Physicochemical, Functional, and Antibacterial Properties of Inulin-Type Fructans Isolated from Dandelion (Taraxacum officinale) Roots by “Green” Extraction Techniques
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
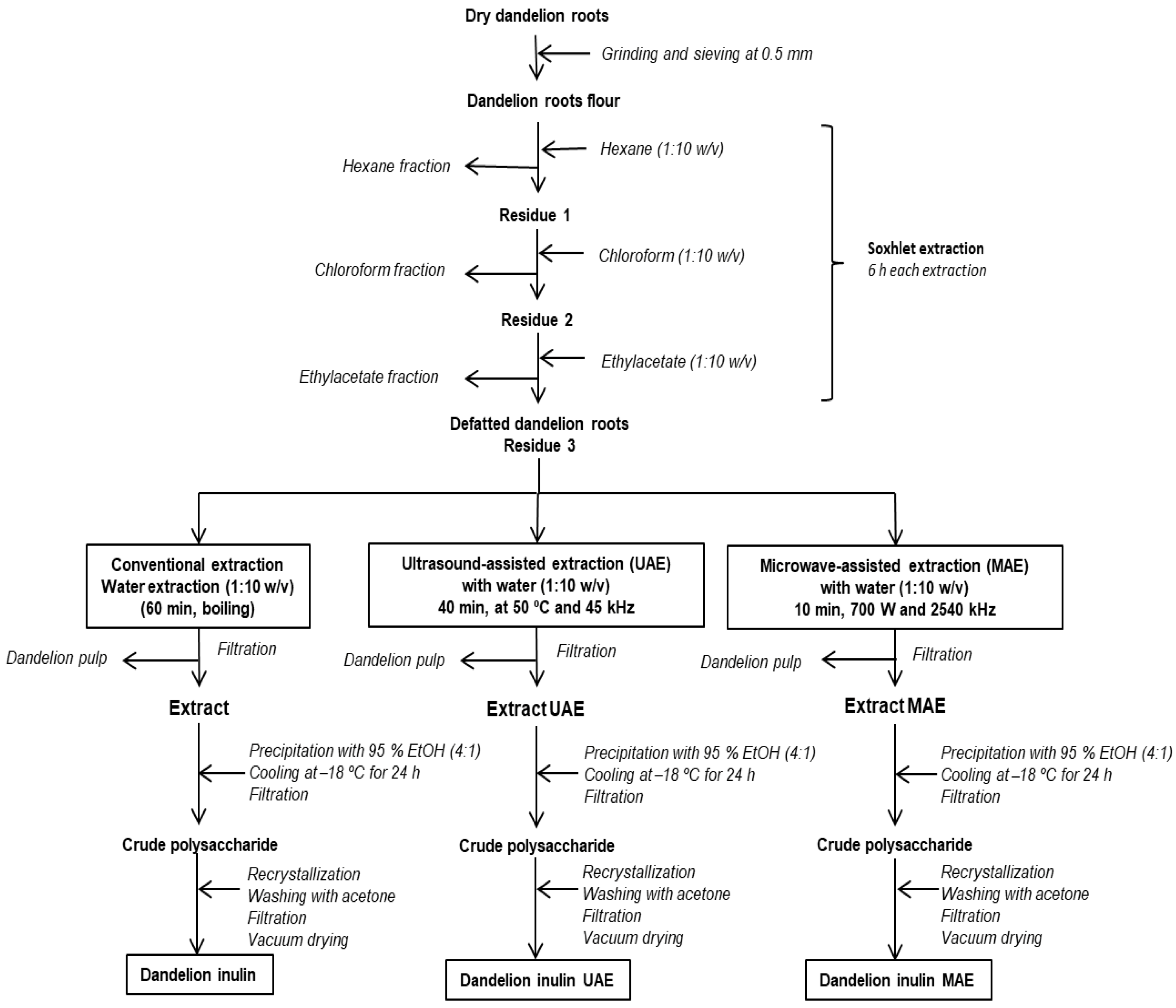
2.2. Fractional Extraction of Dandelion Roots
2.3. Isolation of Inulin from Dandelion Roots
2.4. Characterization of Dandelion Inulin
2.4.1. Yield, Moisture, and Ash Content
2.4.2. pH Values
2.4.3. Melting Point and Angle of Optical Rotation
2.4.4. Protein Content
2.4.5. Reducing Groups and Total Fructose Content
2.4.6. Total Phenolic Content and Antioxidant Potential
2.5. High-Performance Liquid Chromatography Analysis of Dandelion Inulin
2.6. Molecular Weight Distribution Analysis
2.7. Spectroscopic Characterization of Dandelion Inulin
2.7.1. UV Spectrometric Analysis
2.7.2. Fourier Transformation Infrared Spectroscopy
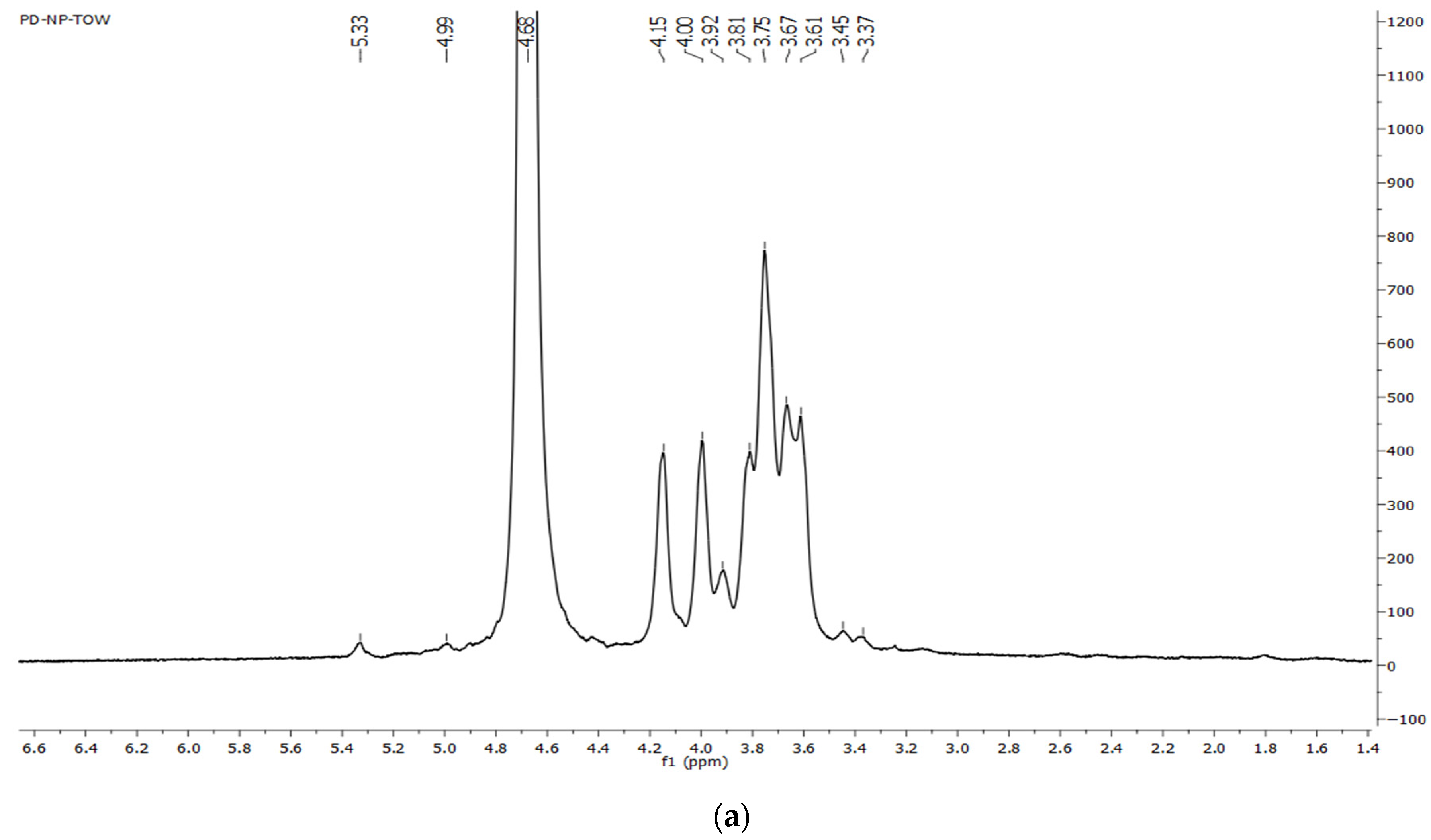
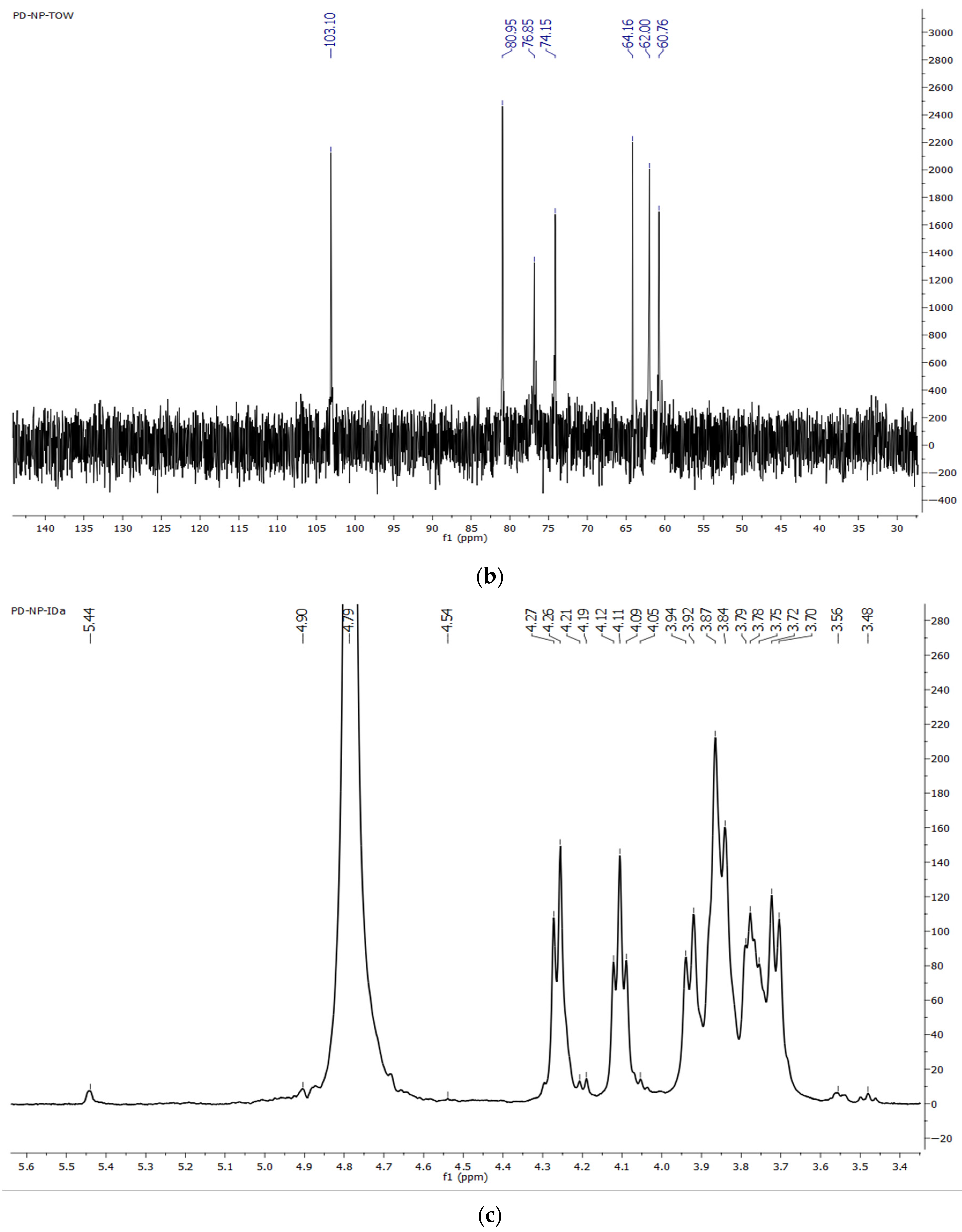
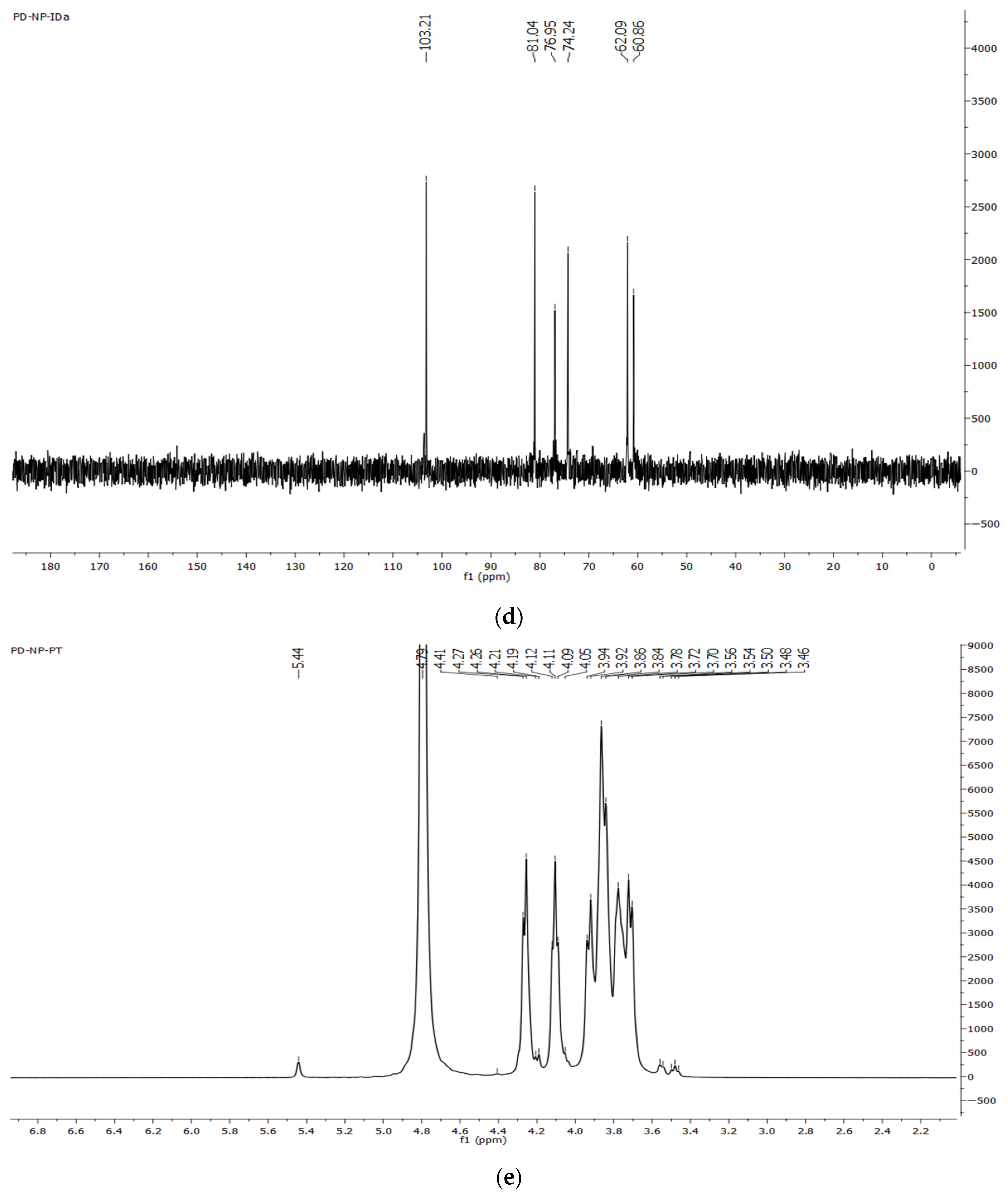
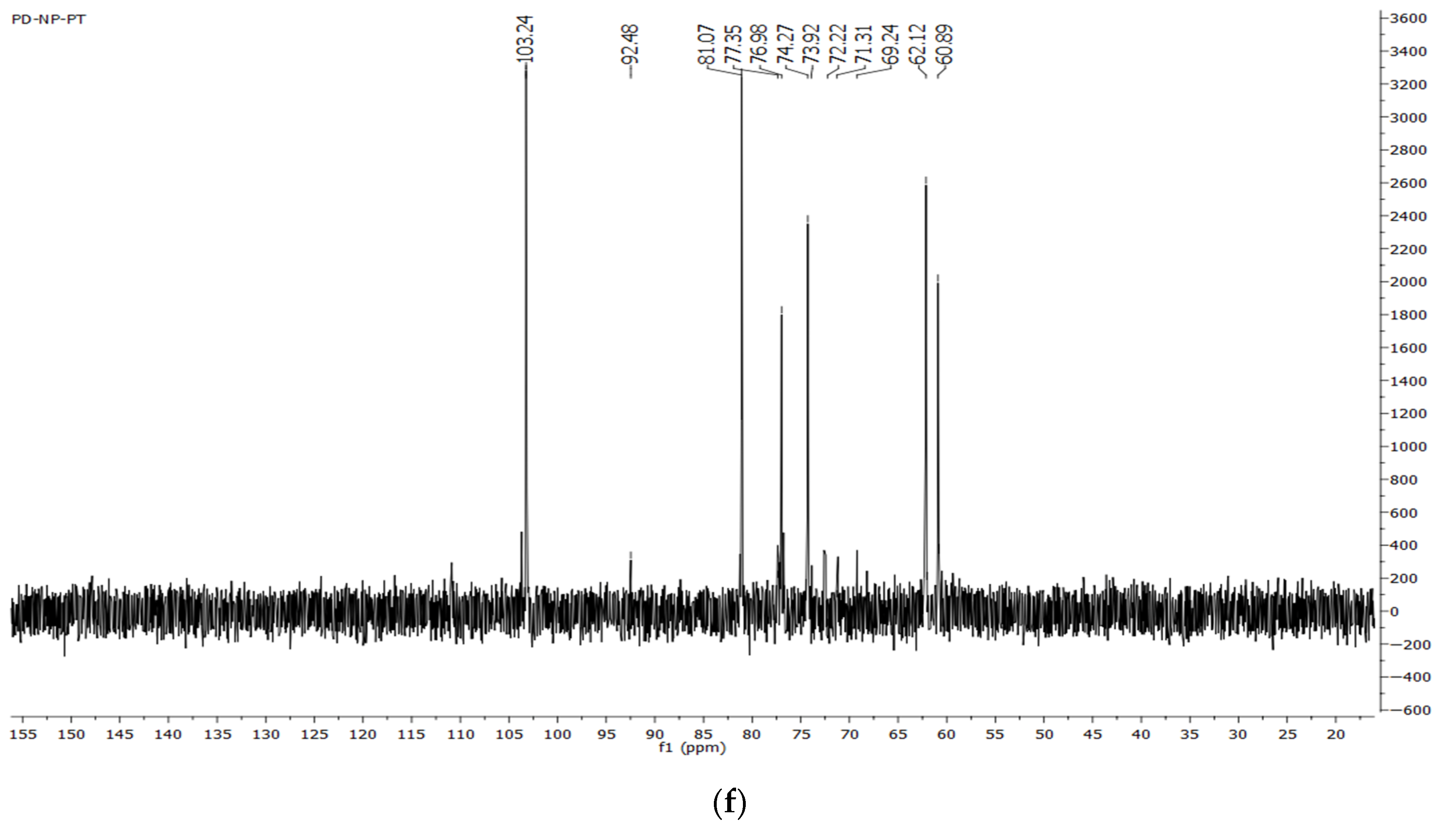
2.7.3. NMR Spectroscopy
2.8. Functional Characterization of Dandelion Inulin
2.8.1. Color
2.8.2. Swelling Properties, Solubility, Water- and Oil-Holding Capacity
2.8.3. Angle of Repose
2.8.4. True, Bulk, Tapped Densities, Bulkiness and Porosity
2.8.5. Flowability and Cohesiveness
2.8.6. Wettability
2.8.7. Hygroscopicity
2.8.8. Taste and Sweetness
2.9. Antimicrobial Activity
2.10. Statistical Analysis
3. Results
3.1. Fructans and Sugar Composition of Defatted Dandelion Roots
3.2. Physicochemical Characterization of Dandelion Inulin
3.3. Spectral Analysis of Dandelion Inulin
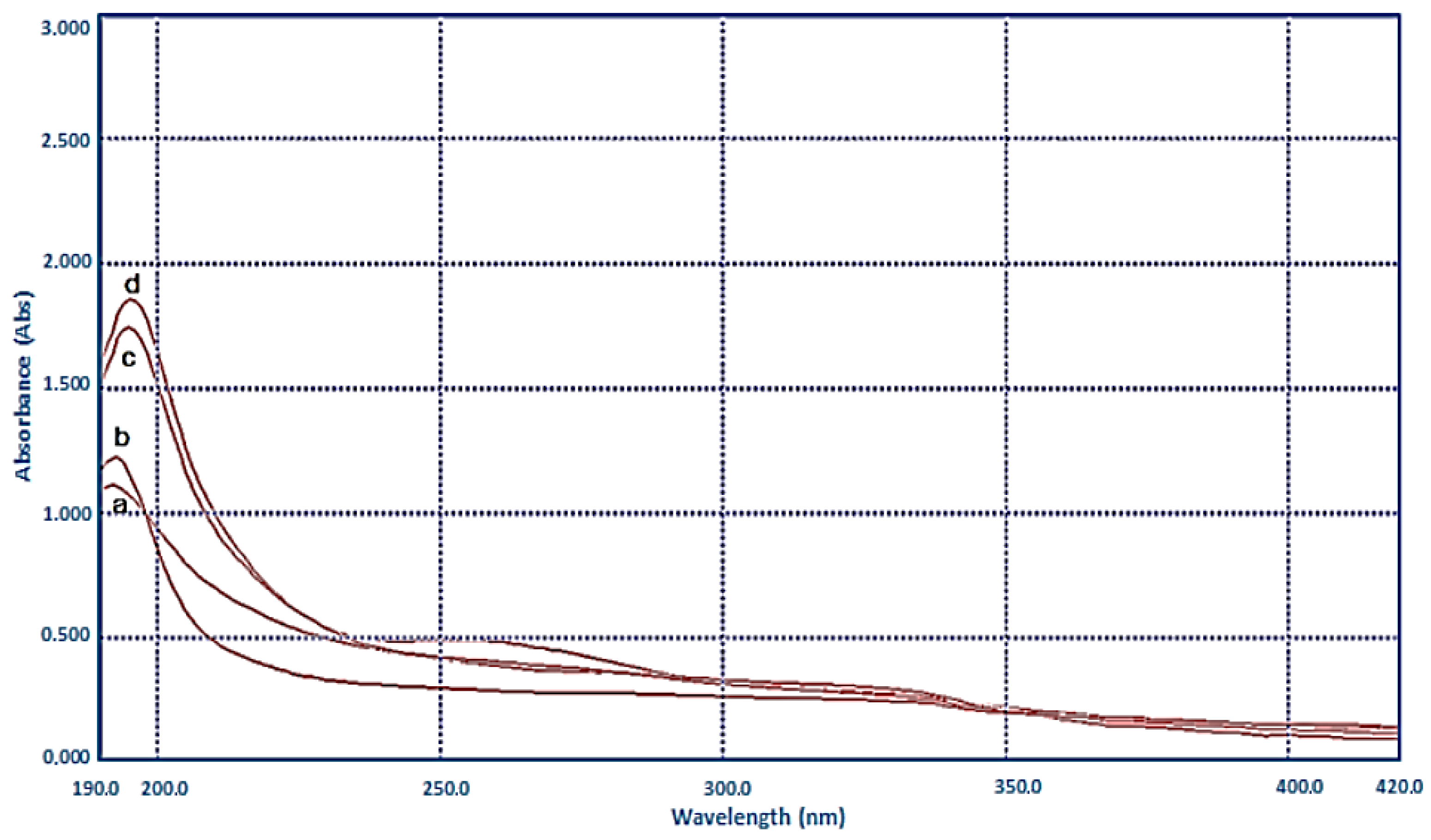
3.3.1. UV-VIS Analysis
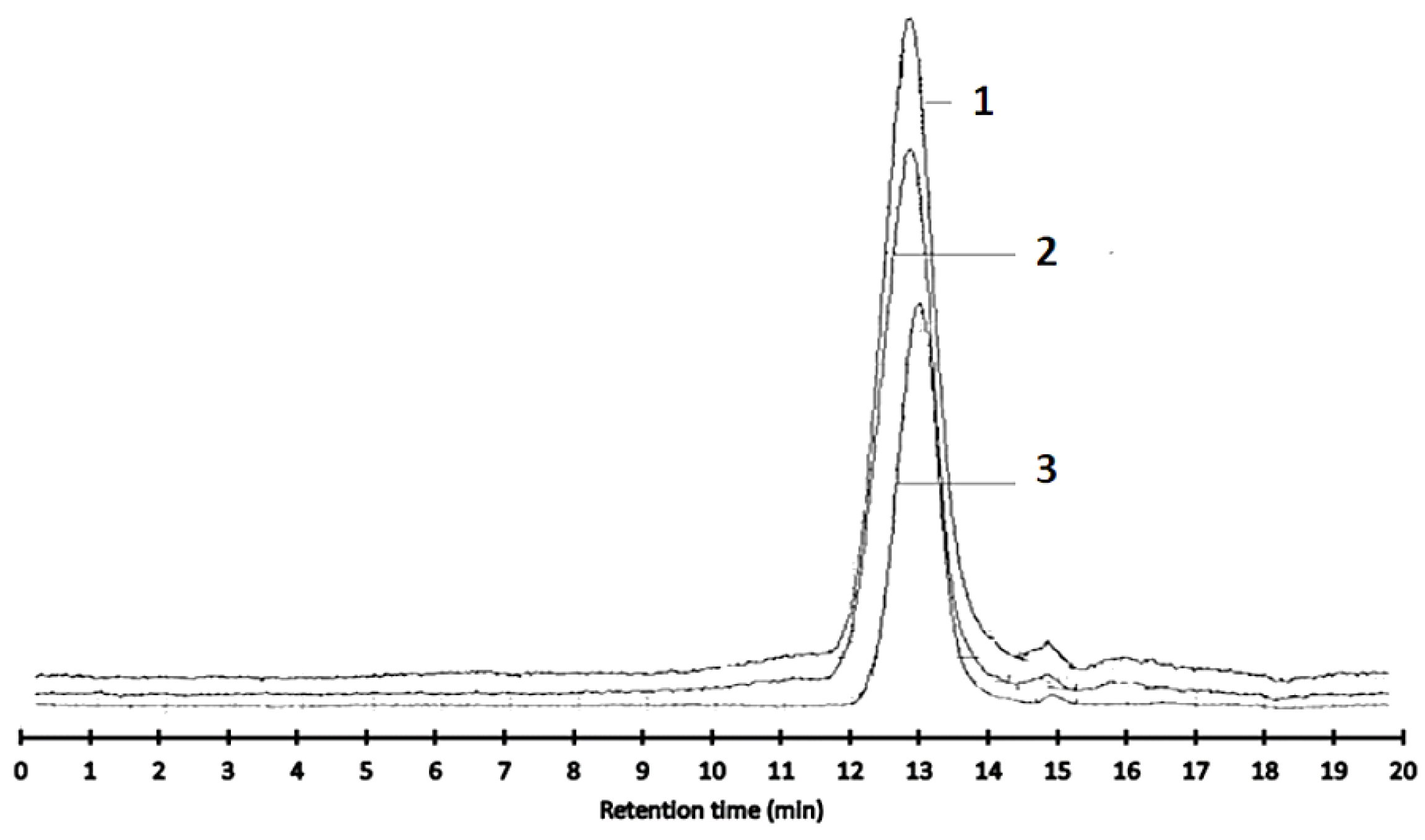
3.3.2. Molecular Weight Distribution Analysis of Dandelion Inulin
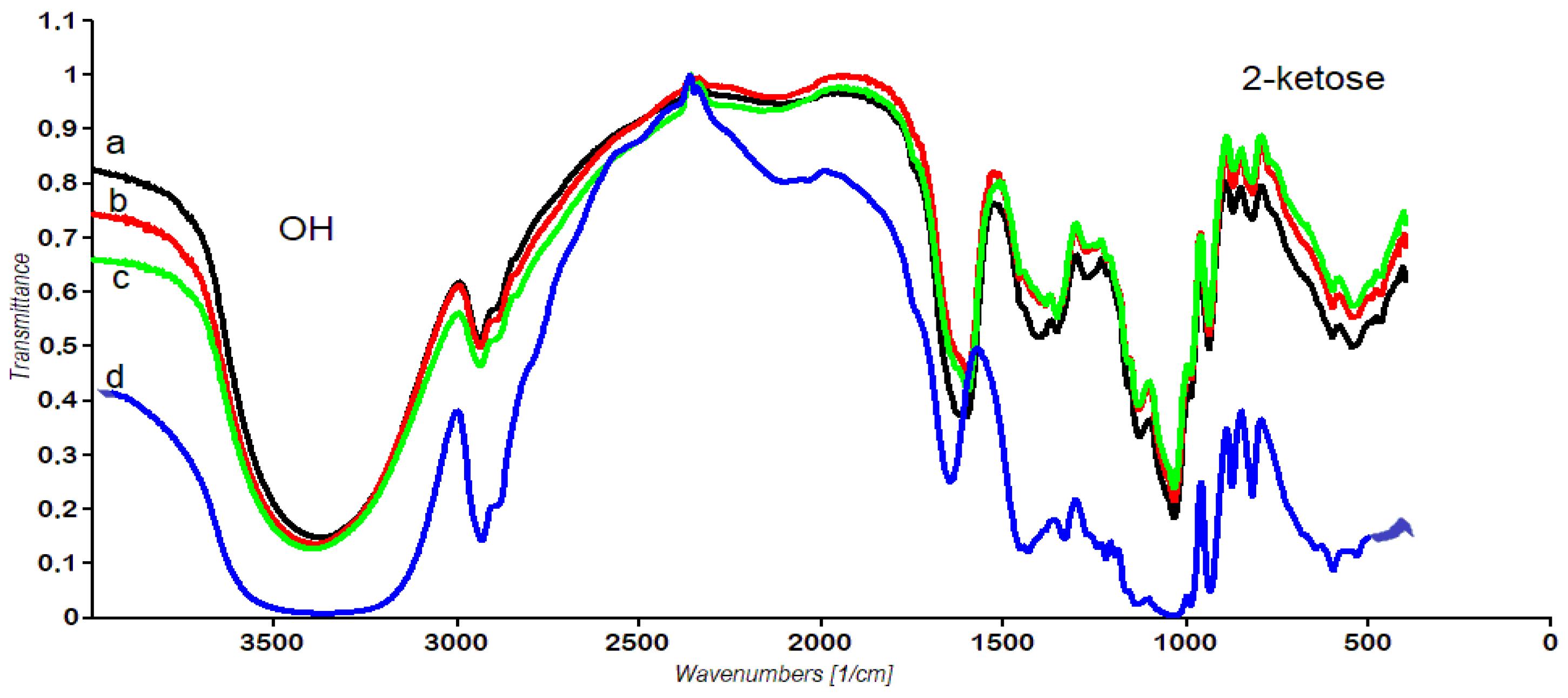
3.3.3. FT-IR Spectra of Dandelion Inulin
3.3.4. NMR Spectra of Dandelion Inulin
3.4. Functional Properties of Dandelion Inulin
3.5. Antimicrobial Activity of Fructan Isolated from Dandelion
4. Discussion
4.1. Physicochemical Characterization of Dandelion Inulin
4.2. FTIR and NMR Spectra of Dandelion Inulin
4.3. Functional Properties
4.4. Antibacterial Activity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MAE | Microwave-assisted extraction |
| UAE | Ultrasound-assisted extraction |
| DP | Degree of polymerization |
| NMR | Nuclear magnetic resonance |
| YI | Yelowness index |
| BI | Browning index |
| OHC | Oil-holding capacity |
| WHC | Water-holding capacity |
References
