Synthesis of GE-EP@OIMs Smart Corrosion Inhibitor
2.3. Characterization Methods for Solid Corrosion Inhibitors
2.3.1. Scanning Electron Microscope
2.3.2. Infrared Spectrum Test
2.3.3. Thermal Stability Test
2.3.4. Mechanical Properties Test
2.3.5. Raman Test
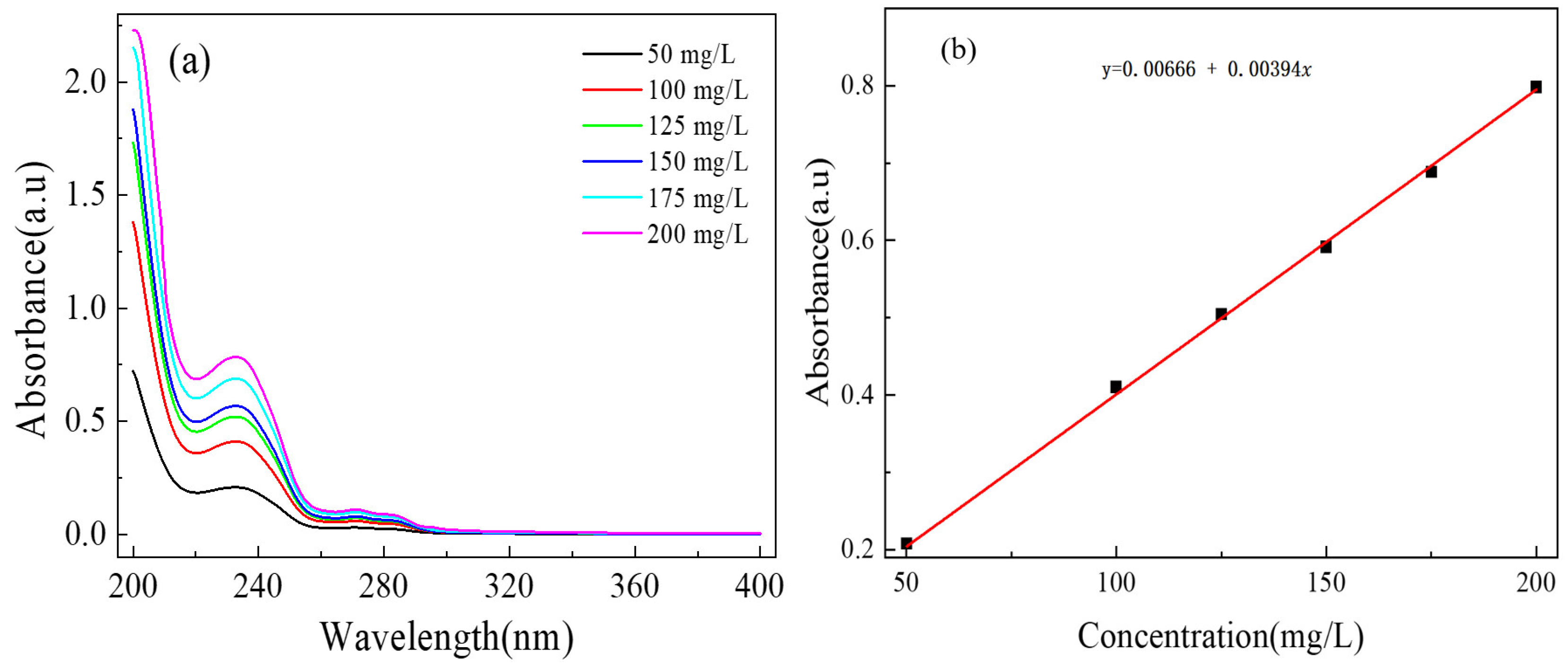
2.4. Release Behavior of OIM from GE-EP@OIMs
2.5. Corrosion Protection Performance of GE-EP@OIMs
2.5.1. Electrochemical Experiment
