Macroissues with Microplastics: A Review on Distribution, Environmental Impacts, Pollutant Interactions, Toxicity, Analytical Methodology and Mitigation Strategies
Abstract
1. Introduction
2. Data Acquisition
3. Occurrence, Distribution and Environmental Impacts of MPs
3.1. MPs in Aquatic Environment
3.2. MPs in Soil
3.3. MPs in Air and Precipitation
4. Interactions of MPs and Other Pollutants
5. Toxicity of MPs
5.1. Impact of MPs on Aquatic Organisms
5.2. Impacts of MPs on Soil Organisms
5.3. Human Exposure and Health Impacts of MPs
6. Methodology for MP Analysis
6.1. Sampling and Sample Handling
6.2. Sample Pre-Treatment and MP Extraction for Analysis
6.3. Analytical Methods for Identification, Quantification and Characterization of MPs
6.3.1. Microscopic Methods
6.3.2. Spectroscopic Methods
6.3.3. Chromatographic Methods
7. Mitigation Strategies for MPs in the Environment
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MP | Microplastic |
| HOCs | Hydrophobic organic pollutants |
| PACs | Plastic additives |
| PA | Polyamide |
| PE | Polyethylene |
| PET | Polyethylene tetraphthalate |
| PP | Polypropylene |
| PVC | Polyvinyl chloride |
| POM | Polyformaldehyde |
| POPs | Persistent organic pollutants |
| PAH | Polyaromatic hydrocarbons |
| PCB | Polychlorinated biphenyls |
| PBDE | Polybrominated diphenyl ethers |
References
- Wang, J.; Zheng, L.; Li, J. A critical review on the sources and instruments of marine microplastics and prospects on the relevant management in China. Waste Manag. Res. 2018, 36, 898–911. [Google Scholar] [CrossRef]
- Rujnić-Sokele, M. Plastični otpad—Globalni ekološki problem (Plastic waste—A global environmental problem). Polimeri 2015, 36, 34–37. [Google Scholar]
- Organisation for Economic Co-Operation and Development (OECD). Plastic Pollution is Growing Relentlessly as Waste Management and Recycling Fall Short. 2022. Available online: www.oecd.org/environment/plastic-pollution-is-growing-relentlessly-as-waste-management-and-recycling-fall-short.htm (accessed on 13 July 2023).
- Sharma, S.; Chatterjee, S. Microplastic pollution, a threat to marine ecosystem and human health: A short review. Environ. Sci. Pollut. Res. 2017, 24, 21530–21547. [Google Scholar] [CrossRef]
- Bule, K.; Grgić, D.K.; Bulatović, V.O.; Miloloža, M.; Radin, E.; Tolić, A.; Zadro, K. Mikroplastika u morskom okolišu Jadrana (Microplastics in the marine environment of the Adriatic). Kem. Ind. 2020, 69, 303–310. [Google Scholar] [CrossRef]
- Ryan, G.P. Plastic and other artefacts on South African beaches: Temporal trends in abundance and composition. S. Afr. J. Sci. 1990, 86, 450–452. [Google Scholar]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- Frias, J.; Nash, R. Microplastics: Finding a consensus on the definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef]
- Jiang, B.; E Kauffman, A.; Li, L.; McFee, W.; Cai, B.; Weinstein, J.; Lead, J.R.; Chatterjee, S.; I Scott, G.; Xiao, S. Health impacts of environmental contamination of micro- and nanoplastics: A review. Environ. Health Prev. Med. 2020, 25, 29. [Google Scholar] [CrossRef]
- Sarijan, S.; Azman, S.; Said, M.I.M.; Jamal, M.H. Microplastics in freshwater ecosystems: A recent review of occurrence, analysis, potential impacts, and research needs. Environ. Sci. Pollut. Res. 2021, 28, 1341–1356. [Google Scholar] [CrossRef]
- Ziani, K.; Ioniță-Mîndrican, C.-B.; Mititelu, M.; Neacșu, S.M.; Negrei, C.; Moroșan, E.; Drăgănescu, D.; Preda, O.-T. Microplastics: A Real Global Threat for Environment and Food Safety: A State of the Art Review. Nutrients 2023, 15, 617. [Google Scholar] [CrossRef]
- De Souza Machado, A.A.; Kloas, W.; Zarfl, C.; Hempel, S.; Rillig, M.C. Microplastics as an emerging threat to terrestrial ecosystems. Glob. Change Biol. 2018, 24, 1405–1416. [Google Scholar] [CrossRef]
