RACF: A Multimodal Deep Learning Framework for Parkinson’s Disease Diagnosis Using SNP and MRI Data
Abstract
1. Introduction
2. Materials and Methods
2.1. Dataset and Preprocessing
2.1.1. SNP Preprocessing
2.1.2. MRI Preprocessing
2.2. Feature Extraction Networks
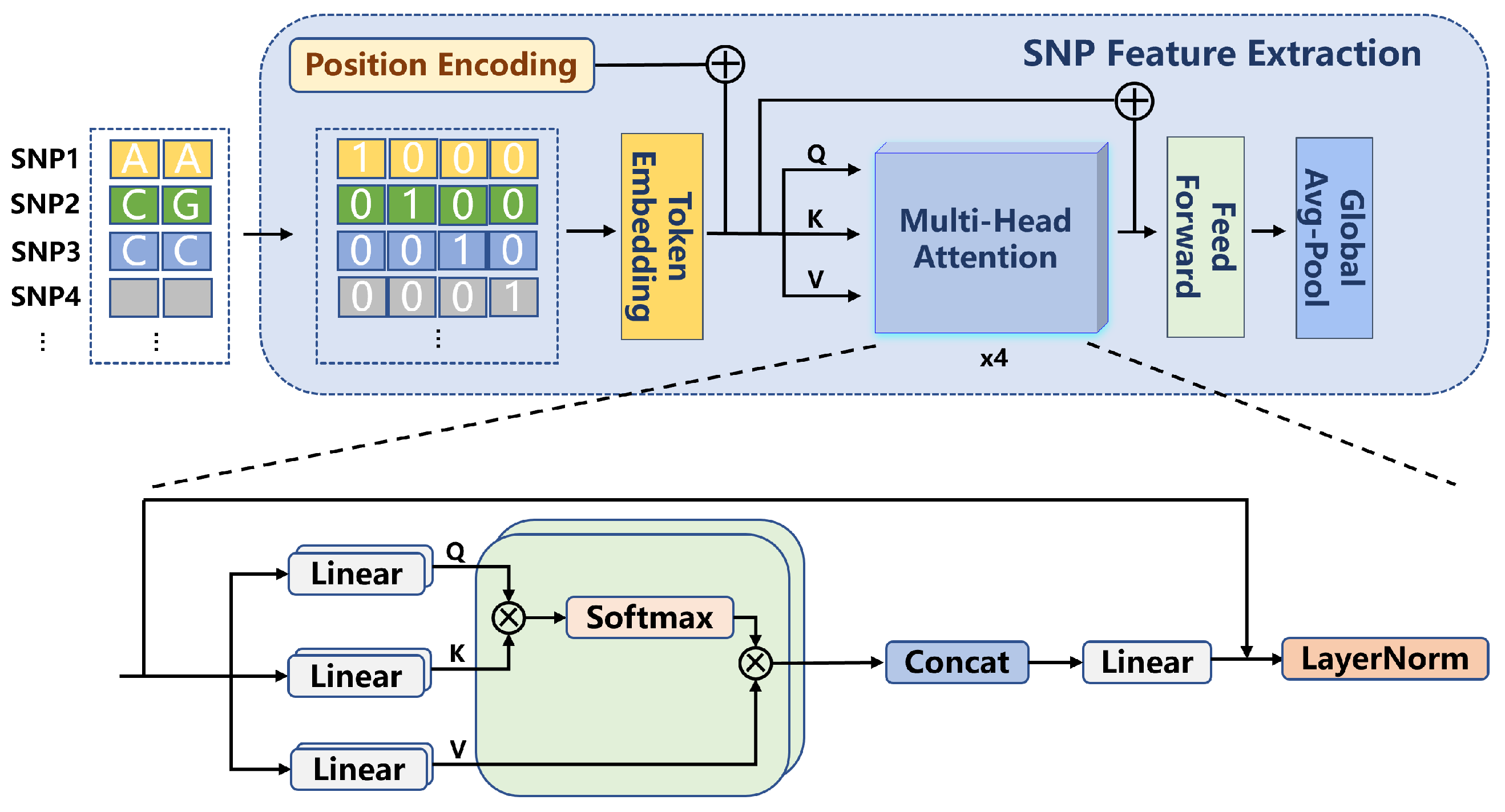
2.2.1. SNP Feature Extraction
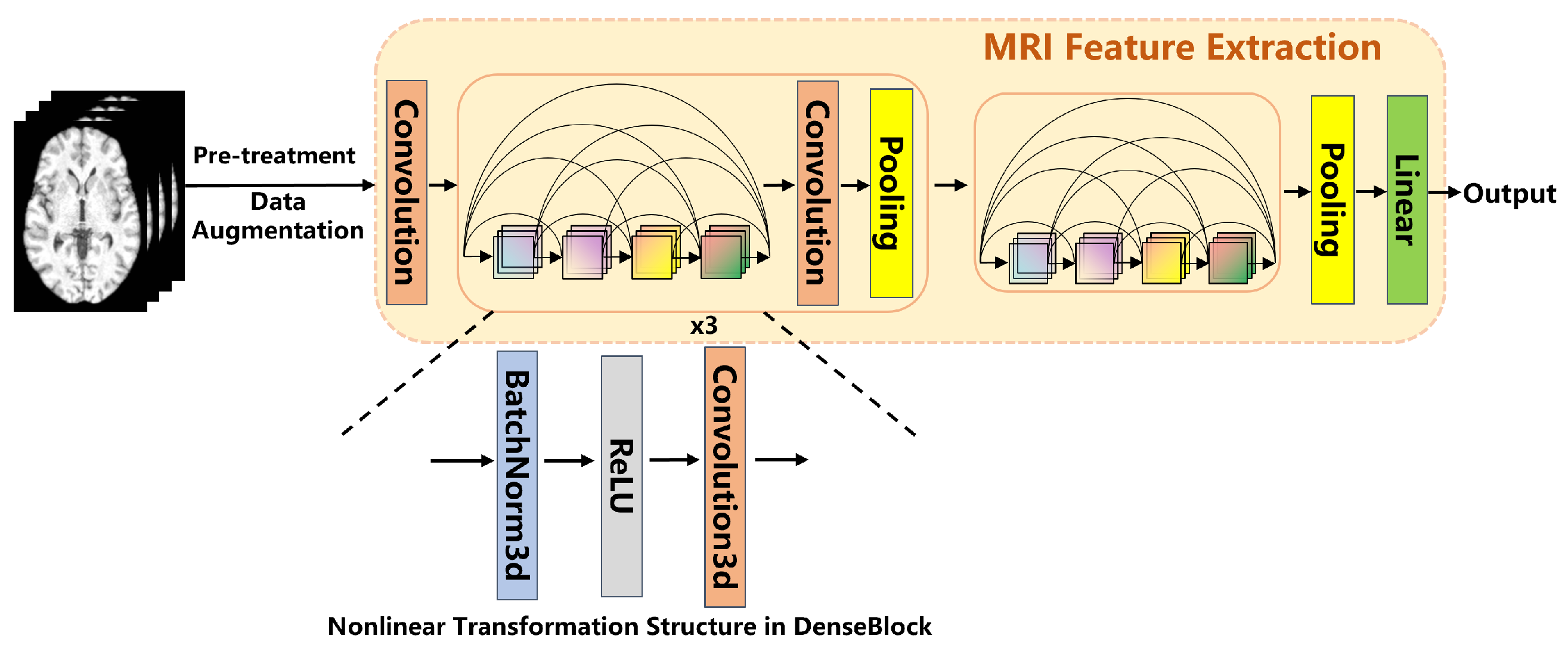
