From Vine to Wine: Non-Colored Flavonoids as Fingerprints
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Red Wine Samples
2.2. Analytical Methods
2.3. Statistical Analysis
3. Results
3.1. Univariate Analysis
3.1.1. Grape Cultivar
3.1.2. Geographical Origin
Island of Precedence
Denomination of Origin in Tenerife Island
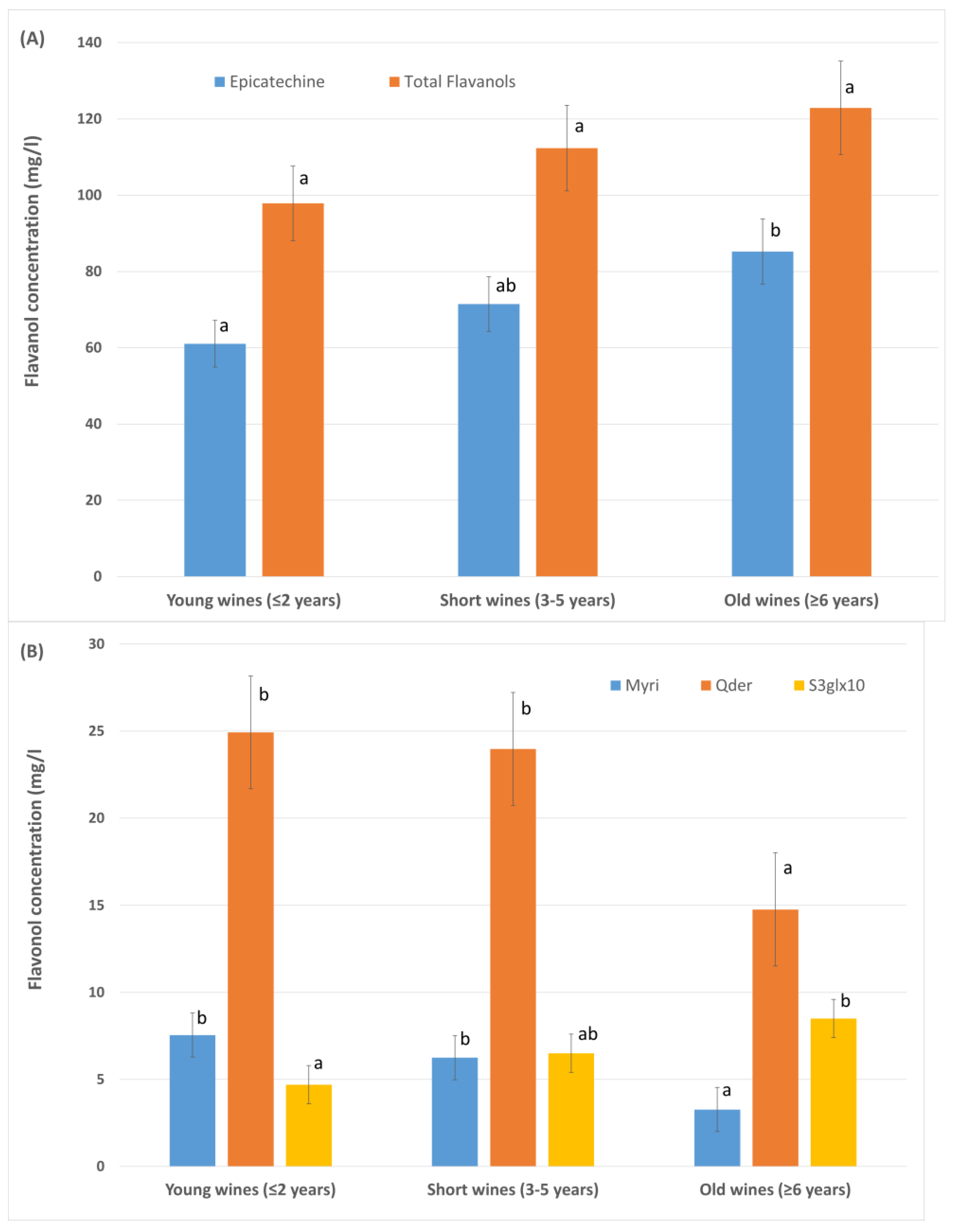
3.1.3. Aging
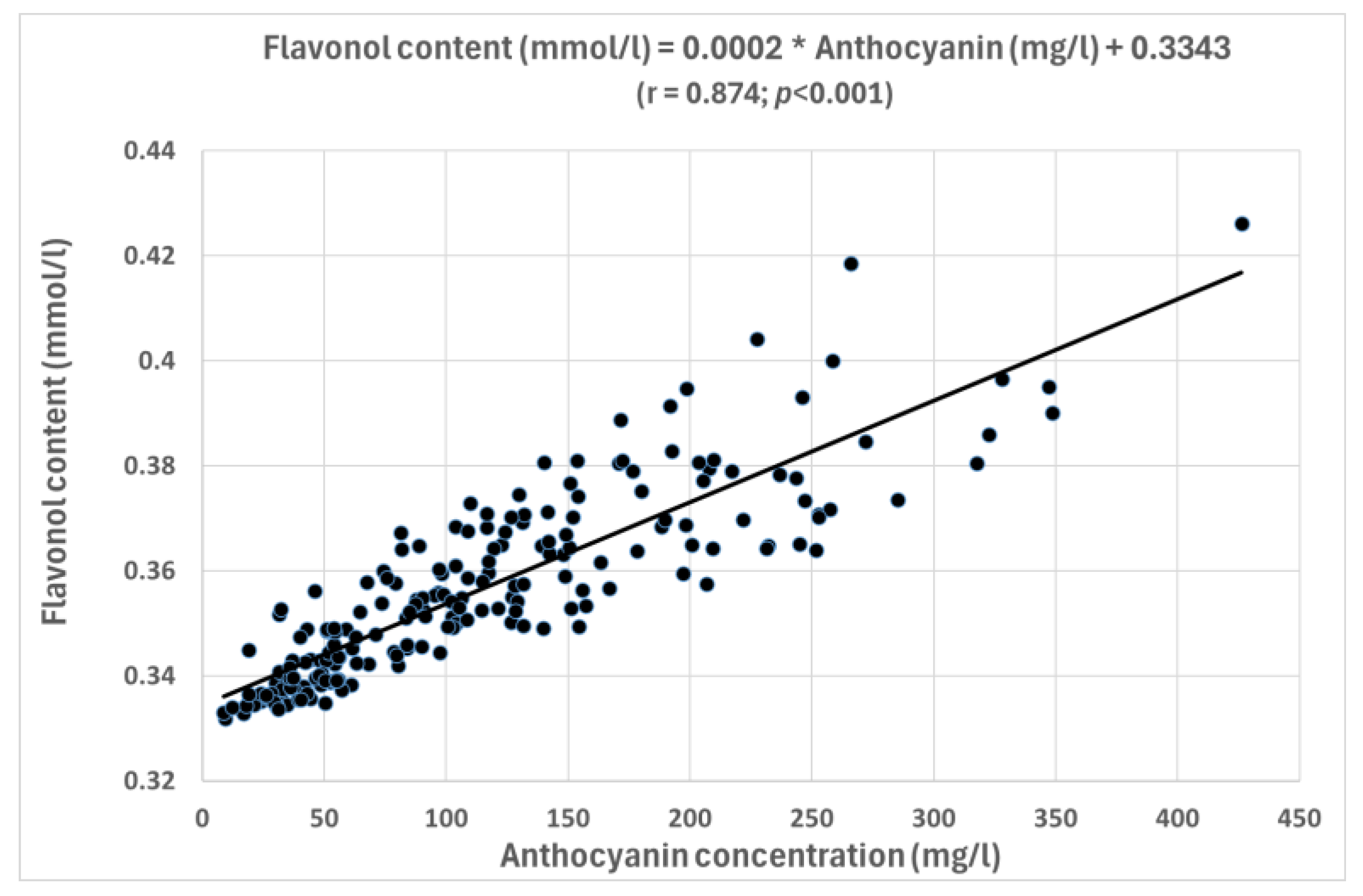
3.2. Correlation Study
3.3. Multivariate Analysis
3.3.1. Principal Compound Analysis
3.3.2. Linear Discriminant Analysis
4. Discussion
4.1. Univariate Analyses
4.1.1. Cultivar
4.1.2. Geographical Origin
4.1.3. Aging
4.2. Bivariate Analysis
4.3. Multivariate Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gutiérrez-Escobar, R.; Aliaño-González, M.J.; Cantos-Villar, E. Wine polyphenol content and its influence on wine quality and properties: A review. Molecules 2021, 26, 718. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; LaRocca, C.A.; Bernat, J.D.; Lindsey, J.S. Digital database of absorption spectra of diverse flavonoids enables structural comparisons and quantitative evaluations. J. Nat. Prod. 2023, 86, 1087–1119. [Google Scholar] [CrossRef]
- He, F.; Mu, L.; Yan, G.; Liang, N.; Pan, Q.; Wang, J.; Reeves, M.J.; Duan, C. Biosynthesis of anthocyanins and their regulation in colored grapes. Molecules 2010, 15, 9057–9091. [Google Scholar] [CrossRef] [PubMed]
- Cheynier, V. Polyphenols in foods are more complex than often thought. Am. J. Clin. Nutr. 2005, 81, 223S–229S. [Google Scholar] [CrossRef]
- Dixon, R.A.; Paiva, N.L. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085. [Google Scholar] [CrossRef]
- Xie, D.; Sharma, S.B.; Paiva, N.L.; Ferreira, D.; Dixon, R.A. Role of anthocyanidin reductase, encoded by BANYULS in plant flavonoid biosynthesis. Science 2003, 299, 396–399. [Google Scholar] [CrossRef]
- Bogs, J.; Downey, M.O.; Harvey, J.S.; Ashton, A.R.; Tanner, G.J.; Robinson, S.P. Proanthocyanidin synthesis and expression of genes encoding leucoanthocyanidin reductase and anthocyanidin reductase in developing grape berries and grapevine leaves. Plant Physiol. 2005, 139, 652–663. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, J.; Austin, M.B.; Stewart Jr, C.; Noel, J.P. Structure and function of enzymes involved in the biosynthesis of phenylpropanoids. Plant Physiol. Biochem. 2008, 46, 356–370. [Google Scholar] [CrossRef]
- Mattivi, F.; Guzzon, R.; Vrhovsek, U.; Stefanini, M.; Velasco, R. Metabolite profiling of grape: Flavonols and anthocyanins. J. Agric. Food Chem. 2006, 54, 7692–7702. [Google Scholar] [CrossRef]
