Mathematical Correlations for Volumetric (Density and Specific Gravity) Properties of Diesel/Biodiesel Blends
Abstract
:1. Introduction
2. Experimental Study
| Parameter | Method—Reference | Value | Method | Value | Method | Value |
|---|---|---|---|---|---|---|
| Diesel | Biodiesel Vegetable (Sunflower) | Biodiesel Animal Fat | ||||
| Density 15°C (g/mL) | ISO 12185 [32] | 0.821 | ISO 12185 [32] | 0.884 | D1298 | 0.870 |
| Ignition point (°C) | ISO 2719 [33] | 56.0 | ISO 3679 [34] | 167 | D93 | 172 |
| Kinematic Viscosity (cSt) | ISO 3104 [35] | 2.393 | ISO 3104 [35] | 6.0 | D445 | 6.0 |
| Cetane index | ISO 4264 [36] | 55.1 | ISO 5165 [37] | 52.3 | D613 | 52.5 |
| Water Κ-F in products (mg/kg) | ISO 12937 [38] | 105 | ISO 14214 [39] | 187 | D6304 | 287 |
| Conductivity (pS/cm) | ISO 6297 [40] | 2 | ISO 6297 [40] | 4 | D2624 | 122 |
| Acidity (mgKOH/gr) | ISO 660 [41] | 0.03 | EN 14104 [42] | 0.24 | D1980 | 0.92 |
3. Mathematical Modelling
3.1. Kay’s Mixing Rule for Density Behaviour in Biodiesel/Diesel Blends
3.2. Tammann–Tait Equation for Density Estimation of Blends Versus Temperature
3.3. Empirical Equation for Density Estimation of Blends Versus Temperature
3.4. Specific Gravity (Sg) and API Behaviour Versus Temperature
4. Theoretical Estimations
4.1. Theoretical Estimations Tool
4.2. Experimental Data for Parameter Estimation
4.3. Evaluation of the Model
5. Results and Discussion
5.1. Density Evaluation for Biodiesel/Diesel Blends at Constant Temperature
5.2. Density Evaluation for Biodiesel/Diesel Blends at Varying Temperatures Using Tammann–Tait Equation
5.3. Density Evaluation for Biodiesel/Diesel Blends at Varying Temperatures Using Empirical Equation
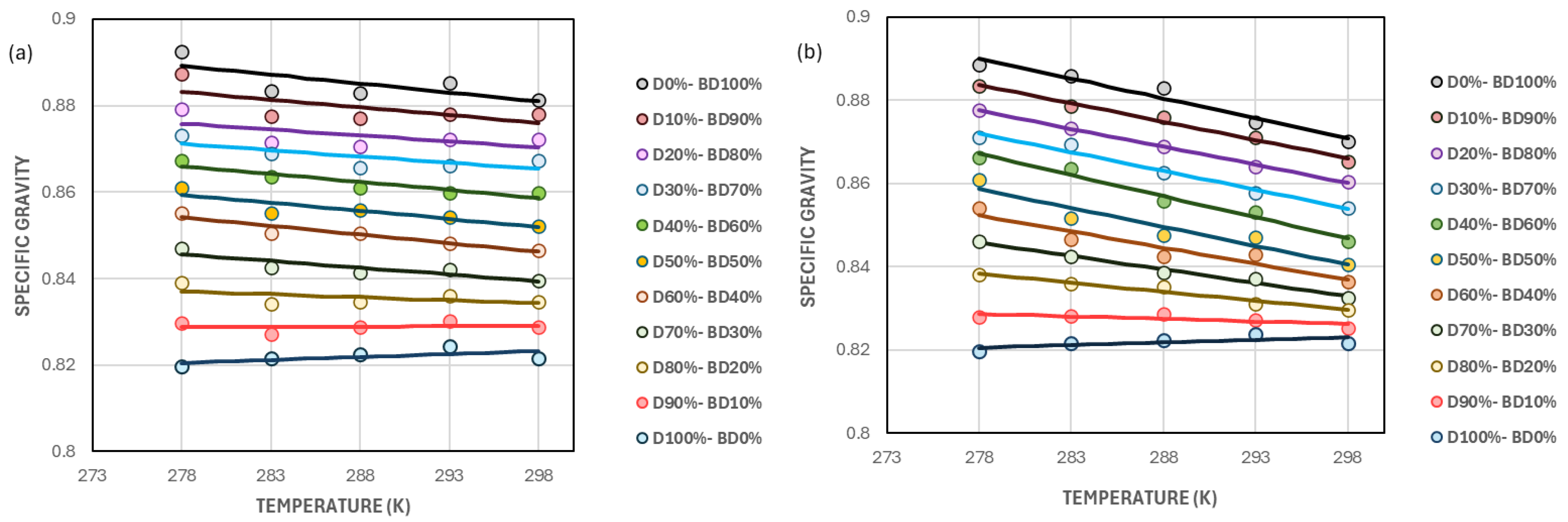
5.4. Estimation of Specific Gravity for Biodiesel/Diesel Blends at Varying Temperatures Using Empirical Equation
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Canabarro, N.I.; Silva-Ortiz, P.; Nogueira, L.A.H.; Cantarella, H.; Maciel-Filho, R.; Souza, G.M. Sustainability Assessment of Ethanol and Biodiesel Production in Argentina, Brazil, Colombia, and Guatemala. Renew. Sustain. Energy Rev. 2023, 171, 113019. [Google Scholar] [CrossRef]
- Aljaafari, A.; Fattah, I.M.R.; Jahirul, M.I.; Gu, Y.; Mahlia, T.M.I.; Islam, M.A.; Islam, M.S. Biodiesel Emissions: A State-of-the-Art Review on Health and Environmental Impacts. Energies 2022, 15, 6854. [Google Scholar] [CrossRef]
- Vasileiadis, V.; Kyriklidis, M.E.; Kyriklidis, C.; Loizou, E.; Tsanaktsidis, C.G. New Biodiesel Production from Diesel and Vegetables Sources Biodiesel by Evolutionary Computation Application. In Proceedings of the 15th International Conference Economies of the Balkan and Eastern European Countries, Chios, Greece, 12–14 May 2023. [Google Scholar]