2.2.2. MRI Feature Extraction
2.3. Multimodal Fusion Strategy
2.4. Training and Evaluation
2.5. Comparative Experiments
3. Experimental Results
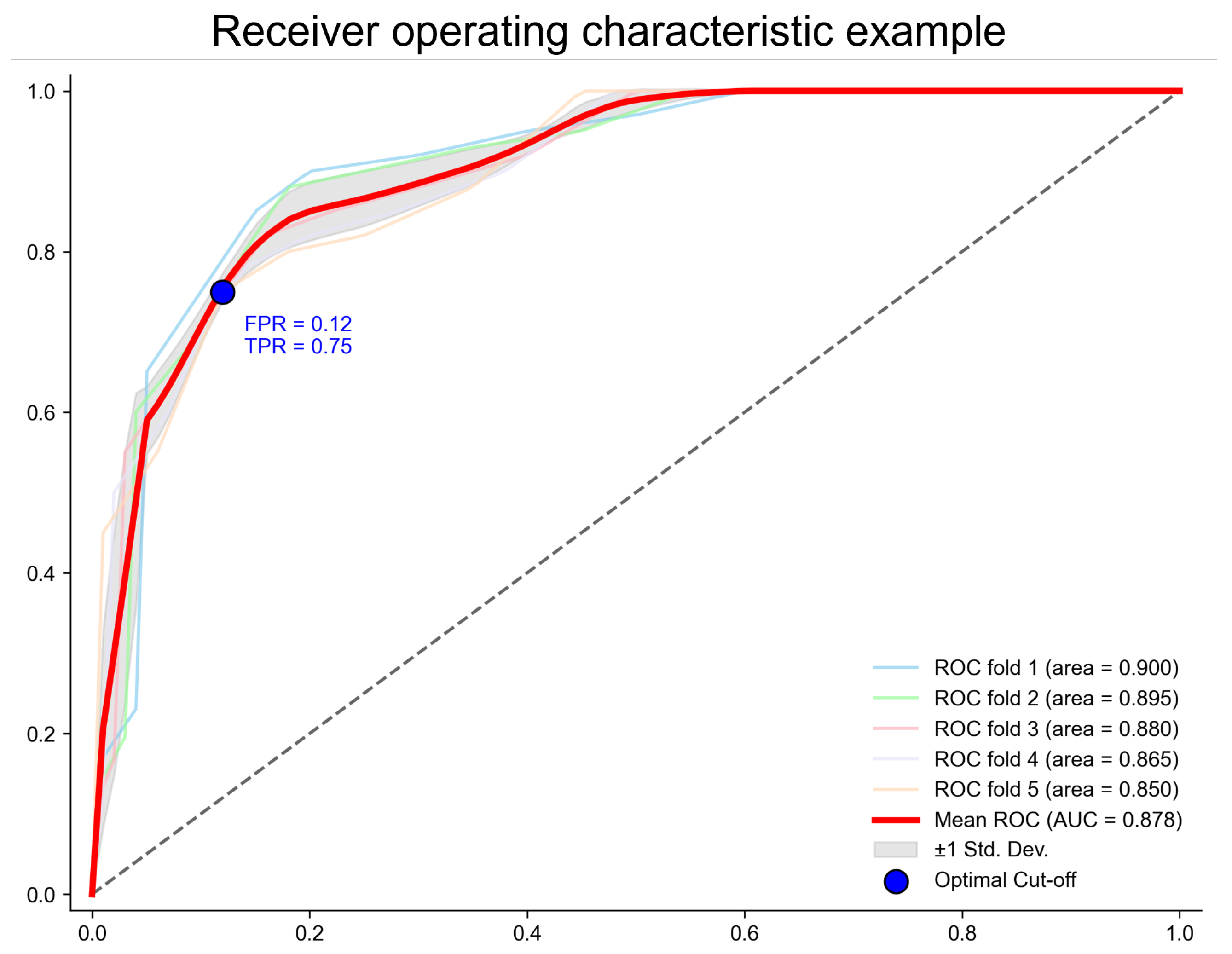
3.1. Model Performance and Method Comparison
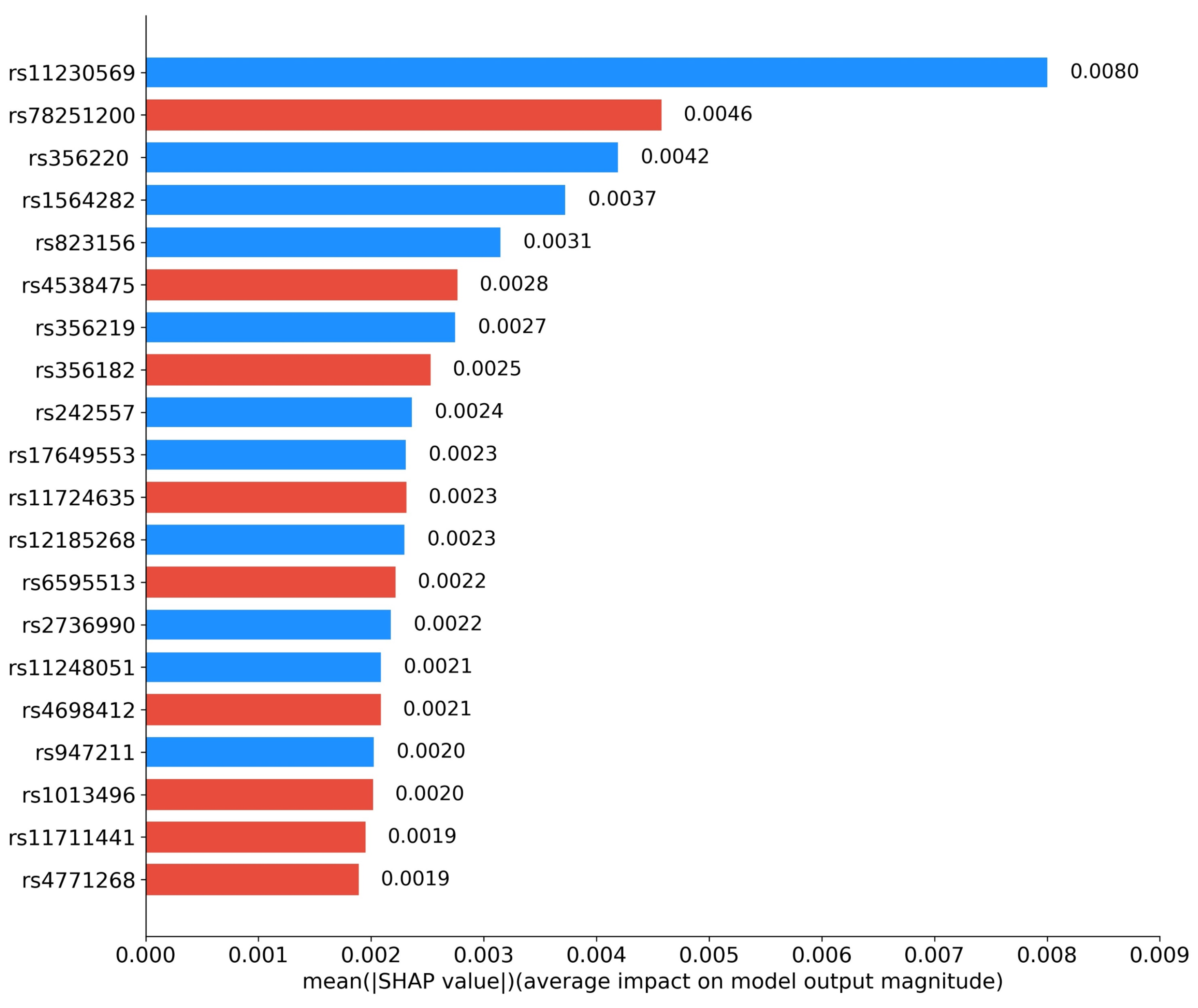
3.2. Abnormal Brain Regions and Pathogenic Gene Analysis
3.3. Ablation Studies
4. Discussion
4.1. Advantages and Innovations of the RACF Framework for Multimodal Fusion
4.2. Biological Validation from Genetic and Imaging Perspectives
4.2.1. Genetic Analysis
4.2.2. Imaging Analysis
4.3. Study Limitations and Future Directions
5. Conclusions
- Unbiased SNP Feature Extraction: In the genetic feature extraction stage, the GWAS-Transformer architecture enables unbiased SNP screening without relying on prior knowledge of known risk loci.
- Cross-Modal Fusion Strategy: The proposed residual-attention contrastive fusion strategy facilitates the efficient fusion of multimodal features, fully leveraging the complementary nature of SNP and sMRI data, thereby significantly enhancing classification performance.