- Kania-Dobrowolska, M.; Baraniak, J. Dandelion (Taraxacum officinale L.) as a source of biologically active compounds supporting the therapy of co-existing diseases in metabolic syndrome. Foods 2022, 11, 2858. [Google Scholar] [CrossRef] [PubMed]
- Schütz, K.; Muks, E.; Carle, R.; Schieber, A. Separation and quantification of inulin in selected artichoke (Cynara scolymus L.) cultivars and dandelion (Taraxacum officinale WEB. ex WIGG.) roots by high-performance anion exchange chromatography with pulsed amperometric detection. Biomed. Chromatogr. 2006, 20, 1295–1303. [Google Scholar] [PubMed]
- Du, G.; Liu, Y.; Zhang, J.; Fang, S.; Wang, C. Microwave-assisted extraction of dandelion root polysaccharides: Extraction process optimization, purification, structural characterization, and analysis of antioxidant activity. Int. J. Biol. Macromol. 2025, 299, 139732. [Google Scholar] [CrossRef]
- Lis, B.; Olas, B. Pro-health activity of dandelion (Taraxacum officinale L.) and its food products—History and present. J. Funct. Foods 2019, 59, 40–48. [Google Scholar] [CrossRef]
- Petkova, N.; Ivanov, I.; Topchieva, S.; Denev, P.; Pavlov, A. Biologically active substances and in vitro antioxidant activity of different extracts from dandelion (Taraxacum officinale) roots. Sci. Bull. F Biotechnol. 2015, 19, 190–197. [Google Scholar]
- Guo, H.; Zhang, W.; Jiang, Y.; Wang, H.; Chen, G.; Guo, M. Physicochemical, structural, and biological properties of polysaccharides from dandelion. Molecules 2019, 24, 1485. [Google Scholar] [CrossRef]
- Zhang, S.; Song, Z.; Shi, L.; Zhou, L.; Zhang, J.; Cui, J.; Li, Y.; Jin, D.-Q.; Ohizumi, Y.; Xu, J.; et al. A dandelion polysaccharide and its selenium nanoparticles: Structure features and evaluation of anti-tumor activity in zebrafish models. Carbohydr. Polym. 2021, 270, 118365. [Google Scholar] [CrossRef]
- Van den Ende, W.; Michiels, A.; Van Wonterghem, D.; Vergauwen, R.; Van Laere, A. Cloning, developmental, and tissue-specific expression of sucrose: Sucrose 1-fructosyl transferase from Taraxacum officinale. Fructan localization in roots. Plant Physiol. 2000, 123, 71–80. [Google Scholar] [CrossRef]
- Bokov, D.O.; Karabeshkin, D.I.; Samylina, I.A.; Potanina, O.G.; Krasnyuk, I.I.; Malinkin, A.D.; Sergunova, E.V.; Kovaleva, T.Y.; Bobkova, N.V.; Antsyshkina, A.M.; et al. Pharmacopoeial analysis of inulin-containing medicinal plant raw materials and drugs. Pharmacog. J. 2020, 12, 415–421. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Tankhaeva, L.M.; Rokhin, A.V. Glucofructans from Taraxacum officinale roots. Chem. Nat. Compd. 2009, 45, 141–144. [Google Scholar] [CrossRef]