- Sönmez, V.Z.; Akarsu, C.; Sivri, N. Impact of coastal wastewater treatment plants on microplastic pollution in surface seawater and ecological risk assessment. Environ. Pollut. 2023, 318, 120922. [Google Scholar] [CrossRef]
- He, D.; Luo, Y.; Lu, S.; Liu, M.; Song, Y.; Lei, L. Microplastics in soils: Analytical methods, pollution characteristics and ecological risks. TrAC Trends Anal. Chem. 2018, 109, 163–172. [Google Scholar] [CrossRef]
- GESAMP. Sources, Fate and Effects of Microplastics in the Marine Environment: A Global Assessment; UNESCO-IOC Report No. 90; GESAMP: London, UK, 2016. [Google Scholar]
- Blettler, M.C.M.; Abrial, E.; Khan, R.F.; Sivri, N.; Espinola, L.A. Rivers as plastic reservoirs and pathways to the oceans. Environ. Pollut. 2018, 239, 1000–1014. [Google Scholar] [CrossRef]
- Qianh, N.; Gao, Q.; Lang, X.; Deng, H.; Bratu, T.M.; Chen, Q.; Stapleton, P.; Yan, B.; Min, W. Rapid single-particle chemical imaging of nanoplastics by SRS microscopy. Proc. Natl. Acad. Sci. USA 2024, 121, e2300582121. [Google Scholar] [CrossRef]
- European Chemicals Agency (ECHA). Estimated 63,000–430,000 Tons/Year of MPs Enter EU Soils; ECHA: Helsinki, Finland, 2021. [Google Scholar]
- Nizzetto, L.; Futter, M.; Langaas, S. Are agricultural soils dumps for microplastics of urban origin? Environ. Sci. Technol. 2016, 50, 10777–10779. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Mirande, C.; Mandin, C.; Guerrouache, M.; Langlois, V.; Tassin, B. A first overview of textile fibers, including microplastics, in indoor and outdoor environments. Environ. Pollut. 2017, 221, 453–458. [Google Scholar] [CrossRef]
- Allen, S.; Allen, D.; Phoenix, V.R.; Le Roux, G.; Jiménez, P.D.; Simonneau, A.; Binet, S.; Galop, D. Atmospheric transport and deposition of microplastics in a remote mountain catchment. Nat. Geosci. 2019, 12, 339–344. [Google Scholar] [CrossRef]
- Derraik, J.G.B. The pollution of the marine environment by plastic debris: A review. Mar. Pollut. Bull. 2002, 44, 842–852. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Cverenkárová, K.; Valachovičová, M.; Mackuľak, T.; Žemlička, L.; Bírošová, L. Microplastics in the Food Chain. Life 2021, 11, 1349. [Google Scholar] [CrossRef]
- Parać, M.; Cukrov, N.; Bulat, T.; Cukrov, N.; Lovrić, M. Microplastics assessment in the Krka river estuary surface water. Environ. Eng. 2022, 9, 29–34. [Google Scholar] [CrossRef]
- Zeri, C.; Adamopoulou, A.; Varezić, D.B.; Fortibuoni, T.; Viršek, M.K.; Kržan, A.; Mandic, M.; Mazziotti, C.; Palatinus, A.; Peterlin, M.; et al. Floating plastics in Adriatic waters (Mediterranean Sea): From the macro- to the micro-scale. Mar. Pollut. Bull. 2018, 136, 341–350. [Google Scholar] [CrossRef]
- Eriksen, M.; Lebreton, L.C.M.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic Pollution in the World′s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef]
- Kovač, N.; Serdarušić, A. Onečišćenje mora plastičnim otpadom (Sea pollution with plastic waste). Paragraf 2017, 1, 57–76. [Google Scholar]
- Mishra, A.K.; Singh, J.; Mishra, P.P. Microplastics in freshwater ecosystem: A serious threat for freshwater environment. Int. J. Environ. Sci. Technol. 2021, 20, 9189–9204. [Google Scholar] [CrossRef]
- Shahul Hamid, F.; Sanam Bhatti, M.; Anuar, N.; Mohan, P.; Periathamby, A. Worldwide distribution and abundance of microplastic: How dire is the situation? Waste Manag. Res. 2018, 36, 873–897. [Google Scholar] [CrossRef]
- Nerland, I.L.; Halsband, C. Microplastics in Marine Environments Occurrence, Distribution and Effects; Report for the Norwegian Environment Agency. Report no. M319 and 6754-2014; NIVA: Oslo, Norway, 2014. [Google Scholar]
