Spectroscopic Techniques Application for Wine and Wine Byproduct Authentication
Abstract
:1. Introduction
2. Food Spectral Print Approaches
2.1. UV–Vis Spectroscopy
2.2. Infrared (IR) Spectroscopy
2.2.1. Mid-Infrared (MIR) Spectroscopy
2.2.2. Near-Infrared (NIR) Spectroscopy
2.3. Fluorescence
2.4. Nuclear Magnetic Resonance (NMR)
2.5. Raman
3. Statistical Techniques in Spectroscopy
3.1. Data Processing
3.2. Data Modeling
4. Emerging Trends in Wine and Byproduct Authentication
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geană, E.; Ciucure, C.; Apetrei, C.; Artem, V. Application of Spectroscopic UV-Vis and FT-IR Screening Techniques Coupled with Multivariate Statistical Analysis for Red Wine Authentication: Varietal and Vintage Year Discrimination. Molecules 2019, 24, 4166. [Google Scholar] [CrossRef] [PubMed]
- Kamiloglu, S. Authenticity and traceability in beverages. Food Chem. 2019, 277, 12–24. [Google Scholar] [CrossRef]
- International Organization of Vine and Wine (OIV). OIV Statistical Report on World Vitiviniculture. Available online: https://www.oiv.int/sites/default/files/2024-04/OIV_STATE_OF_THE_WORLD_VINE_AND_WINE_SECTOR_IN_2023.pdf (accessed on 2 March 2025).
- Ranaweera, R.; Capone, D.; Bastian, S.; Cozzolino, D.; Jeffery, D. A Review of Wine Authentication Using Spectroscopic Approaches in Combination with Chemometrics. Molecules 2021, 26, 4334. [Google Scholar] [CrossRef]
- Koljančić, N.; Furdíková, K.; Araújo Gomes, A.; Spánik, I. Wine authentication: Current progress and state of the art. Trends Food Sci. Technol. 2024, 150, 104598. [Google Scholar] [CrossRef]
- Liveri, M.; Tsantili-Kakoulidou, A.; Tsopelas, F. Identification of white wine adulteration with apple juice and apple cider using cyclic voltammetry on a screen-printed electrode aided by chemometric analysis. J. Food Compost. Anal. 2024, 136, 106751. [Google Scholar] [CrossRef]
- Gallego, L.; Del Alamo, M.; Nevares, I.; Fernandez, J.A.; de Simon, B.F.; Cadahia, E. Phenolic compounds and sensorial characterization of wines aged with alternative to barrel products made of Spanish oak wood (Quercus pyrenaica Willd). Food Sci. Technol. Int. 2012, 18, 151–165. [Google Scholar] [CrossRef]
- Proestos, C.; Bakogiannis, A.; Psarianos, C.; Koutinas, A.A.; Kanellaki, M.; Komaitis, M. High-performance liquid chromatography analysis of phenolic substances in Greek wines. Food Control 2005, 16, 319–323. [Google Scholar] [CrossRef]
- Kallithraka, S.; Tsoutsouras, E.; Tzourou, E.; Lanaridis, P. Principal phenolic compounds in Greek red wines. Food Chem. 2006, 99, 784–793. [Google Scholar] [CrossRef]
- Amargianitaki, M.; Spyros, A. NMR-based metabolomics in wine quality control and authentication. Chem. Biol. Technol. Agric. 2017, 4, 9. [Google Scholar] [CrossRef]
- Cassino, C.; Tsolakis, C.; Bonello, F.; Gianotti, V.; Osella, D. Wine evolution during bottle aging, studied by 1H NMR spectroscopy and multivariate statistical analysis. Food Res. Int. 2019, 116, 566–577. [Google Scholar] [CrossRef]
- Philippidis, A.; Poulakis, E.; Kontzedaki, R.; Orfanakis, E.; Symianaki, A.; Zoumi, A.; Velegrakis, M. Application of Ultraviolet-Visible Absorption Spectroscopy with Machine Learning Techniques for the Classification of Cretan Wines. Foods 2021, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, D.; Smyth, H.E.; Gishen, M. Feasibility study on the use of visible and near-infrared spectroscopy together with chemometrics to discriminate between commercial white wines of different varietal origins. J. Agric. Food Chem. 2003, 51, 7703–7708. [Google Scholar] [CrossRef] [PubMed]
- Popîrdă, A.; Luchian, C.E.; Cotea, V.V.; Colibaba, C.; Scutarașu, E.C.; Toader, M. A review of representative methods used in wine authentication. Agriculture 2021, 11, 225. [Google Scholar] [CrossRef]
- Cozzolino, D.; Cynkar, W.; Shah, N.; Smith, P. Quantitative analysis of minerals and electric conductivity of red grape homogenates by near infrared reflectance spectroscopy. Comput. Electron. Agric. 2011, 77, 81–85. [Google Scholar] [CrossRef]
- Riovanto, R.; Cynkar, W.U.; Berzaghi, P.; Cozzolino, D. Discrimination between Shiraz wines from different Australian regions: The role of spectroscopy and chemometrics. J. Agric. Food Chem. 2011, 59, 10356–10360. [Google Scholar] [CrossRef]
- Ranaweera, K.R.; Gilmore, A.M.; Capone, D.L.; Bastian, S.E.P.; Jeffery, D.W. Authentication of the geographical origin of Australian Cabernet Sauvignon wines using spectrofluorometric and multi-element analyses with multivariate statistical modeling. Food Chem. 2021, 335, 127592. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhao, Y.; Cui, K.; Wang, F.; Zhang, J.; Zhang, A. Wine characterization according to geographical origin using analysis of mineral elements and rainfall correlation of oxygen isotope values. Int. J. Food Sci. Technol. 2021, 57, 552–565. [Google Scholar] [CrossRef]
- Solovyev, P.; Fauhl-Hassek, C.; Riedl, J.; Esslinger, S.; Bontempo, L.; Camin, F. NMR spectroscopy in wine authentication: An official control perspective. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2040–2062. [Google Scholar] [CrossRef]
- Ehlers, M.; Horn, B.; Raeke, J.; Fauhl-Hassek, C.; Hermann, A.; Brockmeyer, J.; Riedl, J. Towards harmonization of non-targeted 1H NMR spectroscopy-based wine authentication: Instrument comparison. Food Control 2022, 132, 8508. [Google Scholar] [CrossRef]
- Gilmore, A.M.; Sui, Q.; Blair, B.; Pan, B.S. Accurate varietal classification and quantification of key quality compounds of grape extracts using the absorbance-transmittance fluorescence excitation emission matrix (A-TEEM) method and machine learning. OENO One 2022, 56, 107–115. [Google Scholar] [CrossRef]
- Ranaweera, R.; Gilmore, A.M.; Bastian, S.E.P.; Capone, D.L.; Jeffery, D.W. Spectrofluorometric analysis to trace the molecular fingerprint of wine during the winemaking process and recognize the blending percentage of different varietal wines. OENO One 2022, 56, 189–196. [Google Scholar] [CrossRef]
