Development of Antifungal Packaging Based on Pectin/Gelatin Containing Azadirachta indica Bioactive Extracts for Carica papaya L. Fruit Coating
Abstract
1. Introduction
2. Materials and Methods
2.1. List of Materials
2.2. Obtaining and Preparation of Material Containing Antifungal Biocompounds
2.3. Extraction Processes
2.3.1. Pressurized Liquid Extraction (PLE)
2.3.2. Microwave-Assisted Extraction Process (MAE)
2.4. Determination of Polyphenols by Spectrophotometric Methods
2.4.1. Total Phenolic Compounds (TPC)
2.4.2. Total Flavonoid Content (TFC)
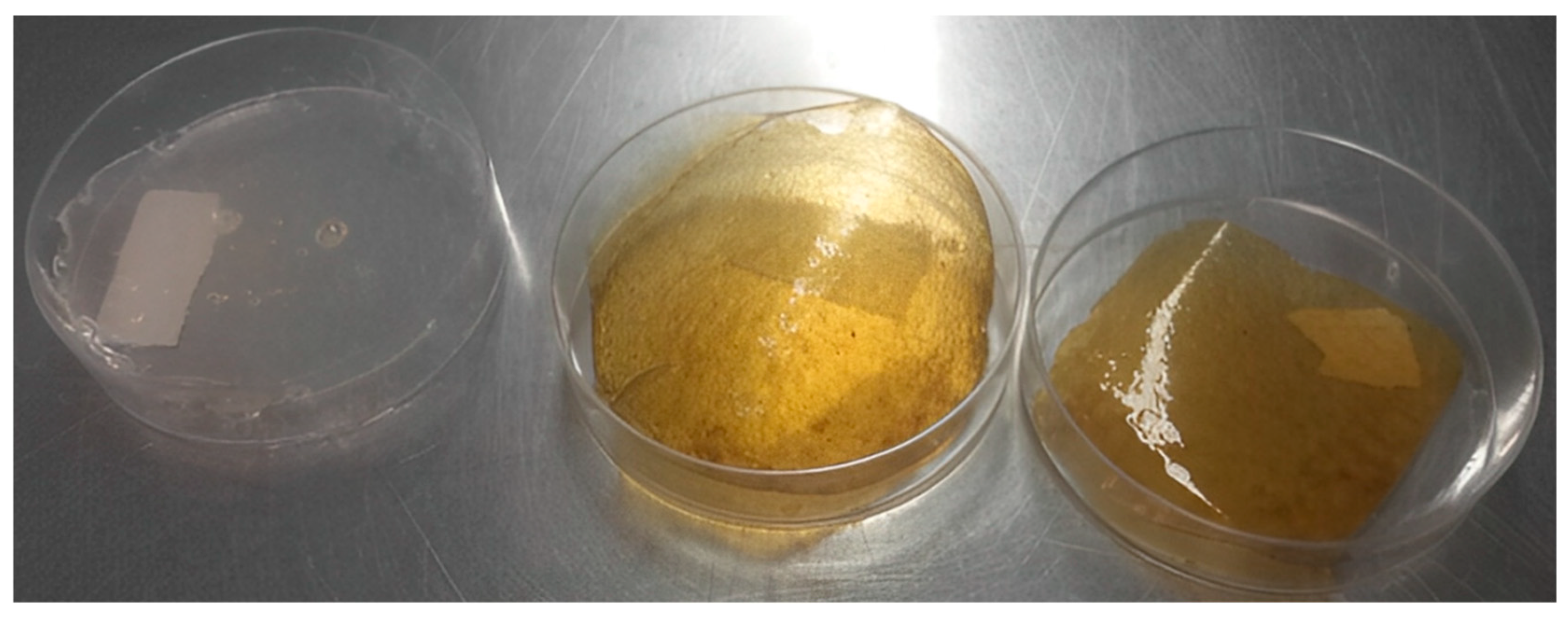
2.5. Development of the Antifungal Food Packaging
2.6. Characterization of the Food Packaging
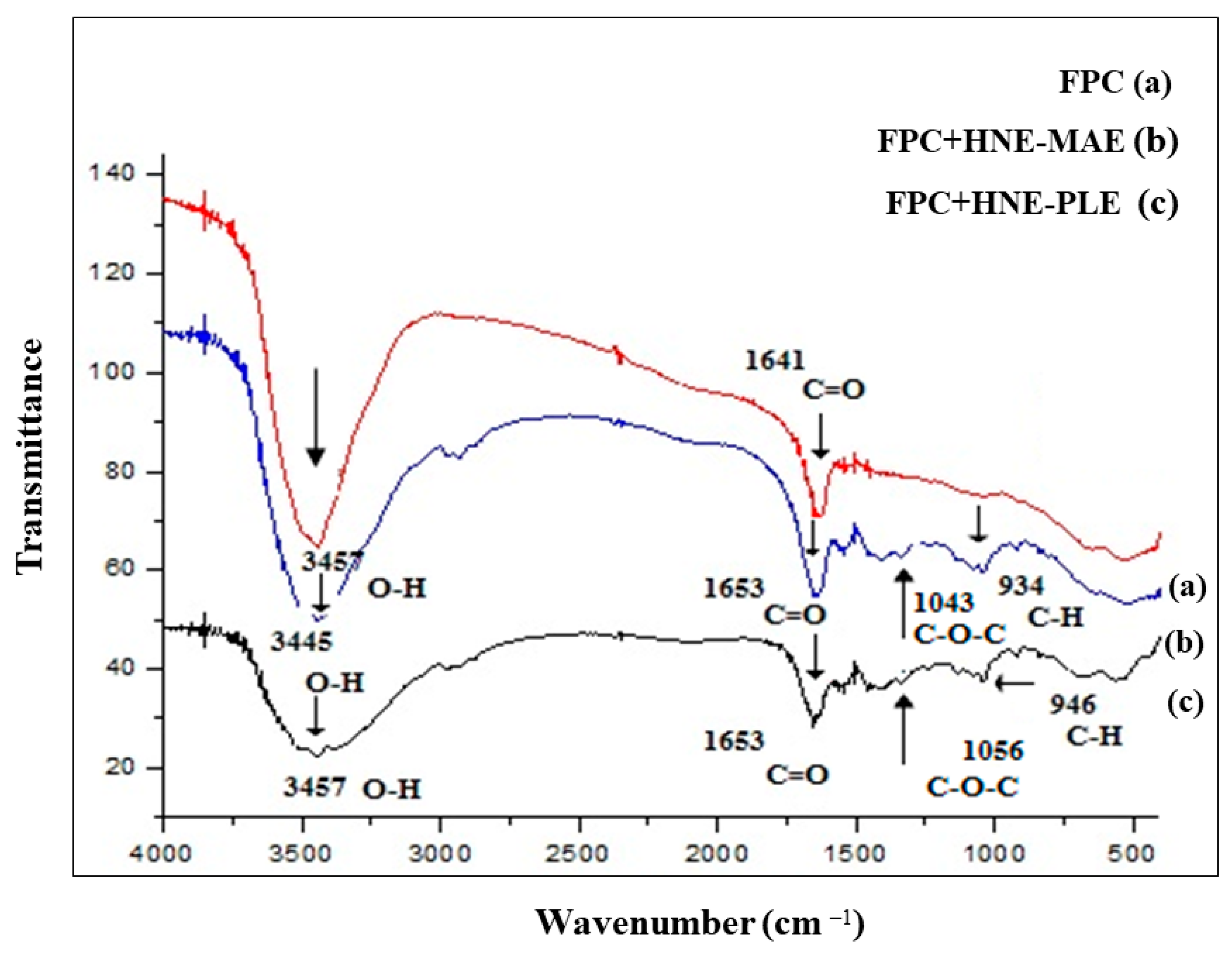
2.6.1. Fourier Transform Infrared (FTIR) Spectroscopy
2.6.2. Mechanical Properties
2.6.3. Water Vapor Permeability (WVP)
2.7. In Vitro Evaluation of Antifungal Food Packaging
2.8. Evaluation of Antifungal Packaging on Postharvest Quality of Papaya Fruit
2.9. Statistical Analysis
3. Results
3.1. Obtaining and Determination of Polyphenols from Neem
3.2. Fourier Transform Infrared (FTIR) Spectroscopy
3.3. Mechanical Properties and Water Vapor Permeability (WVP)
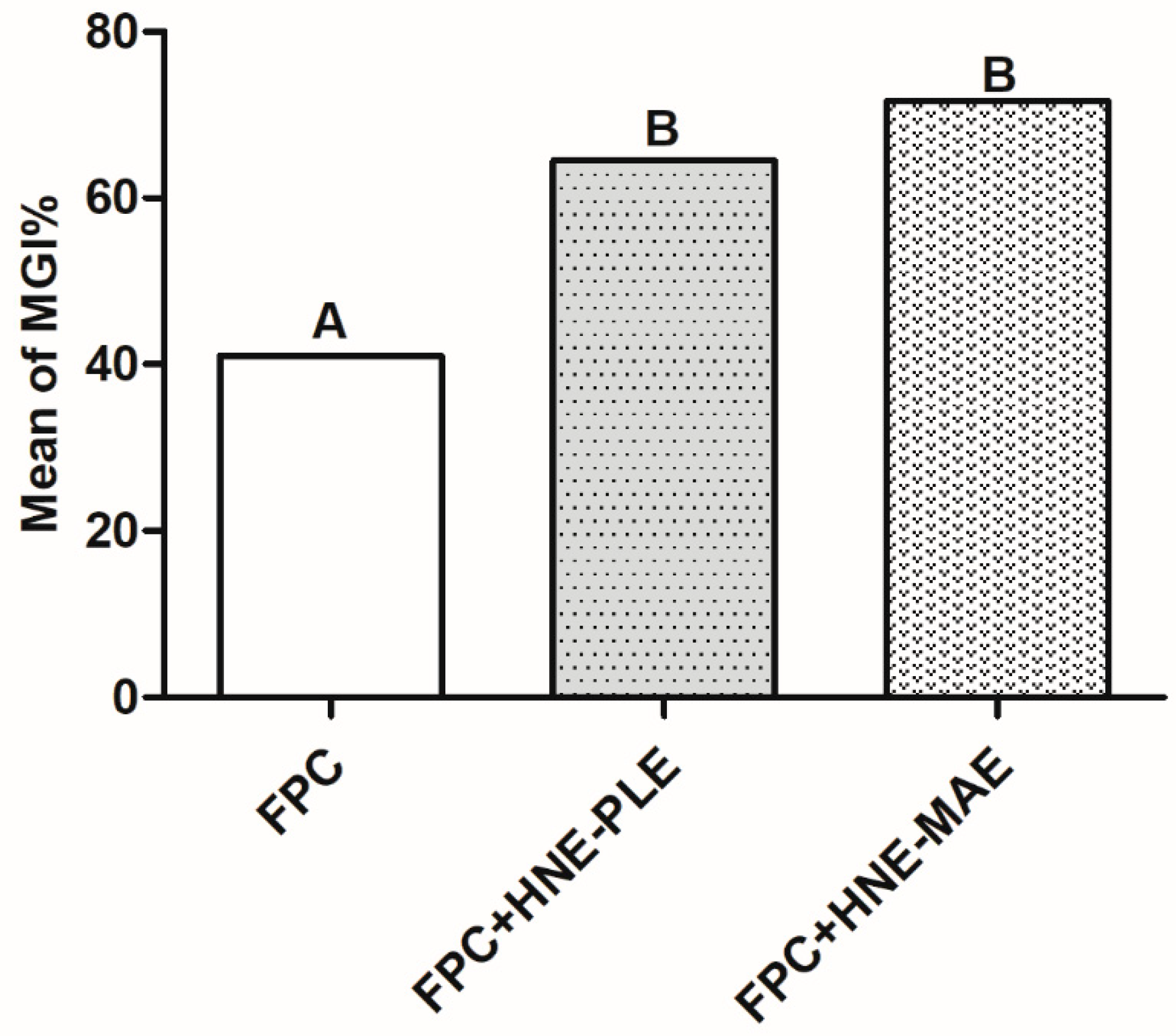
3.4. Antifungal Activity of Food Packaging
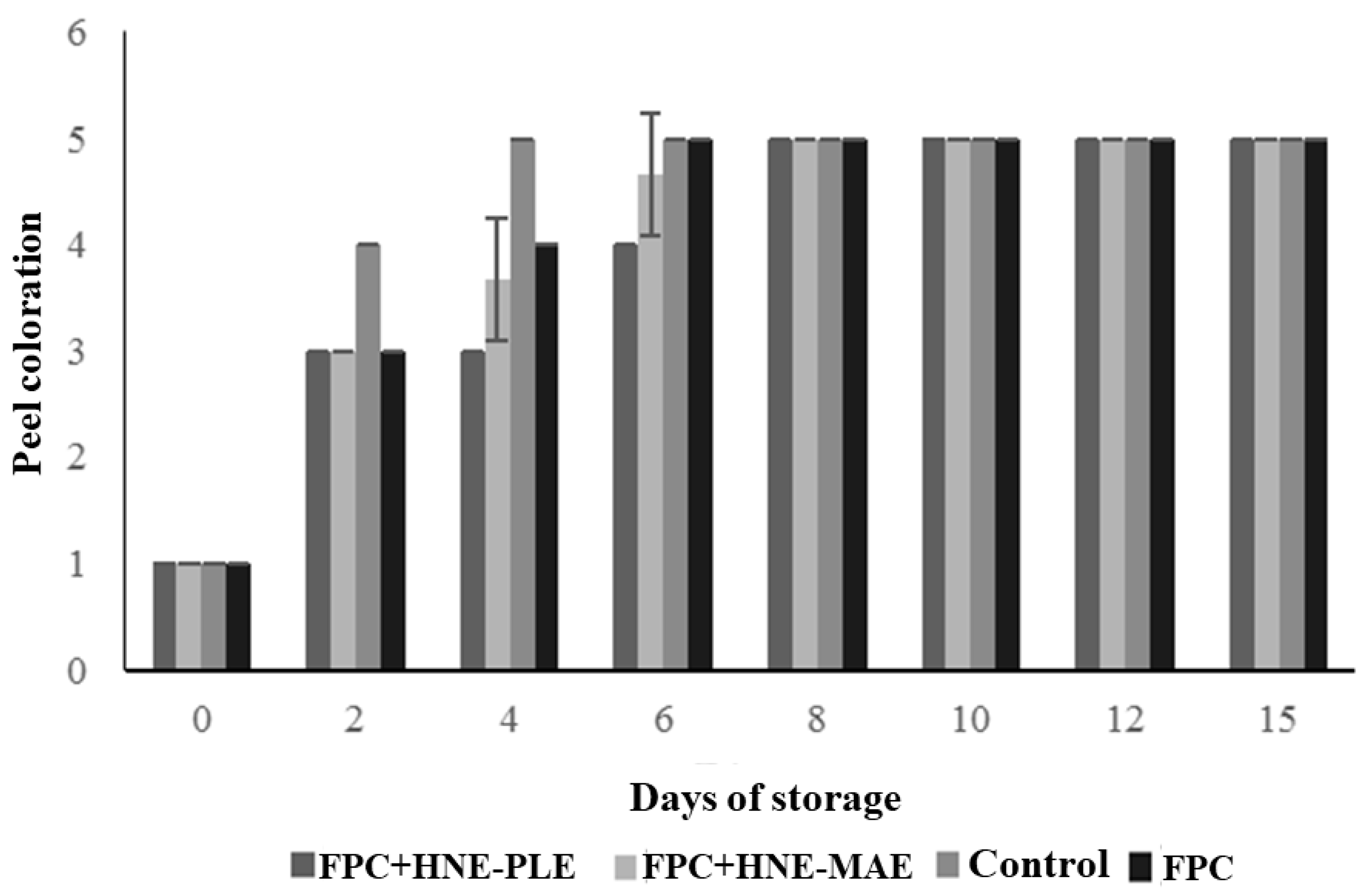
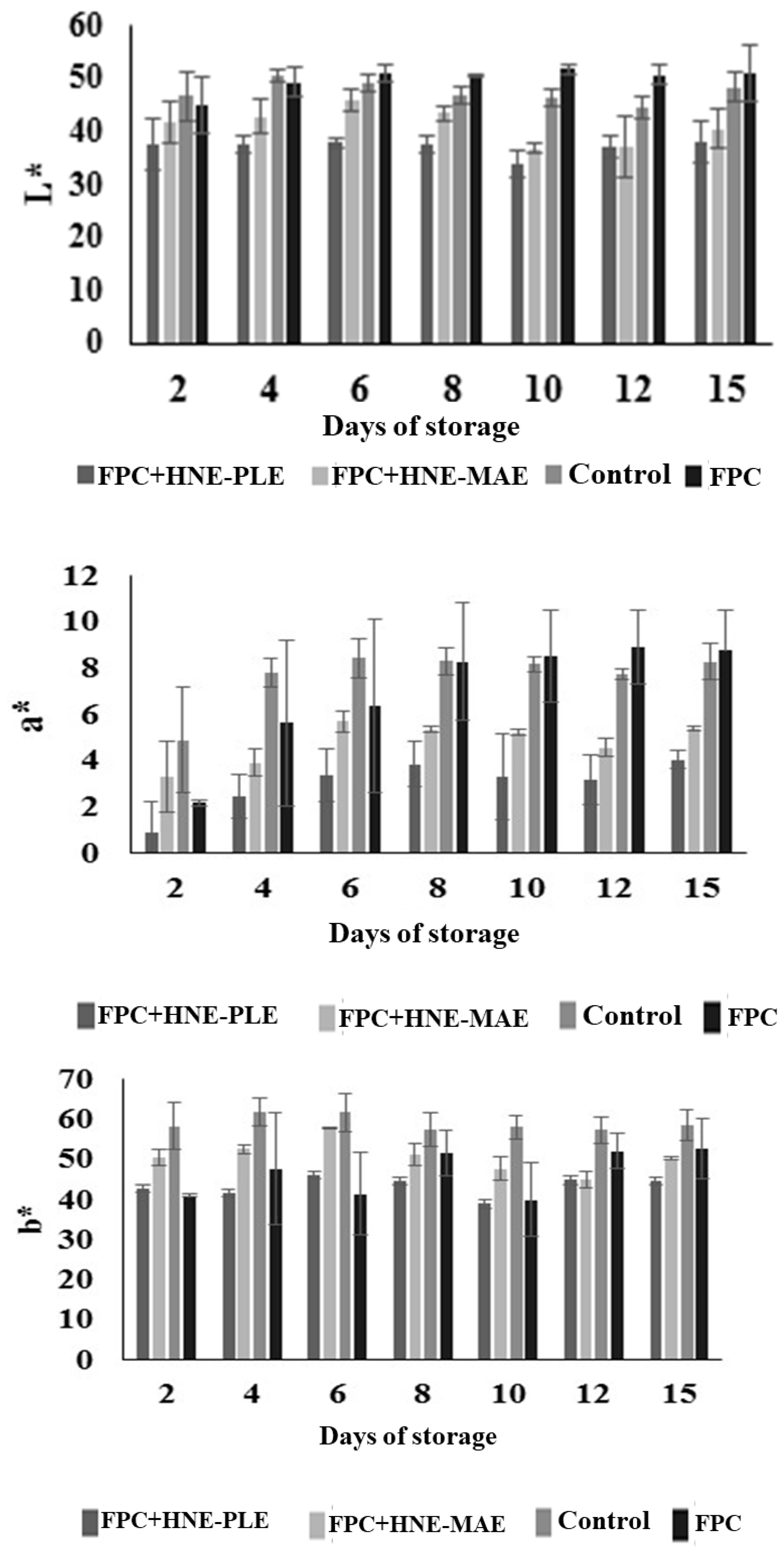
3.5. Evaluation of Antifungal Packaging on Postharvest Quality of Papaya Fruit
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PLE | Pressurized liquid extraction |
| MAE | Microwave-assisted extraction process |
| TPC | Total phenolic compounds |
| mg GAE/100 g | Milligrams of gallic acid equivalents per 100 g of extract |
| TFC | Total flavonoid contents |
| mg QE/100 g | Milligrams of quercetin equivalents per 100 g of extract |
| HNE-PLE | Hydroethanolic neem extracts obtained through PLE |
| HNE-MAE | Hydroethanolic neem extracts obtained through MAE |
| PC | Packaging control |
| HNE | Hydroethanolic neem extract |
| MAE | Microwave-assisted extraction |
| PEC | Pectin |
| GEL | Gelatin |
| PROP | Propylene glycol |
| VE | Vegetable extract |
| AA-0.5 | Acetic acid (0.5 mol/L) |
| TS | Tensile strength |
| EAB | Elongation at break |
| WVP | Water vapor permeability |
| MGI% | Mycelial growth inhibition percentage |
| OEY | Overall extraction yield |
| T | Thickness |
Appendix A

References
- Engel, J.B.; Ambrosi, A.; Tessaro, I.C. Development of Biodegradable Starch-Based Foams Incorporated with Grape Stalks for Food Packaging. Carbohydr. Polym. 2019, 225, 115234. [Google Scholar] [CrossRef] [PubMed]
- dos Muchangos, L.S.; Ito, L.; Tokai, A. Life Cycle Assessment of Plastic Waste Management in Mozambique. J. Mater. Cycles Waste Manag. 2024, 27, 624–637. [Google Scholar] [CrossRef]
- Zhao, X.; You, F. Decarbonizing Energy: Plastic Waste Trade for Zero Waste 2040. Adv. Appl. Energy 2025, 17, 100216. [Google Scholar] [CrossRef]