2.5.2. Weight Loss Experiment
3. Results and Discussion
3.1. Characteristic of GE-EP@OIMs
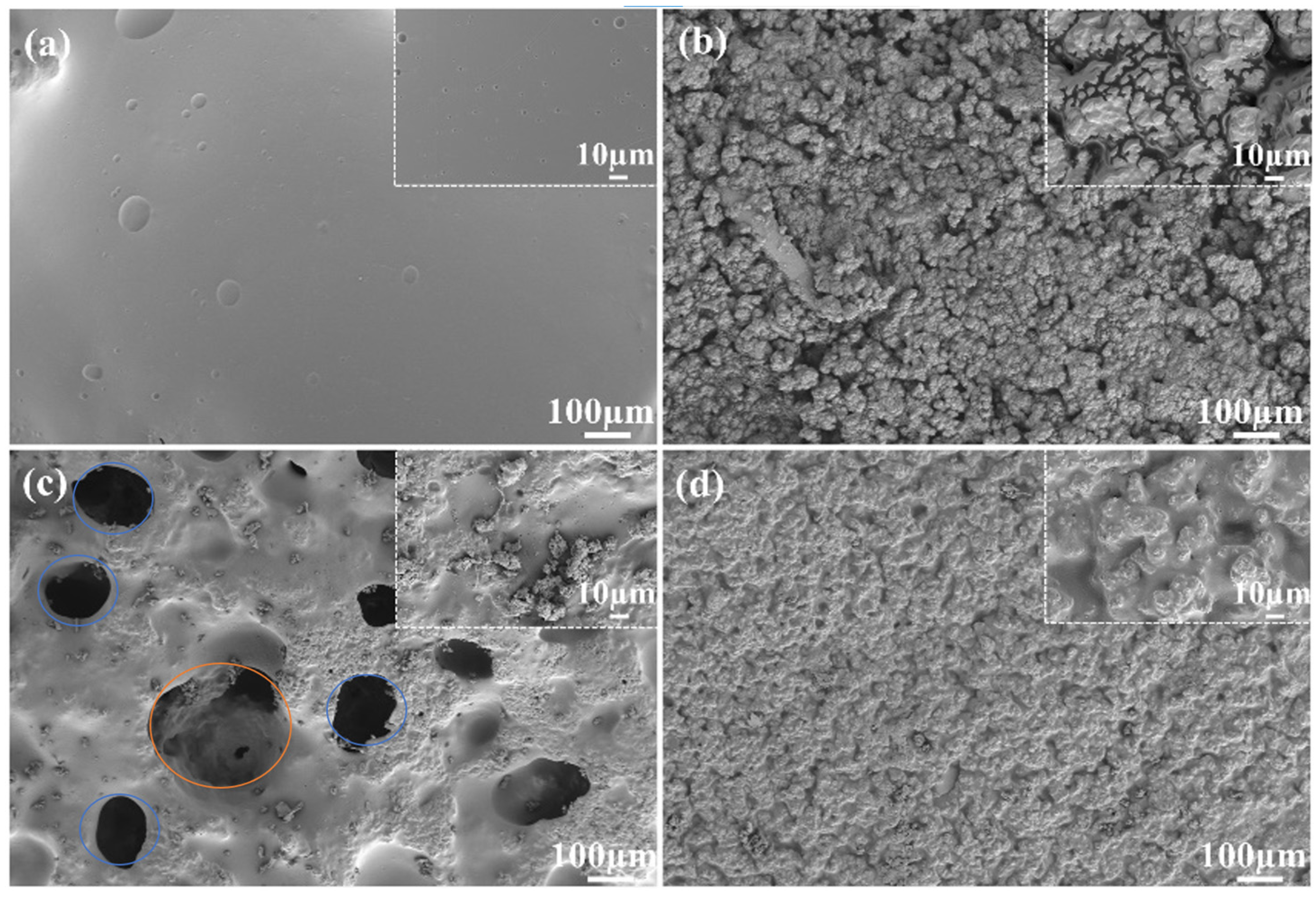
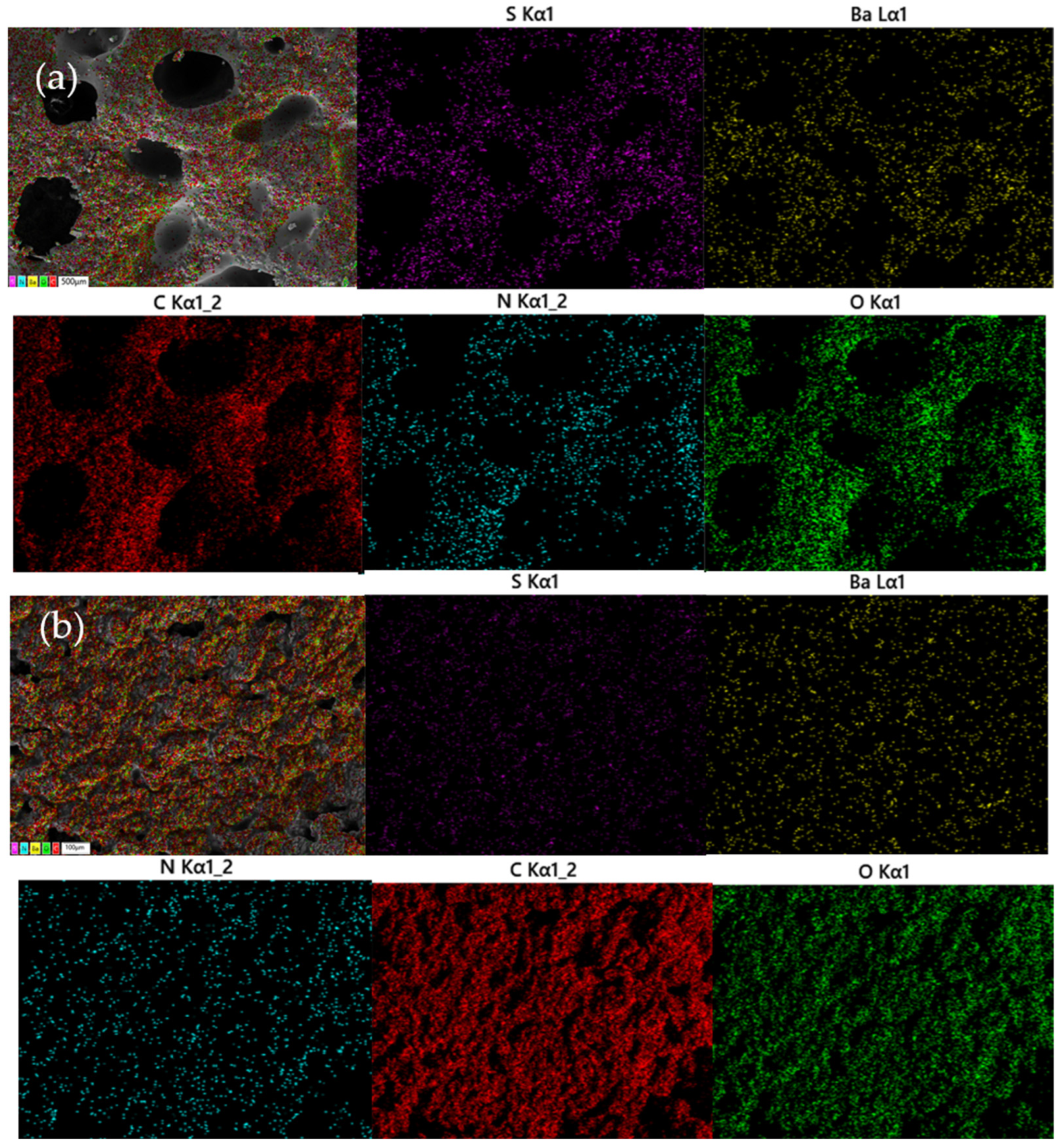
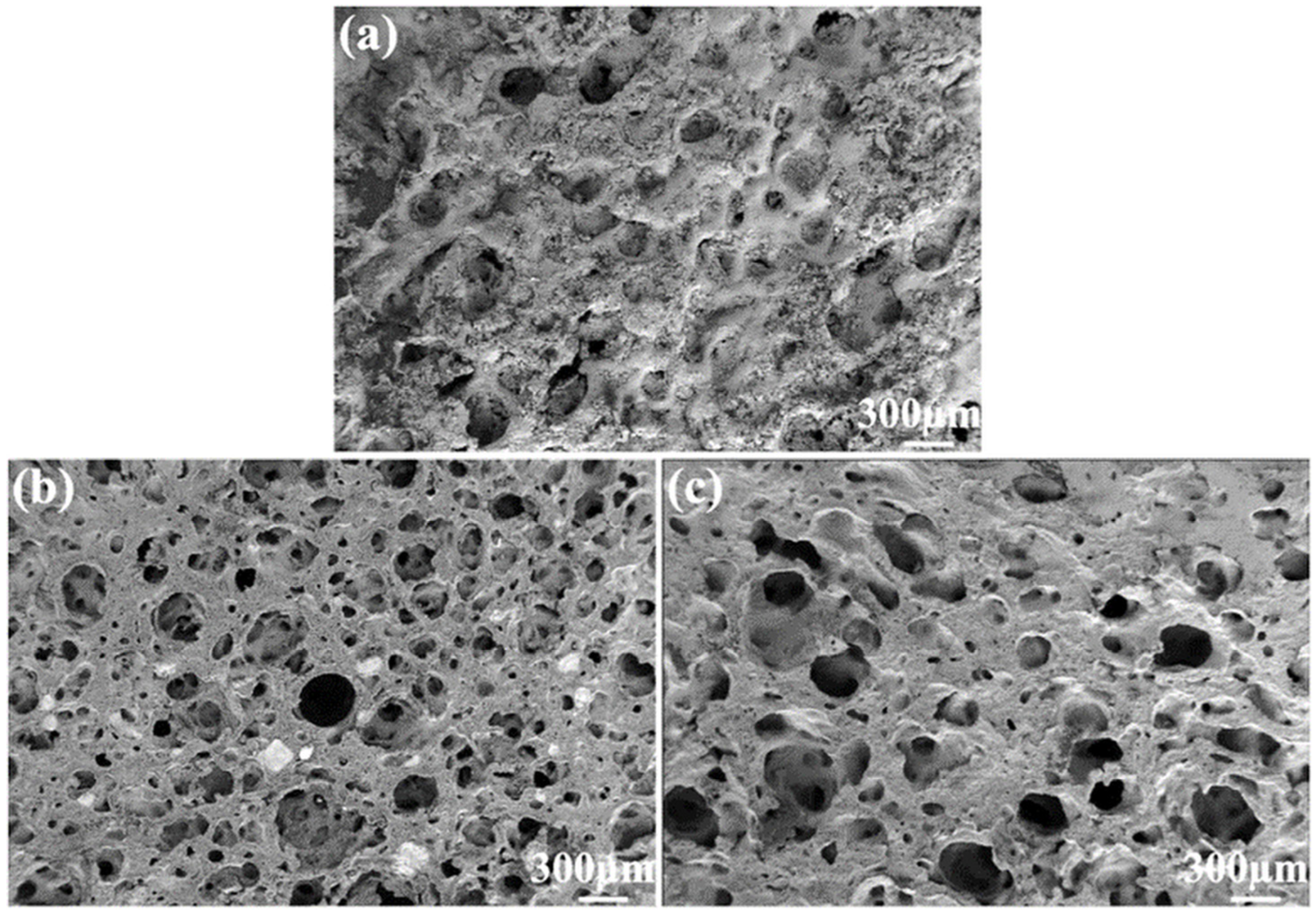
3.1.1. Surface Morphology of GE-EP@OIMs
3.1.2. FT-IR Analysis
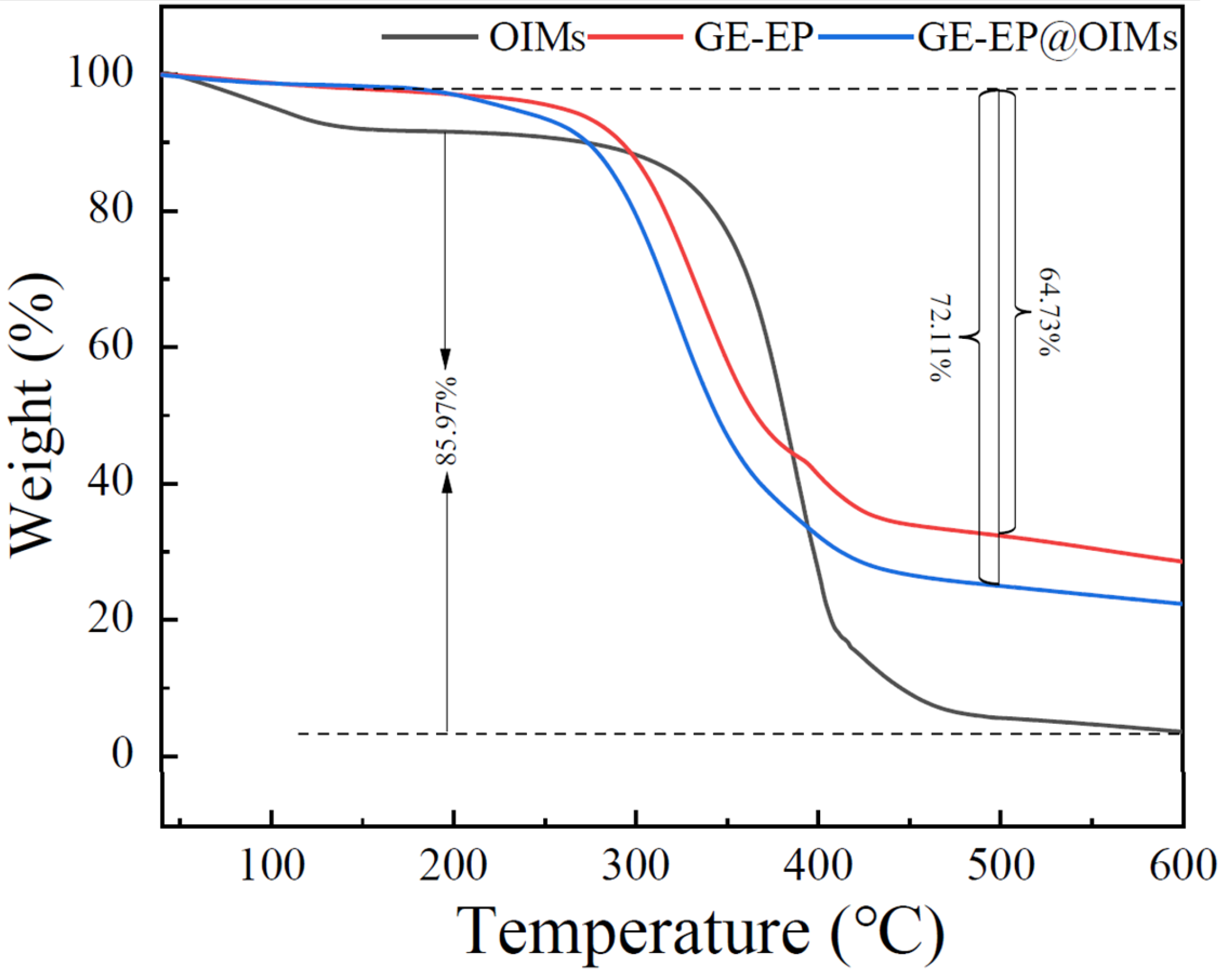
3.1.3. Thermostability and Inhibitor Loading Content of GE-EP@OIMs
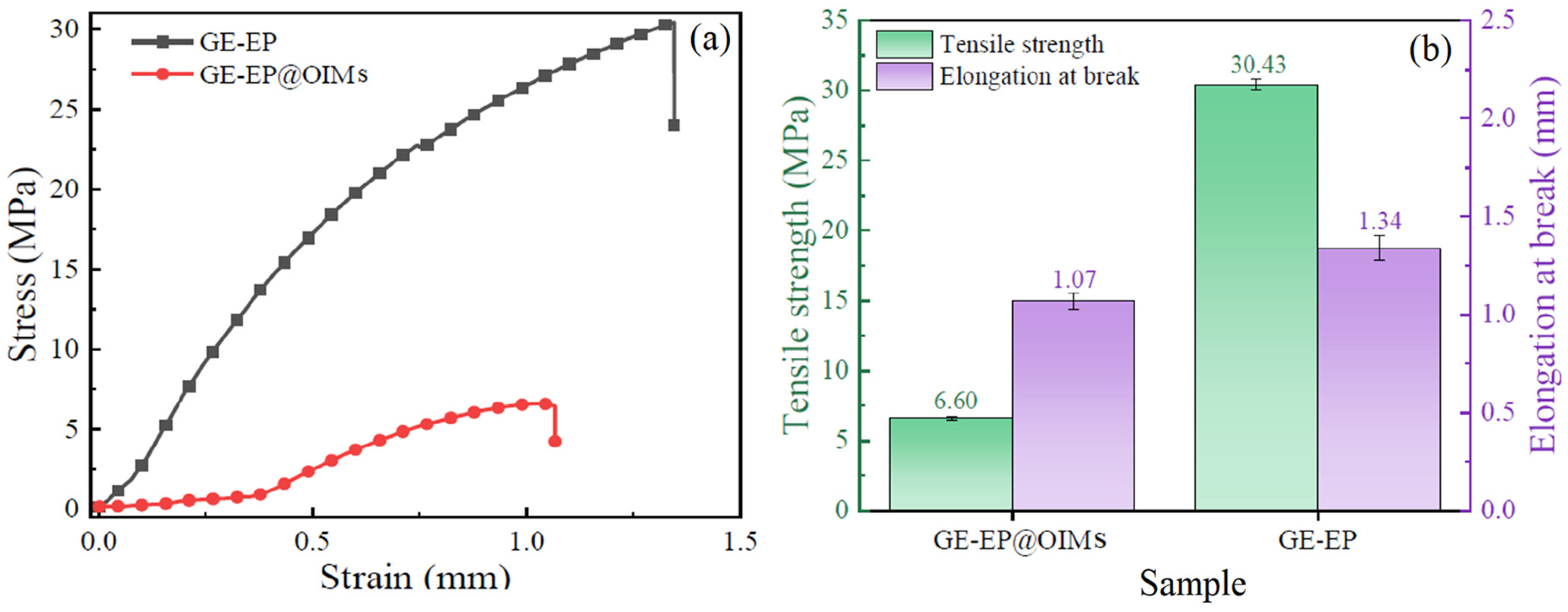