- Grijalba, N.; Maguregui, M.; Unceta, N.; Morillas, H.; Médina, B.; Barrio, R.; Pécheyran, C. Direct non-invasive molecular analysis of packaging label to assist wine-bottle authentication. Microchem. J. 2020, 154, 104564. [Google Scholar] [CrossRef]
- Ríos-Reina, R.; Azcarate, S.; Camiña, J.; Callejón, R.; Amigo, J. Application of hierarchical classification models and reliability estimation by bootstrapping, for authentication and discrimination of wine vinegars by UV–vis spectroscopy. Chemom. Intell. Lab. Syst. 2019, 191, 42–53. [Google Scholar] [CrossRef]
- González-Domínguez, R. Food Authentication: Techniques, Trends and Emerging Approaches (Second Issue). Foods 2022, 11, 1926. [Google Scholar] [CrossRef]
- Chen, Z.; Deutsch, T.; Dinh, H.; Domen, K.; Emery, K.; Forman, A.; Gaillard, N.; Garland, R.; Heske, C.; Jaramillo, T.; et al. UV-Vis Spectroscopy. In Photoelectrochemical Water Splitting. SpringerBriefs in Energy; Springer: New York, NY, USA, 2013; pp. 49–62. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Rehman, K. Ultraviolet-Visible (UV-VIS) Spectroscopy. In Essentials of Pharmaceutical Analysis; Springer: Singapore, 2020; pp. 29–56. [Google Scholar] [CrossRef]
- Mäntele, W.; Deniz, E. UV-VIS absorption spectroscopy: Lambert-Beer reloaded. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 965–968. [Google Scholar] [CrossRef]
- Casale, M.; Oliveri, P.; Armanino, C.; Lanteri, S.; Forina, M. NIR and UV-vis spectroscopy, artificial nose and tongue: Comparison of four fingerprinting techniques for the characterisation of Italian red wines. Anal. Chim. Acta 2010, 668, 143–148. [Google Scholar] [CrossRef]
- Ríos-Reina, R.; Caballero, D.; Azcarate, S.; García-González, D.; Callejón, R.; Amigo, J. VinegarScan: A Computer Tool Based on Ultraviolet Spectroscopy for A Rapid Authentication of Wine Vinegars. Chemosensors 2021, 9, 296. [Google Scholar] [CrossRef]
- Aleixandré-Tudo, J.; Nieuwoudt, H.; Aleixandre, J.; Du Toit, W. Chemometric compositional analysis of phenolic compounds in fermenting samples and wines using different infrared spectroscopy techniques. Talanta 2018, 176, 526–536. [Google Scholar] [CrossRef]
- Miramont, C.; Jourdes, M.; Teissèdre, P. Development of UV-vis and FTIR Partial Least Squares models: Comparison and combination of two spectroscopy techniques with chemometrics for polyphenols quantification in red wine. OENO One 2020, 54, 779–792. [Google Scholar] [CrossRef]
- Martelo-Vidal, M.; Vázquez, M. Determination of polyphenolic compounds of red wines by UV-VIS-NIR spectroscopy and chemometrics tools. Food Chem. 2014, 158, 28–34. [Google Scholar] [CrossRef]
- Chapman, J.; Gangadoo, S.; Truong, V.; Cozzolino, D. Spectroscopic approaches for rapid beer and wine analysis. Curr. Opin. Food Sci. 2019, 28, 67–73. [Google Scholar] [CrossRef]
- Yu, J.; Wang, H.; Zhan, J.; Huang, W. Review of recent UV–Vis and infrared spectroscopy researches on wine detection and discrimination. Appl. Spectrosc. Rev. 2018, 53, 65–86. [Google Scholar] [CrossRef]
- Scano, P. Characterization of the medium infrared spectra of polyphenols of red and white wines by integrating FT IR and UV–Vis spectral data. LWT 2021, 89, 108–116. [Google Scholar] [CrossRef]
- Wu, Z.; Li, H.; Long, J.; Xu, E.; Xu, X.; Jin, Z.; Jiao, A. Discrimination of Chinese rice wines of different geographical origins by UV–vis spectroscopy and chemometrics. J. Inst. Brew. 2015, 121, 167–174. [Google Scholar] [CrossRef]
- Edelmann, A.; Diewok, J.; Schuster, C.; Lendl, B. Rapid method for the discrimination of red wine cultivars based on mid-infrared spectroscopy of phenolic wine extracts. J. Agric. Food Chem. 2001, 49, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Li, R.; Jiang, Z.; Zhang, Y.; Hou, Y.; Wang, Y.; Wu, X.; Gong, L. Geographical classification of Chinese Cabernet Sauvignon wines by data fusion of ultraviolet-visible and synchronous fluorescence spectroscopies: The combined use of multiple wavelength differences. Aust. J. Grape Wine Res. 2016, 22, 358–365. [Google Scholar] [CrossRef]
- Theophile, T. Introduction to infrared spectroscopy. In Infrared Spectroscopy; Theophile, T., Ed.; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef]
- López-Lorente, Á.; Mizaikoff, B. Mid-infrared spectroscopy for protein analysis: Potential and challenges. Anal. Bioanal. Chem. 2016, 408, 2875–2889. [Google Scholar] [CrossRef] [PubMed]
- Tidemand-Lichtenberg, P.; Dam, J.; Andersen, H.; Høgstedt, L.; Pedersen, C. Mid-infrared upconversion spectroscopy. J. Opt. Soc. Am. B-Opt. Phys. 2016, 33, D28–D35. [Google Scholar] [CrossRef]
- Cai, Y.; Chen, Y.; Dorfman, K.; Xin, X.; Wang, X.; Huang, K.; Wu, E. Mid-infrared single-photon upconversion spectroscopy enabled by nonlocal wavelength-to-time mapping. Sci. Adv. 2024, 10, eadl3503. [Google Scholar] [CrossRef]
- Liu, M.; Gray, R.; Costa, L.; Markus, C.; Roy, A.; Marandi, A. Mid-infrared cross-comb spectroscopy. Nat. Commun. 2021, 14, 1044. [Google Scholar] [CrossRef]
- Lou, H.; Deng, Z.; Luo, D.; Pan, J.; Zhou, L.; Xie, G.; Gu, C.; Li, W. High-SNR mid-infrared dual-comb spectroscopy using active phase control cooperating with CWs-dependent phase correction. Opt. Express 2024, 32, 5826–5836. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Fan, X.; Xu, B.; Zhu, Y.; He, Z. Digitally generated high-resolution mid-infrared dual-comb spectroscopy system based on electro-optic modulation. Opt. Lett. 2024, 49, 5711–5714. [Google Scholar] [CrossRef]
- Bevin, C.; Dambergs, R.; Fergusson, A.; Cozzolino, D. Varietal discrimination of Australian wines by means of mid-infrared spectroscopy and multivariate analysis. Anal. Chim. Acta 2008, 621, 19–23. [Google Scholar] [CrossRef]
- Santos, C.; Páscoa, R.; Porto, P.; Cerdeira, A.; González-Sáiz, J.; Pizarro, C.; Lopes, J. Raman spectroscopy for wine analyses: A comparison with near and mid-infrared spectroscopy. Talanta 2018, 186, 306–314. [Google Scholar] [CrossRef]