- Goldstein, M.C.; Titmus, A.J.; Ford, M. Scales of Spatial Heterogeneity of Plastic Marine Debris in the Northeast Pacific Ocean. Heterogeneity of Plastic Debris in North Pacific. PLoS ONE 2013, 8, e80020. [Google Scholar] [CrossRef]
- Tutman, P.; Bojanić-Varezić, D.; Prvan, M. Integrirano planiranje u cilju smanjivanja utjecaja morskog otpada-projekt DeFishGear (Integrated planning aimed at reducing the impact of marine litter-DeFishGear project). In Proceedings of the Conference on Environmental Protection-Visions of Waste Management: Book of Abstracts, Opatija, Croatia, 9–10 July 2016; Milanović, Z., Sinčić, D., Jeftić, L., Kalambura, S., Žunec, N., Eds.; Business Media Croatia: Zagreb, Croatia, 2016; pp. 26–27. [Google Scholar]
- Gajšt, T.; Bizjak, T.; Palatinus, A.; Liubartseva, S.; Kržan, A. Sea surface microplastics in Slovenian part of the Northern Adriatic. Mar. Pollut. Bull. 2016, 113, 392–399. [Google Scholar] [CrossRef]
- Sajjad, M.; Huang, Q.; Khan, S.; Khan, M.A.; Liu, Y.; Wang, J.; Lian, F.; Wang, Q.; Guo, G. Microplastics in the soil environment: A critical review. Environ. Technol. Innov. 2022, 27, 102408. [Google Scholar] [CrossRef]
- Qi, Y.; Yang, X.; Pelaez, A.M.; Lwanga, E.H.; Beriot, N.; Gertsen, H.; Garbeva, P.; Geissen, V. Macro- and micro- plastics in soil-plant system: Effects of plastic mulch film residues on wheat (Triticum aestivum) growth. Sci. Total Environ. 2018, 645, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Lozano, Y.M.; Rillig, M.C. Microplastics Increase Soil pH and Decrease Microbial Activities as a Function of Microplastic Shape, Polymer Type, and Exposure Time. Front. Environ. Sci. 2021, 9, 675803. [Google Scholar] [CrossRef]
- Boots, B.; Russell, C.W.; Green, D.S. Effects of Microplastics in Soil Ecosystems: Above and Below Ground. Environ. Sci. Technol. 2019, 53, 11496–11506. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Effects of microplastics on microalgae populations: A critical review. Sci. Total Environ. 2019, 665, 400–405. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Liu, X.; Qu, F.; Wang, X.; Wang, X.; Li, Y.; Sun, Y. Microplastics in the environment: A review of analytical methods, distribution, and biological effects. TrAC Trends Anal. Chem. 2019, 111, 62–72. [Google Scholar] [CrossRef]
- Li, L.; Luo, Y.; Li, R.; Zhou, Q.; Peijnenburg, W.J.G.M.; Yin, N.; Yang, J.; Tu, C.; Zhang, Y. Effective uptake of submicrometre plastics by crop plants via a crack-entry mode. Nat. Sustain. 2020, 3, 929–937. [Google Scholar] [CrossRef]
- Bergmann, M.; Mützel, S.; Primpke, S.; Tekman, M.B.; Trachsel, J.; Gerdts, G. White and wonderful? Microplastics prevail in snow from the Alps to the Arctic. Sci. Adv. 2019, 5, eaax1157. [Google Scholar] [CrossRef]
- Kaliszewicz, A.; Winczek, M.; Karaban, K.; Kurzydłowski, D.; Górska, M.; Koselak, W.; Romanowski, J. The contamination of inland waters by microplastic fibres under different anthropogenic pressure: Preliminary study in Central Europe (Poland). Waste Manag. Res. J. Sustain. Circ. Econ. 2020, 38, 1231–1238. [Google Scholar] [CrossRef]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Wetherbee, G.A.; Baldwin, A.K.; Ranville, J.F. It Is Raining Plastic; Open-File Report. Report no. 2019-1048; U.S. Geological Survey: Reston, VA, USA, 2019. [CrossRef]
- Rodrigues, J.P.; Duarte, A.C.; Santos-Echeandía, J.; Rocha-Santos, T. Significance of interactions between microplastics and POPs in the marine environment: A critical overview. TrAC Trends Anal. Chem. 2019, 111, 252–260. [Google Scholar] [CrossRef]
- Verla, A.W.; Enyoh, C.E.; Verla, E.N.; Nwarnorh, K.O. Microplastic–toxic chemical interaction: A review study on quantified levels, mechanism and implication. SN Appl. Sci. 2019, 1, 1400. [Google Scholar] [CrossRef]