- Savych, A.; Bilyk, O.; Vaschuk, V.; Humeniuk, I. Analysis of inulin and fructans in Taraxacum officinale L. roots as the main inulin-containing component of antidiabetic herbal mixture. Pharmacia 2021, 68, 527. [Google Scholar] [CrossRef]
- Bagaoutdinova, R.; Fedoseyeva, G.; Okoneshnikova, T. Fructose-containing carbohydrates in plants of different families localization and content. Chem. Comput. Simul. Butlerov Commun. 2001, 2, 13–16. [Google Scholar]
- Zhu, Z.; He, J.; Liu, G.; Barba, F.J.; Koubaa, M.; Ding, L.; Bals, O.; Grimi, N.; Vorobiev, E. Recent insights for the green recovery of inulin from plant food materials using non-conventional extraction technologies: A review. Innov. Food Sci. Emerg. Technol. 2016, 33, 1–9. [Google Scholar] [CrossRef]
- Petkova, N.; Petrova, A.; Ivanov, I.; Hambarlyiska, I.; Tumbarski, Y.; Dincheva, I.; Ognyanov, M.; Denev, P. Chemical composition of different extracts from Echinacea purpurea (L.) Moench roots and evaluation of their antimicrobial activity. ChemEngineering 2023, 7, 94. [Google Scholar] [CrossRef]
- López-Molina, D.; Navarro-Martínez, M.D.; Rojas-Melgarejo, F.; Hiner, A.N.P.; Chazarra, S.; Rodríguez-López, J.N. Molecular properties and prebiotic effect of inulin obtained from artichoke (Cynara scolymus L.). Phytochemistry 2005, 66, 1476–1484. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Wang, D.; Zhang, Y. Preparation of inulin and phenols-rich dietary fiber powder from burdock roots. Carbohydr. Polym. 2009, 78, 666–671. [Google Scholar] [CrossRef]
- Petkova, N.; Ivanov, I.; Vrancheva, R.; Denev, P.; Pavlov, A. Ultrasound and microwave-assisted extraction of elecampane (Inula helenium) roots. Nat. Prod. Commun. 2017, 12, 171–174. [Google Scholar] [CrossRef]
- Mudannayake, D.C.; Wimalasiri, K.M.; Silva, K.F.; Ajlouni, S. Comparison of properties of new sources of partially purified inulin to those of commercially pure chicory inulin. J. Food Sci. 2015, 80, C950–C960. [Google Scholar] [CrossRef]
- Shang, H.; Zhao, J.; Guo, Y.; Zhang, H.; Duan, M.; Wu, H. Extraction, purification, emulsifying property, hypoglycemic activity, and antioxidant activity of polysaccharides from comfrey. Ind. Crops Prod. 2020, 146, 112183. [Google Scholar] [CrossRef]
- Zhou, P.; Xiao, W.; Wang, X.; Wu, Y.; Zhao, R.; Wang, Y. A comparison study on polysaccharides extracted from Atractylodes chinensis (DC.) Koidz. using different methods: Structural characterization and anti-SGC-7901 effect of combination with apatinib. Molecules 2022, 27, 4727. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists—AOAC. Official Methods of Analysis, 17th ed.; AOAC: Rockville, MD, USA, 2007. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Petkova, N.T.; Sherova, G.; Denev, P.P. Characterization of inulin from dahlia tubers isolated by microwave and ultrasound-assisted extractions. Int. Food Res. J. 2018, 25, 1876–1884. [Google Scholar]
