Microplastics in Water Resources: Threats and Challenges
Abstract
Featured Application
Abstract
1. Introduction
2. Microplastics in the Aquatic Environment
2.1. Sources and Transport Pathways of Microplastics to Aquatic Ecosystems
2.2. Threats Resulting from the Presence of MPs in Aquatic Ecosystems
2.3. MPs in Drinking Water Sources
3. Characterization MPs
3.1. Size and Nomenclature
3.2. Shape and Color
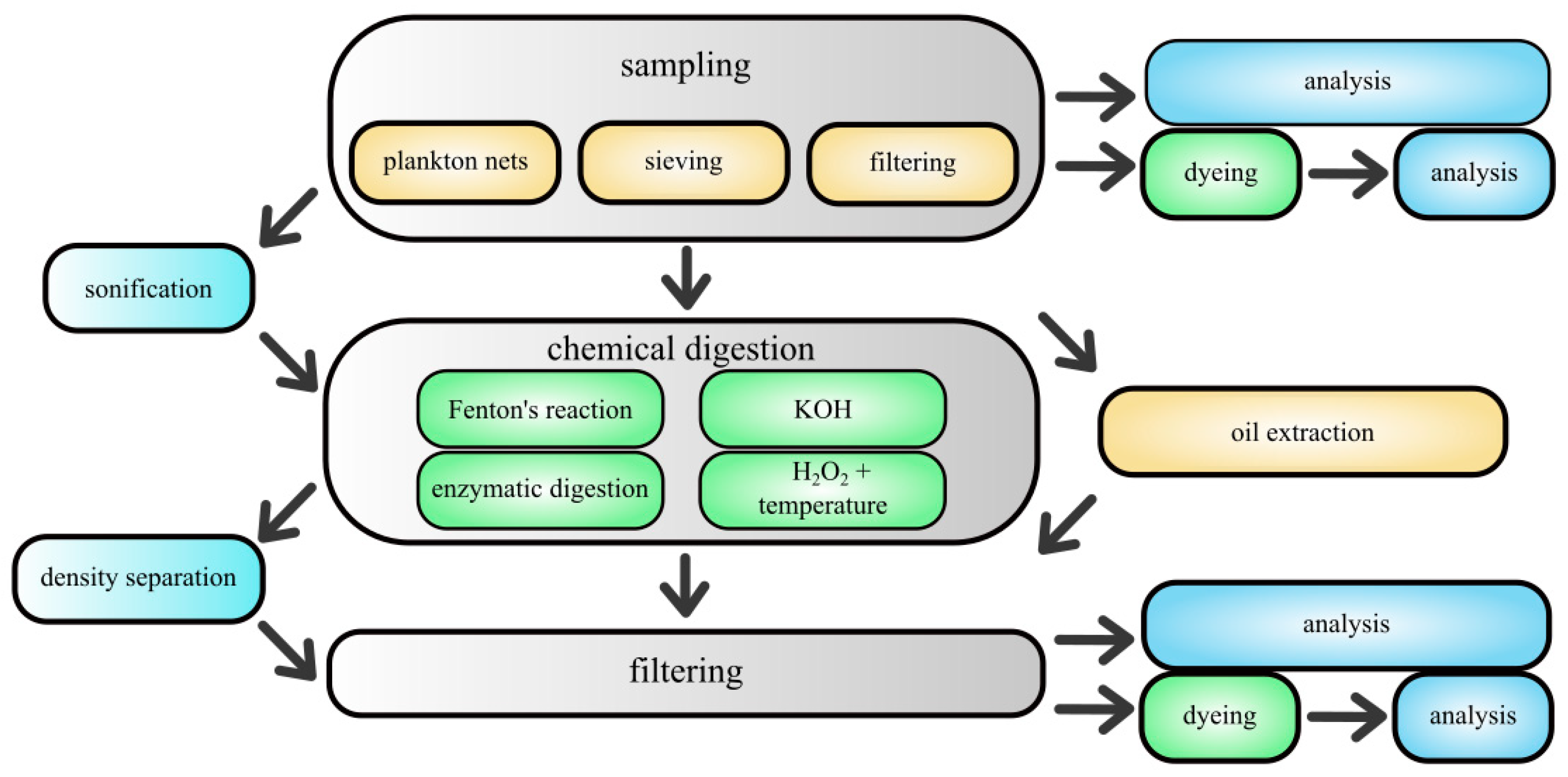
4. Methods for Separation and Extraction of MPs from Environmental Matrices
4.1. The Importance of MP Separation for the Reliability of the Study
4.2. Sieving
4.3. Flotation
4.4. Density Separation
4.5. Oil Extraction
4.6. Chemical and Enzymatic Digestion
5. Methods for Determining Microplastics
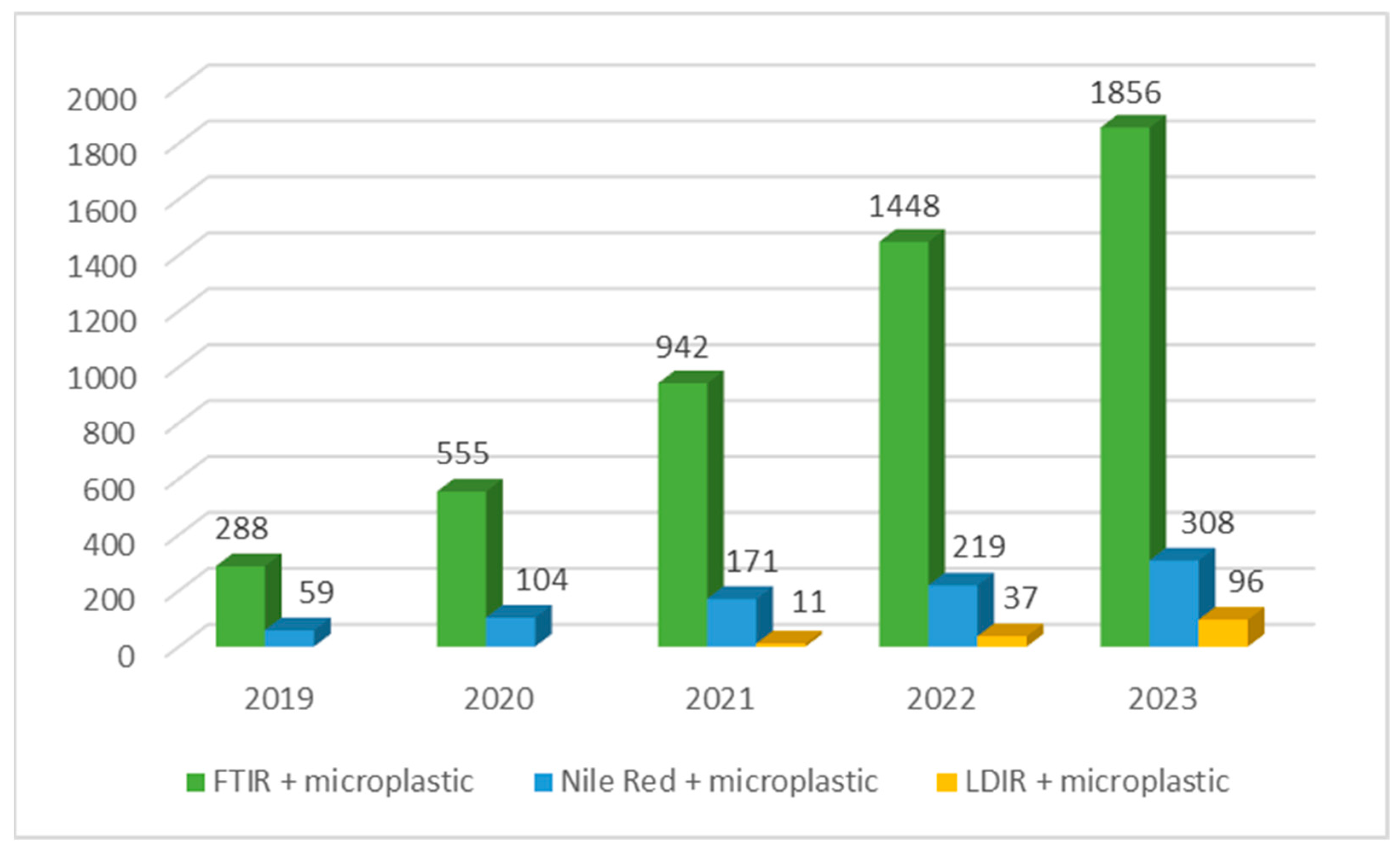
5.1. Trends in MPs Analytics
5.2. Optical Microscopy Method
5.3. FT IR and RS
5.4. Fluorescence Microscopy
5.5. LDIR
6. Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Baekeland, L.H. Address of Acceptance: The Chemical Constitution of Resinous Phenolic Condensation Products. J. Ind. Eng. Chem. 1913, 5, 506–511. [Google Scholar] [CrossRef][Green Version]
- Crespy, D.; Bozonnet, M.; Meier, M. 100 Years of Bakelite, the Material of a 1000 Uses. Angew. Chem. Int. Ed. 2008, 47, 3322–3328. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Moore, C.J.; vom Saal, F.S.; Swan, S.H. Plastics, the Environment and Human Health: Current Consensus and Future Trends. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2153–2166. [Google Scholar] [CrossRef]
- Shams, M.; Alam, I.; Mahbub, M.S. Plastic Pollution during COVID-19: Plastic Waste Directives and Its Long-Term Impact on the Environment. Environ. Adv. 2021, 5, 100119. [Google Scholar] [CrossRef] [PubMed]
- Julienne, F.; Delorme, N.; Lagarde, F. From Macroplastics to Microplastics: Role of Water in the Fragmentation of Polyethylene. Chemosphere 2019, 236, 124409. [Google Scholar] [CrossRef] [PubMed]
- Frias, J.P.G.L.; Nash, R. Microplastics: Finding a Consensus on the Definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef] [PubMed]
- da Costa, J.P.; Santos, P.S.M.; Duarte, A.C.; Rocha-Santos, T. (Nano)Plastics in the Environment—Sources, Fates and Effects. Sci. Total Environ. 2016, 566–567, 15–26. [Google Scholar] [CrossRef]
- Ziccardi, L.M.; Edgington, A.; Hentz, K.; Kulacki, K.J.; Kane Driscoll, S. Microplastics as Vectors for Bioaccumulation of Hydrophobic Organic Chemicals in the Marine Environment: A State-of-the-Science Review. Environ. Toxicol. Chem. 2016, 35, 1667–1676. [Google Scholar] [CrossRef]
- Eerkes-Medrano, D.; Thompson, R.C.; Aldridge, D.C. Microplastics in Freshwater Systems: A Review of the Emerging Threats, Identification of Knowledge Gaps and Prioritisation of Research Needs. Water Res. 2015, 75, 63–82. [Google Scholar] [CrossRef]
- Carpenter, E.J.; Smith, K.L. Plastics on the Sargasso Sea Surface. Science (1979) 1972, 175, 1240–1241. [Google Scholar] [CrossRef]
- Yan, Y.C.; Yang, Z.F. Sources, Distribution, Behavior, and Detection Techniques of Microplastics in Soil: A Review. China Geol. 2023, 6, 695–715. [Google Scholar]
- Strojny, W.; Gruca-Rokosz, R.; Cieśla, M. Preliminary Study of the Occurrence of Microplastics in the Sediments of the Rzeszów Reservoir Using the Laser Direct Infrared (LDIR) Method. Sustainability 2023, 15, 16653. [Google Scholar] [CrossRef]
- Rosati, L.; Carraturo, F.; Capozzi, F.; Chianese, T.; La Pietra, A.; Salamone, M.; Spagnuolo, V.; Ferrandino, I.; Giordano, S. Microplastics’ Impact on the Environment and the Challenging Selection of Reliable Key Biomonitors. Water 2024, 16, 2637. [Google Scholar] [CrossRef]
- Huang, W.; Song, B.; Liang, J.; Niu, Q.; Zeng, G.; Shen, M.; Deng, J.; Luo, Y.; Wen, X.; Zhang, Y. Microplastics and Associated Contaminants in the Aquatic Environment: A Review on Their Ecotoxicological Effects, Trophic Transfer, and Potential Impacts to Human Health. J. Hazard Mater. 2021, 405, 124187. [Google Scholar] [CrossRef] [PubMed]
- Asensio-Montesinos, F.; Blaya-Valencia, G.; Corbí, H.; Beltrán-Sanahuja, A.; Sanz-Lázaro, C. Microplastic Accumulation Dynamics in Two Mediterranean Beaches with Contrasting Inputs. J. Sea Res. 2022, 188, 102269. [Google Scholar] [CrossRef]
- Pilechi, A.; Mohammadian, A.; Murphy, E. A Numerical Framework for Modeling Fate and Transport of Microplastics in Inland and Coastal Waters. Mar. Pollut. Bull. 2022, 184, 114119. [Google Scholar] [CrossRef]
- Mutshekwa, T.; Munyai, L.F.; Mugwedi, L.; Cuthbert, R.N.; Dondofema, F.; Dalu, T. Seasonal Occurrence of Microplastics in Sediment of Two South African Recreational Reservoirs. Water Biol. Secur. 2023, 2, 100185. [Google Scholar] [CrossRef]
- Hee, Y.Y.; Hanif, N.M.; Weston, K.; Latif, M.T.; Suratman, S.; Rusli, M.U.; Mayes, A.G. Atmospheric Microplastic Transport and Deposition to Urban and Pristine Tropical Locations in Southeast Asia. Sci. Total Environ. 2023, 902, 166153. [Google Scholar] [CrossRef]
