Shape-Stabilized Phase Change Materials with Expanded Graphite for Thermal Management of Photovoltaic Cells: Selection of Materials and Preparation of Panels
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- Rubitherm® RT35HC (RT35), whose melting peak temperature is 35 °C, characterized by a melting enthalpy of 240 J/g (±7.5%) and density in the solid state of 0.88 g/cm3, was provided by Rubitherm GmbH (Berlin, Germany);
- Rubitherm® RT44HC (RT44), whose melting peak temperature is 44 °C, characterized by a melting enthalpy of 250 J/g (±7.5%) and density in the solid state of 0.8 g/cm3, was provided by Rubitherm GmbH (Berlin, Germany);
- A fatty acid mixture of myristic and palmitic acids (MPAs), mixed at a 61:39 weight ratio to finetune the melting temperature to around 50 °C, yielded a eutectic composition, whose precursors (purity ≥ 95%) were provided by Merck KGaA (Darmstadt, Germany).
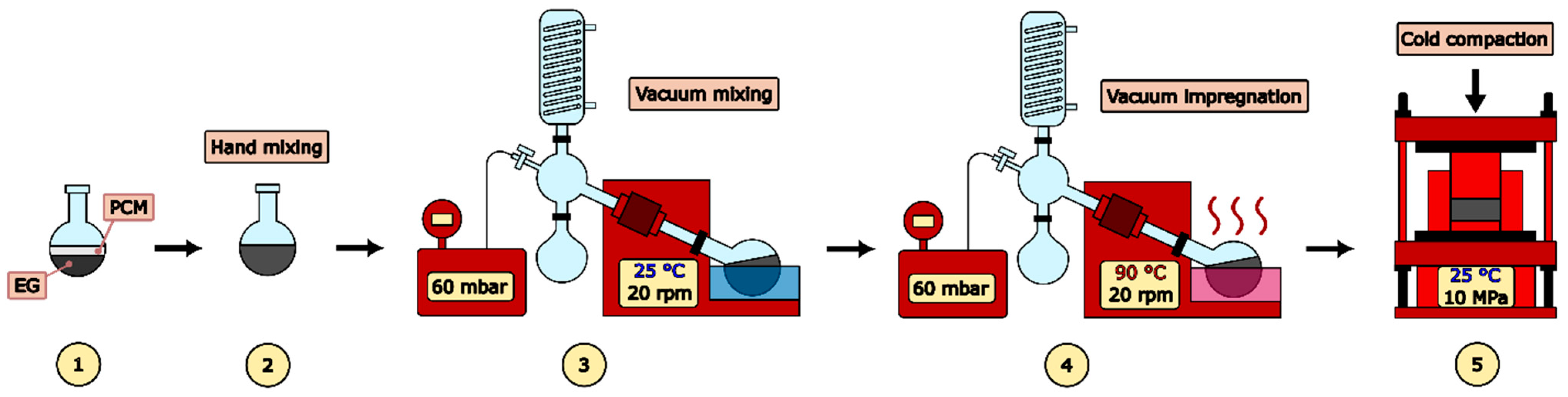
2.2. Sample Preparation
2.3. Experimental Methodologies
3. Results and Discussion
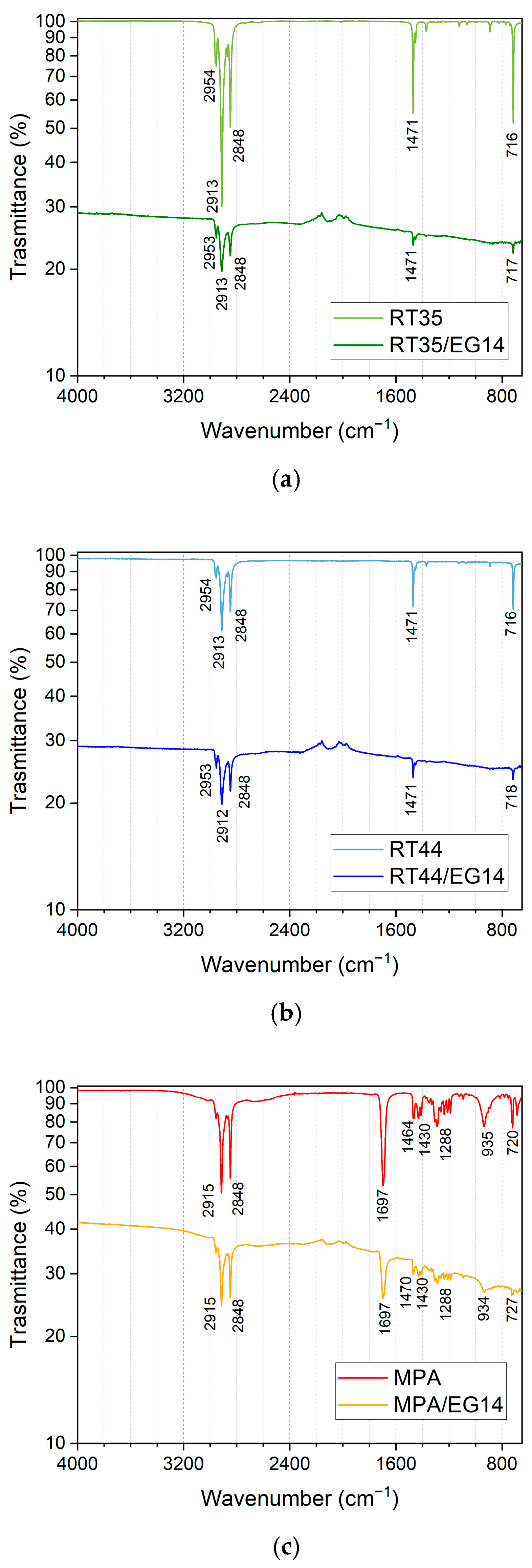
3.1. Fourier-Transform Infrared (FTIR) Spectroscopy
3.2. Leaking Test
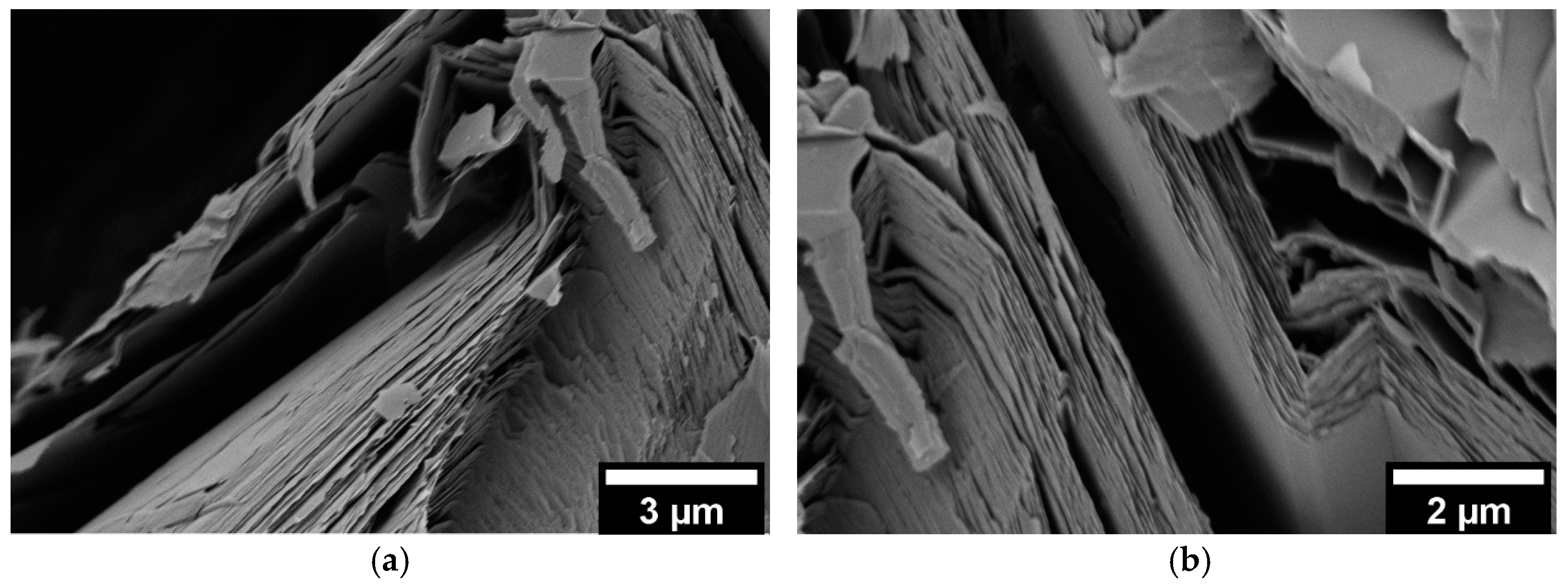
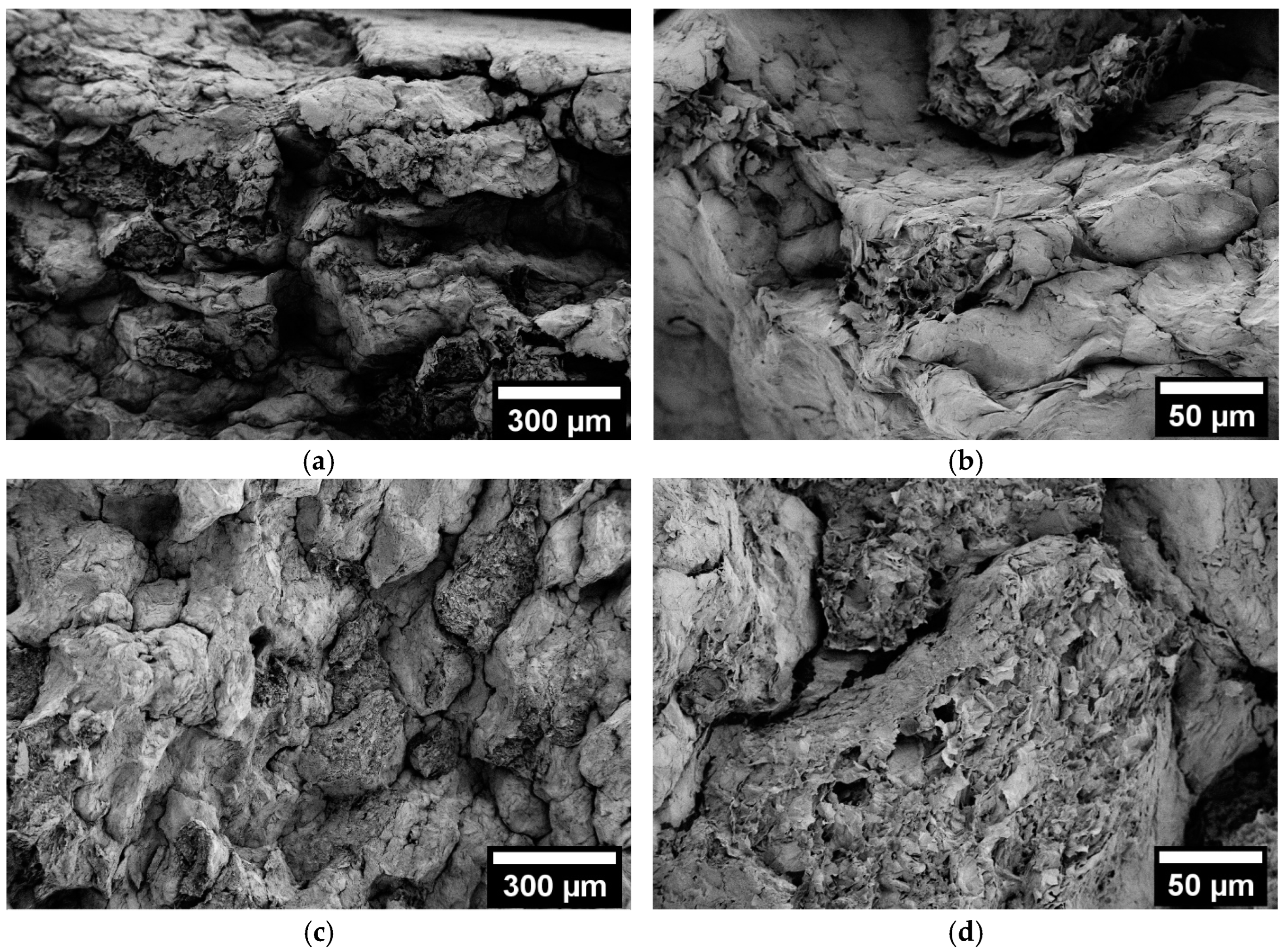
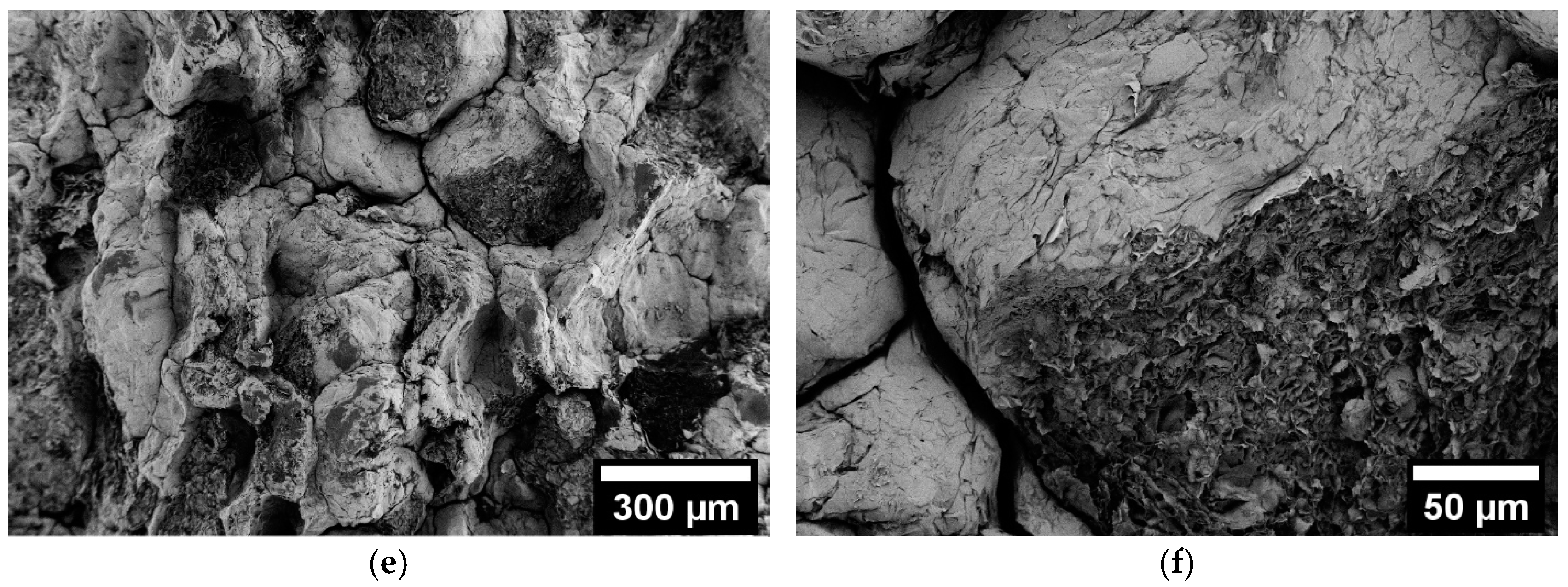
3.3. Scanning Electron Microscopy (SEM)
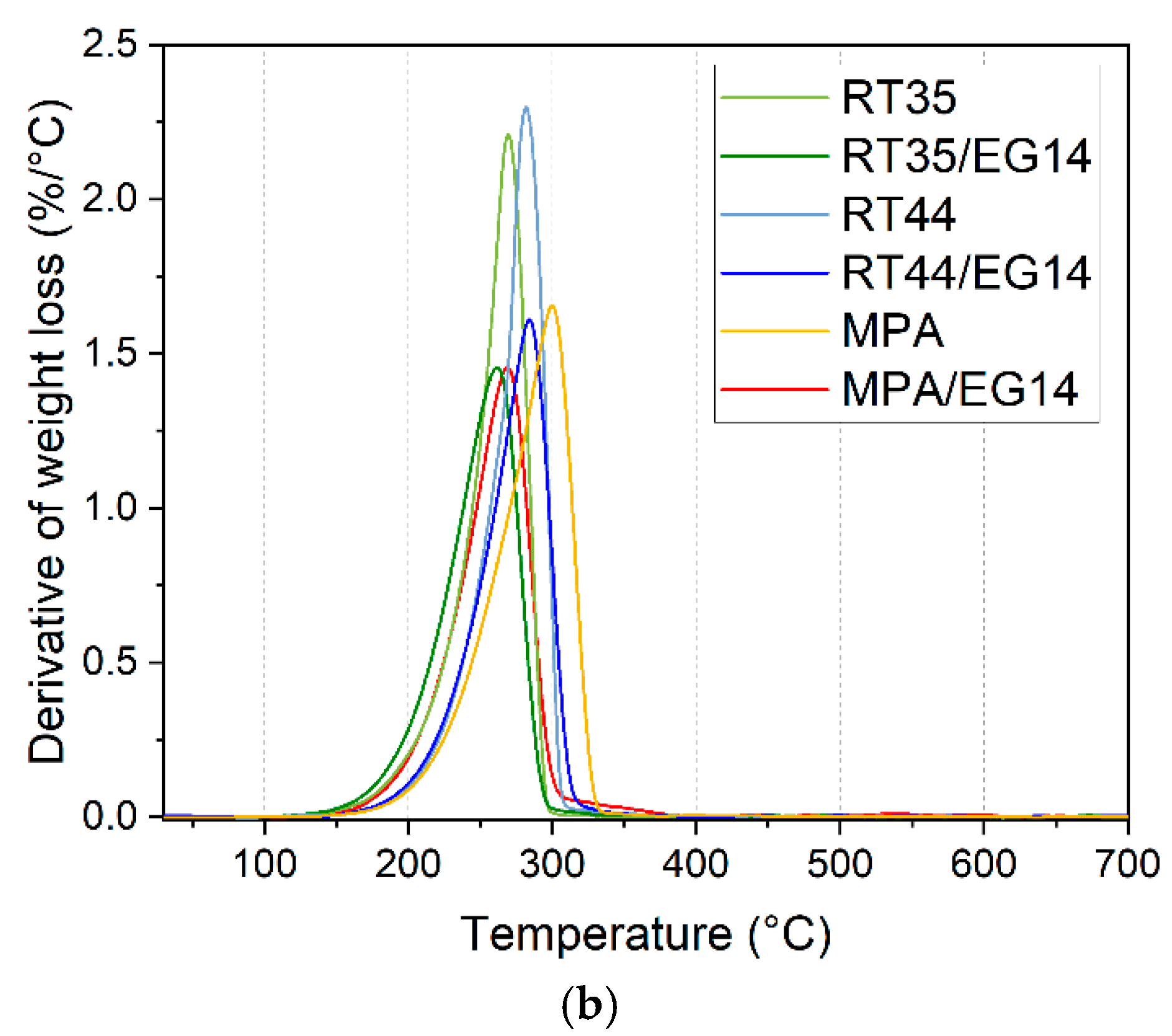
3.4. Thermogravimetric Analysis (TGA)
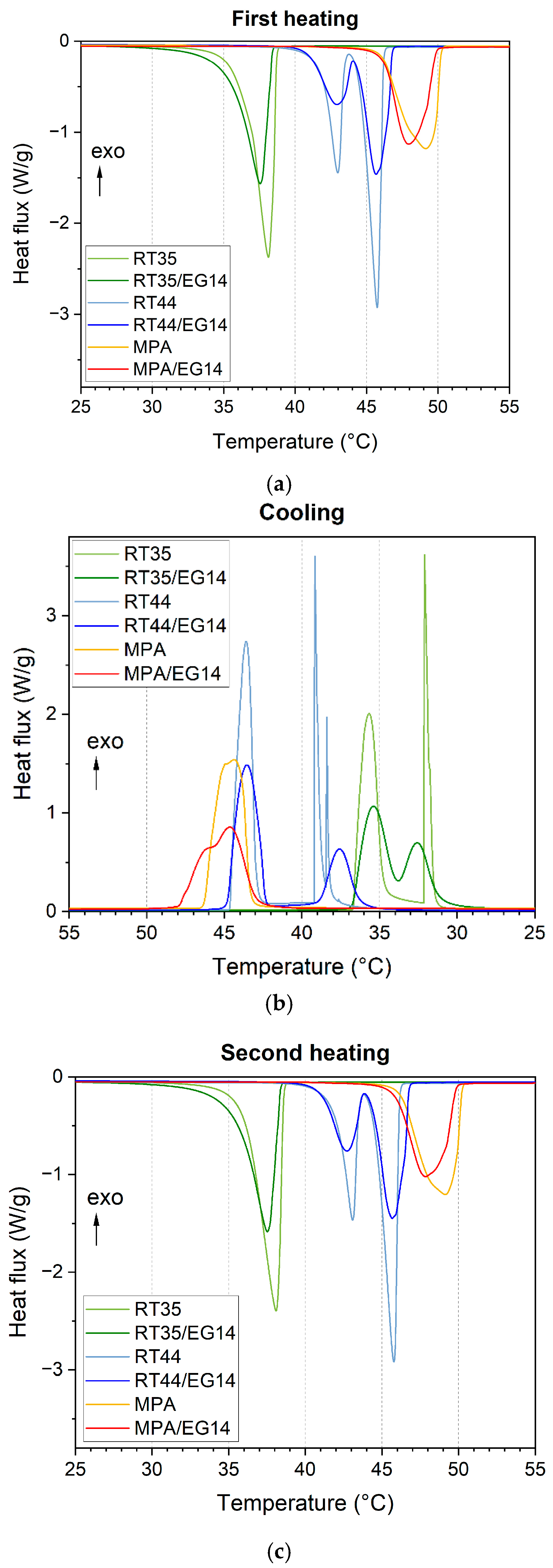
3.5. Differential Scanning Calorimetry (DSC)
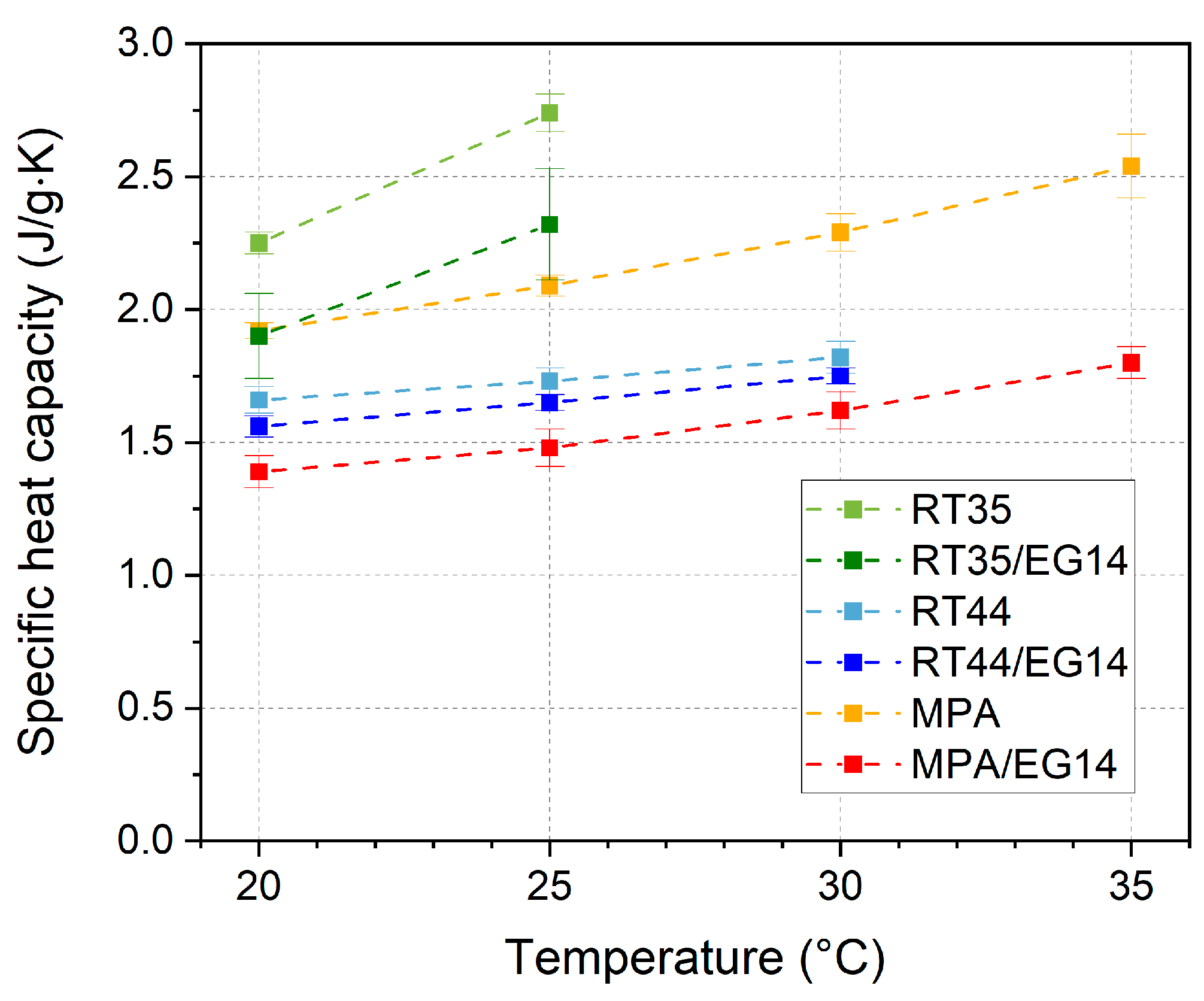
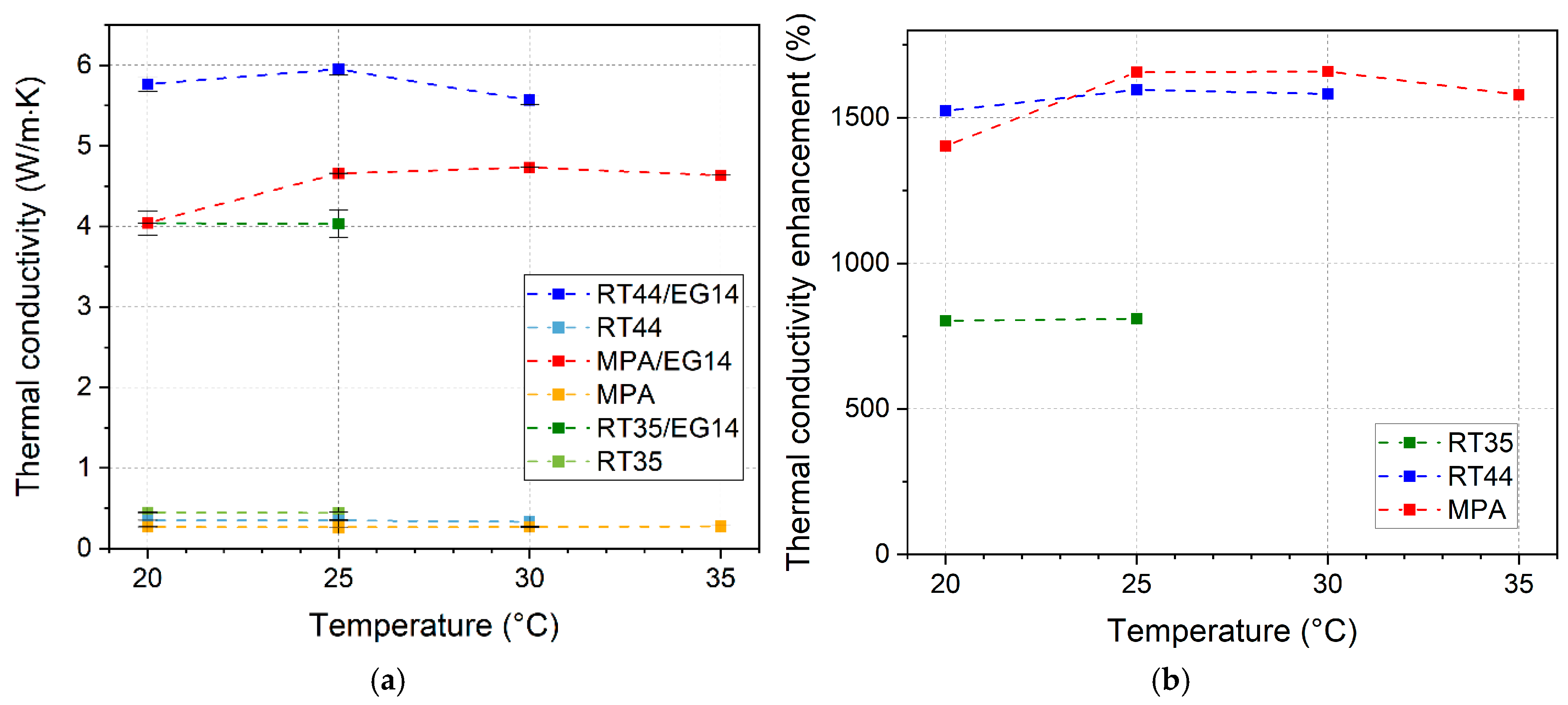
3.6. Specific Heat Capacity, Thermal Diffusivity, and Thermal Conductivity
3.7. Comparative Thermal Management System Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Photovoltaics Report. Available online: https://www.ise.fraunhofer.de/en/publications/studies/photovoltaics-report.html (accessed on 1 August 2024).