- Smith, M.; Love, D.C.; Rochman, C.M.; Neff, R.A. Microplastics in Seafood and the Implications for Human Health. Curr. Environ. Health Rep. 2018, 5, 375–386. [Google Scholar] [CrossRef]
- Lambert, S.; Scherer, C.; Wagner, M. Ecotoxicity testing of microplastics: Considering the heterogeneity of physicochemical properties. Integr. Environ. Assess. Manag. 2017, 13, 470–475. [Google Scholar] [CrossRef]
- Hua, J.; Vijver, M.G.; Richardson, M.K.; Ahmad, F.; Peijnenburg, W.J. Particle-specific toxic effects of differently shaped zinc oxide nanoparticles to zebrafish embryos (Danio rerio). Environ. Toxicol. Chem. 2014, 33, 2859–2868. [Google Scholar] [CrossRef]
- Ivleva, N.P. Chemical Analysis of Microplastics and Nanoplastics: Challenges, Advanced Methods, and Perspectives. Chem. Rev. 2021, 121, 11886–11936. [Google Scholar] [CrossRef]
- Park, H.; Park, B. Review of Microplastic Distribution, Toxicity, Analysis Methods, and Removal Technologies. Water 2021, 13, 2736. [Google Scholar] [CrossRef]
- Lamichhane, G.; Acharya, A.; Marahatha, R.; Modi, B.; Paudel, R.; Adhikari, A.; Raut, B.K.; Aryal, S.; Parajuli, N. Microplastics in environment: Global concern, challenges, and controlling measures. Int. J. Environ. Sci. Technol. 2023, 20, 4673–4694. [Google Scholar] [CrossRef]
- Walker, T.R.; Fequet, L. Current trends of unsustainable plastic production and micro(nano)plastic pollution. TrAC Trends Anal. Chem. 2023, 160, 116984. [Google Scholar] [CrossRef]
- Gouin, T. Addressing the importance of microplastic particles as vectors for long-range transport of chemical contaminants: Perspective in relation to prioritizing research and regulatory actions. Microplast. Nanoplast. 2021, 1, 14. [Google Scholar] [CrossRef]
- Pfohl, P.; Roth, C.; Meyer, L.; Heinemeyer, U.; Gruendling, T.; Lang, C.; Nestle, N.; Hofmann, T.; Wohlleben, W.; Jessl, S. Microplastic extraction protocols can impact the polymer structure. Microplast. Nanoplast. 2021, 1, 8. [Google Scholar] [CrossRef]
- Martinho, S.D.; Fernandes, V.C.; Figueiredo, S.A.; Delerue-Matos, C. Microplastic Pollution Focused on Sources, Distribution, Contaminant Interactions, Analytical Methods, and Wastewater Removal Strategies: A Review. Int. J. Environ. Res. Public Health 2022, 19, 5610. [Google Scholar] [CrossRef]
- Fu, L.; Li, J.; Wang, G.; Luan, Y.; Dai, W. Adsorption behavior of organic pollutants on microplastics. Ecotoxicol. Environ. Saf. 2021, 217, 112207. [Google Scholar] [CrossRef]
- Frias, J.; Sobral, P.; Ferreira, A. Organic pollutants in microplastics from two beaches of the Portuguese coast. Mar. Pollut. Bull. 2010, 60, 1988–1992. [Google Scholar] [CrossRef]
- Singh, S.; Trushna, T.; Kalyanasundaram, M.; Tamhankar, A.J.; Diwan, V. Microplastics in drinking water: A macro issue. Water Supply 2022, 22, 5650–5674. [Google Scholar] [CrossRef]
- Atugoda, T.; Vithanage, M.; Wijesekara, H.; Bolan, N.; Sarmah, A.K.; Bank, M.S.; You, S.; Ok, Y.S. Interactions between microplastics, pharmaceuticals and personal care products: Implications for vector transport. Environ. Int. 2021, 149, 106367. [Google Scholar] [CrossRef] [PubMed]
- Koelmans, A.A.; Redondo-Hasselerharm, P.E.; Nor, N.H.M.; de Ruijter, V.N.; Mintenig, S.M.; Kooi, M. Risk assessment of microplastic particles. Nat. Rev. Mater. 2022, 7, 138–152. [Google Scholar] [CrossRef]
- Chaudhry, A.K.; Sachdeva, P. Microplastics’ origin, distribution, and rising hazard to aquatic organisms and human health: Socio-economic insinuations and management solutions. Reg. Stud. Mar. Sci. 2021, 48, 102018. [Google Scholar] [CrossRef]
- Kazour, M.; Terki, S.; Rabhi, K.; Jemaa, S.; Khalaf, G.; Amara, R. Sources of microplastics pollution in the marine environment: Importance of wastewater treatment plant and coastal landfill. Mar. Pollut. Bull. 2019, 146, 608–618. [Google Scholar] [CrossRef]