- Interpretable Risk Locus Discovery: By analyzing the contribution of model decisions, the framework successfully identifies potential PD-associated risk loci. These findings provide novel insights into the genetic mechanisms of PD and validate the interpretability advantages of RACF.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tysnes, O.B.; Storstein, A. Epidemiology of Parkinson’s Disease. J. Neural Transm. 2017, 124, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the Diagnosis of Parkinson’s Disease. Lancet Neurol. 2021, 20, 385–397. [Google Scholar] [CrossRef]
- De Virgilio, A.; Greco, A.; Fabbrini, G.; Inghilleri, M.; Rizzo, M.I.; Gallo, A.; Conte, M.; Rosato, C.; Appiani, M.C.; de Vincentiis, M. Parkinson’s Disease: Autoimmunity and Neuroinflammation. Autoimmun. Rev. 2016, 15, 1005–1011. [Google Scholar] [CrossRef]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson Disease. Nat. Rev. Dis. Prim. 2017, 3, 17013. [Google Scholar] [CrossRef]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale Presentation and Clinimetric Testing Results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef]
- Gupta, U.; Bansal, H.; Joshi, D. An Improved Sex-Specific and Age-Dependent Classification Model for Parkinson’s Diagnosis Using Handwriting Measurement. Comput. Methods Programs Biomed. 2020, 189, 105305. [Google Scholar] [CrossRef]
- Giannopoulos, A.E.; Zioga, I.; Papageorgiou, P.C.; Kapsali, F.; Spantideas, S.T.; Kapsalis, N.C.; Capsalis, C.N.; Kontoangelos, K.; Papageorgiou, C.C. Early auditory-evoked potentials in body dysmorphic disorder: An ERP/sLORETA study. Psychiatry Res. 2021, 299, 113865. [Google Scholar] [CrossRef]
- Rangaprakash, D.; Tadayonnejad, R.; Deshpande, G.; O’Neill, J.; Feusner, J.D. FMRI hemodynamic response function (HRF) as a novel marker of brain function: Applications for understanding obsessive-compulsive disorder pathology and treatment response. Brain Imaging Behav. 2021, 15, 1622–1640. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Li, F.; Niu, G.; Chen, X. PET imaging of inflammation biomarkers. Theranostics 2013, 3, 448. [Google Scholar] [CrossRef]
- Cirrincione, G.; Cannata, S.; Cicceri, G.; Prinzi, F.; Currieri, T.; Lovino, M.; Militello, C.; Pasero, E.; Vitabile, S. Transformer-based approach to melanoma detection. Sensors 2023, 23, 5677. [Google Scholar] [CrossRef]
- Bektaş, B.; Emre, İ.E.; Kartal, E.; Gulsecen, S. Classification of mammography images by machine learning techniques. In Proceedings of the 2018 3rd International Conference on Computer Science and Engineering (UBMK), Sarajevo, Bosnia and Herzegovina, 20–23 September 2018; IEEE: New York, NY, USA, 2018; pp. 580–585. [Google Scholar]
- Chakraborty, S.; Aich, S.; Kim, H.C. Detection of Parkinson’s Disease from 3T T1 Weighted MRI Scans Using 3D Convolutional Neural Network. Diagnostics 2020, 10, 402. [Google Scholar] [CrossRef]
- Solana-Lavalle, G.; Rosas-Romero, R. Classification of PPMI MRI Scans with Voxel-Based Morphometry and Machine Learning to Assist in the Diagnosis of Parkinson’s Disease. Comput. Methods Programs Biomed. 2021, 198, 105793. [Google Scholar] [CrossRef]
- Sangeetha, S.; Baskar, K.; Kalaivaani, P.C.D.; Kumaravel, T. Deep Learning-Based Early Parkinson’s Disease Detection from Brain MRI Image. In Proceedings of the 2023 7th International Conference on Intelligent Computing and Control Systems (ICICCS), Madurai, India, 17–19 May 2023; pp. 490–495. [Google Scholar]
