Effect of Aging on Class G High Sulfate-Resistant Oil Well Cement Under High Relative Air Humidity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Aging of Cement
2.3. Preparation and Properties of the Cement Paste
2.4. Particle Size and Specific Surface Area
2.5. Mineral Compositions
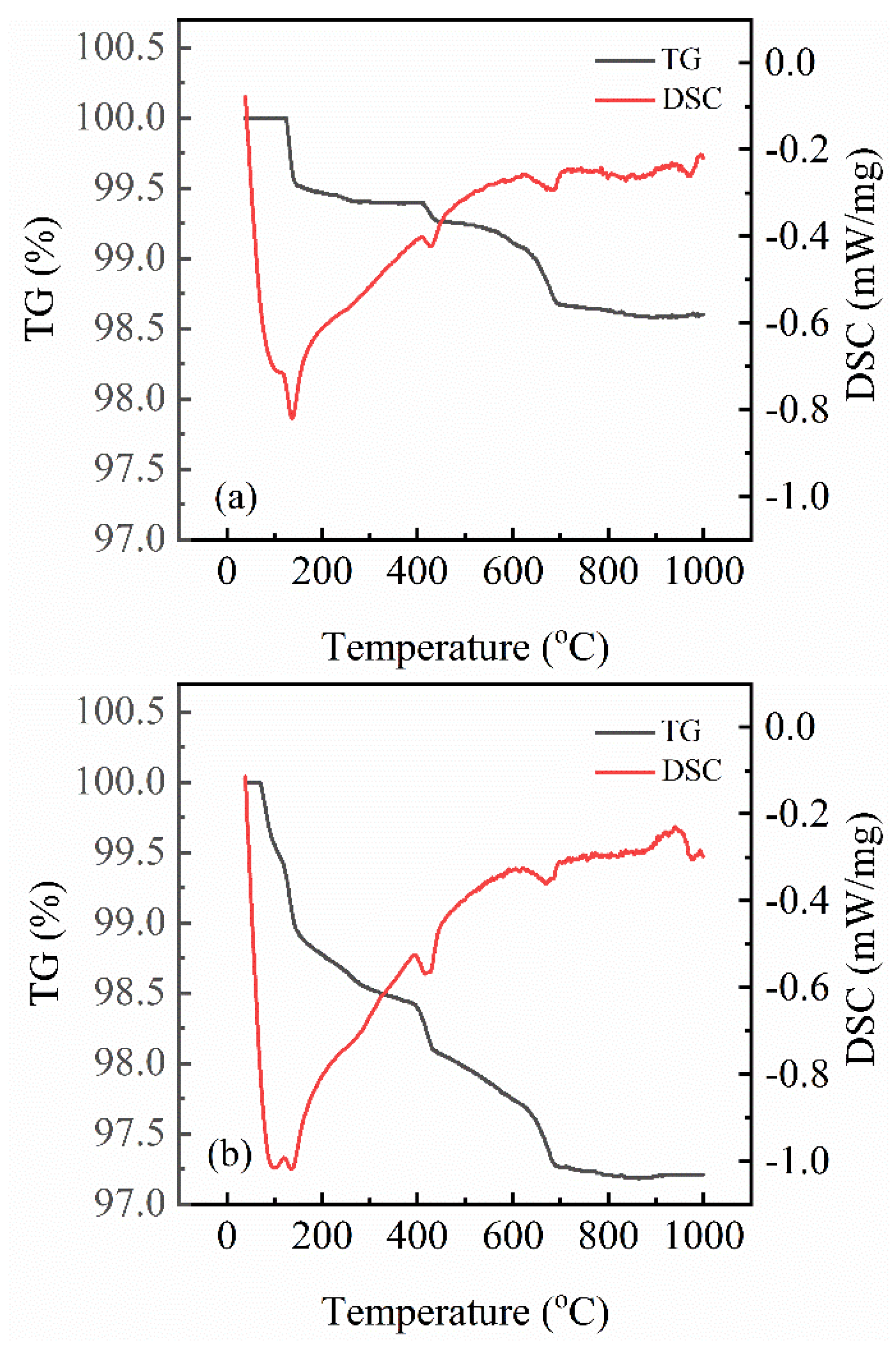
2.6. Thermal Analysis
2.7. Hydration Behavior
3. Results and Discussion