- Fox, S.; Stefánsson, H.; Peternell, M.; Zlotskiy, E.; Ásbjörnsson, E.J.; Sturkell, E.; Wanner, P.; Konrad-Schmolke, M. Physical Characteristics of Microplastic Particles and Potential for Global Atmospheric Transport: A Meta-Analysis. Environ. Pollut. 2024, 342, 122938. [Google Scholar] [CrossRef]
- Brahney, J.; Mahowald, N.; Prank, M.; Cornwell, G.; Klimont, Z.; Matsui, H.; Prather, K.A. Constraining the Atmospheric Limb of the Plastic Cycle. Proc. Natl. Acad. Sci. USA 2021, 118, e2020719118. [Google Scholar] [CrossRef]
- Vogelsang, C.; Lusher, A.L.; Dadkhah, M.E.; Sundvor, I.; Umar, M.; Ranneklev, S.B.; Eidsvoll, D.; Meland, S. Environmental Contaminants-Freshwater Distribution; Norsk institutt for vannforskning: Oslo, Norway, 2018; ISBN 9788257769666. [Google Scholar]
- O’Brien, S.; Rauert, C.; Ribeiro, F.; Okoffo, E.D.; Burrows, S.D.; O’Brien, J.W.; Wang, X.; Wright, S.L.; Thomas, K.V. There’s Something in the Air: A Review of Sources, Prevalence and Behaviour of Microplastics in the Atmosphere. Sci. Total Environ. 2023, 874, 162193. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, S.; Wu, L.; Yang, Y.; Yu, X.; Liu, Q.; Liu, X.; Li, Y.; Wang, X. Vertical Distribution and River-Sea Transport of Microplastics with Tidal Fluctuation in a Subtropical Estuary, China. Sci. Total Environ. 2022, 822, 153603. [Google Scholar] [CrossRef] [PubMed]
- Idowu, G.A.; Oriji, A.Y.; Olorunfemi, K.O.; Sunday, M.O.; Sogbanmu, T.O.; Bodunwa, O.K.; Shokunbi, O.S.; Aiyesanmi, A.F. Why Nigeria Should Ban Single-Use Plastics: Excessive Microplastic Pollution of the Water, Sediments and Fish Species in Osun River, Nigeria. J. Hazard. Mater. Adv. 2024, 13, 100409. [Google Scholar] [CrossRef]
- Li, S.; Wang, H.; Liang, D.; Li, Y.; Shen, Z. How the Yangtze River Transports Microplastic to the East China Sea. Chemosphere 2022, 307, 136112. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Corvianawatie, C.; Cordova, M.R.; Surinati, D.; Li, Y.; Wang, Z.; Li, X.; Li, R.; Wang, J.; He, L.; et al. Microplastics in the Tropical Northwestern Pacific Ocean and the Indonesian Seas. J. Sea Res. 2023, 194, 102406. [Google Scholar] [CrossRef]
- Thushari, G.G.N.; Miyazono, K.; Sato, T.; Yamashita, R.; Takasuka, A.; Watai, M.; Yasuda, T.; Kuroda, H.; Takahashi, K. Floating Plastic Accumulation and Distribution around Kuroshio Current, Western North Pacific. Mar. Pollut. Bull. 2023, 188, 114604. [Google Scholar] [CrossRef] [PubMed]
- Dautel, S.L. Transoceanic Trash: International and United States Strategies For the Great Pacific Garbage Patch. Gold. Gate Univ. Environ. Law J. 2009, 3, 181. [Google Scholar]
- Lebreton, L.; Slat, B.; Ferrari, F.; Sainte-Rose, B.; Aitken, J.; Marthouse, R.; Hajbane, S.; Cunsolo, S.; Schwarz, A.; Levivier, A.; et al. Evidence That the Great Pacific Garbage Patch Is Rapidly Accumulating Plastic. Sci. Rep. 2018, 8, 4666. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Yang, H.; He, W.; Li, B.; Zhu, X.; Liu, S.; Jia, S.; Li, R.; Tang, K.H.D. Leaching of Chemicals from Microplastics: A Review of Chemical Types, Leaching Mechanisms and Influencing Factors. Sci. Total Environ. 2024, 906, 167666. [Google Scholar] [CrossRef]
- Yan, Y.; Zhu, F.; Zhu, C.; Chen, Z.; Liu, S.; Wang, C.; Gu, C. Dibutyl Phthalate Release from Polyvinyl Chloride Microplastics: Influence of Plastic Properties and Environmental Factors. Water Res. 2021, 204, 117597. [Google Scholar] [CrossRef]
- Kida, M.; Pochwat, K.; Ziembowicz, S. Assessment of Machine Learning-Based Methods Predictive Suitability for Migration Pollutants from Microplastics Degradation. J. Hazard Mater. 2024, 461, 132565. [Google Scholar] [CrossRef]
- Gulizia, A.M.; Philippa, B.; Zacharuk, J.; Motti, C.A.; Vamvounis, G. Plasticiser Leaching from Polyvinyl Chloride Microplastics and the Implications for Environmental Risk Assessment. Mar. Pollut. Bull. 2023, 195, 115392. [Google Scholar] [CrossRef]
- Andrady, A.L. The Plastic in Microplastics: A Review. Mar. Pollut. Bull. 2017, 119, 12–22. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, M.; Song, Z.; Wang, G.; Zhao, C.; Shu, Q.; Zhang, Y.; Qiao, F. A Quantitative Analysis of Marine Heatwaves in Response to Rising Sea Surface Temperature. Sci. Total Environ. 2023, 881, 163396. [Google Scholar] [CrossRef] [PubMed]
- Naveen, K.V.; Saravanakumar, K.; Zhang, X.; Sathiyaseelan, A.; Wang, M.H. Impact of Environmental Phthalate on Human Health and Their Bioremediation Strategies Using Fungal Cell Factory—A Review. Environ. Res. 2022, 214, 113781. [Google Scholar] [CrossRef]
- Neuvonen, R.; Huovinen, M.; Dorman, D.C.; Laitinen, H.; Sahlman, H. Phthalates and Polycystic Ovary Syndrome—Systematic Literature Review. Reprod. Toxicol. 2023, 121, 108473. [Google Scholar] [CrossRef] [PubMed]
- Mérida, D.M.; Moreno-Franco, B.; Marquès, M.; León-Latre, M.; Laclaustra, M.; Guallar-Castillón, P. Phthalate Exposure and the Metabolic Syndrome: A Systematic Review and Meta-Analysis. Environ. Pollut. 2023, 333, 121957. [Google Scholar] [CrossRef] [PubMed]