- Alliance, G.R.; Presidency, C. Tripling Renewable Power and Doubling Energy Efficiency by 2030: Crucial Steps Towards 1.5 °C. 2023. Available online: https://www.irena.org/Publications/2023/Oct/Tripling-renewable-power-and-doubling-energy-efficiency-by-2030 (accessed on 25 March 2025).
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The emergence of perovskite solar cells. Nat. Photonics 2014, 8, 506–514. [Google Scholar] [CrossRef]
- Bisconti, F.; Giuri, A.; Suhonen, R.; Kraft, T.M.; Ylikunnari, M.; Holappa, V.; Po’, R.; Biagini, P.; Savoini, A.; Marra, G.; et al. One-step polymer assisted roll-to-roll gravure-printed perovskite solar cells without using anti-solvent bathing. Cell Rep. Phys. Sci. 2021, 2, 100639. [Google Scholar] [CrossRef]
- Di Sabatino, M.; Hendawi, R.; Garcia, A.S. Silicon Solar Cells: Trends, Manufacturing Challenges, and AI Perspectives. Crystals 2024, 14, 167. [Google Scholar] [CrossRef]
- Best Efficiency Cells Chart. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 13 January 2025).
- Hamzat, A.K.; Sahin, A.Z.; Omisanya, M.I.; Alhems, L.M. Advances in PV and PVT cooling technologies: A review. Sustain. Energy Technol. Assess. 2021, 47, 101360. [Google Scholar] [CrossRef]
- Utomo, B.; Darkwa, J.; Du, D.; Worall, M. Solar photovoltaic cooling and power enhancement systems: A review. Renew. Sustain. Energy Rev. 2025, 216, 115644. [Google Scholar] [CrossRef]
- Harmailil, I.O.; Sultan, S.M.; Tso, C.P.; Fudholi, A.; Mohammad, M.; Ibrahim, A. A review on recent photovoltaic module cooling techniques: Types and assessment methods. Results Eng. 2024, 22, 102225. [Google Scholar] [CrossRef]
- Ahmed, Y.E.; Maghami, M.R.; Pasupuleti, J.; Danook, S.H.; Basim Ismail, F. Overview of Recent Solar Photovoltaic Cooling System Approach. Technologies 2024, 12, 171. [Google Scholar] [CrossRef]