3.1. Influence of Aging on the Engineering Performances of Oil Well Cement
3.1.1. Compressive Strength
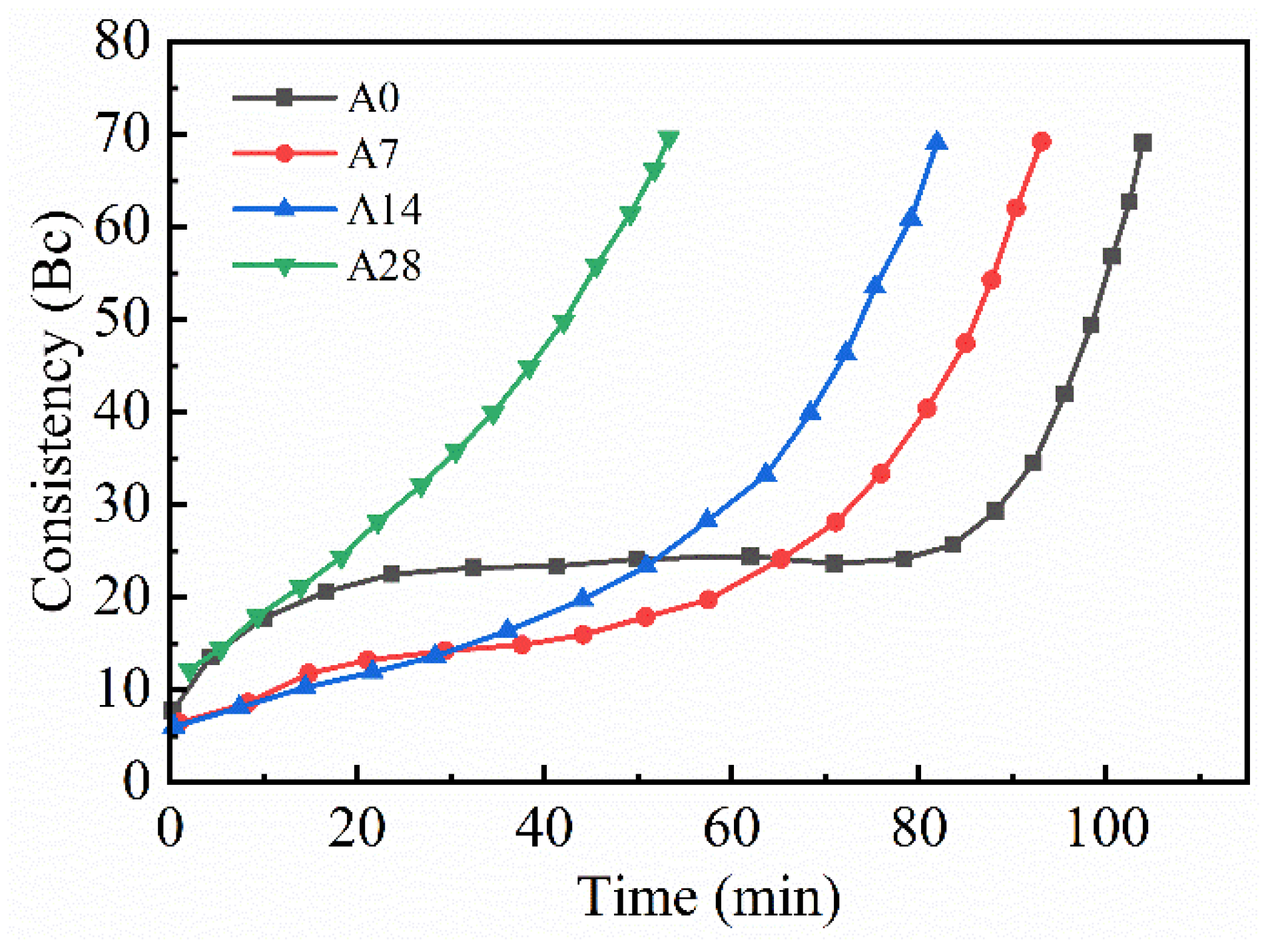
3.1.2. Thickening Time
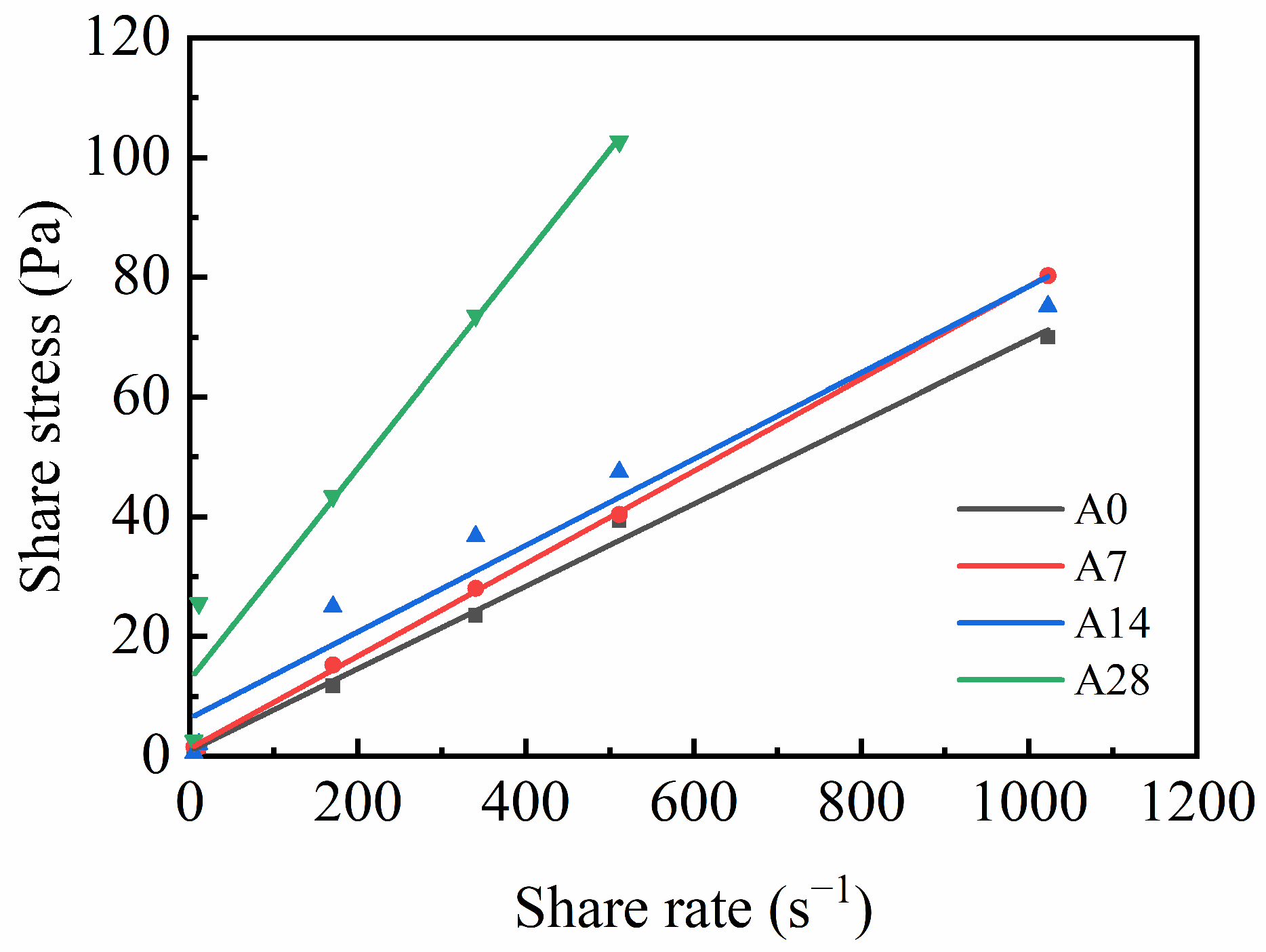
3.1.3. Rheological Properties
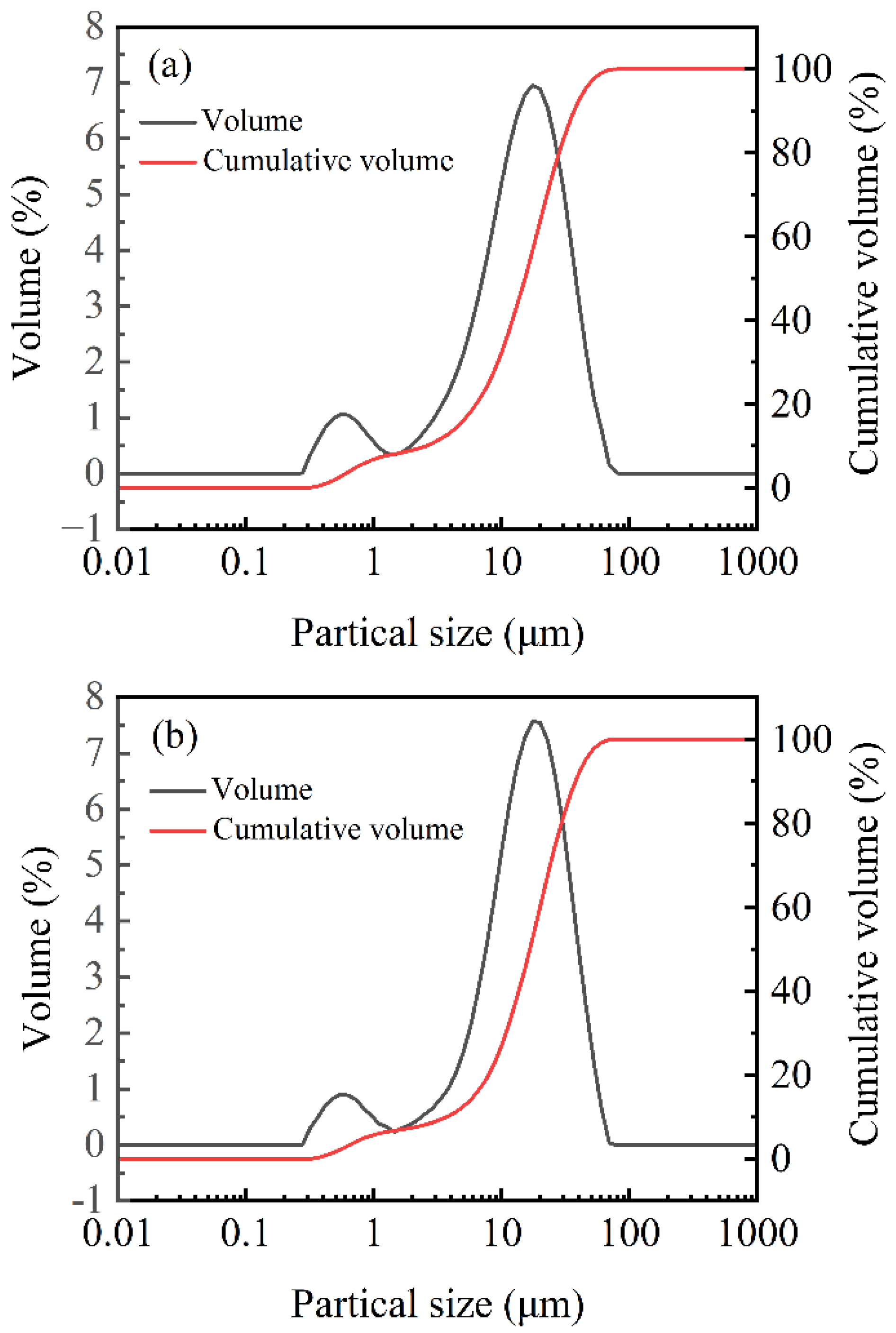
3.2. Influence of Aging on the Particle Size of Oil Well Cement
3.3. Influence of Aging on the Mineral Composition of Oil Well Cement