- Lau, W.W.Y.; Shiran, Y.; Bailey, R.M.; Cook, E.; Stuchtey, M.R.; Koskella, J.; Velis, C.A.; Godfrey, L.; Boucher, J.; Murphy, M.B.; et al. Evaluating scenarios toward zero plastic pollution. Science 2020, 369, 1455–1461. [Google Scholar] [CrossRef]
- Harmon, S.M. The Effects of Microplastic Pollution on Aquatic Organisms. In Microplastic Contamination in Aquatic Environments; Zeng, E.I., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 249–270. [Google Scholar] [CrossRef]
- Pellini, G.; Gomiero, A.; Fortibuoni, T.; Ferrà, C.; Grati, F.; Tassetti, A.; Polidori, P.; Fabi, G.; Scarcella, G. Characterization of microplastic litter in the gastrointestinal tract of Solea solea from the Adriatic Sea. Environ. Pollut. 2018, 234, 943–952. [Google Scholar] [CrossRef]
- Othman, A.R.; Abu Hasan, H.; Muhamad, M.H.; Ismail, N. Abdullah, S.R.S. Microbial degradation of microplastics by enzymatic processes: A review. Environ. Chem. Lett. 2021, 19, 3057–3073. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, R.; Zhang, S.; Sun, Y.; Wang, F. Uptake and translocation of nano/microplastics by rice seedlings: Evidence from a hydroponic experiment. J. Hazard. Mater. 2022, 421, 126700. [Google Scholar] [CrossRef]
- Huerta Lwanga, E.; Gertsen, H.; Gooren, H.; Peters, P.; Salánki, T.; Van Der Ploeg, M.; Besseling, E.; Koelmans, A.A.; Geissen, V. Microplastics in the Terrestrial Ecosystem: Implications for Lumbricus terrestris (Oligochaeta, Lumbricidae). Environ. Sci. Technol. 2016, 50, 2685–2691. [Google Scholar] [CrossRef]
- Chia, R.W.; Lee, J.-Y.; Kim, H.; Jang, J. Microplastic pollution in soil and groundwater: A review. Environ. Chem. Lett. 2021, 19, 4211–4224. [Google Scholar] [CrossRef]
- Li, Y.; Tao, L.; Wang, Q.; Wang, F.; Li, G.; Song, M. Potential Health Impact of Microplastics: A Review of Environmental Distribution, Human Exposure, and Toxic Effects. Environ. Health 2023, 1, 249–257. [Google Scholar] [CrossRef]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human Consumption of Microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef]
- Osman, A.I.; Hosny, M.; Eltaweil, A.S.; Omar, S.; Elgarahy, A.M.; Farghali, M.; Yap, P.-S.; Wu, Y.-S.; Nagandran, S.; Batumalaie, K.; et al. Microplastic sources, formation, toxicity and remediation: A review. Environ. Chem. Lett. 2023, 21, 2129–2169. [Google Scholar] [CrossRef]
- Kwon, J.-H.; Kim, J.-W.; Pham, T.D.; Tarafdar, A.; Hong, S.; Chun, S.-H.; Lee, S.-H.; Kang, D.-Y.; Kim, J.-Y.; Kim, S.-B.; et al. Microplastics in Food: A Review on Analytical Methods and Challenges. Int. J. Environ. Res. Public Health 2020, 17, 6710. [Google Scholar] [CrossRef]
- Peixoto, D.; Pinheiro, C.; Amorim, J.; Oliva-Teles, L.; Guilhermino, L.; Vieira, M.N. Microplastic pollution in commercial salt for human consumption: A review. Estuar. Coast. Shelf Sci. 2019, 219, 161–168. [Google Scholar] [CrossRef]
- Liebezeit, G.; Liebezeit, E. Origin of Synthetic Particles in Honeys. Pol. J. Food Nutr. Sci. 2015, 65, 143–147. [Google Scholar] [CrossRef]
- Liebezeit, G.; Liebezeit, E. Synthetic particles as contaminants in German beers. Food Addit. Contam. Part A 2014, 31, 1574–1578. [Google Scholar] [CrossRef]
- Makhdoumi, P.; Pirsaheb, M.; Amin, A.A.; Kianpour, S.; Hossini, H. Microplastic pollution in table salt and sugar: Occurrence, qualification and quantification and risk assessment. J. Food Compos. Anal. 2023, 119, 105261. [Google Scholar] [CrossRef]
- Basaran, B.; Özçifçi, Z.; Akcay, H.T.; Aytan, Ü. Microplastics in branded milk: Dietary exposure and risk assessment. J. Food Compos. Anal. 2023, 123, 105611. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, Z.; Chen, Y.; Yang, F.; Yao, W.; Xie, Y. Microplastics contamination in eggs: Detection, occurrence and status. Food Chem. 2022, 397, 133771. [Google Scholar] [CrossRef] [PubMed]