- Stintzing, F.C.; Herbach, K.M.; Mosshammer, M.R.; Carle, R.; Yi, W.; Sellappan, S.; Akoh, C.C.; Bunch, R.; Felker, P. Color, betalain pattern, and antioxidant properties of cactus pear (Opuntia spp.) clones. J. Agric. Food Chem. 2005, 53, 442–451. [Google Scholar] [CrossRef]
- Petkova, N.; Vrancheva, R.; Denev, P.; Ivanov, I.; Pavlov, A. HPLC-RID method for determination of inulin and fructooligosacharides. ASN 2014, 1, 99–107. [Google Scholar]
- Caleffi, E.R.; Krausova, G.; Hyrslova, I.; Paredes, L.L.; dos Santos, M.M.; Sassaki, G.L.; de Oliveira, A.J. Isolation and prebiotic activity of inulin-type fructan extracted from Pfaffia glomerata (Spreng) Pedersen roots. Int. J. Biol. Macromol. 2015, 80, 392–399. [Google Scholar] [CrossRef]
- Barclay, T.; Ginic-Markovic, M.; Johnston, M.; Cooper, P.; Petrovsky, N. Analysis of the hydrolysis of inulin using real-time 1H NMR spectroscopy. Carbohydr. Res. 2012, 352, 117–125. [Google Scholar] [CrossRef]
- Nistor, O.V.; Mocanu, G.D.; Andronoiu, D.G.; Barbu, V.V.; Ceclu, L. A complex characterization of pumpkin and quince purees obtained by a combination of freezing and conventional cooking. Foods 2022, 11, 2038. [Google Scholar] [CrossRef]
- Robertson, F.; de Monredon, D.; Dysseler, P.; Guillon, F.; Amado, R.; Thibault, J.-F. Hydration properties of dietary fibre and resistant starch: A European collaborative study. LWT—Food Sci. Technol. 2000, 33, 72–79. [Google Scholar] [CrossRef]
- Hahnm, T.; Klemmm, A.; Ziesse, P.; Harms, K.; Wach, W.; Rupp, S.; Hirth, T.; Zibek, S. Optimization and scale-up of inulin extraction from Taraxacum kok-saghyz roots. Nat. Prod. Commun. 2016, 11, 689–692. [Google Scholar]
- Sharma, A.; Bhushette, P.R.; Annapure, U.S. Purification and physicochemical characterization of Prunus domestica exudate gum polysaccharide. Carbohydr. Polym. Technol. Appl. 2020, 1, 100003. [Google Scholar] [CrossRef]
- Jinapong, N.; Suphantharika, M.; Jamnong, P. Production of instant soymilk powders by ultrafiltration, spray drying and fluidized bed agglomeration. J. Food Eng. 2008, 84, 194–205. [Google Scholar] [CrossRef]
- Farooq, U.; Sharma, P.K.; Malviya, R. Extraction and characterization of almond (Prunus sulcis) gum as pharmaceutical excipient. Am. Eurasian J. Agric. Environ. Sci. 2014, 14, 269–274. [Google Scholar]