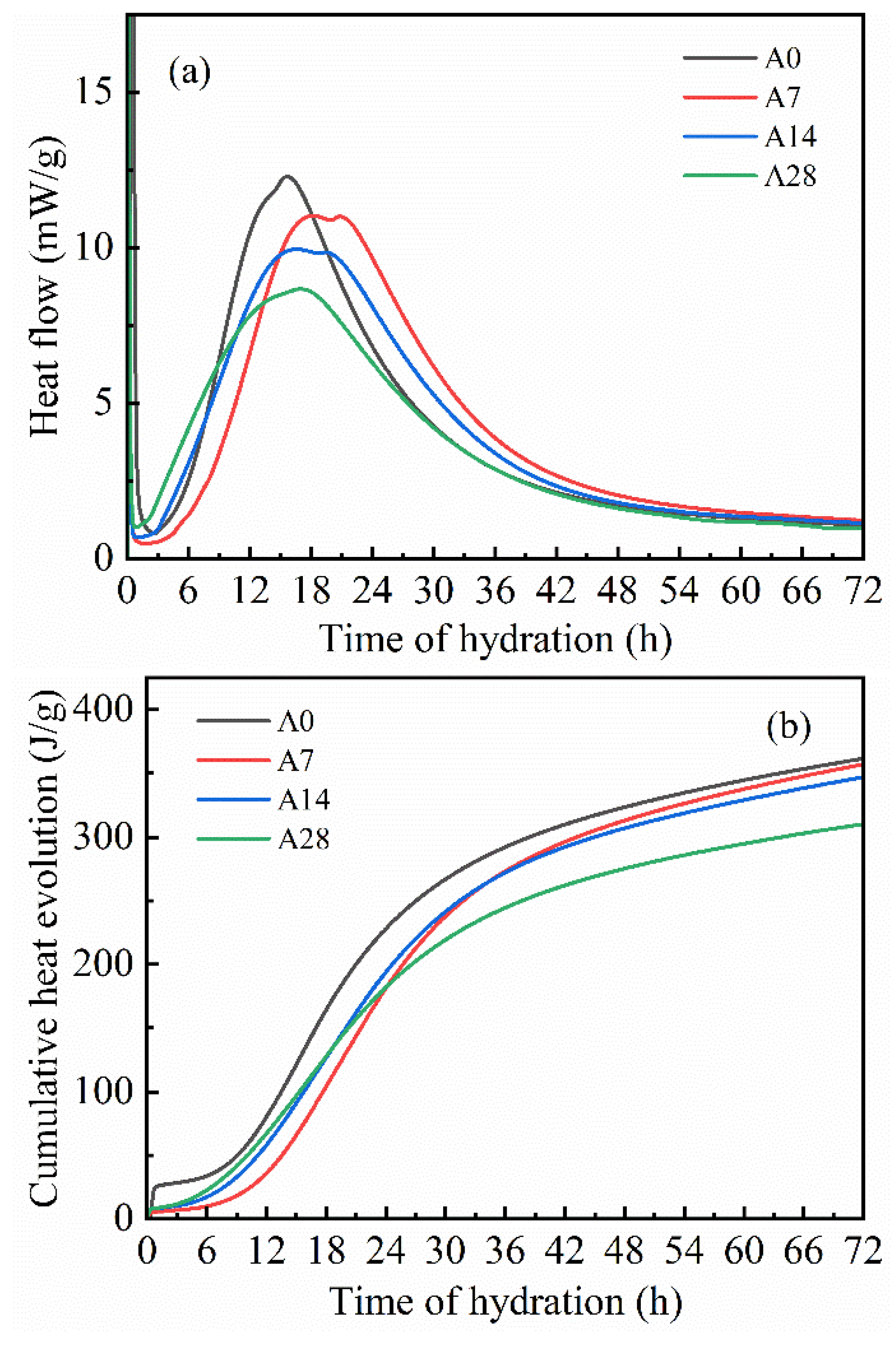
3.4. Influence of Aging on the Hydration Behavior of Oil Well Cement
4. Conclusions and Observations
4.1. Conclusions
- In an aging environment with 90% RH relative air humidity, the cement sorbed H2O and CO2 from the air, led to a reduction in the specific surface area and surface energy of the powder. The contents of the clinker phases C3S, C3A, and gypsum decreased, whereas the quantities of the hydration products AFt and CH increased. This process hindered the reaction between the cement and the water, leading to a decrease in both the hydration rate and heat release.
- The compressive strength, thickening time, and rheological properties of aged cement were affected. As the hydration of the cement slowed, the surface energy of the cement powder decreased, which negatively impacted the compressive strength. The reduction in the gypsum content was insufficient to adequately limit the hydration of C3A, leading to a shortened thickening time. Additionally, the increase in hydration products increased the friction between the particles, further degrading the rheological properties.