- Lawag, R.A.; Ali, H.M. Phase change materials for thermal management and energy storage: A review. J. Energy Storage 2022, 55, 105602. [Google Scholar] [CrossRef]
- Cao, Y.; Su, J.; Xiao, Y.; Ren, J.; Algadi, H.; Yeszhanova, E.; Sartayeva, A.; Huang, J.; Guo, Z.; Tynybekov, B.; et al. Functional biomass/biological macromolecular phase change composites and their applications in different scenarios: A review. Int. J. Biol. Macromol. 2025, 306, 141377. [Google Scholar] [CrossRef]
- Valentini, F.; Grigiante, M.; Prada, A.; Fambri, L.; Dorigato, A.; Pegoretti, A. Experimental dynamic thermal properties determination of EPDM/NBR panels with a shape stabilized Phase Change Material based on summer daily temperatures of four cities in Italy. Energy Build. 2024, 318, 114503. [Google Scholar] [CrossRef]
- Maqbool, Z.; Hanief, M.; Parveez, M. Review on performance enhancement of phase change material based heat sinks in conjugation with thermal conductivity enhancers for electronic cooling. J. Energy Storage 2023, 60, 106591. [Google Scholar] [CrossRef]
- Khan, M.M.; Alkhedher, M.; Ramadan, M.; Ghazal, M. Hybrid PCM-based thermal management for lithium-ion batteries: Trends and challenges. J. Energy Storage 2023, 73, 108775. [Google Scholar] [CrossRef]
- Ji, W.; Dang, Y.; Yu, Y.; Zhou, X.; Li, L. Combination of Phase Change Composite Material and Liquid-Cooled Plate Prevents Thermal Runaway Propagation of High-Specific-Energy Battery. Appl. Sci. 2025, 15, 1274. [Google Scholar] [CrossRef]
- Du, K.; Calautit, J.; Eames, P.; Wu, Y. A state-of-the-art review of the application of phase change materials (PCM) in Mobilized-Thermal Energy Storage (M-TES) for recovering low-temperature industrial waste heat (IWH) for distributed heat supply. Renew. Energy 2021, 168, 1040–1057. [Google Scholar] [CrossRef]