3.1.4. Mechanical Properties of GE-EP@OIMs
3.2. Inhibitor Releasing Characteristics and Mechanism of OIMs@PAM
3.2.1. Release Behavior of GE-EP@OIMs in Different pH Environment
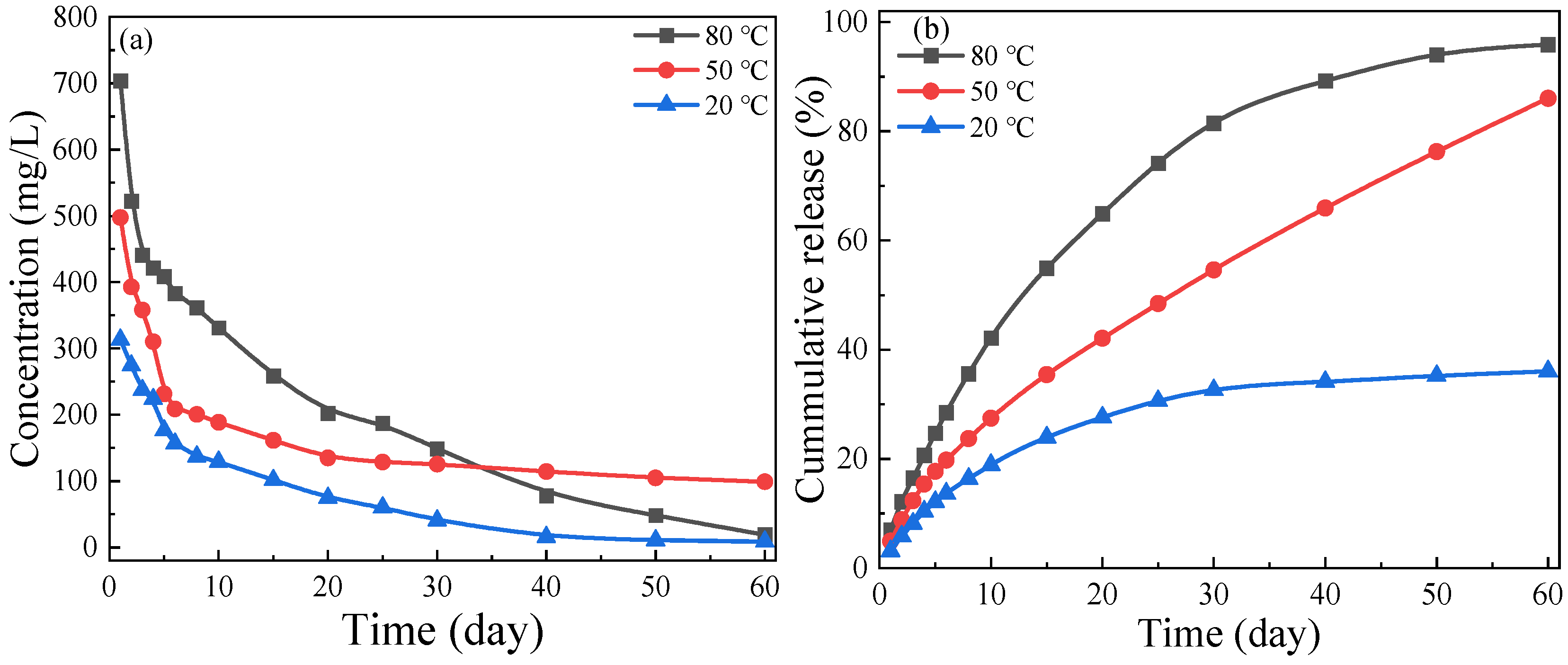
3.2.2. Long-Term Release Behavior of Solid Corrosion Inhibitor at Different Temperatures
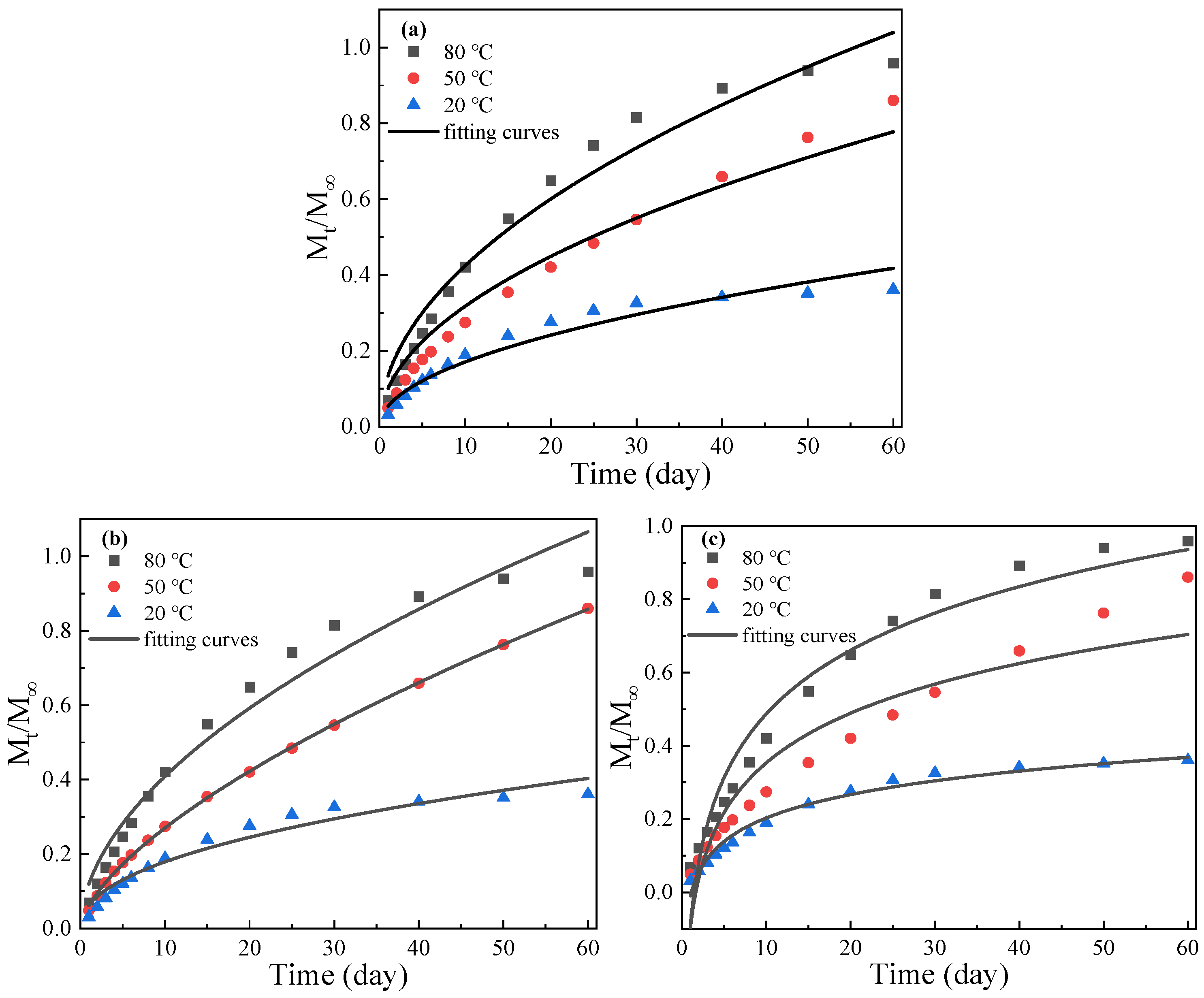
3.2.3. The Release Kinetics of GE-EP@OIMs
3.2.4. GE-EP@OIMs Morphology After Inhibitor Release
3.3. Corrosion Protection Effect of GE-EP@OIMs in Various pH NaCl Solutions
3.3.1. Weight Loss Measurements
- The corrosion rate of L80 carbon steel is positively correlated with the solution temperature. The higher corrosion rate at 80 °C can be attributed to the fact that elevated temperatures promote the migration of corrosive ions at the electrochemical reaction interface, thereby accelerating the electrochemical reaction process;
- The corrosion rate of L80 steel decreases gradually over time, eventually stabilizing. According to the NACE-RP0775 standard [41], the long-term (60 days) corrosion rate of L80 steel at 20 °C is highly corroded, while at 50 °C and 80 °C, it is severely corroded. The fact that corrosion rate of L80 carbon steel in various temperature environments exceeds 0.076 mm/a indicates the need for corrosion protection measures in oil-and-gas fields.