- For Class G oil well cement aged in an environment with 90% relative humidity and at a temperature of 25 °C, the permissible time to avoid significant changes in cement properties should be less than 7 d, with a maximum aging period not exceeding 14 d. Furthermore, at a usage temperature of 80 °C, the performance of the cement paste aged for 28 d was found to be inadequate to meet the necessary operation requirements.
4.2. Observation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, W.; Bao, B. Trend and progress in global oil and gas exploration. Pet. Explor. Dev. 2013, 40, 439–443. [Google Scholar] [CrossRef]
- Dubina, E.; Wadsö, L.; Plank, J. A sorption balance study of water vapour sorption on anhydrous cement minerals and cement constituents. Cem. Concr. Res. 2011, 41, 1196–1204. [Google Scholar] [CrossRef]
- Mounanga, P.; Khelidj, A.; Loukili, A.; Baroghel-Bouny, V. Predicting Ca(OH)2 content and chemical shrinkage of hydrating cement pastes using analytical approach. Cem. Concr. Res. 2004, 34, 255–265. [Google Scholar] [CrossRef]
- Flatt, R.J.; Scherer, G.W.; Bullard, J.W. Why alite stops hydrating below 80% relative humidity. Cem. Concr. Res. 2011, 41, 987–992. [Google Scholar] [CrossRef]
- Wu, S.; Wan, J.; Tian, Z.; Qu, T. Research on logistics simulation of bulk cement terminal handling system. Port Water. Eng. 2022, 12, 210–214+231. [Google Scholar] [CrossRef]
- Huang, M.; Du, H.; Wang, Q.; Wang, L. Extreme wind speed prediction of typhoon for southeast coastal cities and offshore areas of China. J. Build. Str. 2024, 45, 104–114. [Google Scholar] [CrossRef]
- Hartmann, F.A.; Plank, J. New insights into the effects of aging on Portland cement hydration and on retarder performance. Constr. Build. Mater. 2021, 274, 122104. [Google Scholar] [CrossRef]
- Wistuba, S.; Stephan, D.; Raudaschl-Sieber, G.; Plank, J. Hydration and hydration products of two-phase Portland cement clinker doped with Na2O. Adv. Cem. Res. 2007, 19, 125–131. [Google Scholar] [CrossRef]
- Meier, M.R.; Napharatsamee, T.; Plank, J. Dispersing performance of superplasticizers admixed to aged cement. Constr. Build. Mater. 2017, 139, 232–240. [Google Scholar] [CrossRef]
- Myers, R.J.; Geng, G.; Rodriguez, E.D.; Rosa, P.D.; Kirchheim, A.P.; Monteiro, P.J.M. Solution chemistry of cubic and orthorhombic tricalcium aluminate hydration. Cem. Concr. Res. 2017, 100, 176–185. [Google Scholar] [CrossRef]
- Quennoz, A.; Scrivener, K.L. Interactions between alite and C3A-gypsum hydrations in model cements. Cem. Concr. Res. 2013, 44, 46–54. [Google Scholar] [CrossRef]
- Dubina, E.; Black, L.; Sieber, R.; Plank, J. Interaction of water vapour with anhydrous cement minerals. Adv. Appl. Ceram. 2013, 109, 260–268. [Google Scholar] [CrossRef]
- Black, L.; Garbev, K.; Gee, I. Surface carbonation of synthetic C-S-H samples: A comparison between fresh and aged C-S-H using X-ray photoelectron spectroscopy. Cem. Concr. Res. 2008, 38, 745–750. [Google Scholar] [CrossRef]
- Stoian, J.; Oey, T.; Bullard, J.W.; Huang, J.; Kumar, A.; Balonis, M.; Terrill, J.; Neithalath, N.; Sant, G. New insights into the prehydration of cement and its mitigation. Cem. Concr. Res. 2015, 70, 94–103. [Google Scholar] [CrossRef]
- Scherer, G.W.; Zhang, J.; Thomas, J.J. Nucleation and growth models for hydration of cement. Cem. Concr. Res. 2012, 42, 982–993. [Google Scholar] [CrossRef]
- Nicoleau, L.; Nonat, A. A new view on the kinetics of tricalcium silicate hydration. Cem. Concr. Res. 2016, 86, 1–11. [Google Scholar] [CrossRef]
- API RP 10B-2-2013; Recommended Practice for Testing Well Cements. American Petroleum Institute: Washington, DC, USA, 2013.