- Agati, G.; Tattini, M. Multiple functional roles of flavonoids in photoprotection. New Phytol. 2010, 186, 786–793. [Google Scholar] [CrossRef]
- Obreque-Slier, E.; Peña-Neira, Á.; López-Solís, R.; Zamora-Marín, F.; Ricardo-da Silva, J.M.; Laureano, O. Comparative study of the phenolic composition of seeds and skins from Carménère and Cabernet Sauvignon grape varieties (Vitis vinifera L.) during ripening. J. Agric. Food Chem. 2010, 58, 3591–3599. [Google Scholar] [CrossRef]
- Versari, A.; Laurie, V.F.; Ricci, A.; Laghi, L.; Parpinello, G.P. Progress in authentication, typification and traceability of grapes and wines by chemometric approaches. Food Res. Int. 2014, 60, 2–18. [Google Scholar] [CrossRef]
- Badia-Melis, R.; Mishra, P.; Ruiz-García, L. Food traceability: New trends and recent advances. A review. Food Control 2015, 57, 393–401. [Google Scholar] [CrossRef]
- Koljančić, N.; Furdíková, K.; de Araújo Gomes, A.; Špánik, I. Wine authentication: Current progress and state of the art. Trends Food Sci. Technol. 2024, 150, 104598. [Google Scholar] [CrossRef]
- Heras-Roger, J.; Díaz-Romero, C. From Vine to Wine: Coloured Phenolics as Fingerprints. Appl. Sci. 2025, 15, 1755. [Google Scholar] [CrossRef]
- Ibern-Gómez, M.; Andrés-Lacueva, C.; Lamuela-Raventós, R.M.; Waterhouse, A.L. Rapid HPLC analysis of phenolic compounds in red wines. Am. J. Enol. Vitic. 2002, 53, 218–221. [Google Scholar] [CrossRef]
- Baiano, A.; Terracone, C. Varietal differences among the phenolic profiles and antioxidant activities of seven table grape cultivars grown in the south of Italy based on chemometrics. J. Agric. Food Chem. 2011, 59, 9815–9826. [Google Scholar] [CrossRef]
- Ginjom, I.; D’Arcy, B.; Caffin, N.; Gidley, M. Phenolic compound profiles in selected Queensland red wines at all stages of the wine-making process. Food Chem. 2011, 125, 823–834. [Google Scholar] [CrossRef]
- Meng, J.; Fang, Y.; Qin, M.; Zhuang, X.; Zhang, Z. Varietal differences among the phenolic profiles and antioxidant properties of four cultivars of spine grape (Vitis davidii Foex) in Chongyi County (China). Food Chem. 2012, 134, 2049–2056. [Google Scholar] [CrossRef]
- Hernández, T.; Estrella, I.; Carlavilla, D.; Martín-Álvarez, P.J.; Moreno-Arribas, M.V. Phenolic compounds in red wine subjected to industrial malolactic fermentation and ageing on lees. Anal. Chim. Acta 2006, 563, 116–125. [Google Scholar] [CrossRef]