- Petric, I.; Duralija, B.; Leder, R. Analytical Techniques for the Authenticity Evaluation of Chokeberry, Blackberry and Raspberry Fruit Wines: Exploring FT-MIR Analysis and Chemometrics. Horticulturae 2024, 10, 1043. [Google Scholar] [CrossRef]
- Sen, I.; Ozturk, B.; Tokatli, F.; Ozen, B. Combination of visible and mid-infrared spectra for the prediction of chemical parameters of wines. Talanta 2016, 161, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Niimi, J.; Liland, K.; Tomic, O.; Jeffery, D.; Bastian, S.; Boss, P. Prediction of wine sensory properties using mid-infrared spectra of Cabernet Sauvignon and Chardonnay grape berries and wines. Food Chem. 2021, 344, 128634. [Google Scholar] [CrossRef]
- Shen, F.; Ying, Y.; Li, B.; Zheng, Y.; Hu, J. Prediction of sugars and acids in Chinese rice wine by mid-infrared spectroscopy. Food Res. Int. 2011, 44, 1521–1527. [Google Scholar] [CrossRef]
- Croce, R.; Malegori, C.; Oliveri, P.; Medici, I.; Cavaglioni, A.; Rossi, C. Prediction of quality parameters in straw wine by means of FT-IR spectroscopy combined with multivariate data processing. Food Chem. 2020, 305, 125512. [Google Scholar] [CrossRef]
- Patz, C.; Blieke, A.; Ristow, R.; Dietrich, H. Application of FT-MIR spectrometry in wine analysis. Anal. Chim. Acta 2004, 513, 81–89. [Google Scholar] [CrossRef]
- Ozturk, B.; Yucesoy, D.; Ozen, B. Application of Mid-infrared Spectroscopy for the Measurement of Several Quality Parameters of Alcoholic Beverages, Wine and Raki. Food Anal. Methods 2012, 5, 1435–1442. [Google Scholar] [CrossRef]
- Santos, C.; Páscoa, R.; Sarraguça, M.; Porto, P.; Cerdeira, A.; González-Sáiz, J.; Pizarro, C.; Lopes, J. Merging vibrational spectroscopic data for wine classification according to the geographic origin. Food Res. Int. 2017, 102, 504–510. [Google Scholar] [CrossRef]
- Parpinello, G.; Ricci, A.; Arapitsas, P.; Curioni, A.; Moio, L.; Riosegade, S.; Ugliano, M.; Versari, A. Multivariate characterisation of Italian monovarietal red wines using MIR spectroscopy. OENO One 2019, 53. [Google Scholar] [CrossRef]
- Jin, X.; Wu, S.; Yu, W.; Xu, X.; Huang, M.; Tang, Y.; Yang, Z. Wine Authentication Using Integration Assay of MIR, NIR, E-tongue, HS-SPME-GC-MS, and Multivariate Analyses: A Case Study for a Typical Cabernet Sauvignon Wine. J. AOAC Int. 2019, 102, 1174–1180. [Google Scholar] [CrossRef] [PubMed]
- Workman, J. Interpretive Spectroscopy for Near Infrared. Appl. Spectrosc. Rev. 1996, 31, 251–320. [Google Scholar] [CrossRef]
- Beć, K.; Grabska, J.; Huck, C. Near-Infrared Spectroscopy in Bio-Applications. Molecules 2020, 25, 2948. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Zhang, L.; Kang, C.; Quan, B. Near-infrared spectroscopy and machine learning-based technique to predict quality-related parameters in instant tea. Sci. Rep. 2022, 12, 3833. [Google Scholar] [CrossRef] [PubMed]
- Reich, G. Near-infrared spectroscopy and imaging: Basic principles and pharmaceutical applications. Adv. Drug Deliv. Rev. 2005, 57, 1109–1143. [Google Scholar] [CrossRef]
- Hu, L.; Yin, C.; Ma, S.; Liu, Z. Rapid detection of three quality parameters and classification of wine based on Vis-NIR spectroscopy with wavelength selection by ACO and CARS algorithms. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 205, 574–581. [Google Scholar] [CrossRef]
- Ríos-Reina, R.; García-González, D.; Callejón, R.; Amigo, J. NIR spectroscopy and chemometrics for the typification of Spanish wine vinegars with a protected designation of origin. Food Control 2018, 89, 108–116. [Google Scholar] [CrossRef]
- Genisheva, Z.; Quintelas, C.; Mesquita, D.; Ferreira, E.; Oliveira, J.; Amaral, A.; Amaral, A. New PLS analysis approach to wine volatile compounds characterization by near-infrared spectroscopy (NIR). Food Chem. 2018, 246, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, D.; Kwiatkowski, M.; Parker, M.; Cynkar, W.; Dambergs, R.; Gishen, M.; Herderich, M. Prediction of phenolic compounds in red wine fermentations by visible and near-infrared spectroscopy. Anal. Chim. Acta 2004, 513, 73–80. [Google Scholar] [CrossRef]
- Rouxinol, M.; Martins, M.; Murta, G.; Barroso, J.M.; Rato, A. Quality Assessment of Red Wine Grapes through NIR Spectroscopy. Agronomy 2022, 12, 637. [Google Scholar] [CrossRef]
- Nardi, T.; Petrozziello, M.; Girotto, R.; Fugaro, M.; Mazzei, R.; Scuppa, S. Wine aging authentication through Near Infrared Spectroscopy: A feasibility study on chips and barrel-aged wines. OENO One 2020, 54, 165–173. [Google Scholar] [CrossRef]
- Cozzolino, D.; Kwiatkowski, M.; Kwiatkowski, M.; Dambergs, R.; Dambergs, R.; Cynkar, W.; Cynkar, W.; Janik, L.; Janik, L.; Skouroumounis, G.; et al. Analysis of elements in wine using near-infrared spectroscopy and partial least squares regression. Talanta 2008, 74, 711–716. [Google Scholar] [CrossRef]
- Fan, K.-J.; Su, W.-H. Applications of Fluorescence Spectroscopy, RGB- and MultiSpectral Imaging for Quality Determinations of White Meat: A Review. Biosensors 2022, 12, 76. [Google Scholar] [CrossRef]
- Danezis, G.P.; Tsagkaris, A.S.; Brusic, V.; Georgiou, C.A. Food authentication: State of the art and prospects. Curr. Opin. Food Sci. 2016, 10, 22–31. [Google Scholar] [CrossRef]
- Danezis, G.P.; Tsagkaris, A.S.; Camin, F.; Brusic, V.; Georgiou, C.A. Food authentication: Techniques, trends & emerging approaches. TrAC Trends Anal. Chem. 2016, 85, 123–132. [Google Scholar] [CrossRef]
- Radotić, K.; Melø, T.B.; Lindgren, M. A fluorescence spectroscopic study of light transmission and adaxial-abaxial distribution of emitting compounds in leaves of Christmas star (Euphorbia pulcherrima). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 303, 123269. [Google Scholar] [CrossRef]
- Callao, M.P.; Ruisánchez, I. An overview of multivariate qualitative methods for food fraud detection. Food Control 2018, 86, 283–293. [Google Scholar] [CrossRef]