- Farouk, S.M.; Tayeb, A.M.; Abdel-Hamid, S.M.S.; Osman, R.M. Recent Advances in Transesterification for Sustainable Biodiesel Production, Challenges, and Prospects: A Comprehensive Review. Environ. Sci. Pollut. Res. 2024, 31, 12722–12747. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, P.; Haider, M.B.; Yusuf, M.; Klemeš, J.J.; Bokhari, A.; Beg, M.; Al-Othman, A.; Kumar, R.; Jaiswal, A.K. A Review on Latest Trends in Cleaner Biodiesel Production: Role of Feedstock, Production Methods, and Catalysts. J. Clean Prod. 2022, 355, 131588. [Google Scholar] [CrossRef]
- ul Haq, M.; Turab Jafry, A.; Ali, M.; Ajab, H.; Abbas, N.; Sajjad, U.; Hamid, K. Influence of Nano Additives on Diesel-Biodiesel Fuel Blends in Diesel Engine: A Spray, Performance, and Emissions Study. Energy Convers. Manag. X 2024, 23, 100574. [Google Scholar] [CrossRef]
- Ahmad, F.B.; Kalam, M.A.; Zhang, Z.; Masjuki, H.H. Sustainable Production of Furan-Based Oxygenated Fuel Additives from Pentose-Rich Biomass Residues. Energy Convers. Manag. X 2022, 14, 100222. [Google Scholar] [CrossRef]
- Kyriklidis, C.; Kyriklidis, M.E.; Loizou, E.; Stimoniaris, A.; Tsanaktsidis, C.G. Optimal Bio Marine Fuel Production Evolutionary Computation: Genetic Algorithm Approach for Raw Materials Mixtures. Fuel 2022, 323, 124232. [Google Scholar] [CrossRef]
- Venkatesan, S.P.; Rahul, R.; Sabbharishi, V.; Purusothamand, M.; Ganesan, S. Study of Emission Characteristics of a Diesel Engine Run by Fuel Blends of Diesel, Jatropha Biodiesel and Cetane Improver. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Kyriklidis, M.E.; Kyriklidis, C.; Vasileiadis, V.; Loizou, E.; Tsanaktsidis, C.G. Can Biodiesel Become a Determinant Bioeconomy Factor towards Sustainability: Mixture Physicochemical Composition and Input-Output (I-O) Indicators of Biodiesel Sector. In Proceedings of the 2nd International Conference on Sustainable Chemical and Environmental Engineering (SUSTENG 2023), Online, 14–18 June 2023. [Google Scholar]
- Dias, J.; Alvim-Ferraz, M.; Almeida, M.; Díaz, J.; Sánchez-Polo, M.; Rivera-Utrilla, J. Selection of Heterogeneous Catalysts for Biodiesel Production from Animal Fat. Fuel 2011, 94, 418–425. [Google Scholar] [CrossRef]
- Ferreira, A.G.M.; Santos, J.B.; Baptista, J.; Khalighi, S.; Brito, R.M.M.; Cruz, P.F. Effect of Isobutanol Addition on the Biodiesel Density. J. Chem. Eng. Data 2021, 66, 4542–4562. [Google Scholar] [CrossRef]
- do Carmo, F.R.; da Silva, M.R.L.; Alves, A.A.A.; Evangelista, N.S. A New Method for Predicting the Isobaric Heat Capacity of Biodiesel-Related Esters Based on the Corresponding States Principle. Fluid Phase Equilibria 2020, 521, 112734. [Google Scholar] [CrossRef]
- Musthafa, B.; Asokan, M.A. Influence of Fuel Filterability, Performance, and Emission Characteristics of Dual Biodiesel Mixture with Diesel Blends in Compression Ignition Engine. Int. J. Automot. Technol. 2024. [Google Scholar] [CrossRef]
- Ahmed, S.; Warne, T.; Smith, E.; Goemann, H.; Linse, G.; Greenwood, M.; Kedziora, J.; Sapp, M.; Kraner, D.; Roemer, K.; et al. Systematic Review on Effects of Bioenergy from Edible versus Inedible Feedstocks on Food Security. NPJ Sci. Food 2021, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Gülüm, M.; Bilgin, A. Density, Flash Point and Heating Value Variations of Corn Oil Biodiesel-Diesel Fuel Blends. Fuel Process. Technol. 2015, 134, 456–464. [Google Scholar] [CrossRef]