- Milbury, P.E.; Chen, C.; Dolnikowski, G.G.; Blumberg, J.B. Determination of flavonoids and phenolics and their distribution in almonds. J. Agric. Food Chem. 2006, 54, 5027–5033. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, D.W.; Parker, M.; Smith, P.A. Flavonol composition of Australian red and white wines determined by high-performance liquid chromatography. Aust. J. Grape Wine Res. 2008, 14, 153–161. [Google Scholar] [CrossRef]
- Castillo-Muñoz, N.; Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutiérrez, I. Flavonol profiles of Vitis vinifera red grapes and their single-cultivar wines. J. Agric. Food Chem. 2007, 55, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Hermosín-Gutiérrez, I.; Castillo-Muñoz, N.; Gómez-Alonso, S.; García-Romero, E. Flavonol profiles for grape and wine authentication. In Progress in Authentication of Food and Wine; ACS Publications: Washington, DC, USA, 2011; pp. 113–129. [Google Scholar]
- Makris, D.P.; Kallithraka, S.; Kefalas, P. Flavonols in grapes, grape products and wines: Burden, profile and influential parameters. J. Food Compos. Anal. 2006, 19, 396–404. [Google Scholar] [CrossRef]
- Martínez-Lüscher, J.; Brillante, L.; Kurtural, S.K. Flavonol profile is a reliable indicator to assess canopy architecture and the exposure of red wine grapes to solar radiation. Front. Plant Sci. 2019, 10, 10. [Google Scholar] [CrossRef]
- Theocharis, S.; Gkrimpizis, T.; Karadimou, C.; Alatzas, A.; Koundouras, S.; Taskos, D. Modulating ‘Xinomavro’(Vitis vinifera L.) Vine Growth and Berry Composition: A Comparative Analysis of Rootstock Effects. Horticulturae 2024, 10, 490. [Google Scholar] [CrossRef]
- Gordillo, B.; Rivero, F.J.; Jara-Palacios, M.J.; González-Miret, M.L.; Heredia, F.J. Impact of a double post-fermentative maceration with ripe and overripe seeds on the phenolic composition and color stability of Syrah red wines from warm climate. Food Chem. 2021, 346, 128919. [Google Scholar] [CrossRef]
- Padilla-González, G.F.; Grosskopf, E.; Sadgrove, N.J.; Simmonds, M.S. Chemical diversity of Flavan-3-Ols in grape seeds: Modulating factors and quality requirements. Plants 2022, 11, 809. [Google Scholar] [CrossRef]
- Castillo-Muñoz, N.; Gómez-Alonso, S.; García-Romero, E.; Gómez, M.V.; Velders, A.H.; Hermosín-Gutiérrez, I. Flavonol 3-O-glycosides series of Vitis vinifera cv. Petit Verdot red wine grapes. J. Agric. Food Chem. 2009, 57, 209–219. [Google Scholar] [CrossRef]
- Casassa, L.F.; Harbertson, J.F. Extraction, evolution, and sensory impact of phenolic compounds during red wine maceration. Annu. Rev. Food Sci. Technol. 2014, 5, 83–109. [Google Scholar] [CrossRef]