- Chi, Z.; Lin, H.; Wang, X.; Meng, X.; Zhou, J.; Xiang, L.; Cao, G.; Wu, P.; Cai, Z.; Zhao, X. Dimethyl Phthalate Induces Blood Immunotoxicity through Oxidative Damage and Caspase-Dependent Apoptosis. Sci. Total Environ. 2022, 838, 156047. [Google Scholar] [CrossRef]
- Martin, L.; Zhang, Y.; First, O.; Mustieles, V.; Dodson, R.; Rosa, G.; Coburn-Sanderson, A.; Adams, C.D.; Messerlian, C. Lifestyle Interventions to Reduce Endocrine-Disrupting Phthalate and Phenol Exposures among Reproductive Age Men and Women: A Review and Future Steps. Environ. Int. 2022, 170, 107576. [Google Scholar] [CrossRef]
- Moura, D.S.; Pestana, C.J.; Moffat, C.F.; Gkoulemani, N.; Hui, J.; Irvine, J.T.S.; Lawton, L.A. Aging Microplastics Enhances the Adsorption of Pharmaceuticals in Freshwater. Sci. Total Environ. 2024, 912, 169467. [Google Scholar] [CrossRef]
- Zong, X.; Zhang, J.; Zhu, J.; Zhang, L.; Jiang, L.; Yin, Y.; Guo, H. Effects of Polystyrene Microplastic on Uptake and Toxicity of Copper and Cadmium in Hydroponic Wheat Seedlings (Triticum aestivum L.). Ecotoxicol. Environ. Saf. 2021, 217, 112217. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Wu, J.; Huang, S.; Xin, L.; Zhao, Q. Competitive Adsorption Behaviors and Mechanisms of Cd, Ni, and Cu by Biochar When Coexisting with Microplastics under Single, Binary, and Ternary Systems. Sci. Total Environ. 2024, 913, 169524. [Google Scholar] [CrossRef]
- Kim, D.; Kim, S.A.; Nam, S.H.; Kwak, J.I.; Kim, L.; Lee, T.Y.; Kim, H.; An, S.; An, Y.J. Microplastic Ingestion in Aquatic and Soil Biota: A Comprehensive Review of Laboratory Studies on Edible Size and Intake Pattern. Mar. Pollut. Bull. 2024, 200, 116056. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.P.; Ding, J.; Loh, K.H.; Sun, C.; Yusoff, S.; Chanthran, S.S.D.; Lim, P.E. First Evidence of Microplastic Ingestion by Crescent Perch (Terapon jarbua) in Malaysia. Reg. Stud. Mar. Sci. 2023, 67, 103202. [Google Scholar] [CrossRef]
- Rose, P.K.; Yadav, S.; Kataria, N.; Khoo, K.S. Microplastics and Nanoplastics in the Terrestrial Food Chain: Uptake, Translocation, Trophic Transfer, Ecotoxicology, and Human Health Risk. TrAC Trends Anal. Chem. 2023, 167, 117249. [Google Scholar] [CrossRef]
- Ross, M.S.; Loutan, A.; Groeneveld, T.; Molenaar, D.; Kroetch, K.; Bujaczek, T.; Kolter, S.; Moon, S.; Huynh, A.; Khayam, R.; et al. Estimated Discharge of Microplastics via Urban Stormwater during Individual Rain Events. Front. Environ. Sci. 2023, 11, 1090267. [Google Scholar] [CrossRef]
- Hooge, A.; Hauggaard-Nielsen, H.; Heinze, W.M.; Lyngsie, G.; Ramos, T.M.; Sandgaard, M.H.; Vollertsen, J.; Syberg, K. Fate of Microplastics in Sewage Sludge and in Agricultural Soils. TrAC Trends Anal. Chem. 2023, 166, 117184. [Google Scholar] [CrossRef]
- Qaiser, Z.; Aqeel, M.; Sarfraz, W.; Fatima Rizvi, Z.; Noman, A.; Naeem, S.; Khalid, N. Microplastics in Wastewaters and Their Potential Effects on Aquatic and Terrestrial Biota. Case Stud. Chem. Environ. Eng. 2023, 8, 100536. [Google Scholar] [CrossRef]
- Kernchen, S.; Löder, M.G.J.; Fischer, F.; Fischer, D.; Moses, S.R.; Georgi, C.; Nölscher, A.C.; Held, A.; Laforsch, C. Airborne Microplastic Concentrations and Deposition across the Weser River Catchment. Sci. Total Environ. 2022, 818, 151812. [Google Scholar] [CrossRef]
- la Cecilia, D.; Philipp, M.; Kaegi, R.; Schirmer, M.; Moeck, C. Microplastics Attenuation from Surface Water to Drinking Water: Impact of Treatment and Managed Aquifer Recharge—And Identification Uncertainties. Sci. Total Environ. 2024, 908, 168378. [Google Scholar] [CrossRef]
- Negrete Velasco, A.; Ramseier Gentile, S.; Zimmermann, S.; Le Coustumer, P.; Stoll, S. Contamination and Removal Efficiency of Microplastics and Synthetic Fibres in a Conventional Drinking Water Treatment Plant in Geneva, Switzerland. Sci. Total Environ. 2023, 880, 163270. [Google Scholar] [CrossRef]
- Pivokonsky, M.; Cermakova, L.; Novotna, K.; Peer, P.; Cajthaml, T.; Janda, V. Occurrence of Microplastics in Raw and Treated Drinking Water. Sci. Total Environ. 2018, 643, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Sangkham, S.; Aminul Islam, M.; Adhikari, S.; Kumar, R.; Sharma, P.; Sakunkoo, P.; Bhattacharya, P.; Tiwari, A. Evidence of Microplastics in Groundwater: A Growing Risk for Human Health. Groundw. Sustain. Dev. 2023, 23, 100981. [Google Scholar] [CrossRef]
- Haque, F.; Fan, C. Fate of Microplastics under the Influence of Climate Change. iScience 2023, 26, 107649. [Google Scholar] [CrossRef]
- Ren, Z.; Gui, X.; Xu, X.; Zhao, L.; Qiu, H.; Cao, X. Microplastics in the Soil-Groundwater Environment: Aging, Migration, and Co-Transport of Contaminants—A Critical Review. J. Hazard. Mater. 2021, 419, 126455. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Tian, L.; Wang, P.; Wang, Z.; Zeng, L.; Hu, J. Microplastic Pollution in the Groundwater under a Bedrock Island in the South China Sea. Environ. Res 2023, 239, 117277. [Google Scholar] [CrossRef]