- Qu, X.; Qi, X.; Fang, D. A Novel Liquid–Solid Fluidized Bed of Large-Scale Phase-Changing Sphere for Thermal Energy Storage. Appl. Sci. 2024, 14, 9828. [Google Scholar] [CrossRef]
- Wongwuttanasatian, T.; Sarikarin, T.; Suksri, A. Performance enhancement of a photovoltaic module by passive cooling using phase change material in a finned container heat sink. Sol. Energy 2020, 195, 47–53. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, X.; Hua, W. Recent progress in photovoltaic thermal phase change material technology: A review. J. Energy Storage 2023, 65, 107317. [Google Scholar] [CrossRef]
- Ortiz Lizcano, J.C.; Ziar, H.; De Mooij, C.; Verheijen, M.P.F.; Van Nierop Sanchez, C.; Ferlito, D.; Connelli, C.; Canino, A.; Zeman, M.; Isabella, O. Long-term experimental testing of phase change materials as cooling devices for photovoltaic modules. Sol. Energy Mater. Sol. Cells 2024, 277, 113133. [Google Scholar] [CrossRef]
- Wieprzkowicz, A.; Heim, D.; Knera, D. Coupled Model of Heat and Power Flow in Unventilated PV/PCM Wall-Validation in a Component Scale. Appl. Sci. 2022, 12, 7764. [Google Scholar] [CrossRef]
- Mousavi Ajarostaghi, S.S.; Amiri, L.; Poncet, S. Application of Thermal Batteries in Greenhouses. Appl. Sci. 2024, 14, 8640. [Google Scholar] [CrossRef]
- Luo, J.; Zou, D.; Wang, Y.; Wang, S.; Huang, L. Battery thermal management systems (BTMs) based on phase change material (PCM): A comprehensive review. Chem. Eng. J. 2022, 430, 132741. [Google Scholar] [CrossRef]
- Kumar, K.; Sharma, K.; Verma, S.; Upadhyay, N. Experimental Investigation of Graphene-Paraffin Wax Nanocomposites for Thermal Energy Storage. Mater. Today Proc. 2019, 18, 5158–5163. [Google Scholar] [CrossRef]
- Kumar, A.; Gupta, A.; Sharma, K.; Singh, M. Effect of graphene oxide on thermal charging and discharging behaviour of paraffin wax as nano-enhanced phase change materials. MRS Adv. 2024, 9, 1213–1218. [Google Scholar] [CrossRef]