- Bergel, B.F.; da Luz, L.M.; Santana, R.M.C. Comparative Study of the Influence of Chitosan as Coating of Thermoplastic Starch Foam from Potato, Cassava and Corn Starch. Prog. Org. Coat. 2017, 106, 27–32. [Google Scholar] [CrossRef]
- Noorbakhsh-Soltani, S.M.; Zerafat, M.M.; Sabbaghi, S. A Comparative Study of Gelatin and Starch-Based Nano-Composite Films Modified by Nano-Cellulose and Chitosan for Food Packaging Applications. Carbohydr. Polym. 2018, 189, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Fang, C.; Wei, N.; Wei, J.; Feng, T.; Liu, F.; Liu, X.; Wu, B. Antimicrobial, Antioxidative, and UV-Blocking Pectin/Gelatin Food Packaging Films Incorporated with Tannic Acid and Silver Nanoparticles for Strawberry Preservation. Int. J. Biol. Macromol. 2025, 308, 142445. [Google Scholar] [CrossRef]
- Saikia, M.; Badwaik, L.S. Characterization and Antimicrobial Property of Casein, Gelatin and Pectin Based Active Composite Films. J. Packag. Technol. Res. 2018, 2, 233–242. [Google Scholar] [CrossRef]
- Šešlija, S.; Nešić, A.; Ružić, J.; Kalagasidis Krušić, M.; Veličković, S.; Avolio, R.; Santagata, G.; Malinconico, M. Edible Blend Films of Pectin and Poly(Ethylene Glycol): Preparation and Physico-Chemical Evaluation. Food Hydrocoll. 2018, 77, 494–501. [Google Scholar] [CrossRef]
- Kalita, P.; Bora, N.S.; Gogoi, B.; Goswami, A.; Pachuau, L.; Das, P.J.; Baishya, D.; Roy, S. Improving the Hydrophobic Nature of Biopolymer Based Edible Packaging Film: A Review. Food Chem. 2025, 479, 143793. [Google Scholar] [CrossRef]
- Bermúdez-Oria, A.; Rodríguez-Gutiérrez, G.; Rubio-Senent, F.; Fernández-Prior, Á.; Fernández-Bolaños, J. Effect of Edible Pectin-Fish Gelatin Films Containing the Olive Antioxidants Hydroxytyrosol and 3,4-Dihydroxyphenylglycol on Beef Meat during Refrigerated Storage. Meat Sci. 2019, 148, 213–218. [Google Scholar] [CrossRef]
- Gomez-Guillen, M.C.; Gimenez, B.; Lopez-Caballero, M.E.; Montero, M.P. Functional and Bioactive Properties of Collagen and Gelatin from Alternative Sources: A Review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Gupta, S.C.; Prasad, S.; Tyagi, A.K.; Kunnumakkara, A.B.; Aggarwal, B.B. Neem (Azadirachta Indica): An Indian Traditional Panacea with Modern Molecular Basis. Phytomedicine 2017, 34, 14–20. [Google Scholar] [CrossRef]
- Hossain, M.A.; Al-Toubi, W.A.S.; Weli, A.M.; Al-Riyami, Q.A.; Al-Sabahi, J.N. Identification and Characterization of Chemical Compounds in Different Crude Extracts from Leaves of Omani Neem. J. Taibah Univ. Sci. 2013, 7, 181–188. [Google Scholar] [CrossRef]
- Santos, K.; Barbosa, A.; Freitas, V.; Muniz, A.; Mendonça, M.; Calhelha, R.; Ferreira, I.; Franceschi, E.; Padilha, F.; Oliveira, M.; et al. Antiproliferative Activity of Neem Leaf Extracts Obtained by a Sequential Pressurized Liquid Extraction. Pharmaceuticals 2018, 11, 76. [Google Scholar] [CrossRef] [PubMed]