- A-sun, K.; Thumthanaruk, B.; Lekhavat, S.; Jumnongpon, R. Effect of spray drying conditions on physical characteristics of coconut sugar powder. Int. Food Res. J. 2016, 23, 1315–1319. [Google Scholar]
- Kuhn, F.; de Azevedo, E.S.; Noreña, C.P.Z. Behavior of inulin, polydextrose, and egg albumin as carriers of Bougainvillea glabra bracts extract: Rheological performance and powder characterization. J. Food Process. Preserv. 2020, 44, e14834. [Google Scholar] [CrossRef]
- Apolinario, A.C.; de Carvalho, E.M.; de Lima Damasceno, B.P.G.; da Silva, P.C.D.; Converti, A.; Pessoa, A., Jr.; da Silva, J.A. Extraction, isolation and characterization of inulin from Agave sisalana boles. Ind. Crops Prod. 2017, 108, 355–362. [Google Scholar] [CrossRef]
- Bayar, N.; Kriaa, M.; Kammoun, R. Extraction and characterization of three polysaccharides extracted from Opuntia ficus indica cladodes. Int. J. Biol. Macromol. 2016, 92, 441–450. [Google Scholar] [CrossRef]
- de Barros Fernandes, R.V.; Botrel, D.A.; Silva, E.K.; Borges, S.V.; de Oliveira, C.R.; Yoshida, M.I.; de Andrade Feitosa, J.P.; de Paula, R.C.M. Cashew gum and inulin: New alternative for ginger essential oil microencapsulation. Carbohydr. Polym. 2016, 153, 133–142. [Google Scholar] [CrossRef]
- Davoudi, Z.; Azizi, M.H.; Barzegar, M.; Bernkop-Schnürch, A. Porous starch-inulin loaded quercetin microcapsules: Characterization, antioxidant activity, in-vitro release, and storage stability. J. Pharm. Sci. 2024, 113, 1228–1238. [Google Scholar] [CrossRef]
- Dai, D.; Nanthkumar, N.N.; Newburg, D.S.; Walker, W.A. Role of oligosaccharides and glycoconjugates in intestinal host defense. J. Pediatr. Gastroenterol. Nutr. 2000, 30, S23–S33. [Google Scholar] [CrossRef]
- Nuridullaeva, K.N.; Karieva, E.S.; Khalilov, R.M. Development of industrial technology of inulin production from dandelion roots (Taraxacum officinale Wigg.). Pharm. Chem. J. 2023, 57, 1298–1303. [Google Scholar] [CrossRef]
- Grube, M.; Bekers, M.; Upite, D.; Kaminska, E. Infrared spectra of some fructans. Spectroscopy 2002, 16, 289–296. [Google Scholar]
- Tipson, R.S. Infrared Spectroscopy of Carbohydrates: A Review of the Literature; National Bureau of Standards: Washington, DC, USA, 1968; p. 11. [Google Scholar]
- Lopes, S.M.; Krausová, G.; Rada, V.; Gonçalves, J.E.; Gonçalves, R.A.; de Oliveira, A.J. Isolation and characterization of inulin with a high degree of polymerization from roots of Stevia rebaudiana (Bert.) Bertoni. Carbohydr. Res. 2015, 411, 15–21. [Google Scholar] [CrossRef] [PubMed]