- Dey, U.; Raj, D.; Mondal, M.; Roy, P.; Mukherjee, A.; Mondal, N.K.; Das, K. Microplastics in Groundwater: An Overview of Source, Distribution, Mobility Constraints and Potential Health Impacts during the Anthropocene. Groundw. Sustain. Dev. 2023, 23, 101036. [Google Scholar] [CrossRef]
- Viaroli, S.; Lancia, M.; Re, V. Microplastics Contamination of Groundwater: Current Evidence and Future Perspectives. A Review. Sci. Total Environ. 2022, 824, 153851. [Google Scholar] [CrossRef]
- Brožová, K.; Halfar, J.; Čabanová, K.; Motyka, O.; Drabinová, S.; Hanus, P.; Heviánková, S. The First Evidence of Microplastic Occurrence in Mine Water: The Largest Black Coal Mining Area in the Czech Republic. Water Res. 2023, 244, 120538. [Google Scholar] [CrossRef]
- Chia, R.W.; Lee, J.Y.; Jang, J.; Cha, J. Errors and Recommended Practices That Should Be Identified to Reduce Suspected Concentrations of Microplastics in Soil and Groundwater: A Review. Environ. Technol. Innov. 2022, 28, 102933. [Google Scholar] [CrossRef]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at Sea: Where Is All the Plastic? Science (1979) 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Gan, Y.; Zhang, C.; He, H.; Fang, J.; Wang, L.; Wang, Y.; Liu, J. “Microplastic Communities” in Different Environments: Differences, Links, and Role of Diversity Index in Source Analysis. Water Res. 2021, 188, 116574. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Guan, B. Separation and Identification of Nanoplastics in Tap Water. Environ. Res. 2022, 204, 112134. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Wen, X.; Huang, D.; Du, C.; Deng, R.; Zhou, Z.; Tao, J.; Li, R.; Zhou, W.; Wang, Z.; et al. Interactions between Microplastics/Nanoplastics and Vascular Plants. Environ. Pollut. 2021, 290, 117999. [Google Scholar] [CrossRef]
- Enfrin, M.; Dumée, L.F.; Lee, J. Nano/Microplastics in Water and Wastewater Treatment Processes—Origin, Impact and Potential Solutions. Water Res. 2019, 161, 621–638. [Google Scholar] [CrossRef] [PubMed]
- Gigault, J.; ter Halle, A.; Baudrimont, M.; Pascal, P.Y.; Gauffre, F.; Phi, T.L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current Opinion: What Is a Nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef]
- Zhang, B.; Chao, J.; Chen, L.; Liu, L.; Yang, X.; Wang, Q. Research Progress of Nanoplastics in Freshwater. Sci. Total Environ. 2021, 757, 143791. [Google Scholar] [CrossRef]
- Sun, M.; Ding, R.; Ma, Y.; Sun, Q.; Ren, X.; Sun, Z.; Duan, J. Cardiovascular Toxicity Assessment of Polyethylene Nanoplastics on Developing Zebrafish Embryos. Chemosphere 2021, 282, 131124. [Google Scholar] [CrossRef]
- Kershaw, P.J.; Rochman, C.M. Sources, Fate and Effects of Microplastics in the Marine Environment: Part 2 of a Global Assessment; GESAMP; International Maritime Organization: London, UK, 2015. [Google Scholar]
- Barboza, L.G.A.; Cózar, A.; Gimenez, B.C.G.; Barros, T.L.; Kershaw, P.J.; Guilhermino, L. Macroplastics Pollution in the Marine Environment. In World Seas: An Environmental Evaluation Volume III: Ecological Issues and Environmental Impacts; Academic Press: Cambridge, MA, USA, 2019; pp. 305–328. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and Fragmentation of Plastic Debris in Global Environments. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef]
- Hanvey, J.S.; Lewis, P.J.; Lavers, J.L.; Crosbie, N.D.; Pozo, K.; Clarke, B.O. A Review of Analytical Techniques for Quantifying Microplastics in Sediments. Anal. Methods 2017, 9, 1369–1383. [Google Scholar] [CrossRef]
- Eriksen, M.; Lebreton, L.C.M.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic Pollution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef]
- Alimi, O.S.; Farner Budarz, J.; Hernandez, L.M.; Tufenkji, N. Microplastics and Nanoplastics in Aquatic Environments: Aggregation, Deposition, and Enhanced Contaminant Transport. Environ. Sci. Technol. 2018, 52, 1704–1724. [Google Scholar] [CrossRef] [PubMed]
- Galgani, F.; Hanke, G.; Werner, S.; De Vrees, L. Marine Litter within the European Marine Strategy Framework Directive. ICES J. Mar. Sci. 2013, 70, 1055–1064. [Google Scholar] [CrossRef]
- Lambert, S.; Sinclair, C.; Boxall, A. Occurrence, Degradation, and Effect of Polymer-Based Materials in the Environment. In Reviews of Environmental Contamination and Toxicology; Springer: Cham, Switzerland, 2014; pp. 1–53. [Google Scholar]
- Koelmans, A.A.; Besseling, E.; Shim, W.J. Nanoplastics in the Aquatic Environment. Critical Review. In Marine Anthropogenic Litter; Springer International Publishing: Cham, Switzerland, 2015; pp. 325–340. [Google Scholar]