- Royani, A.; Hanafi, M.; Mubarak, N.M.; Aigbodion, V.S.; Manaf, A. Exploitation of Azadirachta Indica Extracts for Multifunctional: Antibacterial, Anticorrosive, Antioxidant Activities, and Its Chemical Constituents. Int. J. Environ. Sci. Technol. 2024, 22, 1549–1566. [Google Scholar] [CrossRef]
- Pastor, C.; Sánchez-González, L.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Physical and Antifungal Properties of Hydroxypropylmethylcellulose Based Films Containing Propolis as Affected by Moisture Content. Carbohydr. Polym. 2010, 82, 1174–1183. [Google Scholar] [CrossRef]
- Dudoit, A.; Mertz, C.; Chillet, M.; Cardinault, N.; Brat, P. Antifungal Activity of Brazilian Red Propolis Extract and Isolation of Bioactive Fractions by Thin-Layer Chromatography-Bioautography. Food Chem. 2020, 327, 127060. [Google Scholar] [CrossRef]
- Vergel-Alfonso, A.A.; Arias-Avelenda, R.; Casariego-Año, A.; Giménez, M.J.; Ruíz-Cruz, S.; López-Corona, B.E.; Del-Toro-Sánchez, C.L.; Gonzalez-Bravo, A.L.; Plascencia-Jatomea, M.; Menchaca-Armenta, M.; et al. Development and Characterization of Pectin and Beeswax-Based Coatings Enhanced with Anthocyanins and Its Antioxidant and Antifungal Properties. Processes 2025, 13, 542. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for Extraction of Bioactive Compounds from Plant Materials: A Review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Grisales-Mejía, J.F.; Cedeño-Fierro, V.; Ortega, J.P.; Torres-Castañeda, H.G.; Andrade-Mahecha, M.M.; Martínez-Correa, H.A.; Álvarez-Rivera, G.; Mendiola, J.A.; Cifuentes, A.; Ibañez, E. Advanced NADES-Based Extraction Processes for the Recovery of Phenolic Compounds from Hass Avocado Residues: A Sustainable Valorization Strategy. Sep. Purif. Technol. 2024, 351, 128104. [Google Scholar] [CrossRef]
- Elmas, E.; Şen, F.B.; Kublay, İ.Z.; Baş, Y.; Tüfekci, F.; Derman, H.; Bekdeşer, B.; Aşçı, Y.S.; Capanoglu, E.; Bener, M.; et al. Green Extraction of Antioxidants from Hazelnut By-Products Using Microwave-Assisted Extraction, Ultrasound-Assisted Extraction, and Pressurized Liquid Extraction. Food Bioproc Technol. 2025, 1–19. [Google Scholar] [CrossRef]
- Barbosa, A.M.; Santos, K.S.; Borges, G.R.; Muniz, A.V.C.S.; Mendonça, F.M.R.; Pinheiro, M.S.; Franceschi, E.; Dariva, C.; Padilha, F.F. Separation of Antibacterial Biocompounds from Hancornia Speciosa Leaves by a Sequential Process of Pressurized Liquid Extraction. Sep. Purif. Technol. 2019, 222, 390–395. [Google Scholar] [CrossRef]
- Chinnasamy, K.; Krishnan, N.K.; Balasubramaniam, M.; Balamurugan, R.; Lakshmanan, P.; Karuppasami, K.M.; Karuppannan, M.S.; Thiyagarajan, E.; Alagarswamy, S.; Muthusamy, S. Nutrient Formulation—A Sustainable Approach to Combat PRSV and Enhance Productivity in Papaya. Agriculture 2025, 15, 201. [Google Scholar] [CrossRef]