- Gao, L.; Sun, X.; Sun, B.; Che, D.; Li, S.; Liu, Z. Preparation and thermal properties of palmitic acid/expanded graphite/carbon fiber composite phase change materials for thermal energy storage. J. Therm. Anal. Calorim. 2020, 141, 25–35. [Google Scholar] [CrossRef]
- Ao, C.; Yan, S.; Zhao, S.; Hu, W.; Zhao, L.; Wu, Y. Stearic acid/expanded graphite composite phase change material with high thermal conductivity for thermal energy storage. Energy Rep. 2022, 8, 4834–4843. [Google Scholar] [CrossRef]
- Zhou, D.; Xiao, S.; Xiao, X. Preparation and Thermal Performance of Fatty Acid Binary Eutectic Mixture/Expanded Graphite Composites as Form-Stable Phase Change Materials for Thermal Energy Storage. ACS Omega 2023, 8, 8596–8604. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Wu, J.; Sun, W.; Cai, W. Effects of Pore Structure and Pore Size of Expanded Graphite on the Properties of Paraffin Wax/Expanded Graphite Composite Phase Change Materials. Energies 2022, 15, 4201. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, X.; Venkataraman, M.; Wiener, J.; Palanisamy, S.; Sozcu, S.; Tan, X.; Kremenakova, D.; Zhu, G.; Yao, J.; et al. Structural Analysis of Phase Change Materials (PCMs)/Expanded Graphite (EG) Composites and Their Thermal Behavior under Hot and Humid Conditions. ChemPlusChem 2023, 88, e202300081. [Google Scholar] [CrossRef]
- Islam, A.; Pandey, A.K.; Saidur, R.; Tyagi, V.V. Shape stable composite phase change material with improved thermal conductivity for electrical-to-thermal energy conversion and storage. Mater. Today Sustain. 2024, 25, 100678. [Google Scholar] [CrossRef]
- Palacios, A.; Navarro-Rivero, M.E.; Zou, B.; Jiang, Z.; Harrison, M.T.; Ding, Y. A perspective on Phase Change Material encapsulation: Guidance for encapsulation design methodology from low to high-temperature thermal energy storage applications. J. Energy Storage 2023, 72, 108597. [Google Scholar] [CrossRef]