- Kaplan, E.; Altunisik, E.; Firat, Y.E.; Barua, P.D.; Dogan, S.; Baygin, M.; Demir, F.B.; Tuncer, T.; Palmer, E.; Tan, R.S.; et al. Novel Nested Patch-Based Feature Extraction Model for Automated Parkinson’s Disease Symptom Classification Using MRI Images. Comput. Methods Programs Biomed. 2022, 224, 107030. [Google Scholar] [CrossRef] [PubMed]
- Karthigeyan, C.M.T.; Rani, C. Optimizing Parkinson’s Disease Diagnosis with Multimodal Data Fusion Techniques. Inf. Technol. Control 2024, 53, 262–279. [Google Scholar] [CrossRef]
- Li, W.; Rao, Q.; Dong, S.; Zhu, M.; Yang, Z.; Huang, X.; Liu, G. PIDGN: An Explainable Multimodal Deep Learning Framework for Early Prediction of Parkinson’s Disease. J. Neurosci. Methods 2025, 110363. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.A.; Hu, X.; Xie, Y.; Wu, H. A Novel CERNNE Approach for Predicting Parkinson’s Disease-Associated Genes and Brain Regions Based on Multimodal Imaging Genetics Data. Med. Image Anal. 2021, 67, 101830. [Google Scholar] [CrossRef]
- Kanyal, A.; Mazumder, B.; Calhoun, V.D.; Preda, A.; Turner, J.; Ford, J.; Ye, D.H. Multi-Modal Deep Learning from Imaging Genomic Data for Schizophrenia Classification. Front. Psychiatry 2024, 15, 1384842. [Google Scholar] [CrossRef]
- Sheng, J.; Xin, Y.; Zhang, Q.; Wang, L.; Yang, Z.; Yin, J. Predictive Classification of Alzheimer’s Disease Using Brain Imaging and Genetic Data. Sci. Rep. 2022, 12, 2405. [Google Scholar] [CrossRef]
- Hearst, M.A.; Dumais, S.T.; Osuna, E.; Platt, J.; Scholkopf, B. Support Vector Machines. IEEE Intell. Syst. Their Appl. 1998, 13, 18–28. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. Xgboost: A Scalable Tree Boosting System. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; pp. 785–794. [Google Scholar]
- Popescu, M.C.; Balas, V.E.; Perescu-Popescu, L.; Mastorakis, N. Multilayer Perceptron and Neural Networks. WSEAS Trans. Circuits Syst. 2009, 8, 579–588. [Google Scholar]
- Gu, S.C.; Ye, Q.; Yuan, C.X. Metabolic Pattern Analysis of 18F-FDG PET as a Marker for Parkinson’s Disease: A Systematic Review and Meta-Analysis. Rev. Neurosci. 2019, 30, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Dong, L.; Huang, X.; Zheng, S.; Qiu, P.; Lan, F. Associations of rs823128, rs1572931, and rs823156 Polymorphisms with Reduced Parkinson’s Disease Risks. Neuroreport 2017, 28, 936–941. [Google Scholar] [CrossRef]
- Guo, X.Y.; Chen, Y.P.; Song, W.; Zhao, B.; Cao, B.; Wei, Q.Q.; Ou, R.W.; Yang, Y.; Yuan, L.X.; Shang, H.F. SNCA Variants rs2736990 and rs356220 as Risk Factors for Parkinson’s Disease but Not for Amyotrophic Lateral Sclerosis and Multiple System Atrophy in a Chinese Population. Neurobiol. Aging 2014, 35, 2882.e1–2882.e6. [Google Scholar] [CrossRef]
- Compta, Y.; Ezquerra, M.; Muñoz, E.; Tolosa, E.; Valldeoriola, F.; Rios, J.; Cámara, A.; Fernández, M.; Buongiorno, M.T.; Marti, M.J. High Cerebrospinal Tau Levels Are Associated with the rs242557 Tau Gene Variant and Low Cerebrospinal β-Amyloid in Parkinson Disease. Neurosci. Lett. 2011, 487, 169–173. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, B.; Li, G.; Zhou, L.; Zhang, L.; Liu, J. MAPT rs17649553 T Allele is Associated with Better Verbal Memory and Higher Small-World Properties in Parkinson’s Disease. Neurobiol. Aging 2023, 129, 219–231. [Google Scholar] [CrossRef]
- Xia, H.; Luo, Q.; Li, X.X.; Yang, X.L. Association between PARK16 Gene Polymorphisms and Susceptibility of Parkinson’s Disease in a Chinese Population. Genet. Mol. Res. 2015, 14, 2978–2985. [Google Scholar] [CrossRef]