- Kosgei, J.C.; Asudi, G.O.; Ombwara, F.K.; Kariuki, L.W.; Rimberia, F.K. Evaluation of Growth and Yield of New Papaya (Carica papaya L.) Hybrid Lines and Their Performance against Moroccan Watermelon Mosaic Virus-Causing Disease. Biol. Divers. 2025; early view. [Google Scholar] [CrossRef]
- Ali, A.; Muhammad, M.T.M.; Sijam, K.; Siddiqui, Y. Effect of Chitosan Coatings on the Physicochemical Characteristics of Eksotika II Papaya (Carica papaya L.) Fruit during Cold Storage. Food Chem. 2011, 124, 620–626. [Google Scholar] [CrossRef]
- dos Passos Braga, S.; Lundgren, G.A.; Macedo, S.A.; Tavares, J.F.; dos Santos Vieira, W.A.; Câmara, M.P.S.; de Souza, E.L. Application of Coatings Formed by Chitosan and Mentha Essential Oils to Control Anthracnose Caused by Colletotrichum Gloesporioides and C. Brevisporum in Papaya (Carica papaya L.) Fruit. Int. J. Biol. Macromol. 2019, 139, 631–639. [Google Scholar] [CrossRef]
- Sharma, V. Evaluation of Incidence and Alternative Management of Post Harvest Fungal Diseases of Papaya Fruits (Carica papaya L.) in Western up. Int. J. Theor. Appl. Sci. 2015, 7, 6–12. [Google Scholar]
- Gao, J.; Zhang, S.; Xu, Y.; Zhang, J.; Wu, P.; Luo, L.; Jiang, L. Efficacy of Pterostilbene Inhibition of Postharvest Anthracnose on Papaya Fruit and Antifungal Mechanisms against Colletotrichum Gloeosporioides. Postharvest Biol. Technol. 2025, 221, 113304. [Google Scholar] [CrossRef]
- Santos, K.S.; Costa, C.; Bessa, M.J.; Teixeira, J.P.; Muniz, A.V.C.d.S.; Padilha, F.F.; Dariva, C.; Oliveira, M.B.P.P. Azadirachta Indica A. Juss (Neem) Phenolic Extract Inhibits Human B-Lymphoblastoid Cells Growth via Cell Cycle Arrest, Apoptosis Induction, and DNA Damage. Open Explor. 2023, 1, 130–142. [Google Scholar] [CrossRef]
- Martínez-Castro, R.; Flórez-Santiago, J.; Valle-Molinares, R.; Cabrera-Barraza, J.; Espitia-Almeida, F. Optimized Microwave-Assisted Azadirachtin Extraction Using Response Surface Methodology. Heliyon 2024, 10, e31504. [Google Scholar] [CrossRef]
- Nonglait, D.L.; Gokhale, J.S. Review Insights on the Demand for Natural Pigments and Their Recovery by Emerging Microwave-Assisted Extraction (MAE). Food Bioprocess Technol. 2024, 17, 1681–1705. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, J.; Feng, Y.; Fu, X.; Yue, H.; Li, D.; Liu, C.; Feng, Z. One-Step Simultaneous and Efficient Extraction of Multiple Active Compounds from Lycium Barbarum by Aqueous Three-Phase System: Focusing on Polysaccharides. Ind. Crops Prod. 2024, 222, 119973. [Google Scholar] [CrossRef]
- Liu, C.; Huang, J.; Zheng, X.; Liu, S.; Lu, K.; Tang, K.; Liu, J. Heat Sealable Soluble Soybean Polysaccharide/Gelatin Blend Edible Films for Food Packaging Applications. Food Packag. Shelf Life 2020, 24, 100485. [Google Scholar] [CrossRef]