- Patil, J.R.; Mahanwar, P.A.; Sundaramoorthy, E.; Mundhe, G.S. A review of the thermal storage of phase change material, morphology, synthesis methods, characterization, and applications of microencapsulated phase change material. J. Polym. Eng. 2023, 43, 354–375. [Google Scholar] [CrossRef]
- Sacchet, S.; Valentini, F.; Rizzo, C.; Po, R.; Fambri, L. High density polyethylene with phase change materials for thermal energy management. Energy Mater. 2025, 5, 500042. [Google Scholar] [CrossRef]
- Rigotti, D.; Dorigato, A.; Pegoretti, A. Multifunctional 3D-Printed Thermoplastic Polyurethane (TPU)/Multiwalled Carbon Nanotube (MWCNT) Nanocomposites for Thermal Management Applications. Appl. Sci. 2024, 14, 9614. [Google Scholar] [CrossRef]
- Kaygusuz, K.; Alkan, C.; Sari, A.; Uzun, O. Encapsulated Fatty Acids in an Acrylic Resin as Shape-stabilized Phase Change Materials for Latent Heat Thermal Energy Storage. Energy Sources Part A Recovery Util. Environ. Eff. 2008, 30, 1050–1059. [Google Scholar] [CrossRef]
- Valentini, F.; Dorigato, A.; Fambri, L.; Bersani, M.; Grigiante, M.; Pegoretti, A. Production and characterization of novel EPDM/NBR panels with paraffin for potential thermal energy storage applications. Therm. Sci. Eng. Prog. 2022, 32, 101309. [Google Scholar] [CrossRef]
- Sacchet, S.; Valentini, F.; Benin, A.; Guidolin, M.; Po, R.; Fambri, L. Expanded Graphite (EG) Stabilization of Stearic and Palmitic Acid Mixture for Thermal Management of Photovoltaic Cells. C 2024, 10, 46. [Google Scholar] [CrossRef]
- ASTM E1269-11; Standard Test Method for Determining Specific Heat Capacity by Differential Scanning Calorimetry. ASTM International: West Conshohocken, PA, USA, 2018. Available online: https://www.astm.org/e1269-11.html (accessed on 13 May 2024).