- El-Kholy, W.; Bisar, G.; Aamer, R. Impact of inulin extracted, purified from (chicory and globe artichoke) roots and the combination with maltodextrin as prebiotic dietary fiber on the functional properties of stirred bio-yogurt. Food Nutr. Sci. 2023, 14, 70–89. [Google Scholar] [CrossRef]
- Bouaziz, M.A.; Rassaoui, R.; Besbes, S. Chemical composition, functional properties, and effect of inulin from Tunisian Agave americana L. leaves on textural qualities of pectin gel. J. Chem. 2014, 2014, 758697. [Google Scholar] [CrossRef]
- Nandi, K.; Sen, D.J.; Patra, F.; Nandy, B.; Bera, K.; Mahanti, B. Angle of repose walks on its two legs: Carr index and Hausner ratio. World J. Pharm. Pharm. Sci. 2020, 9, 1565–1579. [Google Scholar]
- Martínez-Ortega, E.A.; López-Briones, J.S.; Rodríguez-Hernández, G.; Ramírez-Orozco, R.E.; Franco-Robles, E. Antibacterial activity of agave fructans against Salmonella typhimurium. Nat. Prod. Res. 2020, 34, 2639–2641. [Google Scholar] [CrossRef]
- Wang, H.-B. Cellulase-assisted extraction and antibacterial activity of polysaccharides from the dandelion Taraxacum officinale. Carbohydr. Polym. 2014, 103, 140–142. [Google Scholar] [CrossRef]
- Lin, L.; Zhu, Y.; Li, C.; Liu, L.; Surendhiran, D.; Cui, H. Antibacterial activity of PEO nanofibers incorporating polysaccharide from dandelion and its derivative. Carbohydr. Polym. 2018, 198, 225–232. [Google Scholar] [CrossRef]
- Li, M.; Zhang, H.; Hu, X.; Liu, Y.; Liu, Y.; Song, M.; Wu, R.; Wu, J. Isolation of a new polysaccharide from dandelion leaves and evaluation of its antioxidant, antibacterial, and anticancer activities. Molecules 2022, 27, 7641. [Google Scholar] [CrossRef]
- Xu, W.; Huang, K.; Jin, W.; Luo, D.; Liu, H.; Li, Y.; Liu, X. Catalytic and anti-bacterial properties of biosynthesized silver nanoparticles using native inulin. RSC Adv. 2018, 8, 28746–28752. [Google Scholar] [CrossRef]
| Carbohydrate Content, g/100 g Dry weight | Dandelion Roots |
|---|---|
| Total fructans | 25.35 ± 0.14 |
| Inulin | 16.10 ± 0.01 |
| Nystose | 3.10 ± 0.02 |
| 1-Kestose | 1.62 ± 0.80 |
| Sucrose | 3.36 ± 0.50 |
| Glucose | 0.48 ± 0.23 |
| Fructose | 1.43 ± 0.20 |
| Characteristics | Classical Extraction | Ultrasound-Assisted Extraction | Microwave-Assisted Extraction | Chicory Inulin Raftiline HPX (DP = 25) |
|---|---|---|---|---|
| Yield, % | 20.0 ± 1.1 a | 15.2 ± 0.5 b | 11.1 ± 0.6 c | - |
| Purity, % | 71.0 ± 0.9 c | 82.0 ± 0.5 b | 89.0 ± 0.1 a | 85.0 ± 0.1 b |
| Moisture, % | 10.8 ± 0.5 ns | 11.0 ± 0.1 ns | 11.3 ± 0.2 a | 11.3 ± 0.1 a |
| Ash content, % | 3.3 ± 0.2 a | 1.6 ± 0.1 b | 1.7 ± 0.1 b | 0.1 ± 0.1 c |