- Jung, J.W.; Park, J.W.; Eo, S.; Choi, J.; Song, Y.K.; Cho, Y.; Hong, S.H.; Shim, W.J. Ecological Risk Assessment of Microplastics in Coastal, Shelf, and Deep Sea Waters with a Consideration of Environmentally Relevant Size and Shape. Environ. Pollut. 2021, 270, 116217. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.; Deng, Y.; Zhang, S.; Wolosker, M.B.; Zhu, Q.; Ren, H.; Zhang, Y. Accumulation of Different Shapes of Microplastics Initiates Intestinal Injury and Gut Microbiota Dysbiosis in the Gut of Zebrafish. Chemosphere 2019, 236, 124334. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the Marine Environment: A Review of the Methods Used for Identification and Quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The Physical Impacts of Microplastics on Marine Organisms: A Review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef]
- Xiong, X.; Tu, Y.; Chen, X.; Jiang, X.; Shi, H.; Wu, C.; Elser, J.J. Ingestion and Egestion of Polyethylene Microplastics by Goldfish (Carassius Auratus): Influence of Color and Morphological Features. Heliyon 2019, 5, e03063. [Google Scholar] [CrossRef]
- ISO Search. Available online: https://www.iso.org/search.html?prod_isoorg_en%5bquery%5d=microplastic (accessed on 24 February 2025).
- Reineccius, J.; Bresien, J.; Waniek, J.J. Separation of Microplastics from Mass-Limited Samples by an Effective Adsorption Technique. Sci. Total Environ. 2021, 788, 147881. [Google Scholar] [CrossRef]
- Mári, Á.; Bordós, G.; Gergely, S.; Büki, M.; Háhn, J.; Palotai, Z.; Besenyő, G.; Szabó, É.; Salgó, A.; Kriszt, B.; et al. Validation of Microplastic Sample Preparation Method for Freshwater Samples. Water Res. 2021, 202, 117409. [Google Scholar] [CrossRef]
- Prata, J.C.; Sequeira, I.F.; Monteiro, S.S.; Silva, A.L.P.; da Costa, J.P.; Dias-Pereira, P.; Fernandes, A.J.S.; da Costa, F.M.; Duarte, A.C.; Rocha-Santos, T. Preparation of Biological Samples for Microplastic Identification by Nile Red. Sci. Total Environ. 2021, 783, 147065. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wu, H.; Wu, W.; Wang, L.; Liu, J.; An, L.; Xu, Q. Microplastic Characteristics in Organisms of Different Trophic Levels from Liaohe Estuary, China. Sci. Total Environ. 2021, 789, 148027. [Google Scholar] [CrossRef]
- Tirkey, A.; Upadhyay, L.S.B. Microplastics: An Overview on Separation, Identification and Characterization of Microplastics. Mar. Pollut. Bull. 2021, 170, 112604. [Google Scholar] [CrossRef]
- Schönlau, C.; Karlsson, T.M.; Rotander, A.; Nilsson, H.; Engwall, M.; van Bavel, B.; Kärrman, A. Microplastics in Sea-Surface Waters Surrounding Sweden Sampled by Manta Trawl and in-Situ Pump. Mar. Pollut. Bull. 2020, 153, 111019. [Google Scholar] [CrossRef]
- Tokai, T.; Uchida, K.; Kuroda, M.; Isobe, A. Mesh Selectivity of Neuston Nets for Microplastics. Mar. Pollut. Bull. 2021, 165, 112111. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, J.; Sun, C.; Cao, W.; Wang, M.; Jiang, F.; Ju, P. Comparative Study of Three Sampling Methods for Microplastics Analysis in Seawater. Sci. Total Environ. 2021, 765, 144495. [Google Scholar] [CrossRef]
- Prume, J.A.; Gorka, F.; Löder, M.G.J. From Sieve to Microscope: An Efficient Technique for Sample Transfer in the Process of Microplastics’ Quantification. MethodsX 2021, 8, 101341. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, Y.; Wang, C.; Wang, H. A Clean and Efficient Flotation towards Recovery of Hazardous Polyvinyl Chloride and Polycarbonate Microplastics through Selective Aluminum Coating: Process, Mechanism, and Optimization. J. Environ. Manag. 2021, 299, 113626. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, J.; Kang, Z.; Wu, X.; Tang, L.; Qiang, Z.; Zhang, D.; Pan, X. Removal of Micron-Scale Microplastic Particles from Different Waters with Efficient Tool of Surface-Functionalized Microbubbles. J. Hazard. Mater. 2021, 404, 124095. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Wang, L.; Wang, H. Application of Froth Flotation in the Separation of Polyvinyl Chloride and Polycarbonate for Recycling of Waste Plastic Based on a Novel Surface Modification. Waste Manag. 2020, 110, 43–52. [Google Scholar] [CrossRef]
- Imhof, H.K.; Schmid, J.; Niessner, R.; Ivleva, N.P.; Laforsch, C. A Novel, Highly Efficient Method for the Separation and Quantification of Plastic Particles in Sediments of Aquatic Environments. Limnol. Ocean. Methods 2012, 10, 524–537. [Google Scholar] [CrossRef]
- Morét-Ferguson, S.; Law, K.L.; Proskurowski, G.; Murphy, E.K.; Peacock, E.E.; Reddy, C.M. The Size, Mass, and Composition of Plastic Debris in the Western North Atlantic Ocean. Mar. Pollut. Bull. 2010, 60, 1873–1878. [Google Scholar] [CrossRef]
- Honaker, R.Q.; Saracoglu, M.; Huang, Q. Application of Hydrophobic and Magnetic Plastic Particles for Enhanced Flotation Recovery. Min. Eng. 2016, 98, 223–231. [Google Scholar] [CrossRef]
- Sun, B.; Yang, W.; He, M.; Wang, X. An Integrated Multi-Mode Model of Froth Flotation Cell Based on Fusion of Flotation Kinetics and Froth Image Features. Min. Eng. 2021, 172, 107169. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, H.; Bian, K.; Wang, H.; Wang, C. Is Froth Flotation a Potential Scheme for Microplastics Removal? Analysis on Flotation Kinetics and Surface Characteristics. Sci. Total Environ. 2021, 792, 148345. [Google Scholar] [CrossRef]