- Pan, S.J.; Zhou, J.B.; Li, H.T.; Quan, C. Particle Formation by Supercritical Fluid Extraction and Expansion Process. Sci. World J. 2013, 2013, 538584. [Google Scholar] [CrossRef]
- Shulyak, V.A.; Morozov, N.S.; Makhina, V.S.; Klyukova, K.E.; Gracheva, A.V.; Chebotarev, S.N.; Avdeev, V.V. Intercalation of Large Flake Graphite with Fuming Nitric Acid. C 2024, 10, 108. [Google Scholar] [CrossRef]
- Shi, Y.; Zhao, Y.; Zhang, Y.; Fan, Z.; Jiang, D. Study on the Reliability and Stability of Fatty Acid-Paraffin Ternary Phase Change Materials as Energy-saving Materials. ChemistrySelect 2024, 9, e202303114. [Google Scholar] [CrossRef]
- Matuszek, K.; Kar, M.; Pringle, J.M.; Macfarlane, D.R. Phase Change Materials for Renewable Energy Storage at Intermediate Temperatures. Chem. Rev. 2023, 123, 491–514. [Google Scholar] [CrossRef]
- Divi, S.; Chellappa, R.; Chandra, D. Heat capacity measurement of organic thermal energy storage materials. J. Chem. Thermodyn. 2006, 38, 1312–1326. [Google Scholar] [CrossRef]
- Ghasemi, K.; Tasnim, S.H.; Mahmud, S. Fabrication and evaluating thermophysical properties of microencapsulated organic and eutectic phase change material. Colloids Surf. A Physicochem. Eng. Asp. 2024, 686, 133395. [Google Scholar] [CrossRef]
- Duquesne, M.; Mailhé, C.; Doppiu, S.; Dauvergne, J.-L.; Santos-Moreno, S.; Godin, A.; Fleury, G.; Rouault, F.; Palomo Del Barrio, E. Characterization of Fatty Acids as Biobased Organic Materials for Latent Heat Storage. Materials 2021, 14, 4707. [Google Scholar] [CrossRef]
- Nazir, H.; Batool, M.; Ali, M.; Kannan, A.M. Fatty acids based eutectic phase change system for thermal energy storage applications. Appl. Therm. Eng. 2018, 142, 466–475. [Google Scholar] [CrossRef]
- Lachheb, M.; Karkri, M.; Albouchi, F.; Mzali, F.; Ben Nasrallah, S. Thermophysical properties estimation of paraffin/graphite composite phase change material using an inverse method. Energy Convers. Manag. 2014, 82, 229–237. [Google Scholar] [CrossRef]
- Noël, J.A.; White, M.A. Heat capacities of potential organic phase change materials. J. Chem. Thermodyn. 2019, 128, 127–133. [Google Scholar] [CrossRef]
- Sarı, A.; Karaipekli, A. Preparation, thermal properties and thermal reliability of palmitic acid/expanded graphite composite as form-stable PCM for thermal energy storage. Sol. Energy Mater. Sol. Cells 2009, 93, 571–576. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, H.; Zhao, Z.; Sun, L.; Xu, F.; Zhang, J.; Jiao, Q.; Bao, Y.; Ma, J. Preparation, Encapsulation and Thermal Properties of Fatty Acid/Expanded Graphite Composites as Shape-stabilized Phase Change Materials. Chem. J. Chin. Univ.-Chin. Ed. 2012, 33, 526–530. [Google Scholar] [CrossRef]
- Zhang, N.; Yuan, Y.; Wang, X.; Cao, X.; Yang, X.; Hu, S. Preparation and characterization of lauric–myristic–palmitic acid ternary eutectic mixtures/expanded graphite composite phase change material for thermal energy storage. Chem. Eng. J. 2013, 231, 214–219. [Google Scholar] [CrossRef]
- Zhang, N.; Yuan, Y.; Du, Y.; Cao, X.; Yuan, Y. Preparation and properties of palmitic-stearic acid eutectic mixture/expanded graphite composite as phase change material for energy storage. Energy 2014, 78, 950–956. [Google Scholar] [CrossRef]
- Wu, S.; Li, T.X.; Yan, T.; Dai, Y.J.; Wang, R.Z. High performance form-stable expanded graphite/stearic acid composite phase change material for modular thermal energy storage. Int. J. Heat Mass Transf. 2016, 102, 733–744. [Google Scholar] [CrossRef]
- Zhang, J.-L.; Wu, N.; Wu, X.-W.; Chen, Y.; Zhao, Y.; Liang, J.-F.; Bai, X.-B. High latent heat stearic acid impregnated in expanded graphite. Thermochim. Acta 2018, 663, 118–124. [Google Scholar] [CrossRef]
- Li, C.; Zhang, B.; Xie, B.; Zhao, X.; Chen, J.; Chen, Z.; Long, Y. Stearic acid/expanded graphite as a composite phase change thermal energy storage material for tankless solar water heater. Sustain. Cities Soc. 2019, 44, 458–464. [Google Scholar] [CrossRef]
- Zhou, D.; Yuan, J.; Zhou, Y.; Liu, Y. Preparation and characterization of myristic acid/expanded graphite composite phase change materials for thermal energy storage. Sci. Rep. 2020, 10, 10889. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, X.; Zhu, X.; Xin, S. A Low-Temperature Phase Change Material Based on Capric-Stearic Acid/Expanded Graphite for Thermal Energy Storage. ACS Omega 2021, 6, 17988–17998. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, G.; Jia, Z.; Gao, Q.; Li, Y.; Gu, Z. Eutectic Fatty Acids Phase Change Materials Improved with Expanded Graphite. Materials 2022, 15, 6856. [Google Scholar] [CrossRef]
- Gao, H.; Bing, N.; Xie, H.; Yu, W. Energy harvesting and storage blocks based on 3D oriented expanded graphite and stearic acid with high thermal conductivity for solar thermal application. Energy 2022, 254, 124198. [Google Scholar] [CrossRef]
- Li, J.; Wang, W.; Deng, Y.; Gao, L.; Bai, J.; Xu, L.; Chen, J.; Yuan, Z. Thermal Performance Analysis of Composite Phase Change Material of Myristic Acid-Expanded Graphite in Spherical Thermal Energy Storage Unit. Energies 2023, 16, 4527. [Google Scholar] [CrossRef]
- Zhou, D.; Xiao, S.; Liu, Y. The Effect of Expanded Graphite Content on the Thermal Properties of Fatty Acid Composite Materials for Thermal Energy Storage. Molecules 2024, 29, 3146. [Google Scholar] [CrossRef]