| Protein content, % | 1.8 ± 0.1 a | 0.6 ± 0.1 b | 0.6 ± 0.1 b | 0.2 ± 0.1 c |
| Total fructose content, % | 72.0 ± 0.5 b | 74.0 ± 0.2 a | 62.0 ± 0.1 c | 75.0 ± 0.5 a,b |
| Reducing groups, % | 3.8 ± 0.9 b | 4.9 ± 0.5 a | 2.2 ± 0.5 c | 2.3 ± 0.2 c |
| pH | 6–7 | 6–7 | 6–7 | 6–7 |
| Melting point, °C | 178–178.5 | 179–180 | 177–179 | 175–179 |
| Angle of rotation, ° | −24 | −25 | −25 | −25 |
| Molecular weight Mw, Da Mn, Da | 2885 | 3809 | 3345 | 4020 |
| 2762 | 3624 | 3192 | 2890 | |
| Polydispersity index | 1.04 | 1.05 | 1.04 | 1.03 |
| Degree of polymerization (HPLC-SEC) | 17 | 22 | 19 | 25 |
| Degree of polymerization spectrophotometric | 20 | 16 | 29 | 33 |
| Degree of polymerization by NMR | 16 | 25 | 20 | 31 |
| Total phenolic content, mg GAE/g dry weight | 0.82 ± 0.06 a | 0.71 ± 0.05 a,b | 0.60 ± 0.06 b | - |
| Antioxidant activity | - | |||
| FRAP assay, mM/TE g | 1.54 ± 0.15 a | 1.45 ± 0.11 a,b | 1.34 ± 0.16 b | |
| ABTS | 75.02 ± 0.56 | 70.55 ± 0.45 b | 62.79 ± 0.25 c |
| Samples | Swelling Properties, mL Water/g Sample | Solubility, % | WHC g Water/g Sample | OHC g Oil/g Sample |
|---|---|---|---|---|
| Dandelion inulin, classical extraction | 4.40 ± 0.11 b | 30.02 ± 1.20 a | 1.42 ± 0.30 b | 3.03 ± 0.10 c |
| Dandelion inulin, UAE | 5.60 ± 0.12 a | 25.25 ± 0.51 b | 2.29 ± 0.30 a | 3.92 ± 0.15 a |
| Dandelion inulin, MAE | 4.71 ± 0.11 b | 21.12 ± 0.32 c | 2.58 ± 0.17 a | 3.18 ± 0.10 a,b |
| Chicory inulin Raftiline HPX (DP 25) | 2.01 ± 0.12 c | 25.05 ± 0.26 b | 1.81 ± 0.12 b | 3.32 ± 0.15 b |
| Functional Characteristics | Classical Extraction | Ultrasound-Assisted Extraction | Microwave-Assisted Extraction | Chicory Inulin |
|---|---|---|---|---|
| L | 88.16 ± 1.84 b | 82.81 ± 2.10 c | 96.25 ± 0.64 a | 97.82 ± 0.37 a |
| a | 5.97 ± 0.46 a | 4.81 ± 0.53 a | 1.97 ± 0.65 b | 0.18 ± 0.13 c |
| b | 15.46 ± 0.23 a | 10.61 ± 0.78 b | 4.29 ± 0.74 c | 0.75 ±0.48 d |
| C | 16.58 ± 0.38 a | 11.65 ± 0.92 b | 4.73 ± 0.70 c | 0.79 ± 0.45 d |
| hº | 68.80 ± 1.28 a,b | 65.65 ± 1.07 b | 66.82 ± 1.79 a,b | 72.14 ± 0.53 a |
| ΔE | 22.17 ± 0.97 a | 19.04 ± 0.25 b | 4.65 ± 1.00 c | - |
| YI | 25.05 | 18.30 | 6.37 | 1.10 |
| BI | 23.53 | 17.34 | 5.63 | 0.59 |
| Appearance | Faint brown powder | White powder | White powder | White powder |
| Angle of repose (°) | 27.47 | 28.37 | 27.42 | 42.61 |
| Wettability, s | 367 ± 12 a | 270 ± 15 b | 119 ± 11 c | 9 ± 1 d |
| Hygroscopicity, % | 7.0 | 6.7 | 5.3 | 3.7 |
| True density (g/mL) | 1.44 ± 0.01 a | 1.02 ± 0.02 c | 1.14 ± 0.03 b,c | 1.25 ± 0.05 b |
| Bulk density (g/mL) | 0.38 ± 0.02 b | 0.30 ± 0.03 c | 0.47 ± 0.04 a,b | 0.50 ± 0.02 a |