- Vendramin, V.; Pizzinato, D.; Sparrow, C.; Pagni, D.; Cascella, F.; Carapelli, C.; Vincenzi, S. Prevention of quercetin precipitation in red wines: A promising enzymatic solution. OENO One 2022, 56, 41–51. [Google Scholar] [CrossRef]
- El Rayess, Y.; Nehme, N.; Azzi-Achkouty, S.; Julien, S.G. Wine phenolic compounds: Chemistry, functionality and health benefits. Antioxidants 2024, 13, 1312. [Google Scholar] [CrossRef]
- Ma, W.; Guo, A.; Zhang, Y.; Wang, H.; Liu, Y.; Li, H. A review on astringency and bitterness perception of tannins in wine. Trends Food Sci. Technol. 2014, 40, 6–19. [Google Scholar] [CrossRef]
- Rolle, L.; Torchio, F.; Ferrandino, A.; Guidoni, S. Influence of wine-grape skin hardness on the kinetics of anthocyanin extraction. Int. J. Food Prop. 2012, 15, 249–261. [Google Scholar] [CrossRef]
- Blancquaert, E.H.; Oberholster, A.; Ricardo-da-Silva, J.M.; Deloire, A.J. Grape flavonoid evolution and composition under altered light and temperature conditions in Cabernet Sauvignon (Vitis vinifera L.). Front. Plant Sci. 2019, 10, 1062. [Google Scholar] [CrossRef]
- Bouderias, S.; Teszlák, P.; Jakab, G.; Kőrösi, L. Age-and season-dependent pattern of flavonol glycosides in Cabernet Sauvignon grapevine leaves. Sci. Rep. 2020, 10, 14241. [Google Scholar] [CrossRef]
- Heras-Roger, J.; Darias-Rosales, J.; Díaz-Romero, C. Red wine color: The role of polymeric pigments and pyranoanthocyanins in SO2 bleachable methods. Ciência E Técnica Vitivinícola 2024, 39, 84–92. [Google Scholar] [CrossRef]
- T Escribano-Bailon, M.; Santos-Buelga, C. Anthocyanin copigmentation-evaluation, mechanisms and implications for the colour of red wines. Curr. Org. Chem. 2012, 16, 715–723. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, J.; Ren, Y.; Yuan, C.; Wang, Z. The effects of the grape varieties and the wine aging periods on the tannin profiles and the astringency perceptions of wines. J. Food Meas. Charact. 2022, 16, 2726–2737. [Google Scholar] [CrossRef]
- Gouot, J.C.; Smith, J.P.; Holzapfel, B.P.; Walker, A.R.; Barril, C. Grape berry flavonoids: A review of their biochemical responses to high and extreme high temperatures. J. Exp. Bot. 2019, 70, 397–423. [Google Scholar] [CrossRef]
- Eder, R.; Šćepanović, R.P.; Raičević, D.; Popović, T.; Korntheuer, K.; Wendelin, S.; Forneck, A.; Philipp, C. Study of the effects of climatic conditions on the phenolic content and antioxidant activity of Austrian and Montenegrin red wines. OENO One 2023, 57, 69–85. [Google Scholar] [CrossRef]
- Spanish Meteorological Agency (AEMET). Available online: https://www.aemet.es/es/serviciosclimaticos/datosclimatologicos/valoresclimatologicos?k=coo (accessed on 12 February 2024).