- Wu, J.; Chen, S.; Ge, S.; Miao, J.; Li, J.; Zhang, Q. Preparation, Properties and Antioxidant Activity of an Active Film from Silver Carp (Hypophthalmichthys Molitrix) Skin Gelatin Incorporated with Green Tea Extract. Food Hydrocoll. 2013, 32, 42–51. [Google Scholar] [CrossRef]
- Narasagoudr, S.S.; Hegde, V.G.; Chougale, R.B.; Masti, S.P.; Vootla, S.; Malabadi, R.B. Physico-Chemical and Functional Properties of Rutin Induced Chitosan/Poly (Vinyl Alcohol) Bioactive Films for Food Packaging Applications. Food Hydrocoll. 2020, 109, 106096. [Google Scholar] [CrossRef]
- Chen, Y.; Zou, C.; Mastalerz, M.; Hu, S.; Gasaway, C.; Tao, X. Applications of Micro-Fourier Transform Infrared Spectroscopy (FTIR) in the Geological Sciences—A Review. Int. J. Mol. Sci. 2015, 16, 30223–30250. [Google Scholar] [CrossRef]
- Mustafa, A.; Turner, C. Pressurized Liquid Extraction as a Green Approach in Food and Herbal Plants Extraction: A Review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Adetunji, L.R.; Adekunle, A.; Orsat, V.; Raghavan, V. Advances in the Pectin Production Process Using Novel Extraction Techniques: A Review. Food Hydrocoll. 2017, 62, 239–250. [Google Scholar] [CrossRef]
- Chan, C.H.; Yusoff, R.; Ngoh, G.C.; Kung, F.W.L. Microwave-Assisted Extractions of Active Ingredients from Plants. J. Chromatogr. A 2011, 1218, 6213–6225. [Google Scholar] [CrossRef]
- Ekezie, F.G.C.; Sun, D.W.; Cheng, J.H. Acceleration of Microwave-Assisted Extraction Processes of Food Components by Integrating Technologies and Applying Emerging Solvents: A Review of Latest Developments. Trends Food Sci. Technol. 2017, 67, 160–172. [Google Scholar] [CrossRef]
- Khamis Al-Jadidi, H.S.; Hossain, M.A. Studies on Total Phenolics, Total Flavonoids and Antimicrobial Activity from the Leaves Crude Extracts of Neem Traditionally Used for the Treatment of Cough and Nausea. Beni Suef Univ. J. Basic. Appl. Sci. 2015, 4, 93–98. [Google Scholar] [CrossRef]
- Widiyana, A.P.; Illian, D.N.; Magelang, U.M. Phytochemical analysis and total flavonoid content on ethanol and ethyl acetate extract from neem (Azadirachta indica Juss.) leaves utilizing UV–Vis spectrophotometric. J. Farm. Sains Dan Prakt. 2022, 8, 60–65. [Google Scholar] [CrossRef]
- Xu, F.; Wang, C.; Wang, H.; Xiong, Q.; Wei, Y.; Shao, X. Antimicrobial Action of Flavonoids from Sedum aizoon L. against Lactic Acid Bacteria in Vitro and in Refrigerated Fresh Pork Meat. J. Funct. Foods 2018, 40, 744–750. [Google Scholar] [CrossRef]
- Larit, F.; León, F.; Benyahia, S.; Cutler, S.J. Total Phenolic and Flavonoid Content and Biological Activities of Extracts and Isolated Compounds of Cytisus Villosus Pourr. Biomolecules 2019, 9, 732. [Google Scholar] [CrossRef] [PubMed]
- Elumalai, K.; Velmurugan, S. Green Synthesis, Characterization and Antimicrobial Activities of Zinc Oxide Nanoparticles from the Leaf Extract of Azadirachta indica (L.). Appl. Surf. Sci. 2015, 345, 329–336. [Google Scholar] [CrossRef]