- Cutroneo, L.; Reboa, A.; Geneselli, I.; Capello, M. Considerations on Salts Used for Density Separation in the Extraction of Microplastics from Sediments. Mar. Pollut. Bull. 2021, 166, 112216. [Google Scholar] [CrossRef]
- Gohla, J.; Bračun, S.; Gretschel, G.; Koblmüller, S.; Wagner, M.; Pacher, C. Potassium Carbonate (K2CO3)—A Cheap, Non-Toxic and High-Density Floating Solution for Microplastic Isolation from Beach Sediments. Mar. Pollut. Bull. 2021, 170, 112618. [Google Scholar] [CrossRef]
- Kendall, M.J.; Siviour, C.R. Rate Dependence of Poly(Vinyl Chloride), the Effects of Plasticizer and Time–Temperature Superposition. Proc. R. Soc. A Math. Phys. Eng. Sci. 2014, 470, 20140012. [Google Scholar] [CrossRef]
- Jahan, S.; Strezov, V.; Weldekidan, H.; Kumar, R.; Kan, T.; Sarkodie, S.A.; He, J.; Dastjerdi, B.; Wilson, S.P. Interrelationship of Microplastic Pollution in Sediments and Oysters in a Seaport Environment of the Eastern Coast of Australia. Sci. Total Environ. 2019, 695, 133924. [Google Scholar] [CrossRef]
- Bläsing, M.; Amelung, W. Plastics in Soil: Analytical Methods and Possible Sources. Sci. Total Environ. 2018, 612, 422–435. [Google Scholar] [CrossRef]
- Möller, J.N.; Löder, M.G.J.; Laforsch, C. Finding Microplastics in Soils: A Review of Analytical Methods. Environ. Sci. Technol. 2020, 54, 2078–2090. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, H.; Cyvin, J.B.; Totland, C.; Lilleeng, Ø.; Wade, E.J.; Castro, V.; Pettersen, A.; Laugesen, J.; Møskeland, T.; Arp, H.P.H. Microplastic Accumulation by Tube-Dwelling, Suspension Feeding Polychaetes from the Sediment Surface: A Case Study from the Norwegian Continental Shelf. Mar. Environ. Res 2020, 161, 105073. [Google Scholar] [CrossRef]
- Nakajima, R.; Tsuchiya, M.; Lindsay, D.J.; Kitahashi, T.; Fujikura, K.; Fukushima, T. A New Small Device Made of Glass for Separating Microplastics from Marine and Freshwater Sediments. PeerJ 2019, 7, e7915. [Google Scholar] [CrossRef]
- Crichton, E.M.; Noël, M.; Gies, E.A.; Ross, P.S. A Novel, Density-Independent and FTIR-Compatible Approach for the Rapid Extraction of Microplastics from Aquatic Sediments. Anal. Methods 2017, 9, 1419–1428. [Google Scholar] [CrossRef]
- Yang, M.; Chen, B.; Xin, X.; Song, X.; Liu, J.; Dong, G.; Lee, K.; Zhang, B. Interactions between Microplastics and Oil Dispersion in the Marine Environment. J. Hazard. Mater. 2021, 403, 123944. [Google Scholar] [CrossRef]
- Scopetani, C.; Chelazzi, D.; Mikola, J.; Leiniö, V.; Heikkinen, R.; Cincinelli, A.; Pellinen, J. Olive Oil-Based Method for the Extraction, Quantification and Identification of Microplastics in Soil and Compost Samples. Sci. Total Environ. 2020, 733, 139338. [Google Scholar] [CrossRef]
- Constant, M.; Billon, G.; Breton, N.; Alary, C. Extraction of Microplastics from Sediment Matrices: Experimental Comparative Analysis. J. Hazard. Mater. 2021, 420, 126571. [Google Scholar] [CrossRef]
- Pagter, E.; Frias, J.; Nash, R.; O’Connor, I. Standardised Protocol for Monitoring Microplastics in Sediments. 2018. Available online: https://repository.oceanbestpractices.org/handle/11329/1206 (accessed on 24 February 2025).
- Chand, R.; Rasmussen, L.A.; Tumlin, S.; Vollertsen, J. The Occurrence and Fate of Microplastics in a Mesophilic Anaerobic Digester Receiving Sewage Sludge, Grease, and Fatty Slurries. Sci. Total Environ. 2021, 798, 149287. [Google Scholar] [CrossRef]
- Alfonso, M.B.; Takashima, K.; Yamaguchi, S.; Tanaka, M.; Isobe, A. Microplastics on Plankton Samples: Multiple Digestion Techniques Assessment Based on Weight, Size, and FTIR Spectroscopy Analyses. Mar. Pollut. Bull. 2021, 173, 113027. [Google Scholar] [CrossRef]
- Tuuri, E.M.; Gascooke, J.R.; Leterme, S.C. Efficacy of Chemical Digestion Methods to Reveal Undamaged Microplastics from Planktonic Samples. Sci. Total Environ. 2024, 947, 174279. [Google Scholar] [CrossRef]
- López-Rosales, A.; Andrade, J.M.; López-Mahía, P.; Muniategui-Lorenzo, S. Development of an Analytical Procedure to Analyze Microplastics in Edible Macroalgae Using an Enzymatic-Oxidative Digestion. Mar. Pollut. Bull. 2022, 183, 114061. [Google Scholar] [CrossRef] [PubMed]
- Löder, M.G.J.; Imhof, H.K.; Ladehoff, M.; Löschel, L.A.; Lorenz, C.; Mintenig, S.; Piehl, S.; Primpke, S.; Schrank, I.; Laforsch, C.; et al. Enzymatic Purification of Microplastics in Environmental Samples. Environ. Sci Technol 2017, 51, 14283–14292. [Google Scholar] [CrossRef]
- Lusher, A.L.; Munno, K.; Hermabessiere, L.; Carr, S. Isolation and Extraction of Microplastics from Environmental Samples: An Evaluation of Practical Approaches and Recommendations for Further Harmonization. Appl. Spectrosc. 2020, 74, 1049–1065. [Google Scholar] [CrossRef]
- ScienceDirect Search. Available online: https://www.sciencedirect.com/ (accessed on 24 February 2025).