- Rienth, M.; Vigneron, N.; Darriet, P.; Sweetman, C.; Burbidge, C.; Bonghi, C.; Walker, R.P.; Famiani, F.; Castellarin, S.D. Grape berry secondary metabolites and their modulation by abiotic factors in a climate change scenario—A review. Front. Plant Sci. 2021, 12, 643258. [Google Scholar] [CrossRef] [PubMed]
- Kilmister, R.L.; Mazza, M.; Baker, N.K.; Faulkner, P.; Downey, M.O. A role for anthocyanin in determining wine tannin concentration in Shiraz. Food Chem. 2014, 152, 475–482. [Google Scholar] [CrossRef]
- Heras-Roger, J.; Díaz-Romero, C.; Darias-Martín, J. What gives a wine its strong red color? Main correlations affecting copigmentation. J. Agric. Food Chem. 2016, 64, 6567–6574. [Google Scholar] [CrossRef] [PubMed]
- Fanzone, M.; Zamora, F.; Jofré, V.; Assof, M.; Gómez-Cordovés, C.; Peña-Neira, Á. Phenolic characterisation of red wines from different grape varieties cultivated in Mendoza province (Argentina). J. Sci. Food Agric. 2012, 92, 704–718. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Liang, N.; Mu, L.; Pan, Q.; Wang, J.; Reeves, M.J.; Duan, C. Anthocyanins and their variation in red wines II. Anthocyanin derived pigments and their color evolution. Molecules 2012, 17, 1483–1519. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Saucier, C.; Glories, Y. Grape and wine phenolics: History and perspective. Am. J. Enol. Vitic. 2006, 57, 239–248. [Google Scholar] [CrossRef]


| Samples Distribution by Different Characteristics | |||||
|---|---|---|---|---|---|
| Geographical Origin (D.O.) | n | Vine Cultivar | n | Wine aging (years) | n |
| Abona (A.) | 46 | Listan Negro (LN) | 93 | Young (≤2) | 125 |
| Tacoronte-Acentejo (TA.) | 45 | Baboso (B) | 30 | Medium (3–5) | 73 |
| Valle de la Orotava (O.) | 27 | Vijariego (V) | 17 | Old (≥6) | 7 |
| Ycoden Daute Isora (Y.) | 18 | Negramoll (N) | 13 | ||
| El Hierro (H.) | 18 | Listán Prieto (LP) | 14 | ||
| La Palma (LP.) | 15 | Syrah (S) | 12 | Total number of samples: | |
| Gran Canaria (GC.) | 13 | Tintilla (T) | 9 | 205 | |
| Valle de Güímar (G.) | 10 | Castellana (C) | 7 | ||
| Lanzarote (LZ.) | 7 | Rubí Cabernet (R) | 5 | ||
| La Gomera (GO.) | 6 | Merlot (M) | 5 | ||
| V | N | LP | B | T | LN | M | S | C | R | |
|---|---|---|---|---|---|---|---|---|---|---|
| Cate | 32.68 ab (9.79) | 34.31 abc (25.93) | 27.12 a (15.26) | 43.84 bc (16.62) | 64.72 d (28.91) | 35.81 abc (11.84) | 37.21 abc (11.05) | 48.96 c (14.54) | 48.13 c (10.45) | 42.04 abc (13.05) |
| Epic | 68.03 a (24.46) | 72.94 a (29.54) | 53.27 a (34.31) | 75.74 ab (33.15) | 99.99 b (48.62) | 60.82 a (23.10) | 62.24 a (22.86) | 62.35 a (38.96) | 53.40 a (10.57) | 49.40 a (17.88) |
| TFla | 100.71 ab (26.89) | 107.25 ab (50.47) | 80.39 a (44.94) | 119.58 b (42.20) | 164.71 c (65.89) | 96.63 ab (28.03) | 99.45 ab (32.12) | 111.31 ab (51.36) | 101.52 ab (12.76) | 91.45 ab (30.86) |
| M3gu | 0.39 b (0.22) | 0.17 a (0.12) | 0.40 b (0.13) | 0.25 ab (0.15) | 0.36 ab (0.31) | 0.28 ab (0.22) | 0.44 b (0.22) | 0.43 b (0.30) | 0.45 b (0.31) | 0.32 ab (0.26) |
| M3gl | 10.10 cd (2.23) | 12.73 de (3.55) | 15.03 e (6.12) | 6.35 ab (3.14) | 4.63 ab (3.84) | 12.03 de (5.23) | 8.30 bcd (3.61) | 5.51 ab (3.65) | 8.28 bcd (6.95) | 3.55 a (1.41) |
| L3gl | 1.81 ab (0.84) | 2.20 abc (0.92) | 1.86 b (0.69) | 1.71 ab (0.71) | 2.59 abc (0.80) | 2.62 bc (1.14) | 1.55 a (0.39) | 3.01 cd (1.76) | 2.36 bc (0.83) | 3.61 d (1.50) |
| K3gl | 0.57 a (0.37) | 0.39 a (0.11) | 0.37 a (0.14) | 0.40 a (0.16) | 0.45 a (0.15) | 0.42 a (0.17) | 0.39 a (0.17) | 0.53 a (0.48) | 0.43 a (0.19) | 0.45 a (0.13) |