- Dzida, M.; Prusakiewicz, P. The Effect of Temperature and Pressure on the Physicochemical Properties of Petroleum Diesel Oil and Biodiesel Fuel. Fuel 2008, 87, 1941–1948. [Google Scholar] [CrossRef]
- Freitas, S.V.D.; Segovia, J.J.; Carmen Martín, M.; Zambrano, J.; Oliveira, M.B.; Lima, Á.S.; Coutinho, J.A.P. Measurement and Prediction of High-Pressure Viscosities of Biodiesel Fuels. Fuel 2014, 122, 223–228. [Google Scholar] [CrossRef]
- Ivaniš, G.R.; Radović, I.R.; Veljković, V.B.; Kijevčanin, M.L. Biodiesel Density and Derived Thermodynamic Properties at High Pressures and Moderate Temperatures. Fuel 2016, 165, 244–251. [Google Scholar] [CrossRef]
- Hicks, W. NREL Research Points Path to Higher Blends of Biodiesel; National Renewable Energy Laboratory, U.S. Department of Energy, Office of Energy Efficiency and Renewable Energy: Washington, DC, USA, 2024. [Google Scholar]
- Zarrinkolah, M.T.; Hosseini, V. Detailed Analysis of the Effects of Biodiesel Fraction Increase on the Combustion Stability and Characteristics of a Reactivity- Controlled Compression Ignition Diesel-Biodiesel/Natural Gas Engine. Energies 2022, 15, 1094. [Google Scholar] [CrossRef]
- Payri, R.; Salvador, F.J.; Gimeno, J.; Bracho, G. The Effect of Temperature and Pressure on Thermodynamic Properties of Diesel and Biodiesel Fuels. Fuel 2011, 90, 1172–1180. [Google Scholar] [CrossRef]
- Yang, Z.; Shah, K.; Pilon-McCullough, C.; Faragher, R.; Azmi, P.; Hollebone, B.; Fieldhouse, B.; Yang, C.; Dey, D.; Lambert, P.; et al. Characterization of Renewable Diesel, Petroleum Diesel and Renewable Diesel/Biodiesel/Petroleum Diesel Blends. Renew Energy 2024, 224, 120151. [Google Scholar] [CrossRef]
- Ivaniš, G.R.; Radović, I.R.; Veljković, V.B.; Kijevčanin, M.L. Thermodynamic Properties of Biodiesel and Petro-Diesel Blends at High Pressures and Temperatures. Experimental and Modeling. Fuel 2016, 184, 277–288. [Google Scholar] [CrossRef]
- Brakora, J.; Reitz, R.D. A Comprehensive Combustion Model for Biodiesel-Fueled Engine Simulations; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2013. [Google Scholar] [CrossRef]
- Alahmer, A.; Rezk, H.; Aladayleh, W.; Mostafa, A.O.; Abu-Zaid, M.; Alahmer, H.; Gomaa, M.R.; Alhussan, A.A.; Ghoniem, R.M. Modeling and Optimization of a Compression Ignition Engine Fueled with Biodiesel Blends for Performance Improvement. Mathematics 2022, 10, 420. [Google Scholar] [CrossRef]
- Bousbaa, H.; Kaid, N.; Alqahtani, S.; Maatki, C.; Naima, K.; Menni, Y.; Kolsi, L. Prediction and Simulation of Biodiesel Combustion in Diesel Engines: Evaluating Physicochemical Properties, Performance, and Emissions. Fire 2024, 7, 364. [Google Scholar] [CrossRef]
- Tat, M.E.; Van Gerpen, J.H. The Specific Gravity of Biodiesel and Its Blends with Diesel Fuel; Iowa State University: Ames, IA, USA, 2000; Volume 77. [Google Scholar]
- DIN ISO 650; Relative Density 60/60 Degrees F Hydrometers for General Purposes. ISO: Berlin, Germany, 1977.