- Wu, M.; Li, M.; Fan, B.; Sun, Y.; Shen, Q. A Rapid and Low-Cost Method for Detection of Nine Kinds of Vegetable Oil Adulteration Based on 3-D Fluorescence Spectroscopy. LWT 2023, 188, 115419. [Google Scholar] [CrossRef]
- Sikorska, E.; Khmelinskii, I.; Sikorski, M. Fluorescence spectroscopy and imaging instruments for food quality evaluation. In Evaluation Technologies for Food Quality; Woodhead Publishing: Sawston, UK, 2019; pp. 491–533. [Google Scholar] [CrossRef]
- Kumar, K.; Tarai, M.; Mishra, A.K. Unconventional steady-state fluorescence spectroscopy as an analytical technique for analyses of complex-multifluorophoric mixtures. TrAC Trends Anal. Chem. 2017, 97, 216–243. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, Y.; Ju, Y.; Meng, X.; Yin, C. Rapid Identification of Rice Geographical Origin and Adulteration by Excitation-Emission Matrix Fluorescence Spectroscopy Combined with Chemometrics Based on Fluorescence Probe. Food Control 2023, 146, 109547. [Google Scholar] [CrossRef]
- Airado-Rodríguez, D.; Durán-Merás, I.; Galeano-Díaz, T.; Wold, J.P. Front-face fluorescence spectroscopy: A new tool for control in the wine industry. J. Food Compos. Anal. 2011, 24, 257–264. [Google Scholar] [CrossRef]
- Dufour, É.; Letort, A.; Laguet, A.; Lebecque, A.; Serra, J.N. Investigation of variety, typicality and vintage of French and German wines using front-face fluorescence spectroscopy. Anal. Chim. Acta 2006, 563, 292–299. [Google Scholar] [CrossRef]
- Mattivi, F.; Monetti, A.; Vrhovsek, U.; Tonon, D.; Andrés-Lacueva, C. High-performance liquid chromatographic determination of the riboflavin concentration in white wines for predicting their resistance to light. J. Chromatogr. A 2000, 888, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Hoenicke, K.; Simat, T.J.; Steinhart, H.; Köhler, H.J.; Schwab, A. Determination of free and conjugated indole-3-acetic acid, tryptophan and tryptophan metabolites in grape must and wine. J. Agric. Food Chem. 2001, 49, 5494–5501. [Google Scholar] [CrossRef]
- Le Moigne, M.; Dufour, E.; Bertrand, D.; Maury, C.; Seraphin, D.; Jourjon, F. Front face fluorescence spectroscopy and visible spectroscopy coupled with chemometrics have the potential to characterise ripening of Cabernet Franc grapes. Anal. Chim. Acta 2008, 621, 8–18. [Google Scholar] [CrossRef]
- Žiak, Ľ.; Sádecká, J.; Májek, P.; Hroboňová, K. Simultaneous determination of phenolic acids and scopoletin in brandies using synchronous fluorescence spectrometry coupled with partial least squares. Food Anal. Methods 2014, 7, 563–570. [Google Scholar] [CrossRef]
- Sádecká, J.; Jakubíková, M. Varietal classification of white wines by fluorescence spectroscopy. J. Food Sci. Technol. 2020, 57, 2545–2553. [Google Scholar] [CrossRef]
- Suciu, R.C.; Zarbo, L.; Guyon, F.; Magdas, D.A. Application of fluorescence spectroscopy using classical right angle technique in white wines classification. Sci. Rep. 2019, 9, 18250. [Google Scholar] [CrossRef] [PubMed]
- Eltemur, D.; Robatscher, P.; Oberhuber, M.; Scampicchio, M.; Ceccon, A. Applications of Solution NMR Spectroscopy in Quality Assessment and Authentication of Bovine Milk. Foods 2023, 12, 3240. [Google Scholar] [CrossRef] [PubMed]
- Pacholczyk-Sienicka, B.; Ciepielowski, G.; Albrecht, Ł. The Application of NMR Spectroscopy and Chemometrics in Authentication of Spices. In Analysis of Food Spices; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar] [CrossRef]
- Rossmann, A. Determination of stable isotope ratios in food analysis. Food Rev. Int. 2001, 17, 347–381. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, L.; Yu, Z. Quantitative 31P NMR Spectroscopy: Principles, Methodologies, and Applications in Phosphorus-Containing Compound Analysis. Appl. Sci. 2025, 15, 323. [Google Scholar] [CrossRef]
- Gougeon, L.; Da Costa, G.; Le Mao, I.; Ma, W.; Teissedre, P.-L.; Guyon, F.; Richard, T. Wine analysis and authenticity using 1H-NMR metabolomics data: Application to Chinese wines. Food Anal. Methods 2018, 11, 3425–3434. [Google Scholar] [CrossRef]
- Godelmann, R.; Kost, C.; Patz, C.-D.; Ristow, R.; Wachter, H. Quantitation of compounds in wine using 1H NMR spectroscopy: Description of the method and collaborative study. J. AOAC Int. 2016, 99, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Ali, K.; Maltese, F.; Fortes, A.M.; Pais, M.S.; Choi, Y.H.; Verpoorte, R. Monitoring biochemical changes during grape berry development in Portuguese cultivars by NMR spectroscopy. Food Chem. 2011, 124, 1760–1769. [Google Scholar] [CrossRef]
- Pereira, G.E.; Gaudillere, J.-P.; Van Leeuwen, C.; Hilbert, G.; Lavialle, O.; Maucourt, M.; Deborde, C.; Moing, A.; Rolin, D. 1H NMR and Chemometrics to Characterize Mature Grape Berries in Four Wine-Growing Areas in Bordeaux, France. J. Agric. Food Chem. 2005, 53, 6382–6389. [Google Scholar] [CrossRef]
- Mazzei, P.; Celano, G.; Palese, A.M.; Lardo, E.; Drosos, M.; Piccolo, A. HRMAS-NMR metabolomics of Aglianicone grapes pulp to evaluate terroir and vintage effects, and, as assessed by the Electromagnetic Induction (EMI) technique, spatial variability of vineyard soils. Food Chem. 2019, 283, 215–223. [Google Scholar] [CrossRef]
- Picone, G.; Trimigno, A.; Tessarin, P.; Donnini, S.; Rombolà, A.D.; Capozzi, F. 1H NMR foodomics reveals that the biodynamic and the organic cultivation managements produce different grape berries (Vitis vinifera L. cv. Sangiovese). Food Chem. 2016, 213, 187–195. [Google Scholar] [CrossRef]
- Hu, B.; Yue, Y.; Zhu, Y.; Wen, W.; Zhang, F.; Hardie, J.W. Proton Nuclear Magnetic Resonance-Spectroscopic Discrimination of Wines Reflects Genetic Homology of Several Different Grape (V. vinifera L.) Cultivars. PLoS ONE 2015, 10, e0142840. [Google Scholar] [CrossRef]
- Valls Fonayet, J.; Loupit, G.; Richard, T. MS- and NMR-metabolomic tools for the discrimination of wines: Applications for authenticity. Adv. Bot. Res. 2021, 98, 297–357. [Google Scholar] [CrossRef]