| Myri | 3.35 a (2.08) | 4.36 ab (1.96) | 5.00 ab (3.62) | 4.31 b (3.23) | 6.53 ab (3.94) | 7.40 bc (3.40) | 11.14 d (4.08) | 12.10 d (3.67) | 10.24 cd (2.54) | 10.74 d (5.34) |
| Q3gu | 6.39 a (2.77) | 10.72 ab (3.67) | 14.90 cd (9.90) | 8.57 ab (4.91) | 9.77 ab (5.31) | 13.68 bcd (5.82) | 19.27 de (7.10) | 22.94 e (9.43) | 15.66 cd (6.83) | 15.65 cd (5.44) |
| Q3gl | 3.37 a (1.34) | 7.57 abc (3.12) | 12.57 def (8.52) | 5.19 ab (3.41) | 5.00 abc (3.73) | 8.93 bcd (4.50) | 14.01 ef (5.48) | 16.43 f (8.46) | 11.34 cde (4.57) | 7.05 abc (2.64) |
| Ruti | 4.04 a (2.96) | 4.35 a (1.91) | 4.50 a (3.48) | 6.08 a (4.35) | 7.98 ab (8.09) | 8.43 abc (5.24) | 7.90 ab (4.69) | 10.90 bc (6.40) | 13.04 c (4.85) | 13.00 c (4.22) |
| I3gl | 3.25 a (1.47) | 3.24 a (1.88) | 3.87 ab (2.39) | 3.67 ab (1.63) | 4.44 abc (1.56) | 3.71 ab (1.58) | 5.82 c (1.56) | 7.73 d (3.76) | 3.78 ab (1.82) | 5.41 bc (2.24) |
| Isor | 2.95 ab (1.58) | 2.91 ab (1.88) | 2.17 a (1.32) | 3.05 ab (1.36) | 3.95 bc (1.45) | 3.08 ab (1.46) | 5.63 de (2.10) | 6.90 e (3.54) | 2.84 ab (1.34) | 5.00 cd (2.95) |
| S3gl | 0.60 ab (0.69) | 0.32 a (0.32) | 0.35 ab (0.34) | 0.57 ab (0.53) | 0.91 b (1.23) | 0.48 ab (0.53) | 0.72 ab (0.50) | 0.87 ab (0.54) | 0.50 ab (0.20) | 0.58 ab (0.23) |
| Quer | 2.40 ab (2.03) | 5.34 c (3.61) | 3.95 abc (3.08) | 2.03 a (1.68) | 2.08 a (2.73) | 2.11 a (2.14) | 4.58 bc (3.20) | 4.01 bc (2.96) | 1.77 a (2.12) | 4.02 abc (3.00) |
| AFlo | 8.35 a (4.02) | 12.24 ab (5.86) | 11.15 ab (7.15) | 9.39 a (4.86) | 12.46 ab (3.58) | 12.41 ab (5.03) | 21.78 d (6.71) | 22.90 d (7.17) | 15.32 bc (4.39) | 19.67 cd (8.27) |
| GFlo | 26.48 a (5.96) | 37.35 ab (9.58) | 49.34 bc (22.21) | 26.70 a (11.36) | 28.31 a (8.92) | 42.16 b (13.71) | 50.50 bc (15.63) | 57.45 c (21.75) | 42.78 b (18.69) | 36.60 ab (9.27) |
| Qder | 12.16 a (4.37) | 23.63 bc (8.88) | 31.41 cd (20.54) | 15.79 ab (8.72) | 16.85 ab (8.84) | 24.72 bc (10.64) | 37.86 de (13.60) | 43.38 e (17.40) | 28.77 cd (12.89) | 26.72 bc (8.10) |
| Mder | 13.84 ab (3.19) | 17.26 bc (4.11) | 20.42 c (7.97) | 10.9 a (5.11) | 11.52 a (4.23) | 19.72 bc (6.54) | 19.88 c (6.46) | 18.04 bc (5.42) | 18.97 bc (7.14) | 14.61 abc (4.07) |
| Ider | 6.20 a (2.97) | 6.16 a (3.72) | 6.62 a (3.61) | 6.72 a (2.83) | 8.40 ab (2.71) | 6.79 a (2.91) | 11.46 b (3.46) | 14.63 c (7.11) | 6.62 a (3.11) | 10.41 b (5.14) |
| TFlo | 35.19 a (8.44) | 49.96 abc (14.03) | 60.45 cd (28.64) | 36.10 a (15.38) | 40.87 ab (11.74) | 54.76 bcd (17.73) | 71.85 de (19.51) | 80.45 e (27.26) | 57.64 bcd (21.97) | 56.37 bcd (15.12) |
| El Hierro | La Gomera | La Palma | Gran Canaria | Lanzarote | Tenerife | |
|---|---|---|---|---|---|---|
| Cate | 32.46 ab (10.60) | 25.17 a (7.40) | 23.47 a (7.66) | 46.59 c (31.63) | 33.76 abc (12.46) | 41.57 bc (15.48) |
| Epic | 86.55 b (25.29) | 36.69 a (13.88) | 63.81 b (19.78) | 63.75 b (34.79) | 65.4 b (22.95) | 64.34 b (29.38) |
| TFla | 119.01 b (29.15) | 61.86 a (19.79) | 88.76 ab (24.88) | 110.35 b (48.51) | 99.16 b (30.47) | 104.90 b (40.88) |
| M3gu | 0.36 ab (0.22) | 0.19 a (0.09) | 0.24 ab (0.21) | 0.4 b (0.25) | 0.3 ab (0.22) | 0.31 ab (0.22) |
| M3gl | 8.69 a (2.67) | 10.08 a (4.51) | 15.32 a (4.31) | 11.91 ab (5.36) | 15.51 a (7.21) | 9.31 a (5.50) |
| L3gl | 1.27 a (0.21) | 1.78 ab (0.80) | 2.28 b (0.84) | 2.31 b (1.04) | 1.93 ab (0.41) | 2.59 b (1.20) |
| K3gl | 0.44 ab (0.12) | 0.36 a (0.15) | 0.36 a (0.09) | 0.64 b (0.44) | 0.43 ab (0.19) | 0.44 ab (0.24) |