- ASTM D 1298; Standard Test Method for Density, Relative Density (Specific Gravity), or API Gravity of Crude Petroleum and Liquid Petroleum Products by Hydrometer Method. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D6751-20a D6751-23; Standard Specification for Biodiesel Fuel Blend Stock (B100) for Middle Distillate Fuels. ASTM: West Conshohocken, PA, USA, 2023.
- ISO 12185:1996; Crude Petroleum and Petroleum Products—Determination of Density—Oscillating Umtube Method. ISO: Geneva, Switzerland, 1996.
- ISO 2719:2016; Determination of Flash Point—Pensky-Martens Closed Cup Method. ISO: Geneva, Switzerland, 2016.
- ISO 3679:2022; Determination of Flash Point—Method for Flash No-Flash and Flash Point by Small Scale Closed Cup Tester. ISO: Geneva, Switzerland, 2022.
- ISO 3104:2020; Petroleum Products—Transparent and Opaque Liquids—Determination of Kinematic Viscosity and Calculation of Dynamic Viscosity. ISO: Geneva, Switzerland, 2020.
- ISO 4264:2007; Petroleum Products—Calculation of Cetane Index of Middle-Distillate Fuels by the Four-Variable Equation. ISO: Geneva, Switzerland, 2007.
- ISO 5165:2020; Petroleum Products—Determination of the Ignition Quality of Diesel Fuels—Cetane Engine Method. ISO: Geneva, Switzerland, 2020.
- ISO 1937:2000; Petroleum Products—Determination of Water—Coulometric Karl Fischer Titration Method. ISO: Geneva, Switzerland, 2000.
- ISO 14214:2019; Liquid Petroleum Products—Fatty Acid Methyl esters (FAME) for Use in Diesel Engines and Heating Applications—Requirements and Test Methods. ISO: Geneva, Switzerland, 2019.
- ISO 6297:1997; Petreleum Products—Aviation and Distilate Fuels- Determination of Electrical Conductivity. ISO: Geneva, Switzerland, 1997.
- ISO 660:2020; Animal and Vegetable Fats and Oils- Determination of Acid Value and Acidity. ISO: Geneva, Switzerland, 2020.
- EN 14104:2003; Fat and Oil Derivatives—Fatty Acid Methyl Esters (FAME)—Determination of Acid Value. EN: Brussels, Belgium, 2003.