| Tapped density (g/mL) | 0.51 ± 0.04 b | 0.55 ± 0.05 a,b | 0.61 ± 0.02 a | 0.61 ± 0.03 a |
| Bulkiness (mL/g) | 2.63 b | 3.33 a | 2.13 c | 2.00 c |
| Porosity, % | 64.58 a | 46.07 c | 46.49 c | 51.20 b |
| Carr’s index, % | 25 | 26 | 24 | 18 |
| Hausner ratio | 1.33 | 1.40 | 1.37 | 1.21 |
| Flowability | Fair | Fair | Fair | Good |
| Cohesiveness | Intermediate | Intermediate | Intermediate | Intermediate |
| Taste | Neutral | Neutral | Neutral | Neutral |
| Sweetness | None | None | None | None |
| Test Microorganism | Classical Extraction Dandelion Inulin | UAE Dandelion Inulin | MAE Dandelion Inulin | Chicory Inulin DP 25 | Positive Control Biseptol, 400 μg/mL |
|---|---|---|---|---|---|
| Listeria monocytogenes 863 | 12.0 ± 0.1 c | 14.9 ± 0.1 b | 11.1 ± 0.1 d | 15.6 ± 0.2 a | 12.0 ± 0.2 c |
| Salmonella thyphy 745 | 13.0 ± 0.2 a | 11.0 ± 0.2 b | 11.0 ± 0.1 b,ns | - | 12.3 ± 0.1 a,b |
| Bacillus subtilis 6633 | 14.6 ± 0.1 a | 12.3 ± 0.1 c | 12.2 ± 0.2 c | - | 13.0 ± 0.1 b |
| E. coli ATCC 3398 | - | - | - | - | 16.2 ± 0.1 |
| St. aureus 745 | - | - | - | - | 10.1 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petkova, N.; Hambarliyska, I.; Ivanov, I.; Ognyanov, M.; Nikolova, K.; Ibryamova, S.; Ignatova-Ivanova, T. Physicochemical, Functional, and Antibacterial Properties of Inulin-Type Fructans Isolated from Dandelion (Taraxacum officinale) Roots by “Green” Extraction Techniques. Appl. Sci. 2025, 15, 4091. https://doi.org/10.3390/app15084091
Petkova N, Hambarliyska I, Ivanov I, Ognyanov M, Nikolova K, Ibryamova S, Ignatova-Ivanova T. Physicochemical, Functional, and Antibacterial Properties of Inulin-Type Fructans Isolated from Dandelion (Taraxacum officinale) Roots by “Green” Extraction Techniques. Applied Sciences. 2025; 15(8):4091. https://doi.org/10.3390/app15084091
Chicago/Turabian StylePetkova, Nadezhda, Ivanka Hambarliyska, Ivan Ivanov, Manol Ognyanov, Krastena Nikolova, Sevginar Ibryamova, and Tsveteslava Ignatova-Ivanova. 2025. "Physicochemical, Functional, and Antibacterial Properties of Inulin-Type Fructans Isolated from Dandelion (Taraxacum officinale) Roots by “Green” Extraction Techniques" Applied Sciences 15, no. 8: 4091. https://doi.org/10.3390/app15084091
APA StylePetkova, N., Hambarliyska, I., Ivanov, I., Ognyanov, M., Nikolova, K., Ibryamova, S., & Ignatova-Ivanova, T. (2025). Physicochemical, Functional, and Antibacterial Properties of Inulin-Type Fructans Isolated from Dandelion (Taraxacum officinale) Roots by “Green” Extraction Techniques. Applied Sciences, 15(8), 4091. https://doi.org/10.3390/app15084091