- Ahmed, S.; Saifullah; Ahmad, M.; Swami, B.L.; Ikram, S. Green Synthesis of Silver Nanoparticles Using Azadirachta indica Aqueous Leaf Extract. J. Radiat. Res. Appl. Sci. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T. Comparative Studies on Properties and Antioxidative Activity of Fish Skin Gelatin Films Incorporated with Essential Oils from Various Sources. Int. Aquat. Res. 2014, 6, 62. [Google Scholar] [CrossRef]
- Bermúdez-Oria, A.; Rodríguez-Gutiérrez, G.; Vioque, B.; Rubio-Senent, F.; Fernández-Bolaños, J. Physical and Functional Properties of Pectin-Fish Gelatin Films Containing the Olive Phenols Hydroxytyrosol and 3,4-Dihydroxyphenylglycol. Carbohydr. Polym. 2017, 178, 368–377. [Google Scholar] [CrossRef]
- Rao, M.S.; Kanatt, S.R.; Chawla, S.P.; Sharma, A. Chitosan and Guar Gum Composite Films: Preparation, Physical, Mechanical and Antimicrobial Properties. Carbohydr. Polym. 2010, 82, 1243–1247. [Google Scholar] [CrossRef]
- Suderman, N.; Isa, M.I.N.; Sarbon, N.M. The Effect of Plasticizers on the Functional Properties of Biodegradable Gelatin-Based Film: A Review. Food Biosci. 2018, 24, 111–119. [Google Scholar] [CrossRef]
- Hong, Y.H.; Lim, G.O.; Song, K.B. Physical Properties of Gelidium Corneum-Gelatin Blend Films Containing Grapefruit Seed Extract or Green Tea Extract and Its Application in the Packaging of Pork Loins. J. Food Sci. 2009, 74, C6–C10. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Cui, Y.F.; Jia, D.M.; Xie, D. Effect of a Complex Plasticizer on the Structure and Properties of the Thermoplastic PVA/Starch Blends. Polym.-Plast. Technol. Eng. 2009, 48, 489–495. [Google Scholar] [CrossRef]
- Gontard, N.; Guilbert, S.; Cuq, J. Edible Wheat Gluten Films: Influence of the Main Process Variables on Film Properties Using Response Surface Methodology. J. Food Sci. 1992, 57, 190–195. [Google Scholar] [CrossRef]
- Vichasilp, C.; Sai-Ut, S.; Benjakul, S.; Rawdkuen, S. Effect of Longan Seed Extract and BHT on Physical and Chemical Properties of Gelatin Based Film. Food Biophys. 2014, 9, 238–248. [Google Scholar] [CrossRef]
- Yadav, S.; Mehrotra, G.K.; Bhartiya, P.; Singh, A.; Dutta, P.K. Preparation, Physicochemical and Biological Evaluation of Quercetin Based Chitosan-Gelatin Film for Food Packaging. Carbohydr. Polym. 2020, 227, 115348. [Google Scholar] [CrossRef]
- Jati, I.R.A.; Elaine, J.; Setijawaty, E.; Utomo, A.R. Development of Bio-Based Smart Edible Food Packaging Using Roselle Flower Extract and Eggshell Powder as Active Agents. BIO Web Conf. 2024, 98, 05001. [Google Scholar] [CrossRef]