- Monakhova, Y.B.; Godelmann, R.; Hermann, A.; Kuballa, T.; Cannet, C.; Schäfer, H.; Spraul, M.; Rutledge, D.N. Synergistic effect of the simultaneous chemometric analysis of 1H NMR spectroscopic and stable isotope (SNIF-NMR, 18O, 13C) data: Application to wine analysis. Anal. Chim. Acta 2014, 833, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Zhong, Q.; Fauhl-Hassek, C.; Pfister, M.K.H.; Horn, B.; Huang, Z. Classification of Chinese wine varieties using 1H NMR spectroscopy combined with multivariate statistical analysis. Food Control 2017, 88, 113–122. [Google Scholar] [CrossRef]
- Anastasiadi, M.; Zira, A.; Magiatis, P.; Haroutounian, S.A.; Skaltsounis, A.L.; Mikros, E. 1H NMR-based metabonomics for the classification of Greek wines according to variety, region, and vintage. Comparison with HPLC data. J. Agric. Food Chem. 2009, 57, 11067–11074. [Google Scholar] [CrossRef]
- Coco, L.; Pascali, S.; Fanizzi, F. NMR-Metabolomic Study on Monocultivar and Blend Salento EVOOs including Some from Secular Olive Trees. Food Sci. Nutr. 2014, 5, 89–95. [Google Scholar] [CrossRef]
- Godelmann, R.; Fang, F.; Humpfer, E.; Schütz, B.; Bansbach, M.; Schäfer, H.; Spraul, M. Targeted and nontargeted wine analysis by 1H NMR spectroscopy combined with multivariate statistical analysis. Differentiation of important parameters: Grape variety, geographical origin, year of vintage. J. Agric. Food Chem. 2013, 61, 5610–5619. [Google Scholar] [CrossRef]
- Le Mao, I.; Da Costa, G.; Richard, T. 1H-NMR metabolomics for wine screening and analysis. OENO One 2023, 57, 15–31. [Google Scholar] [CrossRef]
- Parachalil, D.R.; McIntyre, J.; Byrne, H.J. Potential of Raman spectroscopy for the analysis of plasma/serum in the liquid state: Recent advances. Analy Bioanal. Chem. 2020, 412, 1993–2007. [Google Scholar] [CrossRef]
- Magdas, D.A.; Guyon, F.; Feher, I.; Pinzaru, S.C. Wine discrimination based on chemometric analysis of untargeted markers using FT-Raman spectroscopy. Food Control 2018, 85, 385–391. [Google Scholar] [CrossRef]
- Magdas, D.A.; Cozar, B.I.; Feher, I.; Guyon, F.; Dehelean, A.; Cinta, P.S. Testing the limits of FT-Raman spectroscopy for wine authentication: Cultivar, geographical origin, vintage and terroir effect influence. Sci. Rep. 2019, 9, 19954. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, R.C.A.T.; Páscoa, N.M.J.; Porto, P.A.L.S.; Cerdeira, A.L.; González-Sáiz, J.M.; Pizarro, C.; Lopes, J.A. Raman spectroscopy for wine analyses: A comparison with near and mid infrared spectroscopy. Talanta 2018, 186, 306–314. [Google Scholar] [CrossRef] [PubMed]
- ValiZade, S.; Forooghi, E.; Jannat, B.; Hashempour-baltork, F.; Abdollahi, H. A combined classification modeling strategy for detection and identification of extra virgin olive oil adulteration using Raman spectroscopy Chemometr. Intell. Lab. Syst. 2023, 240, 104903. [Google Scholar] [CrossRef]
- Papaspyridakou, P.; Giannoutsou, P.; Orkoula, M.G. Non-Destructive and Non-Invasive Measurement of Ethanol and Toxic Alcohol Strengths in Beverages and Spirits Using Portable Raman Spectroscopy. Biosensors 2023, 13, 135. [Google Scholar] [CrossRef] [PubMed]
- Puncochova, K.; Vukosavljevic, B.; Hanus, J.; Beranek, J.; Windbergs, M.; Stepanek, F. Non-invasive insight into the release mechanisms of a poorly soluble drug from amorphous solid dispersions by confocal Raman microscopy. Eur. J. Pharm. Biopharm. 2016, 101, 119–125. [Google Scholar] [CrossRef]
- Westley, C.; Fisk, H.; Xu, Y.; Hollywood, K.A.; Carnell, A.J.; Micklefield, J.; Turner, N.J.; Goodacre, R. Real-time monitoring of enzyme-catalysed reactions using deep UV resonance Raman spectroscopy. Chem. Eur. J. 2017, 23, 6983–6987. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, D.-W.; Pu, H.; Wei, Q. Surface-enhanced Raman spectroscopy (SERS): A novel reliable technique for rapid detection of common harmful chemical residues. Trends Food Sci. Technol. 2018, 75, 10–22. [Google Scholar] [CrossRef]
- Neng, J.; Wang, J.; Wang, Y.; Zhang, Y.; Chen, P. Trace Analysis of Food by Surface-Enhanced Raman Spectroscopy Combined with Molecular Imprinting Technology: Principle, Application, Challenges, and Prospects. Food Chem. 2023, 429, 136883. [Google Scholar] [CrossRef]
- Schulz, H.; Baranska, M. Identification and quantification of valuable plant substances by IR and Raman spectroscopy Vibrational. Spectroscopy 2007, 43, 13–25. [Google Scholar] [CrossRef]
- Yang, H.; Irudayaraj, J.; Paradkar, M.M. Discriminant analysis of edible oils fats by FTIR, FT-NIR and FT Raman spectroscopy. Food Chem. 2005, 93, 25–32. [Google Scholar] [CrossRef]
- Ojeda-Galván, H.J.; Hernández-Arteaga, A.C.; Rodríguez-Aranda, M.C.; Toro-Vazquez, J.F.; Cruz-González, N.; Ortíz-Chávez, S.; Comas-García, M.; Rodríguez, A.G.; Navarro-Contreras, H.R. Application of Raman spectroscopy for the determination of proteins denaturation and amino acids decomposition temperature. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 285, 121941. [Google Scholar] [CrossRef] [PubMed]
- Mathlouthi, M.; Koenig, J.L. Vibrational spectra of carbohydrates. Adv. Carbohydr. Chem. Biochem. 1986, 44, 7–89. [Google Scholar] [CrossRef]
- Esteki, M.; Shahsavari, Z.; Simal-Gandara, J. Use of spectroscopic methods in combination with linear discriminant analysis for authentication of food products. Food Control 2018, 91, 100–112. [Google Scholar] [CrossRef]
- Mandrile, L.; Zeppa, G.; Giovannozzi, A.M.; Rossi, A.M. Controlling protected designation of origin of wine by Raman. Food Chem. 2016, 211, 260–307. [Google Scholar] [CrossRef]
- Martin, C.; Bruneel, J.-L.; Castet, F.; Fritsch, A.; Teissedre, P.-R.; Jourdes, M.; Guillaume, F. Spectroscopic and theoretical investigations of phenolic acids in white wines. Food Chem. 2017, 221, 568–575. [Google Scholar] [CrossRef]
- Gautam, R.; Vanga, S.; Madan, A.; Gayathri, N.; Nongthomba, U.; Umapathy, S. Raman spectroscopic studies on screening of myopathies. Anal Chem. 2015, 87, 2187–2194. [Google Scholar] [CrossRef] [PubMed]