3.3.2. Surface Observation of L80 Steel After Immersion Test
4. Conclusions
- Unprecedented corrosion mitigation performance: This is because GE-EP@OIMs demonstrates exceptional corrosion resistance, particularly at elevated temperatures (50 °C), where it forms a robust protective layer that significantly reduces corrosion rates. This is achieved through its unique chemical composition and morphological properties, which synergistically inhibit corrosion reactions and ensure prolonged structural integrity.
- Revolutionary solid-state formulation: Unlike conventional liquid inhibitors, GE-EP@OIMs’s solid-state design eliminates challenges associated with uneven dispersion and high-pressure injection systems. This innovation not only simplifies deployment but also reduces operational costs, making it a practical and economically viable solution for field applications.
- Superior mechanical and thermal resilience: Despite a slight reduction in tensile strength due to OIM incorporation, GE-EP@OIMs maintains excellent mechanical robustness for medium- and long-term use. Its thermal adaptability ensures consistent performance across varying temperatures, a critical feature for oilfield environments. This is enabled by its molecular design, which ensures stability and effectiveness under thermal stress.
- Environmental and economic sustainability: GE-EP@OIMs is designed with environmental sustainability at its core. By optimizing material usage and reducing reapplication frequency, it minimizes environmental impact and aligns with sustainable development principles. This positions GE-EP@OIMs as a cost-effective and eco-friendly alternative to traditional inhibitors.
- Novel synthesis and high OIM-loading capacity: The synthesis process, which achieves an OIM-loading capacity of 34.75% (significantly higher than the typical 20%), represents a major breakthrough in material design. This high loading capacity, combined with the temperature-responsive release behavior, ensures targeted and efficient corrosion inhibition in chlorine-rich environments.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, S.; Dong, B.; Zhao, D.; Yang, J.; Sun, X.; Yan, L. Corrosion of carbon steel by Pseudomonas stutzeri CQ-Z5 in simulated oilfield water. Bioelectrochemistry 2024, 162, 108846. [Google Scholar]
- David, M.; Jasmin, R.; Louis, G. Techno-economic analysis of hybrid solar-assisted geothermal and biomass heating systems in remote subarctic communities (Nunavik, Canada). Energy 2024, 313, 133898. [Google Scholar]
- Li, H.; Liu, H.; Zhu, J.; Wang, X. Overview on plant extracts as green corrosion inhibitors in the oil and gas fields. J. Mater. Res. Technol. 2019, 8, 6376–6389. [Google Scholar]
- Zhao, L.; Yan, Y.; Yan, X. A semi-empirical model for CO2 erosion-corrosion of carbon steel pipelines in wet gas-solid flow. J. Pet. Sci. Eng. 2021, 200, 108203. [Google Scholar]
- Li, L.; Wang, X.; Miao, J.; Aliya, A.; Jing, X.; Ren, N. Carbon neutrality of wastewater treatment: A systematic concept beyond the plant boundary. Environ. Sci. Ecotechnol. 2022, 11, 100180. [Google Scholar] [CrossRef]
- Chen, Z.; Abdolreza, F.; Danial, I.; Alireza, R.; Esmaeil, A.; Chen, C. Highly biodegradable corrosion inhibitors derived from sunflower oil for mild steel corrosion in CO2- and H2S-saturated oilfield-produced water. Energy Fuels 2024, 38, 9529–9545. [Google Scholar] [CrossRef]
- Hughes, A.E.; Winkler, D.A.; Carr, J.; Lee, P.D. Corrosion inhibition, inhibitor environments, and the role of machine learning. Corros. Mater. Degrad. 2022, 3, 672–693. [Google Scholar] [CrossRef]
- Zhang, L.; Ge, L.; He, G.; Tian, Z.; Huang, J. Tuning the linkers in polymer-based cathodes to realize high sulfur content and high-performance potassium–sulfur batteries. J. Phys. Chem. C 2021, 125, 28790–28801. [Google Scholar]
- Chen, C.; Reniers, G. Chemical industry in China: The current status, safety problems, and pathways for future sustainable development. Saf. Sci. 2020, 128, 104741. [Google Scholar]
- Jones, R.; Al Zubaidi, I.; Alzughaibi, M. Crude glycerol as an innovative corrosion inhibitor. Appl. Syst. Innov. 2018, 1, 12. [Google Scholar] [CrossRef]
- Pojtanabuntoeng, T.; Kinsella, B.; Ehsani, H. Assessment of corrosion control by pH neutralisation in the presence of glycol at low temperature. Corros. Sci. 2017, 128, 94–103. [Google Scholar]