- Rozman, U.; Turk, T.; Skalar, T.; Zupančič, M.; Čelan Korošin, N.; Marinšek, M.; Olivero-Verbel, J.; Kalčíková, G. An Extensive Characterization of Various Environmentally Relevant Microplastics—Material Properties, Leaching and Ecotoxicity Testing. Sci. Total Environ. 2021, 773, 145576. [Google Scholar] [CrossRef] [PubMed]
- Tasnim, J.; Ahmed, M.K.; Hossain, K.B.; Islam, M.S. Spatiotemporal Distribution of Microplastic Debris in the Surface Beach Sediment of the Southeastern Coast of Bangladesh. Heliyon 2023, 9, e21864. [Google Scholar] [CrossRef]
- Claessens, M.; De Meester, S.; Van Landuyt, L.; De Clerck, K.; Janssen, C.R. Occurrence and Distribution of Microplastics in Marine Sediments along the Belgian Coast. Mar. Pollut. Bull. 2011, 62, 2199–2204. [Google Scholar] [CrossRef]
- Lenz, R.; Enders, K.; Stedmon, C.A.; MacKenzie, D.M.A.; Nielsen, T.G. A Critical Assessment of Visual Identification of Marine Microplastic Using Raman Spectroscopy for Analysis Improvement. Mar. Pollut. Bull. 2015, 100, 82–91. [Google Scholar] [CrossRef]
- Chen, G.; Fu, Z.; Yang, H.; Wang, J. An Overview of Analytical Methods for Detecting Microplastics in the Atmosphere. TrAC Trends Anal. Chem. 2020, 130, 115981. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J. Investigation of Microplastics in Aquatic Environments: An Overview of the Methods Used, from Field Sampling to Laboratory Analysis. TrAC Trends Anal. Chem. 2018, 108, 195–202. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, D.; Pei, J.; Fei, Y.; Ouyang, D.; Zhang, H.; Luo, Y. Identification and Quantification of Microplastics Using Fourier-Transform Infrared Spectroscopy: Current Status and Future Prospects. Curr. Opin. Environ. Sci. Health 2020, 18, 14–19. [Google Scholar] [CrossRef]
- Xu, J.L.; Thomas, K.V.; Luo, Z.; Gowen, A.A. FTIR and Raman Imaging for Microplastics Analysis: State of the Art, Challenges and Prospects. TrAC Trends Anal. Chem. 2019, 119, 115629. [Google Scholar] [CrossRef]
- Tagg, A.S.; Sapp, M.; Harrison, J.P.; Ojeda, J.J. Identification and Quantification of Microplastics in Wastewater Using Focal Plane Array-Based Reflectance Micro-FT-IR Imaging. Anal. Chem. 2015, 87, 6032–6040. [Google Scholar] [CrossRef]
- Tan, A.; Zhao, J.; Zhao, Y.; Li, X.; Su, H. Determination of Microplastics by FTIR Spectroscopy Based on Quaternion Parallel Feature Fusion and Support Vector Machine. Chemom. Intell. Lab. Syst. 2023, 243, 105018. [Google Scholar] [CrossRef]
- Araujo, C.F.; Nolasco, M.M.; Ribeiro, A.M.P.; Ribeiro-Claro, P.J.A. Identification of Microplastics Using Raman Spectroscopy: Latest Developments and Future Prospects. Water Res. 2018, 142, 426–440. [Google Scholar] [CrossRef]
- Yan, X.; Cao, Z.; Murphy, A.; Qiao, Y. An Ensemble Machine Learning Method for Microplastics Identification with FTIR Spectrum. J. Environ. Chem. Eng. 2022, 10, 108130. [Google Scholar] [CrossRef]
- Corami, F.; Rosso, B.; Morabito, E.; Rensi, V.; Gambaro, A.; Barbante, C. Small Microplastics (<100 Μm), Plasticizers and Additives in Seawater and Sediments: Oleo-Extraction, Purification, Quantification, and Polymer Characterization Using Micro-FTIR. Sci. Total Environ. 2021, 797, 148937. [Google Scholar] [CrossRef]
- Hahn, A.; Gerdts, G.; Völker, C.; Niebühr, V. Using FTIRS as Pre-Screening Method for Detection of Microplastic in Bulk Sediment Samples. Sci. Total Environ. 2019, 689, 341–346. [Google Scholar] [CrossRef]
- Bridson, J.H.; Abbel, R.; Smith, D.A.; Northcott, G.L.; Gaw, S. Release of Additives and Non-Intentionally Added Substances from Microplastics under Environmentally Relevant Conditions. Environ. Adv. 2023, 12, 100359. [Google Scholar] [CrossRef]
- Uurasjärvi, E.; Sainio, E.; Setälä, O.; Lehtiniemi, M.; Koistinen, A. Validation of an Imaging FTIR Spectroscopic Method for Analyzing Microplastics Ingestion by Finnish Lake Fish (Perca fluviatilis and Coregonus albula). Environ. Pollut. 2021, 288, 117780. [Google Scholar] [CrossRef]
- Andoh, C.N.; Attiogbe, F.; Bonsu Ackerson, N.O.; Antwi, M.; Adu-Boahen, K. Fourier Transform Infrared Spectroscopy: An Analytical Technique for Microplastic Identification and Quantification. Infrared. Phys. Technol. 2024, 136, 105070. [Google Scholar] [CrossRef]
- Konings, M.C.; Zada, L.; Schmidt, R.W.; Ariese, F. Optimization of Sample Preparation, Fluorescence- and Raman Techniques for Environmental Microplastics. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 319, 124537. [Google Scholar] [CrossRef]
- Käppler, A.; Fischer, D.; Oberbeckmann, S.; Schernewski, G.; Labrenz, M.; Eichhorn, K.-J.; Voit, B. Analysis of Environmental Microplastics by Vibrational Microspectroscopy: FTIR, Raman or Both? Anal. Bioanal. Chem. 2016, 408, 8377–8391. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Campos, L.C.; Busquets, R. A Novel High-Throughput Analytical Method to Quantify Microplastics in Water by Flow Cytometry. Green Anal. Chem. 2023, 5, 100057. [Google Scholar] [CrossRef]
- Dümichen, E.; Eisentraut, P.; Bannick, C.G.; Barthel, A.-K.; Senz, R.; Braun, U. Fast Identification of Microplastics in Complex Environmental Samples by a Thermal Degradation Method. Chemosphere 2017, 174, 572–584. [Google Scholar] [CrossRef]
- Postma, J.V. An Inexpensive Atmospheric Microplastic Collector for Use in Remote Areas. Atmos. Pollut. Res. 2022, 13, 101550. [Google Scholar] [CrossRef]