- GB/T 8077-2023; Methods for Testing Uniformity of Concrete Admixtures. Standardization Administration of China: Beijing, China, 2023.
- Li, D.; Lin, L.; Wang, Y.; Yang, G. The effect of test conditions on the partical size distribution analtsis in the laser partical size analyzer. Res. Expl. Lab. 2024, 43, 32–35. [Google Scholar] [CrossRef]
- GB/T 8074-2008; Testing Method for Specific Surface of Cement-Blaine Method. Standardization Administration of China: Beijing, China, 2008.
- GB/T 10238-2015; Oil Well Cement. Standardization Administration of China: Beijing, China, 2015.
- SY/T 5504.1; Evaluation Method of Well Cement Additives-Part 1: Retarder. National Energy Administration: Beijing, China, 2014.
- Sun, F.; Pang, X.; Kawashima, S.; Cheng, G.; Guo, S.; Bu, Y. Effect of tartaric acid on the hydration of oil well cement at elevated temperatures between 60 °C and 89 °C. Cem. Concr. Res. 2022, 101, 106952. [Google Scholar] [CrossRef]
- Dai, X.; Aydin, S.; Yardimci, M.Y.; Lesage, K.; Schutter, G.D. Influence of water to binder ratio on the rheology and structural build-up of alkali-activated slag/fly ash mixtures. Constr. Build. Mater. 2020, 264, 120253. [Google Scholar] [CrossRef]
- Dubina, E.; Plank, J.; Black, L.; Wadsö, L. Impact of environmental moisture on C3A polymorphs in the absence and presence of CaSO4·0.5H2O. Adv. Cem. Res. 2014, 26, 29–40. [Google Scholar] [CrossRef]
- Quennoz, A.; Scrivener, K.L. Hydration of C3A–gypsum systems. Cem. Concr. Res. 2012, 42, 1032–1041. [Google Scholar] [CrossRef]
- Naber, C.; Bellmann, F.; Sowoidnich, T.; Goetz-Neunhoeffer, F.; Neubauer, J. Alite dissolution and C-S-H precipitation rates during hydration. Cem. Concr. Res. 2019, 115, 283–293. [Google Scholar] [CrossRef]
- Fu, M.; Zou, X.; Zhang, W.; Guo, G.; Huang, K. Technical measures to reduce the initial consistency of G-grade high sulfur resistant oil well cement. Cem. Eng. 2021, 5, 28–30. [Google Scholar] [CrossRef]
- Plank, J.; Zhimin, D.; Keller, H.; Hössle, F.; Seidl, W. Fundamental mechanisms for polycarboxylate intercalation into C3A hydrate phases and the role of sulfate present in cement. Cem. Concr. Res. 2010, 40, 45–57. [Google Scholar] [CrossRef]
- Huang, H.; Tian, H.; Wang, R.; Bu, Y. Study on the influential factor on cement slurry’s rheology. Oil Drill. Produc. Tech. 2005, 5, 46–48+97. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, H.; Yang, H.; Mu, R.; Chen, H. Rheological Properties of Nanosilica-Modified Cement Paste at Different Temperatures and Hydration Times. J. Mater. Civ. Eng. 2021, 33, 04020404. [Google Scholar] [CrossRef]


| SiO2 | Al2O3 | Fe2O3 | TiO2 | CaO | MgO | SO3 | K2O | Na2O | LOI |
|---|---|---|---|---|---|---|---|---|---|
| 21.47 | 4.11 | 5.48 | 0.35 | 62.75 | 1.34 | 2.24 | 0.48 | 0.05 | 1.07 |
| C3S | C2S | C3A | C4AF | CaSO4·2H2O | CaSO4·0.5H2O | CH |
|---|---|---|---|---|---|---|