- Chen, B.; Ruilong, W.; Xueyou, Y.; Ma, A. Thermal and electrical properties of palmitic acid/expanded graphite composite phase change materials for thermal energy storage. Fuller. Nanotub. Carbon Nanostruct. 2024, 32, 1093–1101. [Google Scholar] [CrossRef]
- Jesus D’oliveira, E.; Azimov, U.; Costa Pereira, S.-C.; Lafdi, K. Effect of particle size on the thermal conductivity of organic phase change materials with expanded graphite. J. Energy Storage 2024, 92, 112090. [Google Scholar] [CrossRef]
- Kenisarin, M.; Mahkamov, K.; Kahwash, F.; Makhkamova, I. Enhancing thermal conductivity of paraffin wax 53–57 °C using expanded graphite. Sol. Energy Mater. Sol. Cells 2019, 200, 110026. [Google Scholar] [CrossRef]
- Li, Y.; Yan, H.; Yang, F.; Li, S.; Jiang, C.; Xie, L. Paraffin Wax–Expanded Graphite Composite Phase Change Materials With Enhancing Thermal Conductivity and Thermal Stability for Thermal Energy Storage. ChemistrySelect 2024, 9, e202404879. [Google Scholar] [CrossRef]



| Sample | RT35 | RT44 | MPA | EG | ||||
|---|---|---|---|---|---|---|---|---|
| [phr] | [wt.%] | [phr] | [wt.%] | [phr] | [wt.%] | [phr] | [wt.%] | |
| RT35/EG14 | 100 | 87.7 | - | - | - | - | 14 | 12.3 |
| RT44/EG14 | - | - | 100 | 87.7 | - | - | 14 | 12.3 |
| MPA/EG14 | - | - | - | - | 100 | 87.7 | 14 | 12.3 |
| Sample | T5% | Tpeak | m700 |
|---|---|---|---|
| [°C] | [°C] | [wt.%] | |
| RT35 | 204.5 | 269.3 | 0.0 |
| RT35/EG14 | 195.3 | 261.5 | 12.5 |
| RT44 | 219.5 | 281.7 | 0.0 |
| RT44/EG14 | 217.8 | 284.0 | 10.7 |
| MPA | 223.2 | 299.8 | 0.0 |
| MPA/EG14 | 204.3 | 268.8 | 12.9 |
| Sample | Tm1 [°C] | ΔHm1 [J/g] | Tc [°C] | ΔHc [J/g] | Tm2 [°C] | ΔHm2 [J/g] | [wt.%] | [wt.%] |
|---|---|---|---|---|---|---|---|---|
| RT35 | 37.5 | 234 | 36.2–36.7 | 234 | 37.4 | 236 | 100 | 100 |
| RT35/EG14 | 37.3 | 204 | 35.6–32.7 | 206 | 37.2 | 206 | 87.4 | 99.7 |
| RT44 | 42.7–45.2 | 272 | 44.2–39.9 | 270 | 45.2–42.8 | 270 | 100 | 100 |
| RT44/EG14 | 42.8–45.4 | 221 | 43.8–37.7 | 219 | 45.4–42.6 | 220 | 81.5 | 92.9 |
| MPA | 48.6 | 193 | 45.2 | 196 | 48.5 | 196 | 100 | 100 |
| MPA/EG14 | 47.7 | 166 | 44.9 | 155 | 47.6 | 159 | 81.1 | 92.5 |
| PCM Type | Tm | EG Content | λ | Year |
|---|---|---|---|---|
| [°C] | [wt.%] | [W/m·K] | [Reference] | |
| Fatty acid (PA) | 68 | 8.0 | 0.8 | 2009 [51] |
| Fatty acid (CA-LA-PA) | 18 | n.a. | 0.7 | 2012 [52] |
| Fatty acid (LA-MA-PA) | 31 | 5.3 | 1.7 | 2013 [53] |
| Fatty acid (PA-SA) | 54 | 7.1 | 2.5 | 2014 [54] |
| Fatty acid (SA) | 69 | 15.0–30.0 | 7.5–23.3 | 2016 [55] |
| Fatty acid (SA base) | 53 | 5.0–20.0 | 1.5–3.2 | 2018 [56] |
| Fatty acid (SA base) | 53 | 2.0–10.0 | 0.8–3.6 | 2019 [57] |
| Fatty acid (MA) | 53 | 6.5 | 2.1 | 2020 [58] |
| Fatty acid (CA-SA) | 25 | 10.0–12.0 | 0.5–0.6 | 2021 [59] |
| Fatty acid (LA-SA) | 31 | 10.0–15.0 | 0.6 | 2022 [60] |
| Fatty acid (SA) | 68 | 8.0–12.0 | 3.3–6.5 | 2022 [28] |
| Fatty acid (SA) | 69 | 5.0–25.0 | 0.7–7.7 | 2022 [61] |
| Fatty acid (MA) | 54 | 4.0–6.0 | 2.3 | 2023 [62] |
| Fatty acid (CA-MA) | 19 | 5.0–20.0 | 0.3–1.2 | 2024 [63] |
| Fatty acid (PA) | 61 | 16.0 | 4.9 | 2024 [64] |
| Fatty acid (PA-SA) | 53 | 9.0–12.3 | 4.0–10.3 | 2024 [39] |
| Fatty acids | 62–66 | 2.0–6.0 | 0.3–0.5 | 2024 [65] |
| Fatty acid (MA-PA) | 48 | 12.3 | 4.7 | 2025 Present study |
| Paraffin | 64 | 2.0–6.0 | 0.3–0.5 | 2024 [65] |
| Paraffin | 53–57 | 6.0 | 1.0–1.3 | 2019 [66] |
| Paraffin | 50 | 8.0 | 0.8 | 2022 [30] |
| Paraffin | 36 | 10.0–30.0 | 5.3–6.0 | 2024 [67] |
| Paraffin | 37 | 12.3 | 4.0 | 2025 Present study |
| Paraffin | 45 | 12.3 | 6.0 | 2025 Present study |
| TMS Panels | ρ [g/cm3] | λ25 [W/(m·K)] | ΔT + [°C] | ΔT− [°C] | ΔHm [J/g] | ΔHm* [J/cm3] | SD [kg/m2] | TMA [MJ/m2] | t [mm] | TEC [kJ/Unit] |
|---|---|---|---|---|---|---|---|---|---|---|
| RT35/EG14 | 0.94 | 4.03 | 30–39 | 37–30 | 206 | 194 | 18.8 | 3.9 | 19.4 | 14.4 |
| RT44/EG14 | 0.99 | 5.95 | 40–47 | 45–35 | 220 | 218 | 19.8 | 4.4 | 18.4 | 15.4 |
| MPA/EG14 | 1.05 | 4.66 | 45–51 | 48–42 | 159 | 167 | 21.0 | 3.3 | 17.3 | 11.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sacchet, S.; Valentini, F.; Guidolin, M.; Po, R.; Fambri, L. Shape-Stabilized Phase Change Materials with Expanded Graphite for Thermal Management of Photovoltaic Cells: Selection of Materials and Preparation of Panels. Appl. Sci. 2025, 15, 4352. https://doi.org/10.3390/app15084352
Sacchet S, Valentini F, Guidolin M, Po R, Fambri L. Shape-Stabilized Phase Change Materials with Expanded Graphite for Thermal Management of Photovoltaic Cells: Selection of Materials and Preparation of Panels. Applied Sciences. 2025; 15(8):4352. https://doi.org/10.3390/app15084352
Chicago/Turabian StyleSacchet, Sereno, Francesco Valentini, Marco Guidolin, Riccardo Po, and Luca Fambri. 2025. "Shape-Stabilized Phase Change Materials with Expanded Graphite for Thermal Management of Photovoltaic Cells: Selection of Materials and Preparation of Panels" Applied Sciences 15, no. 8: 4352. https://doi.org/10.3390/app15084352
APA StyleSacchet, S., Valentini, F., Guidolin, M., Po, R., & Fambri, L. (2025). Shape-Stabilized Phase Change Materials with Expanded Graphite for Thermal Management of Photovoltaic Cells: Selection of Materials and Preparation of Panels. Applied Sciences, 15(8), 4352. https://doi.org/10.3390/app15084352