| Myri | 2.43 a (1.50) | 3.61 a (2.25) | 3.79 a (2.04) | 5.11 ab (3.12) | 9.87 c (3.86) | 7.99 bc (3.99) |
| Q3gu | 6.07 a (3.09) | 8.49 a (5.05) | 9.78 ab (4.41) | 9.41 ab (4.96) | 22.27 c (8.70) | 14.3 b (6.85) |
| Q3gl | 3.5 a (2.06) | 6.4 ab (3.78) | 7.45 ab (3.74) | 6.51 ab (3.13) | 18.02 c (5.46) | 9.11 b (5.60) |
| Rutin | 3.58 a (2.35) | 4.33 a (3.13) | 4.23 a (2.44) | 4.3 a (3.82) | 6.7 ab (4.89) | 9.16 b (5.55) |
| I3gl | 3.23 ab (1.30) | 2.32 a (1.85) | 2.55 a (1.24) | 3.66 ab (1.85) | 4.88 b (2.97) | 4.37 b (2.11) |
| Isor | 3.21 ab (1.34) | 2.13 a (1.72) | 2.3 a (1.37) | 3.04 ab (2.05) | 4.11 b (2.98) | 3.55 ab (1.97) |
| S3gl | 0.49 ab (0.60) | 0.36 a (0.32) | 0.3 a (0.33) | 0.87 b (1.20) | 0.4 ab (0.36) | 0.56 ab (0.49) |
| Quer | 2.46 ab (1.80) | 2.15 ab (2.16) | 1.51 a (3.39) | 3.24 ab (2.85) | 3.91 b (1.09) | 2.54 ab (2.50) |
| AFlo | 7.85 a (3.65) | 7.4 a (4.97) | 9.73 ab (5.02) | 11.17 abc (6.63) | 15.38 c (6.63) | 14.02 bc (6.28) |
| GFlo | 24.04 a (6.82) | 29.97 ab (14.67) | 38.28 b (10.42) | 35.82 ab (11.10) | 63.73 c (17.65) | 41.0 b (16.14) |
| Qder | 12.02 a (5.72) | 17.04 ab (10.48) | 21.15 ab (9.55) | 19.16 ab (9.61) | 41.80 c (13.86) | 25.96 b (13.21) |
| Mder | 11.49 a (3.65) | 13.87 ab (6.41) | 19.35 b (5.09) | 17.42 b (5.70) | 25.67 c (7.93) | 17.61 b (6.89) |
| Ider | 6.44 abc (2.57) | 4.45 a (3.56) | 4.86 ab (2.55) | 6.7 abc (3.70) | 8.99 c (5.87) | 7.92 bc (3.94) |
| TFlo | 32.14 a (9.84) | 37.85 ab (19.73) | 48.29 ab (14.58) | 47.21 ab (16.21) | 79.22 c (23.25) | 55.08 b (20.91) |
| Cate | Epic | M3gu | M3gl | L3gl | K3gl | Myri | Q3gu | Q3gl | Ruti | I3gl | Isor | S3gl | Quer | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cate | 1 | 0.430 ** | 0.069 | −0.234 ** | 0.253 ** | 0.179 * | 0.341 ** | 0.222 ** | 0.098 | 0.339 ** | 0.400 ** | 0.340 ** | 0.287 ** | 0.151 * |
| Epic | 0.000 | 1 | 0.080 | −0.038 | 0.040 | 0.022 | 0.049 | 0.056 | 0.004 | 0.118 | 0.267 ** | 0.334 ** | −0.022 | 0.277 ** |
| M3gu | 0.326 | 0.255 | 1 | −0.072 | 0.196 ** | 0.098 | 0.058 | 0.089 | 0.149 * | −0.053 | 0.131 | 0.191 ** | 0.069 | 0.140 * |
| M3gl | 0.001 | 0.591 | 0.302 | 1 | −0.136 | 0.096 | 0.001 | 0.220 ** | 0.250 ** | 0.002 | −0.059 | −0.157 * | −0.226 ** | 0.042 |
| L3gl | 0.000 | 0.568 | 0.005 | 0.052 | 1 | 0.233 ** | 0.423 ** | 0.308 ** | 0.150 * | 0.457 ** | 0.293 ** | 0.253 ** | 0.237 ** | 0.065 |
| K3gl | 0.010 | 0.758 | 0.162 | 0.172 | 0.001 | 1 | 0.084 | 0.042 | −0.099 | 0.184 ** | 0.113 | 0.054 | 0.240 ** | 0.063 |
| Myri | 0.000 | 0.489 | 0.409 | 0.990 | 0.000 | 0.232 | 1 | 0.832 ** | 0.638 ** | 0.733 ** | 0.573 ** | 0.427 ** | 0.243 ** | 0.076 |
| Q3gu | 0.001 | 0.426 | 0.207 | 0.002 | 0.000 | 0.551 | 0.000 | 1 | 0.900 ** | 0.607 ** | 0.684 ** | 0.473 ** | 0.149 * | 0.271 ** |
| Q3gl | 0.163 | 0.960 | 0.033 | 0.000 | 0.032 | 0.157 | 0.000 | 0.000 | 1 | 0.293 ** | 0.546 ** | 0.374 ** | 0.048 | 0.297 ** |
| Ruti | 0.000 | 0.091 | 0.452 | 0.981 | 0.000 | 0.008 | 0.000 | 0.000 | 0.000 | 1 | 0.471 ** | 0.334 ** | 0.220 ** | −0.010 |
| I3gl | 0.000 | 0.000 | 0.061 | 0.405 | 0.000 | 0.107 | 0.000 | 0.000 | 0.000 | 0.000 | 1 | 0.877 ** | 0.309 ** | 0.524 ** |
| Isor | 0.000 | 0.000 | 0.006 | 0.025 | 0.000 | 0.439 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 1 | 0.233 ** | 0.591 ** |
| S3gl | 0.000 | 0.753 | 0.327 | 0.001 | 0.001 | 0.001 | 0.000 | 0.033 | 0.496 | 0.002 | 0.000 | 0.001 | 1 | 0.112 |
| Quer | 0.031 | 0.000 | 0.045 | 0.545 | 0.355 | 0.372 | 0.281 | 0.000 | 0.000 | 0.890 | 0.000 | 0.000 | 0.109 | 1 |