- Lv, L.; Qu, J.; Yu, Z.; Chen, D.; Zhou, C.; Hong, P.; Sun, S.; Li, C. A Simple Method for Detecting and Quantifying Microplastics Utilizing Fluorescent Dyes—Safranine T, Fluorescein Isophosphate, Nile Red Based on Thermal Expansion and Contraction Property. Environ. Pollut. 2019, 255, 113283. [Google Scholar] [CrossRef]
- Kang, H.; Park, S.; Lee, B.; Ahn, J.; Kim, S. Modification of a Nile Red Staining Method for Microplastics Analysis: A Nile Red Plate Method. Water 2020, 12, 3251. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, L.; Liu, R.; He, M.; Cui, X.; Wang, C. Comprehensive Assessment of Factors Influencing Nile Red Staining: Eliciting Solutions for Efficient Microplastics Analysis. Mar. Pollut. Bull. 2021, 171, 112698. [Google Scholar] [CrossRef]
- Cole, M. A Novel Method for Preparing Microplastic Fibers. Sci. Rep. 2016, 6, 34519. [Google Scholar] [CrossRef]
- Idehara, W.; Haga, Y.; Tsujino, H.; Ikuno, Y.; Manabe, S.; Hokaku, M.; Asahara, H.; Higashisaka, K.; Tsutsumi, Y. Exploring Nile Red Staining as an Analytical Tool for Surface-Oxidized Microplastics. Environ. Res. 2025, 269, 120934. [Google Scholar] [CrossRef]
- Sturm, M.T.; Horn, H.; Schuhen, K. The Potential of Fluorescent Dyes—Comparative Study of Nile Red and Three Derivatives for the Detection of Microplastics. Anal. Bioanal. Chem. 2021, 413, 1059–1071. [Google Scholar] [CrossRef]
- Li, Q.; Zeng, A.; Jiang, X.; Gu, X. Are Microplastics Correlated to Phthalates in Facility Agriculture Soil? J. Hazard. Mater. 2021, 412, 125164. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; She, Z.; Xiong, X.; Ouyang, G.; Luo, Z. Automated Analysis of Microplastics Based on Vibrational Spectroscopy: Are We Measuring the Same Metrics? Anal. Bioanal. Chem. 2022, 414, 3359–3372. [Google Scholar] [CrossRef]
- Tian, X.; Beén, F.; Bäuerlein, P.S. Quantum Cascade Laser Imaging (LDIR) and Machine Learning for the Identification of Environmentally Exposed Microplastics and Polymers. Environ. Res. 2022, 212, 113569. [Google Scholar] [CrossRef]
- López-Rosales, A.; Andrade, J.; Fernández-González, V.; López-Mahía, P.; Muniategui-Lorenzo, S. A Reliable Method for the Isolation and Characterization of Microplastics in Fish Gastrointestinal Tracts Using an Infrared Tunable Quantum Cascade Laser System. Mar. Pollut. Bull. 2022, 178, 113591. [Google Scholar] [CrossRef] [PubMed]
- López-Rosales, A.; Ferreiro, B.; Andrade, J.; Fernández-Amado, M.; González-Pleiter, M.; López-Mahía, P.; Rosal, R.; Muniategui-Lorenzo, S. A Reliable Method to Determine Airborne Microplastics Using Quantum Cascade Laser Infrared Spectrometry. Sci. Total Environ. 2024, 913, 169678. [Google Scholar] [CrossRef]
- Hansen, J.; Hildebrandt, L.; Zimmermann, T.; El Gareb, F.; Fischer, E.K.; Pröfrock, D. Quantification and Characterization of Microplastics in Surface Water Samples from the Northeast Atlantic Ocean Using Laser Direct Infrared Imaging. Mar. Pollut. Bull. 2023, 190, 114880. [Google Scholar] [CrossRef]
- Nalbone, L.; Panebianco, A.; Giarratana, F.; Russell, M. Nile Red Staining for Detecting Microplastics in Biota: Preliminary Evidence. Mar. Pollut. Bull. 2021, 172, 112888. [Google Scholar] [CrossRef]
- Agilent 2019. Microplastics Workflow Frequendly Asked Question (FAQ). Available online: https://www.alphachrom.hr/cms_files/downloads/najcesca-pitanja-o-analizi-mikroplastike-i-ldir-8700-instrumentu-faq-download_news-1588066879.pdf (accessed on 24 February 2025).


| Term | Size | Reference |
|---|---|---|
| megaplastics | >1 m | GESAMP 2015 [70] |
| >100 mm | Barboza et al. 2019 [71] Barnes et.al. 2009 [72] | |
| macroplastics | <1 m | GESAMP 2015 [70] |
| >20 cm | Hanvey et al. 2017 [73] Eriksen et al. 2014 [74] | |
| >25 mm | Alimi et al. 2018 [75] | |
| >20 mm | Barboza et al. 2019 [71] Barnes et.al. 2009 [72] | |
| mesoplastic | 5–20 cm | Hanvey et al. 2017 [73] Eriksen et al. 2014 [74] |
| <2.5 cm | GESAMP 2015 [70] | |
| 5–25 mm | Alimi et al. 2018 [75] | |
| 5–20 mm | Barboza et al. 2019 [71] Barnes et.al. 2009 [72] | |
| large microplastic | 1–5 mm | Hanvey et al. 2017 [73] Eriksen et al. 2014 [74] |
| microplastic | <5 mm | Frias et al. 2019 [6] GESAMP 2015 [70] Barboza et al. 2019 [71] Barnes et.al. 2009 [72] Galgani et al. 2013 [76] Lambert et al. 2014 [77] |
| 0.1–5 mm | Alimi et al. 2018 [75] | |
| small microplastic | 1–1000 um | Hanvey et al. 2017 [73] Eriksen et al. 2014 [74] |
| nanoplastic | <1000 nm | Hanvey et al. 2017 [73] Koelmans et al. 2015 [78] |
| <100 nm | Alimi et al. 2018 [75] | |
| <1 um | GESAMP 2015 [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strojny, W.; Gruca-Rokosz, R.; Cieśla, M. Microplastics in Water Resources: Threats and Challenges. Appl. Sci. 2025, 15, 4118. https://doi.org/10.3390/app15084118
Strojny W, Gruca-Rokosz R, Cieśla M. Microplastics in Water Resources: Threats and Challenges. Applied Sciences. 2025; 15(8):4118. https://doi.org/10.3390/app15084118
Chicago/Turabian StyleStrojny, Wojciech, Renata Gruca-Rokosz, and Maksymilian Cieśla. 2025. "Microplastics in Water Resources: Threats and Challenges" Applied Sciences 15, no. 8: 4118. https://doi.org/10.3390/app15084118
APA StyleStrojny, W., Gruca-Rokosz, R., & Cieśla, M. (2025). Microplastics in Water Resources: Threats and Challenges. Applied Sciences, 15(8), 4118. https://doi.org/10.3390/app15084118