- Zhang, Y.; Yu, M.; Chen, C.; Li, S.; Liu, J. Self-healing coatings based on stimuli-responsive release of corrosion inhibitors: A review. Front. Mater. 2022, 8, 795397. [Google Scholar]
- Zuo, J.; Dong, B.; Xing, F.; Luo, C.; Zhan, J.; Wang, L. Preparation and behavior of sustained-release corrosion inhibitor microcapsules by centrifugation-coating method. Powder Technol. 2021, 389, 32–39. [Google Scholar]
- Wang, T.; Wang, W.; Feng, H.; Sun, T.; Ma, C.; Cao, L. Photothermal nanofiller-based polydimethylsiloxane anticorrosion coating with multiple cyclic self-healing and long-term self-healing performance. Chem. Eng. J. 2022, 446, 137077. [Google Scholar] [CrossRef]
- Tian, Z.; Li, S.; Chen, Y.; Zhang, L.; An, Z. Self-healing coating with a controllable release of corrosion inhibitors by using multifunctional zinc oxide quantum dots as valves. ACS Appl. Mater. Interfaces 2022, 14, 47188–47197. [Google Scholar]
- Zhang, D.; Ren, B.; Zhang, Y.; Xu, L.; Huang, Q.; He, Y.; Li, X.; Wu, J.; Yang, J.; Chen, Q.; et al. From design to applications of stimuli-responsive hydrogel strain sensors. J. Mater. Chem. B 2020, 8, 3171–3191. [Google Scholar]
- Xing, Z.; Li, Z.; Lu, H. Self-assembled topological transition via intra-and inter-chain coupled binding in physical hydrogel towards mechanical toughening. Polymer 2021, 235, 124268. [Google Scholar]
- Li, C.; Zhao, X.; Meng, C.; Zhang, T.; Sun, S.; Hu, S. Application of hollow mesoporous organosilica nanoparticles as pH and redox double stimuli-responsive nanocontainers in the controlled release of corrosion inhibitors. Prog. Org. Coat. 2021, 159, 106437. [Google Scholar]
- Ren, Y.; Qi, X.; He, Y.; Peng, Z.; Yang, H.; Liu, X. Green inhibitor-loaded functional halloysite nanotubes modified coatings for improving corrosion protection of carbon steel. Mater. Today Commun. 2024, 38, 108231. [Google Scholar]
- GBT 27761-2011; Test Method for Weight Loss and Residual Amount of Thermogravimetric Analyzer. Standards Press of China: Beijing, China, 2011.
- GBT 1040; Determination of Tensile Properties of Plastics. Standards Press of China: Beijing, China, 2006.
- GB/T 16545-2015; Corrosion of Metals and Alloys—Removal of Corrosion Products from Corrosion Samples. Standardization Administration of China: Beijing, China, 2015.
- Zhang, Z.; Zhao, Y.; Gong, Q.; Li, Z.; Li, J. MOFs for CO2 capture and separation from flue gas mixtures: The effect of multifunctional sites on their adsorption capacity and selectivity. Chem. Commun. 2013, 49, 653–661. [Google Scholar] [CrossRef]
- Aayisha, S.; Renuga Devi, T.S.; Janani, S.; Muthu, S.; Raja, M.; Sevvanthi, S. DFT, molecular docking and experimental FT-IR, FT-Raman, NMR inquisitions on “4-chloro-N-(4,5-dihydro-1H-imidazol-2-yl)-6-methoxy-2-methylpyrimidin-5-amine”: Alpha-2-imidazoline receptor agonist antihypertensive agent. J. Mol. Struct. 2019, 1186, 468–481. [Google Scholar] [CrossRef]
- Yang, Q.; Lin, B.; Tang, J.; Wang, Y.; Zheng, H.; Zhang, H.; Zhang, Y. A pH-Controlled Solid Inhibitor Based on PAM Hydrogel for Steel Corrosion Protection in Wide Range pH NaCl Medium. Molecules 2023, 28, 1314. [Google Scholar] [CrossRef] [PubMed]
- Arukalam, I.O.; Madu, I.O.; Ishidi, E.Y. High performance characteristics of Lupinus arboreus gum extract as self-healing and corrosion inhibition agent in epoxy-based coating. Prog. Org. Coat. 2021, 151, 106095. [Google Scholar]
- Qiu, C.; Luo, J.; Ling, Y.; Lu, Z.; Ni, L.; Chen, Y.; Liang, M. Thermal Degradation Behavior and Mechanism of Organosilicon Modified Epoxy Resin. Macromol. Chem. Phys. 2022, 223, 2200164. [Google Scholar]
- Correia, D.M.; Padrão, J.; Rodrigues, L.R.; Dourado, F.; Lanceros-Méndez, S.; Sencadas, V. Thermal and hydrolytic degradation of electrospun fish gelatin membranes. Polym. Test. 2013, 32, 995–1000. [Google Scholar]
- Barreto, P.L.M.; Pires, A.T.N.; Soldi, V. Thermal degradation of edible films based on milk proteins and gelatin in inert atmosphere. Polym. Degrad. Stab. 2003, 79, 147–152. [Google Scholar]
- Gou, S.; Luo, S.; Liu, T.; Xia, H.; Jing, D.; Zhang, Q.; Guo, Q. Thermally stable imidazoline-based sulfonate copolymers for enhanced oil recovery. RSC Adv. 2015, 5, 85165–85173. [Google Scholar]