- Alves, A.A.A.; da Costa, M.F.L.; de Medeiros, L.H.G.; Medeiros, H.A.D.; Daridon, J.L.; de Sant’Ana, H.B.; Feitosa, F.X. Investigating the Thermophysical Properties of the 1-Butanol + Biodiesel System: The Impact of Pressure on Volumetric Characteristics. Fuel 2024, 371, 132076. [Google Scholar] [CrossRef]
- Tesfa, B.; Mishra, R.; Gu, F.; Powles, N. Prediction Models for Density and Viscosity of Biodiesel and Their Effects on Fuel Supply System in CI Engines. Renew Energy 2010, 35, 2752–2760. [Google Scholar] [CrossRef]
- Reichert, P. Aquasim 2.0-User Manual, Computer Program for the Identification and Simulation of Aquatic Systems; EAWAG: Dübendorf, Switzerland, 1998; ISBN 3-906484-16-5. [Google Scholar]
- Petzold, L. A description of DASSL: A differential/algebraic system solver. In Scientific Computing; Stepleman, R.E., Ed.; IMACS/North-Holland: Amsterdam, The Netherlands, 1983; pp. 65–68. [Google Scholar]
- Zhang, Y.; Lashermes, G.; Houot, S.; Doublet, J.; Steyer, J.P.; Zhu, Y.G.; Barriuso, E.; Garnier, P. Modelling of Organic Matter Dynamics during the Composting Process. Waste Manag. 2012, 32, 19–30. [Google Scholar] [CrossRef]
- Nash, J.E.; Sutcliffe, J. River Flow Forecasting Through Conceptual Models Part I—A Discussion of Principles; North-Holland Publishing Co.: Amsterdam, The Netherlands, 1970; Volume 10. [Google Scholar]

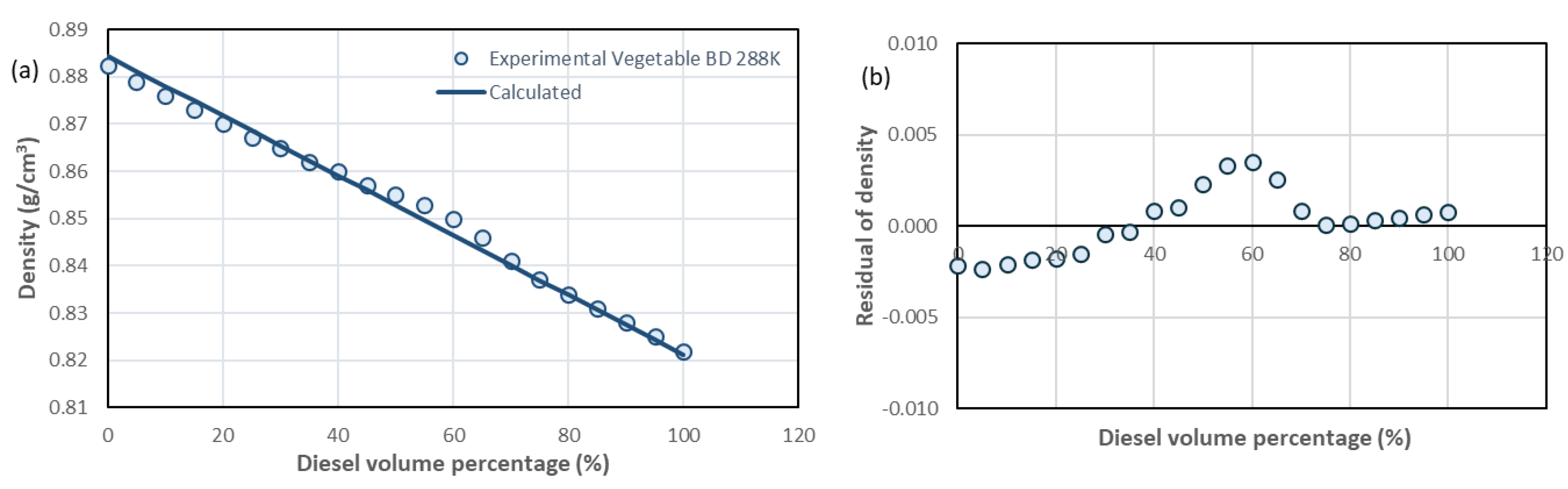
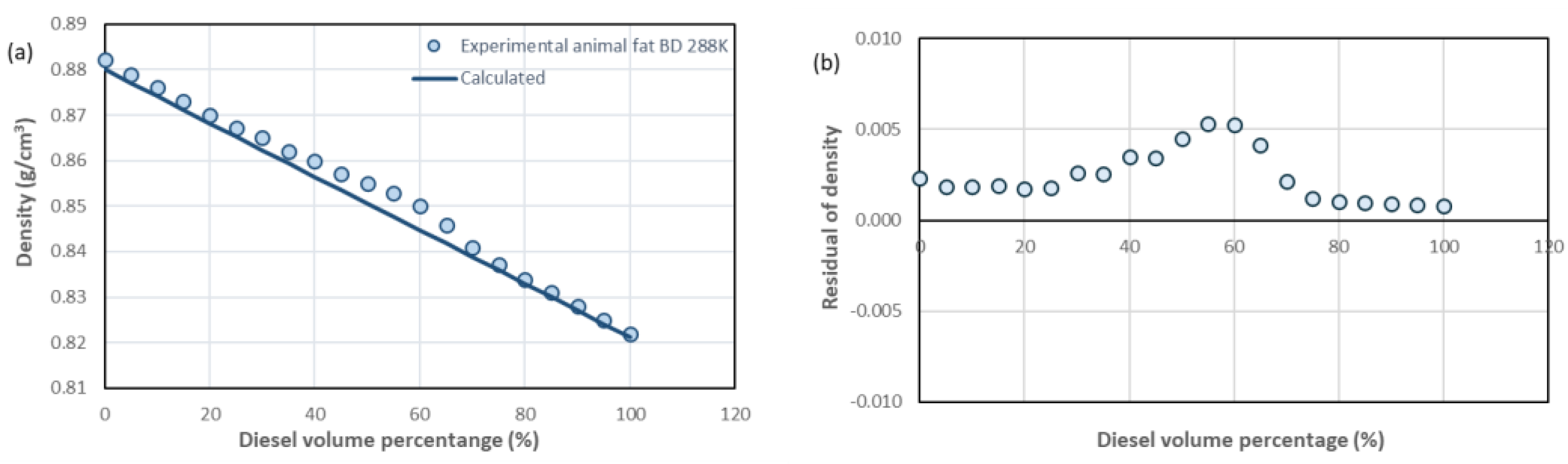
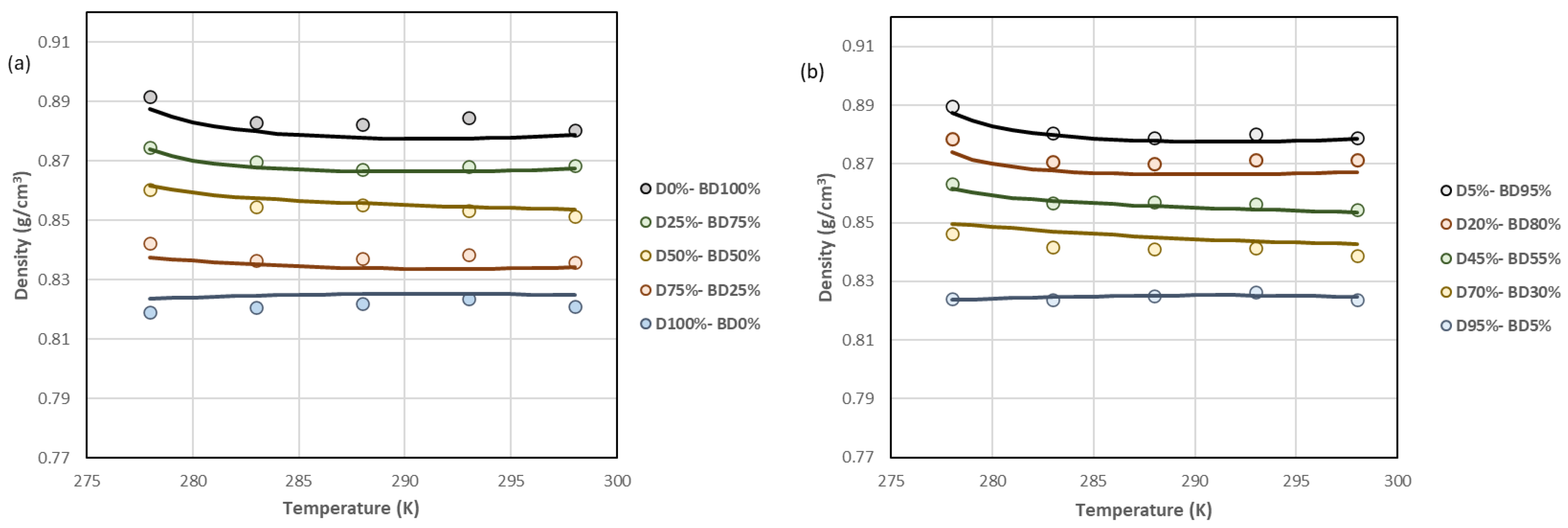