| Influencing Factors | Type of LDA | Correct Classification (% After Cross-Validation) | Selected Variables for F1 and F2 |
|---|---|---|---|
| |||
| All variables | 82.9% | F1: M3gl, Ider, Isor, I3gl | |
| (68.8%) | F2: TFlo, GFlo, Qder | ||
| Stepwise | 62.4% | F1: Myri, Isor, Q3gl | |
| (58.0%) | F2: M3gl, AFl, TFla, GFlo | ||
| |||
| All variables | 84.9% | F1: Myri, Q3gu, AFlo | |
| (73.7%) | F2: L3gl, Quer | ||
| Stepwise | 73.2% | F1: Myri, AFlo, Q3gu | |
| (72.7%) | F2: M3gl, Mder, GFlo | ||
| |||
| All variables | 65.8% | F1: Myri, I3gl, Ider | |
| (49.3%) | F2: Qder, TFlo, GFlo | ||
| Stepwise | 47.3% | F1: Epic, Myri, I3gl | |
| (45.2%) | F2: Qder, Q3gl, AFlo | ||
| |||
| All variables | 81.5% | F1: Isor, Quer, Cate | |
| (76.6%) | F2: L3gl, Ider, AFlo | ||
| Stepwise | 75.4% | F1: K3gl, Isor, AFlo | |
| (74.6%) | F2: Myri, TFlo, Cate |
| Original → ↓ Predicted | LN | N | B | LP | T | C | R | M | V | S |
|---|---|---|---|---|---|---|---|---|---|---|
| LN | 83.9 | 7.7 | 13.3 | 0.0 | 22.2 | 0.0 | 20.0 | 0.0 | 23.5 | 16.7 |
| N | 0.0 | 92.3 | 3.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 5.9 | 0.0 |
| B | 3.2 | 0.0 | 76.7 | 7.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| LP | 0.0 | 0.0 | 0.0 | 92.9 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| T | 3.2 | 0.0 | 0.0 | 0.0 | 77.8 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| C | 1.1 | 0.0 | 0.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| R | 5.4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 80.0 | 0.0 | 0.0 | 8.3 |
| M | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 |
| V | 3.2 | 0.0 | 6.7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 70.6 | 0.0 |
| S | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 75.0 |
| Original → ↓ Predicted | DO T | DO O | DO Y | DO G | DO A | LP | HI | LZ | GO | GC |
|---|---|---|---|---|---|---|---|---|---|---|
| DO T | 84.4 | 3.7 | 5.6 | 10.0 | 15.2 | 0.0 | 0.0 | 0.0 | 0.0 | 23.1 |
| DO O | 6.7 | 92.6 | 16.7 | 0.0 | 6.5 | 6.7 | 0.0 | 0.0 | 0.0 | 0.0 |
| DO Y | 0.0 | 0.0 | 55.6 | 0.0 | 6.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| DO G | 0.0 | 0.0 | 5.6 | 80.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| DO A | 4.4 | 0.0 | 5.6 | 10.0 | 71.7 | 0.0 | 0.0 | 0.0 | 0.0 | 7.7 |
| LP | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 93.3 | 0.0 | 0.0 | 0.0 | 0.0 |
| HI | 0.0 | 0.0 | 5.6 | 0.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 0.0 |
| LZ | 0.0 | 3.7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 |
| GO | 4.4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 100.0 | 0.0 |
| GC | 0.0 | 0.0 | 5.6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 69.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heras-Roger, J.; Benítez-Brito, N.; Díaz-Romero, C. From Vine to Wine: Non-Colored Flavonoids as Fingerprints. Appl. Sci. 2025, 15, 4543. https://doi.org/10.3390/app15084543
Heras-Roger J, Benítez-Brito N, Díaz-Romero C. From Vine to Wine: Non-Colored Flavonoids as Fingerprints. Applied Sciences. 2025; 15(8):4543. https://doi.org/10.3390/app15084543
Chicago/Turabian StyleHeras-Roger, Jesús, Néstor Benítez-Brito, and Carlos Díaz-Romero. 2025. "From Vine to Wine: Non-Colored Flavonoids as Fingerprints" Applied Sciences 15, no. 8: 4543. https://doi.org/10.3390/app15084543
APA StyleHeras-Roger, J., Benítez-Brito, N., & Díaz-Romero, C. (2025). From Vine to Wine: Non-Colored Flavonoids as Fingerprints. Applied Sciences, 15(8), 4543. https://doi.org/10.3390/app15084543