- Chen, Y.P.; Song, W.; Huang, R.; Chen, K.; Zhao, B.; Li, J.; Yang, Y.; Shang, H.F. GAK rs1564282 and DGKQ rs11248060 Increase the Risk for Parkinson’s Disease in a Chinese Population. J. Clin. Neurosci. 2013, 20, 880–883. [Google Scholar] [CrossRef]
- Mogi, M.; Harada, M.; Narabayashi, H.; Inagaki, H.; Minami, M.; Nagatsu, T. Interleukin (IL)-1β, IL-2, IL-4, IL-6 and Transforming Growth Factor-α Levels Are Elevated in Ventricular Cerebrospinal Fluid in Juvenile Parkinsonism and Parkinson’s Disease. Neurosci. Lett. 1996, 211, 13–16. [Google Scholar] [CrossRef]
- Saad, M.; Lesage, S.; Saint-Pierre, A.; Corvol, J.C.; Zelenika, D.; Lambert, J.C.; Vidailhet, M.; Mellick, G.D.; Lohmann, E.; Durif, F.; et al. Genome-Wide Association Study Confirms BST1 and Suggests a Locus on 12q24 as the Risk Loci for Parkinson’s Disease in the European Population. Hum. Mol. Genet. 2011, 20, 615–627. [Google Scholar] [CrossRef]
- Li, N.N.; Tan, E.K.; Chang, X.L.; Mao, X.Y.; Zhao, D.M.; Zhang, J.H.; Liao, Q.; Peng, R. MCCC1/LAMP3 Reduces Risk of Sporadic Parkinson’s Disease in Han Chinese. Acta Neurol. Scand. 2013, 128, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Li, Y.; Niu, M.; Li, G.; Luo, N.; Zhou, L.; Kang, W.; Liu, J. SNPs in SNCA, MCCC1, DLG2, GBF1 and MBNL2 Are Associated with Parkinson’s Disease in Southern Chinese Population. J. Cell. Mol. Med. 2020, 24, 8744–8752. [Google Scholar] [CrossRef]
- Griffanti, L.; Rolinski, M.; Szewczyk-Krolikowski, K.; Menke, R.A.; Filippini, N.; Zamboni, G.; Jenkinson, M.; Hu, M.T.; Mackay, C.E. Challenges in the Reproducibility of Clinical Studies with Resting State fMRI: An Example in Early Parkinson’s Disease. NeuroImage 2016, 124, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Vaillancourt, D.E.; Spraker, M.B.; Prodoehl, J.; Abraham, I.; Corcos, D.M.; Zhou, X.J.; Comella, C.L.; Little, D.M. High-Resolution Diffusion Tensor Imaging in the Substantia Nigra of De Novo Parkinson Disease. Neurology 2009, 72, 1378–1384. [Google Scholar] [CrossRef]
- Zheng, Z.; Shemmassian, S.; Wijekoon, C.; Kim, W.; Bookheimer, S.Y.; Pouratian, N. DTI Correlates of Distinct Cognitive Impairments in Parkinson’s Disease. Hum. Brain Mapp. 2014, 35, 1325–1333. [Google Scholar] [CrossRef]
- Wu, T.; Long, X.; Wang, L.; Hallett, M.; Zang, Y.; Li, K.; Chan, P. Functional Connectivity of Cortical Motor Areas in the Resting State in Parkinson’s Disease. Hum. Brain Mapp. 2011, 32, 1443–1457. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, Y.; Zhang, Y.; Wang, F.; Yu, H.; Zhang, C.; Jiang, Z.; Luo, W. Iron Deposition in Parkinson’s Disease by Quantitative Susceptibility Mapping. BMC Neurosci. 2019, 20, 1–8. [Google Scholar] [CrossRef]
- Cassidy, C.M.; Zucca, F.A.; Girgis, R.R.; Baker, S.C.; Weinstein, J.J.; Sharp, M.E.; Bellei, C.; Valmadre, A.; Vanegas, N.; Kegeles, L.S.; et al. Neuromelanin-Sensitive MRI as a Noninvasive Proxy Measure of Dopamine Function in the Human Brain. Proc. Natl. Acad. Sci. USA 2019, 116, 5108–5117. [Google Scholar] [CrossRef]
- Fearnley, J.M.; Lees, A.J. Ageing and Parkinson’s Disease: Substantia Nigra Regional Selectivity. Brain 1991, 114, 2283–2301. [Google Scholar] [CrossRef]
- Rohrer, J.D.; Lashley, T.; Schott, J.M.; Warren, J.E.; Mead, S.; Isaacs, A.M.; Beck, J.; Hardy, J.; De Silva, R.; Warrington, E.; et al. Clinical and Neuroanatomical Signatures of Tissue Pathology in Frontotemporal Lobar Degeneration. Brain 2011, 134, 2565–2581. [Google Scholar] [CrossRef]