- Acquarelli, J.; Van Laarhoven, T.; Gerretzen, J.; Tran, T.; Buydens, L.; Marchiori, E. Convolutional neural networks for vibrational spectroscopic data analysis. Anal. Chim. Acta 2017, 954, 22–31. [Google Scholar] [CrossRef]
- Stavrakakis, G.; Philippidis, A.; Velegrakis, M. Application of Optical Spectroscopic Techniques and Multivariate Statistical Analysis as a Method of Determining the Percentage and Type of Adulteration of Extra Virgin Olive Oil. Food Anal. Methods 2022, 15, 285–293. [Google Scholar] [CrossRef]
- Ranaweera, K.R.; Gilmore, A.M.; Capone, D.L.; Bastian, S.E.P.; Jeffery, D.W. Spectrofluorometric analysis combined with machine learning for geographical and varietal authentication, and prediction of phenolic compound concentrations in red wine. Food Chem. 2021, 361, 130–149. [Google Scholar] [CrossRef]
- De Jong, T.; Kok, D.; Torren, A.; Schopmans, H.; Tromp, R.; Molen, S.; Jobst, J. Quantitative analysis of spectroscopic low energy electron microscopy data: High-dynamic-range imaging, drift correction, and cluster analysis. Ultramicroscopy 2019, 213, 112913. [Google Scholar] [CrossRef]
- Shah, D.; Wang, J.; He, Q. A feature-based soft sensor for spectroscopic data analysis. J. Process Control. 2019, 78, 98–107. [Google Scholar] [CrossRef]
- Cozzolino, D.; Holdstock, M.; Dambergs, R.; Cynkar, W.; Smith, P. Mid-infrared spectroscopy and multivariate analysis: A tool to discriminate between organic and non-organic wines grown in Australia. Food Chem. 2009, 116, 761–765. [Google Scholar] [CrossRef]
- Radulescu, C.; Olteanu, R.L.; Nicolescu, C.M.; Bumbac, M.; Buruleanu, L.C.; Holban, G.C. Vibrational Spectroscopy Combined with Chemometrics as Tool for Discriminating Organic vs. Conventional Culture Systems for Red Grape Extracts. Foods 2021, 10, 1856. [Google Scholar] [CrossRef]
- Hair, J.F.; Black, W.C.; Babin, B.J.; Anderson, R.E. Multivariate Data Analysis, 7th ed.; Pearson: New York, NY, USA, 2010. [Google Scholar]
- Marôco, J. Análise Estatística com o SPSS Statistics, 7th ed.; Report Number: Pêro Pinheiro, Portugal, 2018. [Google Scholar]
- Nardecchia, A.; Vitale, R.; Duponchel, L. Fusing spectral and spatial information with 2-D stationary wavelet transform (SWT 2-D) for a deeper exploration of spectroscopic images. Talanta 2020, 224, 121835. [Google Scholar] [CrossRef] [PubMed]
- Nieuwoudt, H.H.; Prior, B.A.; Pretorius, I.S.; Marena, M.; Bauer, F.F. Principal Component Analysis Applied to Fourier Transform Infrared Spectroscopy for the Design of Calibration Sets for Glycerol Prediction Models in Wine and for the Detection and Classification of Outlier Samples. J. Agric. Food Chem. 2004, 52, 12. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.R.; Toutenburg, H.; Shalabh, H.C. Linear Models and Generalizations: Least Squares and Alternatives, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Zhang, L.; Henson, M.; Sekulic, S. Multivariate data analysis for Raman imaging of a model pharmaceutical tablet. Anal. Chim. Acta 2005, 545, 262–278. [Google Scholar] [CrossRef]
- Gareth, J.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning with Applications in R; Springer: New York, NY, USA, 2021. [Google Scholar]
- Murru, C.; Chimeno-Trinchet, C.; Díaz-García, M.; Badía-Laíño, R.; Fernández-González, A. Artificial Neural Network and Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy to identify the chemical variables related to ripeness and variety classification of grapes for Protected. Designation of Origin wine production. Comput. Electron. Agric. 2019, 164, 104922. [Google Scholar] [CrossRef]
- Martelo-Vidal, M.; Vázquez, M. Application of artificial neural networks coupled to UV–VIS–NIR spectroscopy for the rapid quantification of wine compounds in aqueous mixtures. CyTA—J. Food. 2014, 3, 32–39. [Google Scholar] [CrossRef]
- Mingione, E.; Leone, C.; Almonti, D.; Menna, E.; Baiocco, G.; Ucciardello, N. Artificial neural networks application for analysis and control of grapes fermentation process. Procedia CIRP 2022, 112, 22–27. [Google Scholar] [CrossRef]
- Boido, E.; Fariña, L.; Carrau, F.; Cozzolino, D.; Dellacassa, E. Application of near-infrared spectroscopy/artificial neural network to quantify glycosylated norisoprenoids in Tannat grapes. Food Chem. 2022, 387, 132927. [Google Scholar] [CrossRef]
- Ríos-Reina, R.; Segura-Borrego, M.P.; Camiña, J.M.; Callejón, R.M.; Azcarate, S.M. Multiplatform spectral print strategies for the authentication of Spanish PDO fortified wines using AHIMBU, an automatic hierarchical classification tool. Chemom. Intell. Lab. Syst. 2025, 257, 105311. [Google Scholar] [CrossRef]
- Galstyan, A.; Semipyatniy, V.; Mikhailova, I.; Gilmanov, K.; Bigaeva, A.; Vafin, R. Methodological Approaches to DNA Authentication of Foods, Wines and Raw Materials for Their Production. Foods 2021, 10, 595. [Google Scholar] [CrossRef]
- Song, J.; De Paolis, C.; Boccacci, P.; Ferrero, L.; Moine, A.; Segade, R.; Giacosa, S.; Gambino, G.; Rolle, L.; Paissoni, M. Influence of filtration treatments on grapevine DNA traceability in wine. Food Biosci. 2023, 57, 103533. [Google Scholar] [CrossRef]
- García-Beneytez, E.; Moreno-Arribas, M.; Borrego, J.; Polo, M.; Ibáñez, J. Application of a DNA analysis method for the cultivar identification of grape musts and experimental and commercial wines of Vitis vinifera L. using microsatellite markers. J. Agric. Food Chem. 2002, 50, 6090–6096. [Google Scholar] [CrossRef] [PubMed]
- Catalano, V.; Moreno-Sanz, P.; Lorenzi, S.; Grando, M. Experimental Review of DNA-Based Methods for Wine Traceability and Development of a Single-Nucleotide Polymorphism (SNP) Genotyping Assay for Quantitative Varietal Authentication. J. Agric. Food Chem. 2016, 64, 6969–6984. [Google Scholar] [CrossRef]
- Barrias, S.; Fernandes, J.; Martins-Lopes, P. Newly developed QCM-DNA biosensor for SNP detection in small DNA fragments: A wine authenticity case study. Food Control 2025, 169, 111036. [Google Scholar] [CrossRef]