- Altunışık, A. Prevalence of microplastics in commercially sold soft drinks and human risk assessment. J. Environ. Manag. 2023, 336, 117720. [Google Scholar] [CrossRef]
- Kadac-Czapska, K.; Trzebiatowska, P.J.; Mazurkiewicz, M.; Kowalczyk, P.; Knez, E.; Behrendt, M.; Mahlik, S.; Zaleska-Medynska, A.; Grembecka, M. Isolation and identification of microplastics in infant formulas—A potential health risk for children. Food Chem. 2024, 440, 138246. [Google Scholar] [CrossRef] [PubMed]
- Conti, G.O.; Ferrante, M.; Banni, M.; Favara, C.; Nicolosi, I.; Cristaldi, A.; Fiore, M.; Zuccarello, P. Micro- and nano-plastics in edible fruit and vegetables. The first diet risks assessment for the general population. Environ. Res. 2020, 187, 109677. [Google Scholar] [CrossRef]
- Karami, A.; Golieskardi, A.; Choo, C.K.; Larat, V.; Karbalaei, S.; Salamatinia, B. Microplastic and mesoplastic contamination in canned sardines and sprats. Sci. Total Environ. 2018, 612, 1380–1386. [Google Scholar] [CrossRef]
- Du, F.; Cai, H.; Zhang, Q.; Chen, Q.; Shi, H. Microplastics in take-out food containers. J. Hazard. Mater. 2020, 399, 122969. [Google Scholar] [CrossRef]
- Kosuth, M.; Wattenberg, E.V.; Mason, S.A. Synthetic Polymer Contamination in Global Drinking Water; OrbMedia Inc.: Washington, DC, USA, 2017. [Google Scholar]
- Makhdoumi, P.; Naghshbandi, M.; Ghaderzadeh, K.; Mirzabeigi, M.; Yazdanbakhsh, A.; Hossini, H. Micro-plastic occurrence in bottled vinegar: Qualification, quantification and human risk exposure. Process. Saf. Environ. Prot. 2021, 152, 404–413. [Google Scholar] [CrossRef]
- Prata, J.C.; Paço, A.; Reis, V.; da Costa, J.P.; Fernandes, A.J.S.; da Costa, F.M.; Duarte, A.C.; Rocha-Santos, T. Identification of microplastics in white wines capped with polyethylene stoppers using micro-Raman spectroscopy. Food Chem. 2020, 331, 127323. [Google Scholar] [CrossRef] [PubMed]
- Kutralam-Muniasamy, G.; Shruti, V.; Pérez-Guevara, F.; Roy, P.D. Microplastic diagnostics in humans: “The 3Ps” Progress, problems, and prospects. Sci. Total Environ. 2023, 856, 159164. [Google Scholar] [CrossRef]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool: A Prospective Case Series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Liu, Y.; Zhang, T.; Zhang, F.; Ren, H.; Zhang, Y. Analysis of Microplastics in Human Feces Reveals a Correlation between Fecal Microplastics and Inflammatory Bowel Disease Status. Environ. Sci. Technol. 2022, 56, 414–421. [Google Scholar] [CrossRef]
- Vianello, A.; Jensen, R.L.; Liu, L.; Vollertsen, J. Simulating human exposure to indoor airborne microplastics using a Breathing Thermal Manikin. Sci. Rep. 2019, 9, 8670. [Google Scholar] [CrossRef]
- Li, Q.; Yuan, M.; Chen, Y.; Jin, X.; Shangguan, J.; Cui, J.; Chang, S.; Guo, M.; Wang, Y. The neglected potential source of microplastics from daily necessities: A study on protective mobile phone cases. J. Hazard. Mater. 2023, 441, 129911. [Google Scholar] [CrossRef]
- Atis, S.; Tutluoglu, B.; Levent, E.; Ozturk, C.; Tunaci, A.; Sahin, K.; Saral, A.; Oktay, I.; Kanik, A.; Nemery, B. The respiratory effects of occupational polypropylene flock exposure. Eur. Respir. J. 2005, 25, 110–117. [Google Scholar] [CrossRef]
- Dong, H.; Wang, X.; Niu, X.; Zeng, J.; Zhou, Y.; Suona, Z.; Yuan, Y.; Chen, X. Overview of analytical methods for the determination of microplastics: Current status and trends. TrAC Trends Anal. Chem. 2023, 167, 117261. [Google Scholar] [CrossRef]
- Razeghi, N.; Hamidian, A.H.; Wu, C.; Zhang, Y.; Yang, M. Microplastic sampling techniques in freshwaters and sediments: A review. Environ. Chem. Lett. 2021, 19, 4225–4252. [Google Scholar] [CrossRef]