- Guo, H.; Shao, C.; Ma, Y.; Zhang, Y.; Lu, P. Development of Active and Intelligent PH Food Packaging Composite Films Incorporated with Litchi Shell Extract as an Indicator. Int. J. Biol. Macromol. 2023, 226, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Xue Mei, L.; Mohammadi Nafchi, A.; Ghasemipour, F.; Mat Easa, A.; Jafarzadeh, S.; Al-Hassan, A.A. Characterization of PH Sensitive Sago Starch Films Enriched with Anthocyanin-Rich Torch Ginger Extract. Int J Biol Macromol 2020, 164, 4603–4612. [Google Scholar] [CrossRef]
- Chen, S.; Wu, M.; Lu, P.; Gao, L.; Yan, S.; Wang, S. Development of PH Indicator and Antimicrobial Cellulose Nanofibre Packaging Film Based on Purple Sweet Potato Anthocyanin and Oregano Essential Oil. Int. J. Biol. Macromol. 2020, 149, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Wahyuningtiyas, N.E.; Suryanto, H. Properties of Cassava Starch Based Bioplastic Reinforced by Nanoclay. J. Mech. Eng. Sci. Technol. 2018, 2, 20–26. [Google Scholar] [CrossRef]
- Akcay, U.C.; Baloglu, M.C.; Altunoglu, Y.C.; Baloglu, P.; Koyuncu, M.A.; Erbas, D.; Horuz, E.; Arslan, B.; Turkoglu, S. Comparative Transcriptomic Analysis Provides Novel Insights on the Hormonal Regulation of Postharvest Apples under Cold Storage. Hortic. Environ. Biotechnol. 2025, 1–17. [Google Scholar] [CrossRef]
- Basiak, E.; Linke, M.; Debeaufort, F.; Lenart, A.; Geyer, M. Dynamic Behaviour of Starch-Based Coatings on Fruit Surfaces. Postharvest Biol. Technol. 2019, 147, 166–173. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.; Sun, Y.; Wang, X.; Li, L. Combined Antioxidant and Sensory Effects of Active Chitosan/Zein Film Containing α-Tocopherol on Agaricus Bisporus. Food Packag. Shelf Life 2020, 24, 100470. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Z.; Bi, Y.; Zhang, J.; Wang, D. Antifungal Effect of Borates against Fusarium Sulphureum on Potato Tubers and Its Possible Mechanisms of Action. Postharvest Biol. Technol. 2012, 74, 55–61. [Google Scholar] [CrossRef]
- Gilles, M.; Zhao, J.; An, M.; Agboola, S. Chemical Composition and Antimicrobial Properties of Essential Oils of Three Australian Eucalyptus Species. Food Chem. 2010, 119, 731–737. [Google Scholar] [CrossRef]
- Mahmoud, D.A.; Hassanein, N.M.; Youssef, K.A.; Abou Zeid, M.A. Antifungal Activity of Different Neem Leaf Extracts and the Nimonol against Some Important Human Pathogens. Braz. J. Microbiol. 2011, 42, 1007–1016. [Google Scholar] [CrossRef]
- Nwachukwu, E.; Umechuruba, C. Antifungal Activities of Some Leaf Extracts on Seed-Borne Fungi of African Yam Bean Seeds, Seed Germination and Seedling Emergence. J. Appl. Sci. Environ. Manag. 2001. [Google Scholar] [CrossRef]
| Packaging | PEC (g) | GEL (g) | PROP (g) | VE (g) | AA-0.5 (mL) | B (%) |
|---|---|---|---|---|---|---|
| FPC | 2.0 | 0.2 | 0.3 | - | 50.0 | 1 |
| FPC + HNE-PLE | 2.0 | 0.2 | 0.3 | 1.0 | 50.0 | 1 |
| FPC + HNE-MAE | 2.0 | 0.2 | 0.3 | 1.0 | 50.0 | 1 |
| Sample | OEY (%) | TPC (mg AGE/100 g) | TFC (mg QE/100 g) | |||
|---|---|---|---|---|---|---|
| PLE | MAE | PLE | MAE | PLE | MAE | |
| Leaves | 20 Aa | 6 Bb | 2893 Ee | 3136 Ee | 542 Hh | 797 Ii |