- Barrias, S.; Fernandes, J.; Eiras-Dias, J.; Brazão, J.; Martins-Lopes, P. Label-free DNA-based optical biosensor as a potential system for wine authenticity. Food Chem. 2019, 270, 299–304. [Google Scholar] [CrossRef]
- Vignani, R.; Lio’, P.; Scali, M. How to integrate wet lab and bioinformatics procedures for wine DNA admixture analysis and compositional profiling: Case studies and perspectives. PLoS ONE 2019, 14, e0211962. [Google Scholar] [CrossRef]
- Villano, C.; Lisanti, M.T.; Gambuti, A.; Vecchio, R.; Moio, L.; Frusciante, L.; Aversano, R.; Carputo, D. Wine varietal authentication based on phenolics, volatiles and DNA markers: State of the art, perspectives and drawbacks. Food Control 2017, 80, 1–10. [Google Scholar] [CrossRef]
- Zambianchi, S.; Soffritti, G.; Stagnati, L.; Patrone, V.; Morelli, L.; Vercesi, A.; Busconi, M. Applicability of DNA traceability along the entire wine production chain in the real case of a large Italian cooperative winery. Food Control 2021, 124, 107929. [Google Scholar] [CrossRef]
- Cichna-Markl, M.; Mafra, I. Techniques for Food Authentication: Trends and Emerging Approaches. Foods 2023, 12, 1134. [Google Scholar] [CrossRef] [PubMed]
- Aiello, G.; Tosi, D. An Artificial Intelligence-based tool to predict “unhealthy” wine and olive oil. J. Agric. Food Res. 2024, 16, 101179. [Google Scholar] [CrossRef]
- Hategan, A.R.; Pirnau, A.; Magdas, D.A. Applications of Machine Learning for Wine Recognition Based on 1H-NMR Spectroscopy. Beverages 2025, 11, 45. [Google Scholar] [CrossRef]
- Adamashvili, N.; Zhizhilashvili, N.; Tricase, C. The Integration of the Internet of Things, Artificial Intelligence, and Blockchain Technology for Advancing the Wine Supply Chain. Computers 2024, 13, 72. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Qiao, L.; Zhang, N.; Sun, B.; Li, H.; Sun, J.; Chen, H. From Traditional to Intelligent, A Review of Application and Progress of Sensory Analysis in Alcoholic Beverage Industry. Food Chem. X 2024, 23, 101542. [Google Scholar] [CrossRef]
- Vilela, A.; Bacelar, E.; Pinto, T.; Anjos, R.; Correia, E.; Gonçalves, B.; Cosme, F. Beverage and Food Fragrance Biotechnology, Novel Applications, Sensory and Sensor Techniques: An Overview. Foods 2019, 8, 643. [Google Scholar] [CrossRef]
- Seesaard, T.; Goel, N.; Kumar, M.; Wongchoosuk, C. Advances in gas sensors and electronic nose technologies for agricultural cycle applications. Comput. Electron. Agric. 2022, 193, 106673. [Google Scholar] [CrossRef]
- Jońca, J.; Pawnuk, M.; Arsen, A.; Sówka, I. Electronic Noses and Their Applications for Sensory and Analytical Measurements in the Waste Management Plants—A Review. Sensors 2022, 22, 1510. [Google Scholar] [CrossRef]
- Rodríguez-Méndez, M.L.; De Saja, J.A.; González-Antón, R.; García-Hernández, C.; Medina-Plaza, C.; García-Cabezón, C.; Martín-Pedrosa, F. Electronic Noses and Tongues in Wine Industry. Front. Bioeng. Biotechnol. 2016, 4, 81. [Google Scholar] [CrossRef]
- Sarlo, L.; Duroux, C.; Clément, Y.; Lanteri, P.; Rossetti, F.; David, O.; Tillement, O. Enhancing wine authentication: Leveraging 12,000+ international mineral wine profiles and artificial intelligence for accurate origin and variety prediction. OENO One 2024, 58. [Google Scholar] [CrossRef]
- Yin, X.-L.; Peng, Z.-X.; Pan, Y.; Lv, Y.; Long, W.; Gu, H.-W.; Fu, H.; She, Y. UHPLC-QTOF-MS-based untargeted metabolomic authentication of Chinese red wines according to their grape varieties. Int. Food Res. 2024, 178, 113923. [Google Scholar] [CrossRef]
- Mac, H.X.; Pham, T.T.; Ha, N.T.T.; Nguyen, L.L.P.; Baranyai, L.; Friedrich, L. Current Techniques for Fruit Juice and Wine Adulterant Detection and Authentication. Beverages 2023, 9, 84. [Google Scholar] [CrossRef]
- Epova, E.; Bérail, S.; Séby, F.; Vacchina, V.; Bareille, G.; Médina, B.; Sarthou, L.; Donard, O. Strontium elemental and isotopic signatures of Bordeaux wines for authenticity and geographical origin assessment. Food Chem. 2019, 294, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Li, Y.; Zhang, J.; Wang, L.; Rengasamy, K.W.; Zhang, A. Analysis of soils, grapes, and wines for Sr isotope characterization in Diqing Tibetan Autonomous Prefecture (China) and combining multiple elements for wine geographical traceability purposes. J. Food Compos. Anal. 2023, 122, 105470. [Google Scholar] [CrossRef]
- Cellier, R.; Bérail, S.; Barre, J.; Epova, E.; Claverie, F.; Ronzani, A.; Milcent, S.; Ors, P.; Donard, O. Analytical strategies for Sr and Pb isotopic signatures by MC-ICP-MS applied to the authentication of Champagne and other sparkling wines. Talanta 2021, 234, 122433. [Google Scholar] [CrossRef] [PubMed]
- Kochergina, Y.; Pavloušek, P.; Šanda, M.; Hora, J. 87Sr/86Sr isotopic composition of wine: Uses and limitations. OENO One 2024, 58. [Google Scholar] [CrossRef]
- Camin, F.; Boner, M.; Bontempo, L.; Fauhl-Hassek, C.; Kelly, S.D.; Riedl, J.; Rossmann, A. Stable isotope techniques for verifying the declared geographical origin of food in legal cases. Trends Food Sci. Technol. 2017, 61, 176–187. [Google Scholar] [CrossRef]
- Geana, E.I.; Popescu, R.; Costinel, D.; Dinca, O.R.; Stefanescu, I.; Ionete, R.E.; Bala, C. Verifying the red wines adulteration through isotopic and chromatographic investigations coupled with multivariate statistic interpretation of the data. Food Control 2016, 62, 1–9. [Google Scholar] [CrossRef]
- Wu, H.; Tian, L.; Chen, B.; Jin, B.; Tian, B.; Xie, L.; Rogers, K.M.; Lin, G. Verification of imported red wine origin into China using multi isotope and elemental analyses. Food Chem. 2019, 301, 125137. [Google Scholar] [CrossRef]
- Wu, H.; Lin, G.; Tian, L.; Yan, Z.; Yi, B.; Bian, X.; Jin, B.; Xie, L.; Zhou, H.; Rogers, K.M. Origin verification of French red wines using isotope and elemental analyses coupled with chemometrics. Food Chem. 2021, 339, 127760. [Google Scholar] [CrossRef]