- Stock, F.; Kochleus, C.; Bänsch-Baltruschat, B.; Brennholt, N.; Reifferscheid, G. Sampling techniques and preparation methods for microplastic analyses in the aquatic environment—A review. TrAC Trends Anal. Chem. 2019, 113, 84–92. [Google Scholar] [CrossRef]
- Campanale, C.; Savino, I.; Pojar, I.; Massarelli, C.; Uricchio, V.F. A Practical Overview of Methodologies for Sampling and Analysis of Microplastics in Riverine Environments. Sustainability 2020, 12, 6755. [Google Scholar] [CrossRef]
- Bäuerlein, P.S.; Erich, M.W.; van Loon, W.M.; Mintenig, S.M.; Koelmans, A.A. A monitoring and data analysis method for microplastics in marine sediments. Mar. Environ. Res. 2023, 183, 105804. [Google Scholar] [CrossRef] [PubMed]
- Obbard, R.W.; Sadri, S.; Wong, Y.Q.; Khitun, A.A.; Baker, I.; Thompson, R.C. Global warming releases microplastic legacy frozen in Arctic Sea ice. Earth′s Future 2014, 2, 315–320. [Google Scholar] [CrossRef]
- Mári, Á.; Bordós, G.; Gergely, S.; Büki, M.; Háhn, J.; Palotai, Z.; Besenyő, G.; Szabó, É.; Salgó, A.; Kriszt, B.; et al. Validation of microplastic sample preparation method for freshwater samples. Water Res. 2021, 202, 117409. [Google Scholar] [CrossRef]
- Müller, Y.K.; Wernicke, T.; Pittroff, M.; Witzig, C.S.; Storck, F.R.; Klinger, J.; Zumbülte, N. Microplastic analysis—Are we measuring the same? Results on the first global comparative study for microplastic analysis in a water sample. Anal. Bioanal. Chem. 2020, 412, 555–560. [Google Scholar] [CrossRef]
- Huang, Z.; Hu, B.; Wang, H. Analytical methods for microplastics in the environment: A review. Environ. Chem. Lett. 2023, 21, 383–401. [Google Scholar] [CrossRef]
- Kühn, S.; van Oyen, A.; Booth, A.M.; Meijboom, A.; van Franeker, J.A. Marine microplastic: Preparation of relevant test materials for laboratory assessment of ecosystem impacts. Chemosphere 2018, 213, 103–113. [Google Scholar] [CrossRef]
- Araujo, C.F.; Nolasco, M.M.; Ribeiro, A.M.P.; Ribeiro-Claro, P.J.A. Identification of microplastics using Raman spectroscopy: Latest developments and future prospects. Water Res. 2018, 142, 426–440. [Google Scholar] [CrossRef]
- Napper, I.E.; Thompson, R.C. Plastic Debris in the Marine Environment: History and Future Challenges. Glob. Chall. 2020, 4, 1900081. [Google Scholar] [CrossRef]
- Pervaiz, M.; Oakley, P.; Sain, M. Extrusion of Thermoplastic Starch: Effect of “Green” and Common Polyethylene on the Hydrophobicity Characteristics. Mater. Sci. Appl. 2014, 5, 845–856. [Google Scholar] [CrossRef]
- Boschmeier, E.; Ipsmiller, W.; Bartl, A. Market assessment to improve fibre recycling within the EU textile sector. Waste Manag. Res. J. Sustain. Circ. Econ. 2024, 42, 135–145. [Google Scholar] [CrossRef]
- Boersma, A.; Grigoriadi, K.; Nooijens, M.G.A.; Henke, S.; Kooter, I.M.; Parker, L.A.; Dortmans, A.; Urbanus, J.H. Microplastic Index—How to Predict Microplastics Formation? Polymers 2023, 15, 2185. [Google Scholar] [CrossRef]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of Microplastic on Shorelines Woldwide: Sources and Sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Gao, S.-H.; Ge, C.; Gao, Q.; Huang, S.; Kang, Y.; Luo, G.; Zhang, Z.; Fan, L.; Zhu, Y.; et al. Removing microplastics from aquatic environments: A critical review. Environ. Sci. Ecotechnol. 2023, 13, 100222. [Google Scholar] [CrossRef]
- Padervand, M.; Lichtfouse, E.; Robert, D.; Wang, C. Removal of microplastics from the environment. A review. Environ. Chem. Lett. 2020, 18, 807–828. [Google Scholar] [CrossRef]
- Agarwal, S. Biodegradable Polymers: Present Opportunities and Challenges in Providing a Microplastic-Free Environment. Macromol. Chem. Phys. 2020, 221, 2000017. [Google Scholar] [CrossRef]