- Wang, L.; Zhang, C.; Xie, H.; Xia, H.; Jing, D.; Zhang, Q.; Li, S.; Li, Z.; Guo, Q. Calcium alginate gel capsules loaded with inhibitor for corrosion protection of downhole tube in oilfields. Corros. Sci. 2015, 90, 296–304. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, H.; Tang, J. Molecular dynamics simulation and experimental investigation on the synergistic mechanism and synergistic effect of oleic acid imidazoline and l-cysteine corrosion inhibitors. Corros. Sci. 2021, 179, 108819. [Google Scholar]
- Williams, G.; Geary, S.; McMurray, H.N. Smart release corrosion inhibitor pigments based on organic ion-exchange resins. Corros. Sci. 2012, 57, 139–147. [Google Scholar]
- Wen, J.; Lei, J.; Chen, J.; Gou, J.; Li, Y.; Li, L. An intelligent coating based on pH-sensitive hybrid hydrogel for corrosion protection of mild steel. Chem. Eng. J. 2020, 392, 123742. [Google Scholar] [CrossRef]
- Imanieh, I.; Afshar, A. Corrosion protection of aluminum by smart coatings containing layered double hydroxide (LDH) nanocontainers. J. Mater. Res. Technol. 2019, 8, 3004–3023. [Google Scholar]
- El Achaby, M.; Fayoud, N.; Figueroa-Espinoza, M.C.; Aboulkas, A. New highly hydrated cellulose microfibrils with a tendril helical morphology extracted from agro-waste material: Application to removal of dyes from waste water. RSC Adv. 2018, 8, 5212–5224. [Google Scholar] [PubMed]
- Koutsoulas, C.; Pippa, N.; Demetzos, C.; Zabka, M. Preparation of liposomal nanoparticles incorporating terbinafine in vitro drug release studies. J. Nanosci. Nanotechnol. 2014, 14, 4529–4533. [Google Scholar] [CrossRef]
- Lynch, I.; Dawson, K.A. Release of model compounds from “plum-pudding”-type gels composed of microgel particles randomly dispersed in a gel matrix. J. Phys. Chem. B 2004, 108, 10893–10898. [Google Scholar]
- Wu, Y.; Duan, Y.; Qiu, J.; Gao, X.; Ma, H. A pH-responsive intelligent coating based on composite CaCO3 microspheres for long-term corrosion protection of Q235 carbon steel. Appl. Surf. Sci. 2022, 578, 151980. [Google Scholar]
- Steyaert, I.; Rahier, H.; Van Vlierberghe, S.; Olijve, J.; De Clerck, K. Gelatin nanofibers: Analysis of triple helix dissociation temperature and cold-water-solubility. Food Hydrocoll. 2016, 57, 200–208. [Google Scholar]
- NACE RP0775-2005; Preparation, Installation, Analysis, and Interpretation of Corrosion Coupons in Oilfield Operations. NACE International: Houston, TX, USA, 2005.




| Element | C | Si | Mn | P | S | Cr | Ni | Mo | Fe |
|---|---|---|---|---|---|---|---|---|---|
| L80 | 0.36 | 0.45 | 1.0 | 0.03 | 0.004 | 0.95 | 0.04 | 0.38 | Balance |
| Time (Days) | Number of Measurements | Mass Before (mg) | Mass After (mg) | Weight Loss (mg) | Corrosion Rate (mm/y) |
|---|---|---|---|---|---|
| 1 | 3 | 120.543 | 120.214 | 0.329 | 0.5464 |
| 5 | 3 | 120.214 | 119.876 | 0.338 | 0.8078 |
| 10 | 3 | 119.876 | 119.532 | 0.344 | 0.4330 |
| 20 | 3 | 119.532 | 119.19 | 0.342 | 0.7149 |
| 30 | 3 | 119.19 | 118.85 | 0.34 | 0.8456 |
| 40 | 3 | 118.85 | 118.51 | 0.34 | 0.6661 |
| 50 | 3 | 118.51 | 118.17 | 0.34 | 0.4582 |
| 60 | 3 | 118.17 | 117.83 | 0.34 | 0.4929 |
| Higuchi | Korsmeyer–Peppas | Elovich | ||||||
|---|---|---|---|---|---|---|---|---|
| Model | R2 | kH | R2 | n | k | R2 | β | α |
| 0.97 | 0.1342 | 0.97 | 0.5345 | 0.1194 | 0.95 | 3.9886 | 0.1749 | |
| 0.96 | 0.1004 | 0.99 | 0.6449 | 0.0612 | 0.89 | 5.1100 | 0.1191 | |
| 0.95 | 0.0539 | 0.95 | 0.4431 | 0.0631 | 0.98 | 10.8334 | 0.0832 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Yang, Q.; Khalaf, A.H.; Lin, B.; Tang, J. Long-Term Anti-Corrosion Performance of Ultra-High Content Inhibitor Loaded Gel-Epoxy Solid Inhibitor with Temperature-Responisve Effect. Appl. Sci. 2025, 15, 3964. https://doi.org/10.3390/app15073964
Zhao Y, Yang Q, Khalaf AH, Lin B, Tang J. Long-Term Anti-Corrosion Performance of Ultra-High Content Inhibitor Loaded Gel-Epoxy Solid Inhibitor with Temperature-Responisve Effect. Applied Sciences. 2025; 15(7):3964. https://doi.org/10.3390/app15073964
Chicago/Turabian StyleZhao, Ying, Qing Yang, Ali Hussein Khalaf, Bing Lin, and Junlei Tang. 2025. "Long-Term Anti-Corrosion Performance of Ultra-High Content Inhibitor Loaded Gel-Epoxy Solid Inhibitor with Temperature-Responisve Effect" Applied Sciences 15, no. 7: 3964. https://doi.org/10.3390/app15073964
APA StyleZhao, Y., Yang, Q., Khalaf, A. H., Lin, B., & Tang, J. (2025). Long-Term Anti-Corrosion Performance of Ultra-High Content Inhibitor Loaded Gel-Epoxy Solid Inhibitor with Temperature-Responisve Effect. Applied Sciences, 15(7), 3964. https://doi.org/10.3390/app15073964