- Grahn, J.A.; Parkinson, J.A.; Owen, A.M. The Cognitive Functions of the Caudate Nucleus. Prog. Neurobiol. 2008, 86, 141–155. [Google Scholar] [CrossRef]


| Label | Number | Age (Mean ± SD) | Gender (M/F) | UPDRS (Mean ± SD) |
|---|---|---|---|---|
| PD | 285 | 61.7 ± 9.6 | 184/101 | 69.86 ± 24.56 |
| HC | 139 | 60.5 ± 11.7 | 95/44 | 2.16 ± 2.84 |
| Data | Model | Accuracy | Precision | Recall | F1 Score |
|---|---|---|---|---|---|
| MRI | 3D DenseNet | 0.841 | 0.864 | 0.822 | 0.831 |
| SNP | Transformer | 0.801 | 0.767 | 0.958 | 0.852 |
| Fusion | Ours | 0.912 | 0.819 | 0.807 | 0.943 |
| Model | Accuracy | Precision | Recall | F1 Score |
|---|---|---|---|---|
| SVM [21] | 0.749 | 0.729 | 0.713 | 0.844 |
| RF [22] | 0.711 | 0.744 | 0.894 | 0.848 |
| XGB [23] | 0.764 | 0.751 | 0.853 | 0.807 |
| MLP [24] | 0.809 | 0.843 | 0.752 | 0.818 |
| Kanyal et al. [19] | 0.864 | 0.814 | 0.802 | 0.815 |
| Sheng et al. [20] | 0.827 | 0.778 | 0.876 | 0.836 |
| PIDGN [17] | 0.895 | 0.848 | 0.903 | 0.912 |
| Ours | 0.912 | 0.859 | 0.895 | 0.943 |
| SNP | Chromosome | Gene | Function |
|---|---|---|---|
| rs11230569 | 11 | SLC41A1 | The balance of magnesium ions in the brain |
| rs78251200 | 11 | SAAL1 | Immune regulation |
| rs356220 | 4 | SNCA | Accumulation of alpha-synuclein |
| rs1564282 | 4 | GAK | Regulation of cell division and microtubule stability |
| rs823156 | 11 | SLC41A1 | The balance of magnesium ions in the brain |
| rs4538475 | 4 | BST1 | Intracellular calcium ion regulation |
| rs356219 | 4 | SNCA | Accumulation of alpha-synuclein |
| rs356182 | 4 | LOC124900602 | Mitochondrial regulation |
| rs242557 | 17 | MAPT | Encoding tau protein |
| rs17649553 | 17 | MAPT | Affecting language memory ability |
| rs11724635 | 4 | BST1 | Intracellular calcium ion regulation |
| rs12185268 | 17 | MAPT | Encoding tau protein |
| rs6595513 | 5 | LINC01170 | Intron variant |
| rs2736990 | 4 | SNCA | Accumulation of alpha-synuclein |
| rs11248051 | 4 | GAK | Regulation of cell division and microtubule stability |
| rs4698412 | 4 | BST1 | Regulation of NAD+ metabolism and immune system activity |
| rs947211 | 1 | PARK16 | Regulation of RAB7L1 gene expression |
| rs1013496 | 5 | LINC01170 | Intron variant |
| rs11711441 | 3 | MCCC1/LAMP3 | Amino acid metabolism / cellular immunity |
| rs4771268 | 13 | MBNL2 | Neural development and neuronal function |
| Method | Accuracy | Precision | Recall | F1 Score |
|---|---|---|---|---|
| without Contrastive Learning | 0.849 | 0.829 | 0.843 | 0.818 |
| without Residual Connection | 0.717 | 0.763 | 0.735 | 0.723 |
| RACF → Simple feature concatenation | 0.697 | 0.751 | 0.667 | 0.641 |
| Ours (Full Model) | 0.912 | 0.859 | 0.895 | 0.943 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, J.; Long, X. RACF: A Multimodal Deep Learning Framework for Parkinson’s Disease Diagnosis Using SNP and MRI Data. Appl. Sci. 2025, 15, 4513. https://doi.org/10.3390/app15084513
Cao J, Long X. RACF: A Multimodal Deep Learning Framework for Parkinson’s Disease Diagnosis Using SNP and MRI Data. Applied Sciences. 2025; 15(8):4513. https://doi.org/10.3390/app15084513
Chicago/Turabian StyleCao, Jiangbo, and Xiaojing Long. 2025. "RACF: A Multimodal Deep Learning Framework for Parkinson’s Disease Diagnosis Using SNP and MRI Data" Applied Sciences 15, no. 8: 4513. https://doi.org/10.3390/app15084513
APA StyleCao, J., & Long, X. (2025). RACF: A Multimodal Deep Learning Framework for Parkinson’s Disease Diagnosis Using SNP and MRI Data. Applied Sciences, 15(8), 4513. https://doi.org/10.3390/app15084513