| 60.0 | 20.1 | 1.2 | 15.8 | 2.2 | 0.3 | 0.4 |
| Cement Sample | Curing Time (d) | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 7 | 28 | |
| A0 | 26.8 ± 1.2 | 32.5 ± 1.3 | 37.2 ± 1.7 | 40.1 ± 1.4 | 35.8 ± 1.6 |
| A7 | 21.8 ± 1.5 | 24.7 ±1.4 | 25.2 ± 1.6 | 32.1 ± 1.3 | 30.5 ± 1.8 |
| A14 | 19.6 ± 1.2 | 23.0 ± 1.3 | 26.7 ± 1.5 | 29.8 ± 1.9 | 29.8 ± 0.8 |
| A28 | 18.9 ± 0.9 | 24.1 ± 0.7 | 24.6 ± 1.5 | 26.8 ± 0.4 | 25.9 ± 0.8 |
| Cement Sample | Rheological Model | Fitting Function | R2 | µp (Pa·s) | τ0 (Pa) | Fluidity (cm, 25 °C) |
|---|---|---|---|---|---|---|
| A0 | Bingham fluid | τ = 0.068γ + 0.920 | 0.995 | 0.068 | 0.920 | 19.0 |
| A7 | Bingham fluid | τ = 0.077γ + 1.333 | 0.999 | 0.077 | 1.333 | 19.0 |
| A14 | Bingham fluid | τ = 0.091γ + 2.467 | 0.998 | 0.091 | 2.467 | 18.0 |
| A28 | Bingham fluid | τ = 0.176γ + 12.864 | 0.947 | 0.176 | 12.864 | 16.0 |
| Cement Sample | Partical Size (μm) | Specific Surface Area (m2·kg−1) | ||
|---|---|---|---|---|
| d10 | d50 | d90 | ||
| A0 | 2.54 | 15.15 | 36.69 | 375 |
| A28 | 3.66 | 16.56 | 37.55 | 310 |
| Cement Sample | C3S | C2S | C3A | C4AF | CaSO4·0.5H2O | CaSO4·2H2O | AFt | CH | CaCO3 |
|---|---|---|---|---|---|---|---|---|---|
| A0 | 60.0 | 20.1 | 1.2 | 15.8 | 0.3 | 2.2 | 0.0 | 0.4 | 0.0 |
| A7 | 59.6 | 20.4 | 1.1 | 15.6 | 0.0 | 2.0 | 0.5 | 0.8 | 0.0 |
| A14 | 59.0 | 20.7 | 0.9 | 15.5 | 0.0 | 1.6 | 1.2 | 1.1 | 0.1 |
| A28 | 58.9 | 20.5 | 0.7 | 15.5 | 0.0 | 1.6 | 1.5 | 1.2 | 0.1 |
| Temperature (°C) | Mass Loss (%) | Added Mass (%) | Origins of Mass Loss | |
|---|---|---|---|---|
| A0 | A28 | |||
| 25~140 | 0.50 | 1.00 | 0.50 | Physically adsorbed water |
| 140~400 | 0.10 | 0.60 | 0.50 | Decomposition of calcium sulfate hemihydrate |
| 400~550 | 0.20 | 0.30 | 0.10 | Decomposition of calcium hydroxide and calcium aluminosulfate hydrate |
| 550~1000 | 0.60 | 0.85 | 0.25 | Decomposition of calcium carbonate and C-S-H |
| 25~1000 | 1.40 | 2.75 | 1.35 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, Y.; Gao, Z.; Geng, C.; Yao, X.; Lu, D. Effect of Aging on Class G High Sulfate-Resistant Oil Well Cement Under High Relative Air Humidity. Appl. Sci. 2025, 15, 4371. https://doi.org/10.3390/app15084371
Lai Y, Gao Z, Geng C, Yao X, Lu D. Effect of Aging on Class G High Sulfate-Resistant Oil Well Cement Under High Relative Air Humidity. Applied Sciences. 2025; 15(8):4371. https://doi.org/10.3390/app15084371
Chicago/Turabian StyleLai, Yang, Zixuan Gao, Chenzi Geng, Xiao Yao, and Duyou Lu. 2025. "Effect of Aging on Class G High Sulfate-Resistant Oil Well Cement Under High Relative Air Humidity" Applied Sciences 15, no. 8: 4371. https://doi.org/10.3390/app15084371
APA StyleLai, Y., Gao, Z., Geng, C., Yao, X., & Lu, D. (2025). Effect of Aging on Class G High Sulfate-Resistant Oil Well Cement Under High Relative Air Humidity. Applied Sciences, 15(8), 4371. https://doi.org/10.3390/app15084371