- Grünzner, M.; Pahl, S.; White, M.P.; Thompson, R.C. Exploring expert perceptions about microplastics: From sources to potential solutions. Microplastics Nanoplastics 2023, 3, 7. [Google Scholar] [CrossRef]
- Plastics Europe (2022) Plastics—the Facts 2022. An Analysis of the Latest Data Related to Plastics Production, Demand, Conversion and Waste management in Europe. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2022 (accessed on 8 June 2023).
- Vroman, I.; Tighzert, L. Biodegradable Polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Jaiswal, K.K.; Dutta, S.; Banerjee, I.; Pohrmen, C.B.; Singh, R.K.; Das, H.T.; Dubey, S.; Kumar, V. Impact of aquatic microplastics and nanoplastics pollution on ecological systems and sustainable remediation strategies of biodegradation and photodegradation. Sci. Total Environ. 2022, 806, 151358. [Google Scholar] [CrossRef]

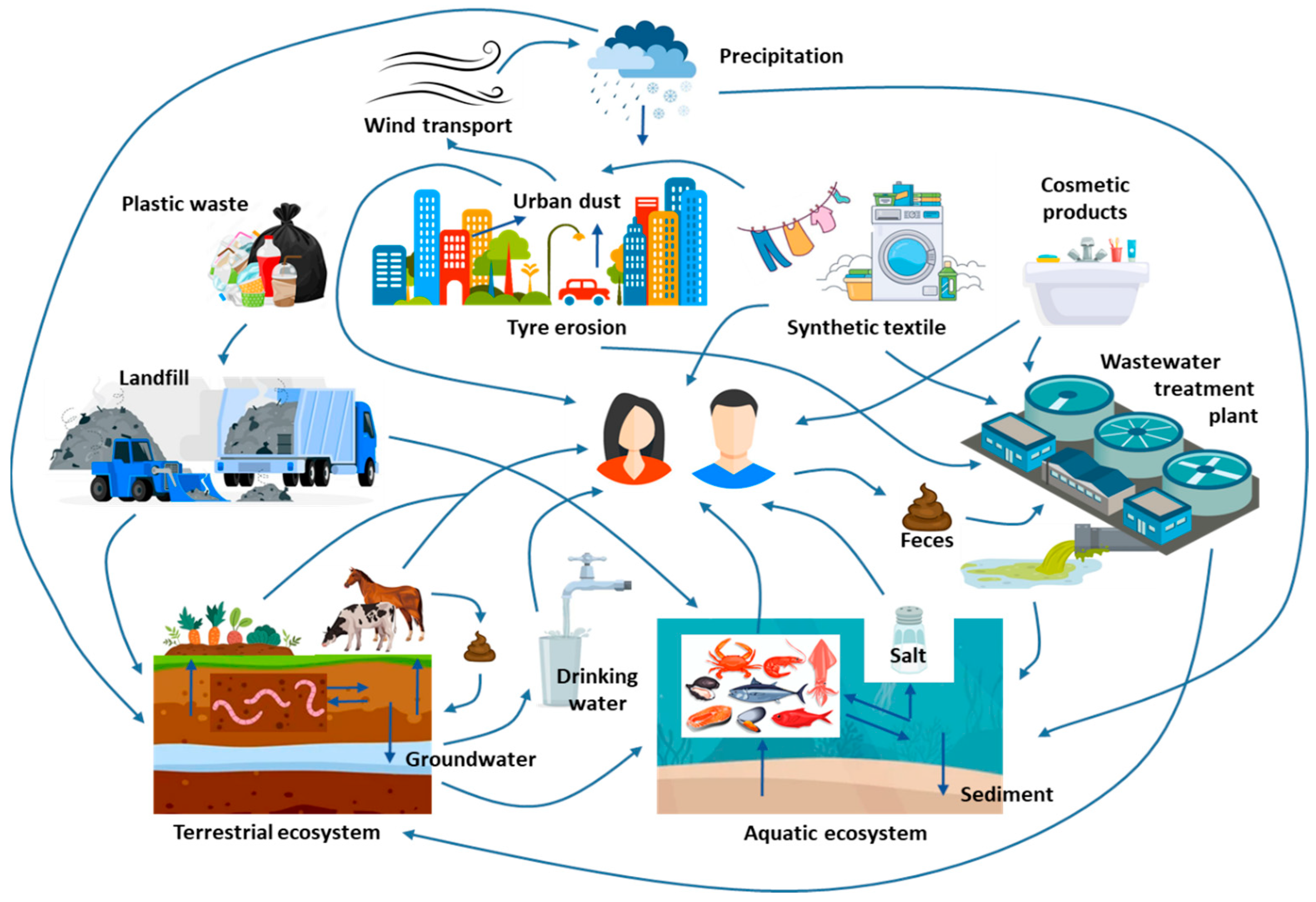
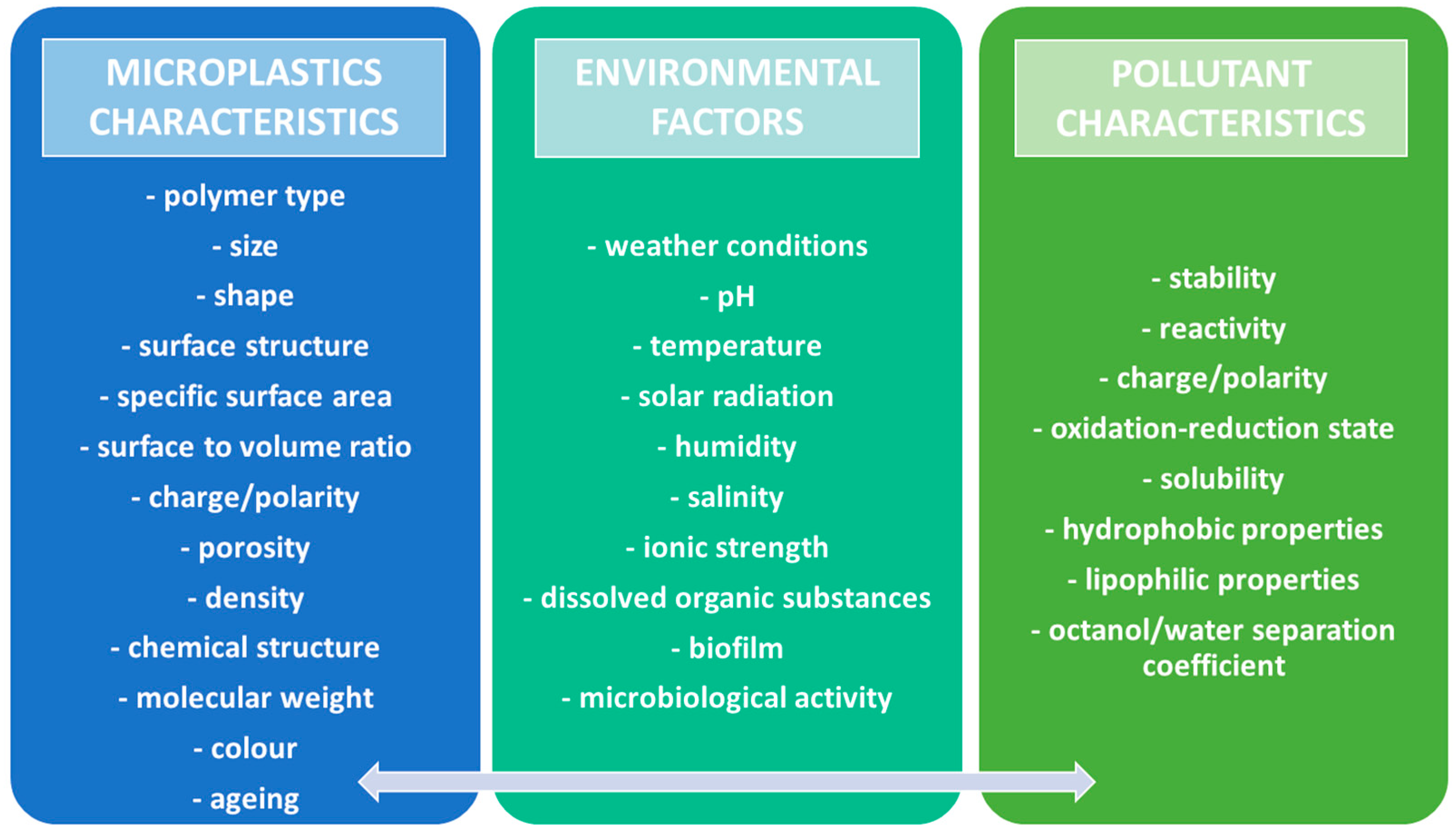
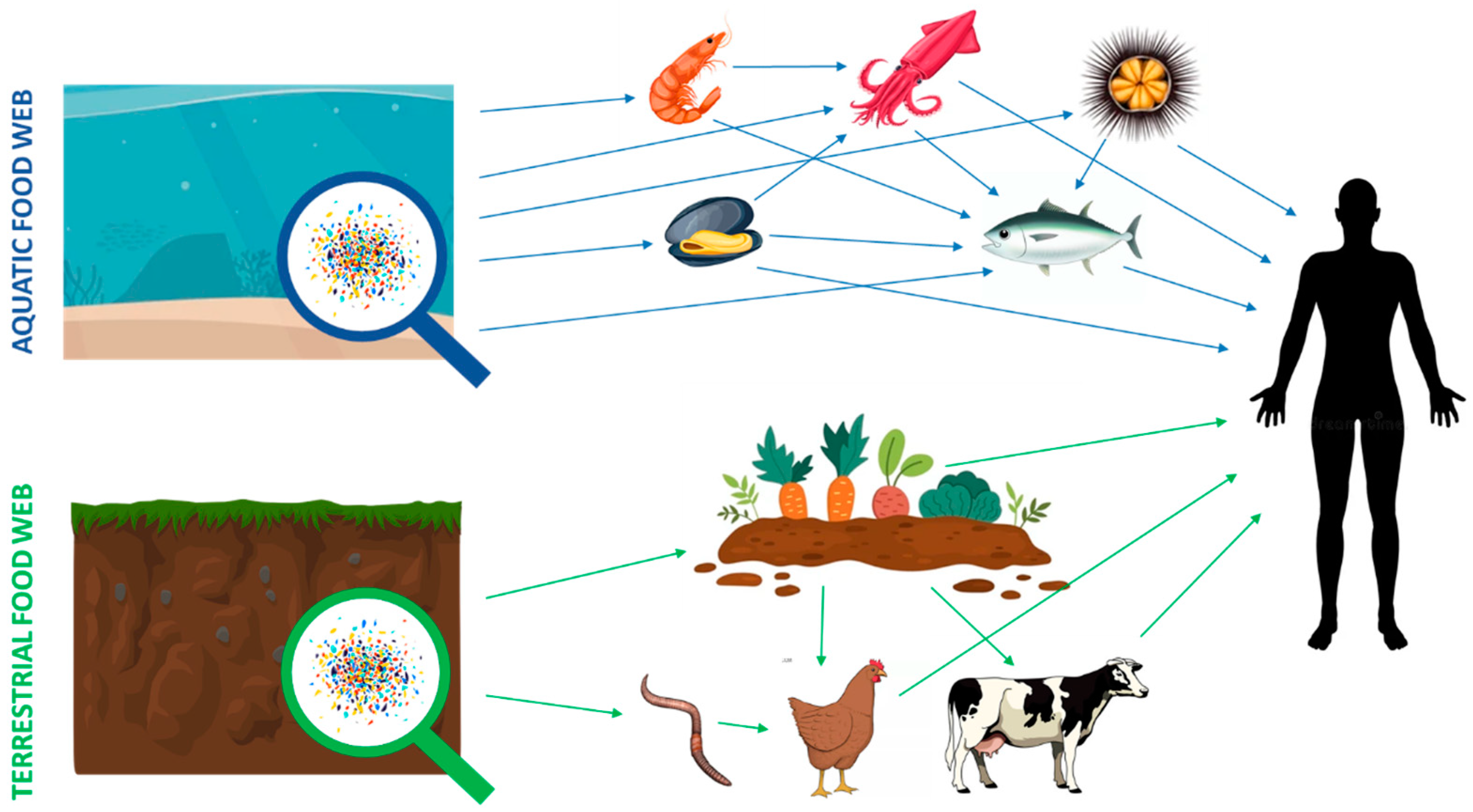


| Environmental Compartment | Estimated Abundance (2024) | Units | Sources | Notes |
|---|---|---|---|---|
| Marine Water | 0.1–10 particles/m3 (surface water) | Particles per cubic meter [m3] | [15] | Higher concentrations near coastlines and urban areas; varies with depth and region. |
| Rivers and Lakes | 10–1000 particles/m3 | Particles per cubic meter [m3] | [16] | Varies significantly depending on proximity to urban discharge and industrial areas. |
| Drinking Water | 110,000–370,000 particles/L (nanoplastics < 1 µm) | Particles per liter [L] | [17] | Most are <1 µm in size; recent findings (2024) using advanced spectroscopic techniques. |
| Soil | 63,000–430,000 tons/year input | Tons per year | [18,19] | From agricultural sources, sludge application, plastic mulching. |
| Atmosphere (Urban Air) | 0.01–5 particles/m3 | Particles per cubic meter (m3) | [20,21] | Highly variable by location and weather; data still emerging. |
| Atmospheric Deposition | Up to 700 particles/m2/day | Particles per m2 per day | [21] |
| Chemical Reaction | Reagents | Reference |
|---|---|---|
| Acidic digestion | HNO3 | [27,99] |
| HCl | [76,99] | |
| HClO4 | [76,99] | |
| H2SO4 | [41] | |
| Alkaline digestion | NaOH | [57,76,99] |
| KOH | [57,76,99] | |
| Oxidizing digestion | H2O2 | [41,57,76,99] |
| Fenton reagent | [41,57,76,99] | |
| Piranha solution | [41,57] | |
| KClO4 | [76] | |
| Enzymatic degradation | Proteinase Trypsin Collagenase | [76] |
| Cellulase Lipase Chitinase | [99] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anić-Vučinić, A.; Turk, D.; Bek, A. Macroissues with Microplastics: A Review on Distribution, Environmental Impacts, Pollutant Interactions, Toxicity, Analytical Methodology and Mitigation Strategies. Appl. Sci. 2025, 15, 4057. https://doi.org/10.3390/app15074057
Anić-Vučinić A, Turk D, Bek A. Macroissues with Microplastics: A Review on Distribution, Environmental Impacts, Pollutant Interactions, Toxicity, Analytical Methodology and Mitigation Strategies. Applied Sciences. 2025; 15(7):4057. https://doi.org/10.3390/app15074057
Chicago/Turabian StyleAnić-Vučinić, Aleksandra, Dunja Turk, and Anja Bek. 2025. "Macroissues with Microplastics: A Review on Distribution, Environmental Impacts, Pollutant Interactions, Toxicity, Analytical Methodology and Mitigation Strategies" Applied Sciences 15, no. 7: 4057. https://doi.org/10.3390/app15074057
APA StyleAnić-Vučinić, A., Turk, D., & Bek, A. (2025). Macroissues with Microplastics: A Review on Distribution, Environmental Impacts, Pollutant Interactions, Toxicity, Analytical Methodology and Mitigation Strategies. Applied Sciences, 15(7), 4057. https://doi.org/10.3390/app15074057