| Seeds | 5 Bb | 4 Dd | 417 Fe | 641 Gg | 365 Jj | 348 Kk |
| Packaging | T [mm] | E [%] | TS [Mpa] | WVP (g·mm/d·m2·kPa) |
|---|---|---|---|---|
| FPC | 0.11 ± 0.00 a | 8.00 a ± 0.00 a | 13.76 ± 0.79 a | 5.2 ± 0.4 a |
| FPC + HNE-PLE | 0.10 ± 0.01 a | 10.33 ± 0.21 b | 16.45 ± 2.25 b | 2.0 ± 0.2 b |
| FPC + HNE-MAE | 0.16 ± 0.04 a | 9.50 ± 0.21 c | 9.24 ± 2.01 c | 6.9 ± 1.7 a |
| Packaging | Mass Loss (%) over Days of Storage | ||||||
|---|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 8 | 10 | 12 | 15 | |
| FPC | 2.56 a | 8.06 a | 10.77 a | 12.33 a | 14.26 b | 17.14 b | 20.73 b |
| FPC + HNE-PLE | 2.55 a | 7.15 a | 9.37 a | 10.91 b | 12.83 b | 15.94 b | 19.67 b |
| FPC + HNE-MAE | 2.89 a | 6.89 a | 9.22 a | 10.62 b | 12.50 b | 15.16 b | 18.56 b |
| CONTROL | 2.80 a | 9.81 a | 13.31 a | 16.75 a | 21.47 a | 28.16 a | 34.31 a |
| Packaging | External Appearance for Fungus Incidence in Papaya Fruit | |||||||
|---|---|---|---|---|---|---|---|---|
| Days of Storage | ||||||||
| 0 | 2 | 4 | 6 | 8 | 10 | 12 | 15 | |
| FPC | 1 | 1 | 1 | 1 | 2 | 2 | 3 | 3 |
| FPC + HNE-PLE | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| FPC + HNE-MAE | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 |
| CONTROL | 1 | 1 | 2 | 4 | 4 | 4 | 5 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, T.T.d.B.; Barbosa, A.M.; Nunes, T.P.; Costa, A.S.G.d.; Oliveira, M.B.P.P.; Borges, G.R.; Padilha, F.F.; Dariva, C.; Santos, K.S. Development of Antifungal Packaging Based on Pectin/Gelatin Containing Azadirachta indica Bioactive Extracts for Carica papaya L. Fruit Coating. Appl. Sci. 2025, 15, 4423. https://doi.org/10.3390/app15084423
Ribeiro TTdB, Barbosa AM, Nunes TP, Costa ASGd, Oliveira MBPP, Borges GR, Padilha FF, Dariva C, Santos KS. Development of Antifungal Packaging Based on Pectin/Gelatin Containing Azadirachta indica Bioactive Extracts for Carica papaya L. Fruit Coating. Applied Sciences. 2025; 15(8):4423. https://doi.org/10.3390/app15084423
Chicago/Turabian StyleRibeiro, Thais Trindade de Brito, Andriele Mendonça Barbosa, Tatiana Pacheco Nunes, Anabela Silvia Guedes da Costa, Maria Beatriz Prior Pinto Oliveira, Gustavo Rodrigues Borges, Francine Ferreira Padilha, Claudio Dariva, and Klebson Silva Santos. 2025. "Development of Antifungal Packaging Based on Pectin/Gelatin Containing Azadirachta indica Bioactive Extracts for Carica papaya L. Fruit Coating" Applied Sciences 15, no. 8: 4423. https://doi.org/10.3390/app15084423
APA StyleRibeiro, T. T. d. B., Barbosa, A. M., Nunes, T. P., Costa, A. S. G. d., Oliveira, M. B. P. P., Borges, G. R., Padilha, F. F., Dariva, C., & Santos, K. S. (2025). Development of Antifungal Packaging Based on Pectin/Gelatin Containing Azadirachta indica Bioactive Extracts for Carica papaya L. Fruit Coating. Applied Sciences, 15(8), 4423. https://doi.org/10.3390/app15084423