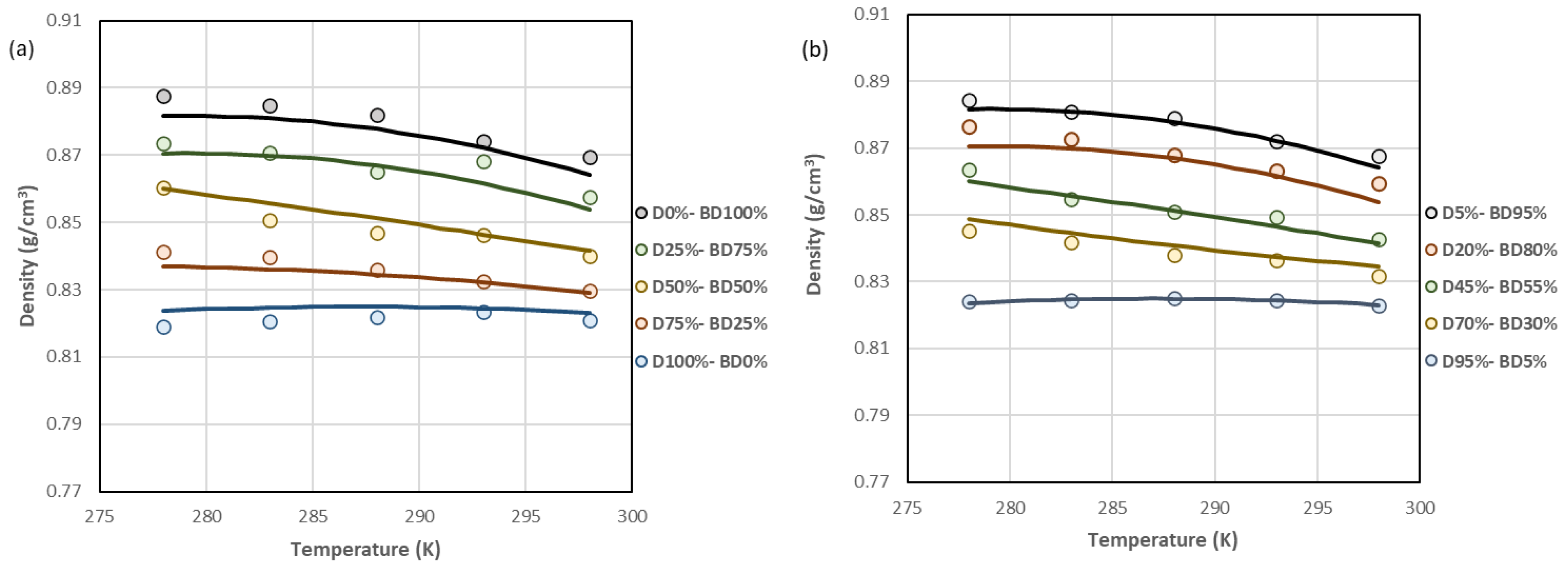

| D100%-BD0%, D95%-BD5%, D90%-BD10% * | D85%-BD15%, D80%-BD20%, D75%- BD25% | D70%-BD30%, D65%-BD35%, D60%-BD40% | D55%-BD45%, D50%-BD50%, D40%-BD60% D45%-BD55% | D35%-BD65%, D30%-BD70%, D25%-BD75%, D20%-BD80% | D15%-BD85%, D10%-BD90%, D5%-BD95%, D0%-BD100% | |
|---|---|---|---|---|---|---|
| Parameters | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 |
| a0 (gr·cm−3) | −0.1543 | 2.4919 | 1.8164 | 2.3069 | 4.5449 | 5.2909 |
| a1 (gr·cm−3·K−1) | 0.0067 | −0.0114 | −0.0064 | −0.0098 | −0.0253 | −0.0303 |
| a2 (gr·cm−3·K−2) | −1.14 × 10−5 | 1.95 × 10−5 | 1.05 × 10−5 | 1.64 × 10−5 | 4.34 × 10−5 | 5.19 × 10−5 |
| b0 (MPa) | 400 | 400 | 400 | 400 | 400 | 400 |
| b1 (MPa·K−1) | −1.31 | −1.31 | −1.31 | −1.31 | −1.31 | −1.31 |
| b2 (MPa·K−2) | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
| C | 0.0831 | 0.0831 | 0.0831 | 0.0831 | 0.0831 | 0.0831 |
| D100%-BD0%, D95%-BD5%, D90%-BD10% * | D85%-BD15%, D80%-BD20%, D75%-BD25% | D70%-BD30%, D65%-BD35%, D60%-BD40% | D55%-BD45%, D50%-BD50%, D40%-BD60% D45%-BD55% | D35%-BD65%, D30%-BD70%, D25%-BD75%, D20%-BD80% | D15%-BD85%, D10%-BD90%, D5%-BD95%, D0%-BD100% | |
|---|---|---|---|---|---|---|
| Parameters | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 |
| a0 (gr·cm−3) | −0.5089 | −0.3161 | 1.7982 | 0.7752 | −2.8150 | −2.9471 |
| a1 (gr·cm−3·K−1) | 0.0093 | 0.0084 | −0.0059 | 0.0015 | 0.0264 | 0.0274 |
| a2 (gr·cm−3·K−2) | −1.62 × 10−5 | −1.52 × 10−5 | 9.07 × 10−6 | −4.13 × 10−6 | −4.73 × 10−5 | −4.92 × 10−5 |