- Martin, G.; Mazure, M.; Jouitteau, C.; Martin, Y.; Aguile, L.; Allain, P. Characterization of the Geographic Origin of Bordeaux Wines by a Combined Use of Isotopic and Trace Element Measurements. AJEV 1999, 50, 409–417. [Google Scholar] [CrossRef]
- Moehring, M.; Harrington, P. Analysis of Wine and Its Use in Tracing the Origin of Grape Cultivation. Crit. Rev. Anal. Chem. 2021, 52, 1901–1912. [Google Scholar] [CrossRef] [PubMed]
- Kokkinofta, R.; Fotakis, C.; Zervou, M.; Zoumpoulakis, P.; Savvidou, C.; Poulli, K.; Louka, C.; Economidou, N.; Tzioni, E.; Damianou, K.; et al. Isotopic and Elemental Authenticity Markers: A Case Study on Cypriot Wines. Food Anal. Methods 2017, 10, 3902–3913. [Google Scholar] [CrossRef]
- Guyon, F.; Douet, C.; Colas, S.; Salagoïty, M.; Médina, B. Effects of must concentration techniques on wine isotopic parameters. J. Agric. Food Chem. 2006, 54, 9918–9923. [Google Scholar] [CrossRef]
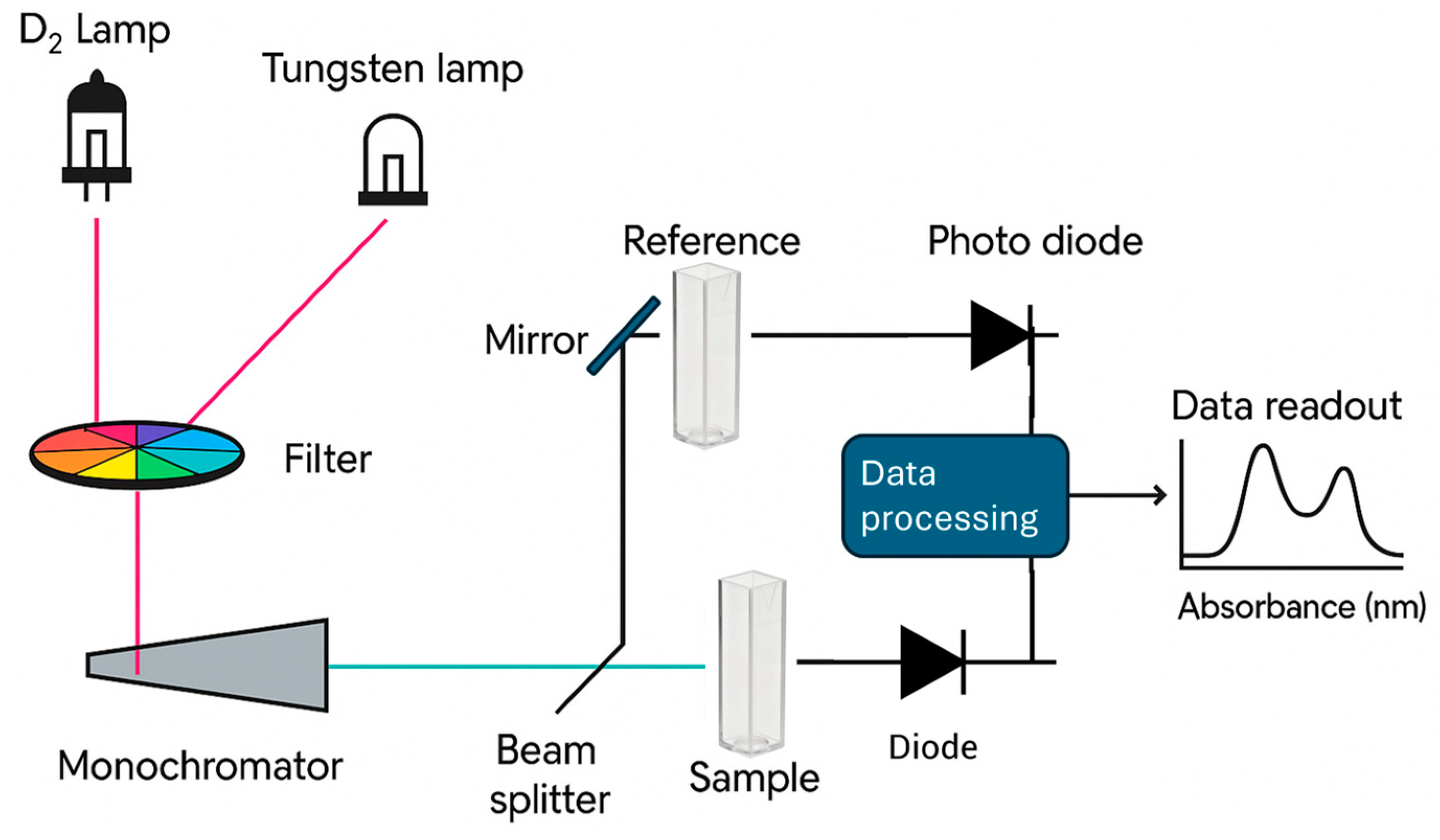


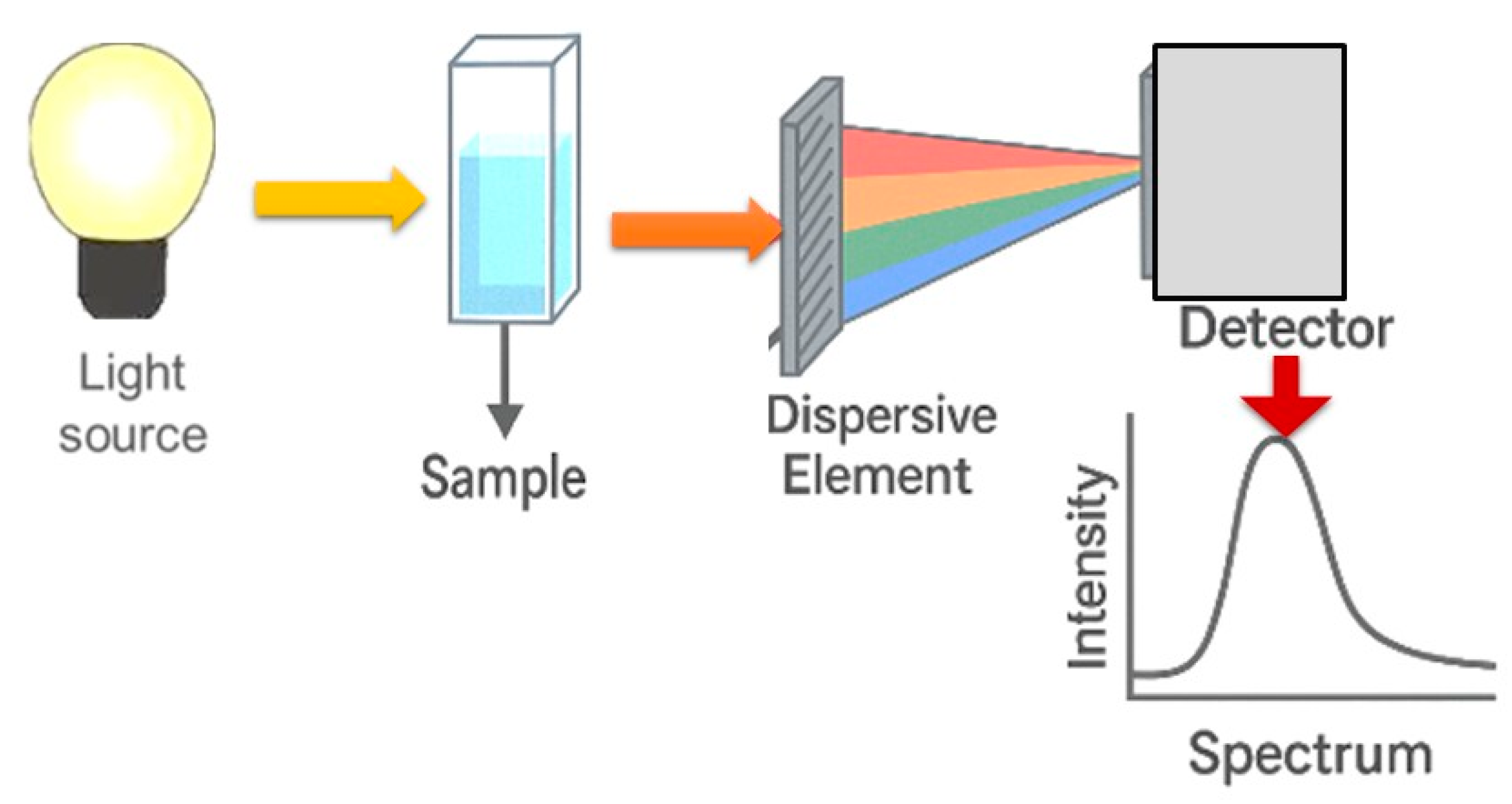
| Spectroscopic Technique | Application | Key Insights |
|---|---|---|
| NMR | Wine ingredient quantification and metabolomic fingerprinting | 1H NMR spectroscopy is gaining attention for its simplicity and speed, although it lacks official recognition. It allows for both targeted and non-targeted analysis, providing a comprehensive profile of wine samples [19,20]. |
| UV–Vis and FT-IR | Varietal and vintage year discrimination | Combined with chemometric analysis, these techniques effectively classify wines by grape variety and vintage year. UV–Vis is more effective for varietal discrimination, while FT-IR excels in vintage classification [1]. |
| Fluorescence Spectroscopy (A-TEEM) | Geographical and varietal authentication | A-TEEM, combined with machine learning, achieves high accuracy in classifying wines by variety and origin. It also effectively predicts phenolic compound concentrations [4,21,22]. |
| Raman and IR | Non-invasive wine-bottle authentication | These techniques analyze packaging labels to differentiate genuine bottles from counterfeit ones, offering a non-destructive authentication method [23]. |
| Data Fusion Approaches | PDO wine vinegar classification | Combining multiple spectroscopic techniques (MIR, NIR, EEM, 1H-NMR) enhances classification accuracy and provides a comprehensive analysis of wine vinegar [24]. |
| Wine Type/Region | Application of UV–Vis Spectroscopy | Ref. |
|---|---|---|
| Spanish Wine Vinegars | Used for the authentication and discrimination of wine vinegars by developing classification models. | [24] |
| Red Wines (DO Rías Baixas and DO Ribeira Sacra, Spain) | Determination of polyphenolic compounds using UV–Vis–NIR spectroscopy and chemometrics tools. | [33] |
| Sardinian Wines (Vermentino and Cannonau) | Characterization of polyphenolic fractions during winemaking using UV–Vis and FTIR spectroscopy. | [36] |
| Industrial and Commercial Wines | Monitoring phenolic composition and levels during winemaking using UV–Vis spectroscopy. | [31] |
| Red Wines (Austrian Cultivars) | Discrimination of wine cultivars using UV–Vis spectroscopy of phenolic extracts. | [38] |
| Chinese Rice Wines | Classification of wines from different geographical origins using UV–Vis spectroscopy. | [39] |
| Chinese Cabernet Sauvignon Wines | Geographical classification using data fusion of UV–Vis and synchronous fluorescence spectroscopies. | [40] |
| Red Wines | Quantification of polyphenols using UV–Vis and FTIR spectroscopy with chemometrics. | [32] |
| Wine Type/Variety | Application of MIR Spectroscopy | Ref. |
|---|---|---|
| Australian Red and White Wines | Varietal classification using MIR spectroscopy combined with PCA and LDA. | [47] |
| White Wines | Routine analysis of alcoholic strength, total acidity, and other parameters using MIR. | [48] |
| Chokeberry, Blackberry, Raspberry Fruit Wines | Authentication and classification of fruit wines using FT-MIR and chemometrics. | [49] |
| Red Wines | Prediction of phenolic compounds during fermentation using ATR-MIR spectroscopy. | [31] |
| Various Grape Varieties | Prediction of chemical compositions using combined visible and MIR spectroscopies. | [50] |
| Cabernet Sauvignon and Chardonnay | Modeling sensory properties of wines using MIR spectra. | [51] |
| Chinese Rice Wine | Determination of sugars and acids using FT-MIR spectroscopy. | [52] |
| Italian Straw Wine | Prediction of alcohol content, sugar levels, and total acidity using FT-MIR spectroscopy. | [53] |
| Wine Type | Application of NIR Spectroscopy | Ref. |
|---|---|---|
| Wine from the Vinho Verde region | Quantification of volatile compounds using FT-NIR transmission spectroscopy. | [65] |
| Red Wine | Prediction of phenolic compounds during fermentation using NIR spectroscopy. | [66] |
| Red Wine Grapes | Quality assessment and quantification of quality attributes like SSC, TA, and tannins. | [67] |
| Various Wines | Classification of wines based on the aging process using NIR spectroscopy and multivariate analysis. | [68] |
| Australian Wines | Measurement of elemental concentrations using VIS-NIR spectroscopy. | [69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, T.; Cosme, F.; Correia, E.; Vilela, A. Spectroscopic Techniques Application for Wine and Wine Byproduct Authentication. Appl. Sci. 2025, 15, 4457. https://doi.org/10.3390/app15084457
Pinto T, Cosme F, Correia E, Vilela A. Spectroscopic Techniques Application for Wine and Wine Byproduct Authentication. Applied Sciences. 2025; 15(8):4457. https://doi.org/10.3390/app15084457
Chicago/Turabian StylePinto, Teresa, Fernanda Cosme, Elisete Correia, and Alice Vilela. 2025. "Spectroscopic Techniques Application for Wine and Wine Byproduct Authentication" Applied Sciences 15, no. 8: 4457. https://doi.org/10.3390/app15084457
APA StylePinto, T., Cosme, F., Correia, E., & Vilela, A. (2025). Spectroscopic Techniques Application for Wine and Wine Byproduct Authentication. Applied Sciences, 15(8), 4457. https://doi.org/10.3390/app15084457