| b0 (MPa) | 400 | 400 | 400 | 400 | 400 | 400 |
| b1 (MPa·K−1) | −1.31 | −1.31 | −1.31 | −1.31 | −1.31 | −1.31 |
| b2 (MPa·K−2) | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
| C | 0.0831 | 0.0831 | 0.0831 | 0.0831 | 0.0831 | 0.0831 |
| Par. | D100%-BD 0% | D95%-BD5% | D90%-BD10% | D85%-BD15% | D80%-BD20% | D75%-BD25% | D70%-BD30% | D65%-BD35% | D60%-BD40% | D55%-BD45% |
|---|---|---|---|---|---|---|---|---|---|---|
| c | 0.8199 | 0.8244 | 0.8286 | 0.8335 | 0.8377 | 0.8427 | 0.8472 | 0.8515 | 0.8562 | 0.8586 |
| d | 0.0001 | 0.0001 | 0.0001 | −0.0001 | −0.0001 | −0.0004 | −0.0003 | −0.0003 | −0.0004 | −0.0004 |
| Par. | D50%-BD50% | D45%-BD55% | D40%-BD60% | D35%-BD65% | D30%-BD70% | D25%-BD75% | D20%-BD80% | D15%-BD85% | D10%-BD90% | D5%-BD95% |
| c | 0.8613 | 0.8637 | 0.8679 | 0.8707 | 0.8726 | 0.8744 | 0.8772 | 0.8813 | 0.8850 | 0.8888 |
| d | −0.0004 | −0.0004 | −0.0004 | −0.0004 | −0.0003 | −0.0003 | −0.0003 | −0.0003 | −0.0004 | −0.0004 |
| Par. | D100%-BD0% | D95%-BD5% | D90%-BD10% | D85%-BD15% | D80%-BD20% | D75%-BD25% | D70%-BD30% | D65%-BD35% | D60%-BD40% | D55%-BD45% |
|---|---|---|---|---|---|---|---|---|---|---|
| c | 0.8199 | 0.8258 | 0.8292 | 0.8358 | 0.8404 | 0.8456 | 0.8526 | 0.8526 | 0.8562 | 0.8605 |
| d | 0.0001 | −0.0001 | −0.0001 | −0.0003 | −0.0004 | −0.0006 | −0.0007 | −0.0007 | −0.0008 | −0.0009 |
| Par. | D50%-BD50% | D45%-BD55% | D40%-BD60% | D35%-BD65% | D30%-BD70% | D25%-BD75% | D20%-BD80% | D15%-BD85% | D10%-BD90% | D5%-BD95% |
| c | 0.8632 | 0.8668 | 0.8722 | 0.8756 | 0.8765 | 0.8789 | 0.8818 | 0.8845 | 0.8881 | 0.8901 |
| d | −0.0009 | −0.0009 | −0.0010 | −0.0010 | −0.0009 | −0.0009 | −0.0009 | −0.0009 | −0.0009 | −0.0008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasileiadis, V.; Papageorgiou, I.T.; Kyriklidis, C.; Vasiliadou, I.A.; Tsanaktsidis, C.G. Mathematical Correlations for Volumetric (Density and Specific Gravity) Properties of Diesel/Biodiesel Blends. Appl. Sci. 2025, 15, 4404. https://doi.org/10.3390/app15084404
Vasileiadis V, Papageorgiou IT, Kyriklidis C, Vasiliadou IA, Tsanaktsidis CG. Mathematical Correlations for Volumetric (Density and Specific Gravity) Properties of Diesel/Biodiesel Blends. Applied Sciences. 2025; 15(8):4404. https://doi.org/10.3390/app15084404
Chicago/Turabian StyleVasileiadis, Vasileios, Ioanna Th. Papageorgiou, Christos Kyriklidis, Ioanna A. Vasiliadou, and Constantinos G. Tsanaktsidis. 2025. "Mathematical Correlations for Volumetric (Density and Specific Gravity) Properties of Diesel/Biodiesel Blends" Applied Sciences 15, no. 8: 4404. https://doi.org/10.3390/app15084404
APA StyleVasileiadis, V., Papageorgiou, I. T., Kyriklidis, C., Vasiliadou, I. A., & Tsanaktsidis, C. G. (2025). Mathematical Correlations for Volumetric (Density and Specific Gravity) Properties of Diesel/Biodiesel Blends. Applied Sciences, 15(8), 4404. https://doi.org/10.3390/app15084404








