Reduction and Immobilization of Potassium Permanganate on Iron Oxide Catalyst by Fluidized-Bed Crystallization Technology
Abstract
:1. Introduction
2. Material and Methods
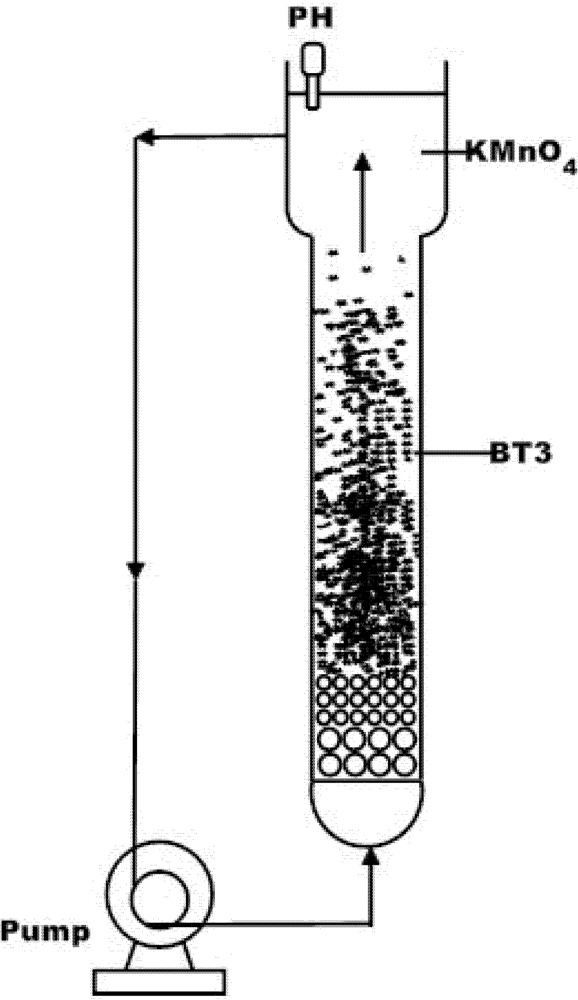
3. Results and Discussion
3.1. Characterization of BT-3
| Properties | BT-3 |
|---|---|
| Color | Black |
| Material | Iron oxide |
| Bulk density (g·cm−1) | 1.56 |
| True density (g·cm−1) | 2.38 |
| Total iron content (g·Kg−1) | 649 |
| Specific surface area (BET) (m2/g-solid) | 174 |
| Pore vol. (cm3/g) | 0.14 |
| Average grain size (mm) | 0.5–1 |


3.2. Effect of KMnO4 Concentration

3.3. Effect of BT-3 Dosage

3.4. Effect of the Operational pH Level

3.5. The Possible Mechanism of KMnO4 Immobilization on BT-3
 ≡FeOOH2+ (2)
≡FeOOH2+ (2) ≡FeOOH2-MnO4 (3)
≡FeOOH2-MnO4 (3) Fe2++ 2H2O (4)
Fe2++ 2H2O (4) 3Fe (OH) 3(s) + MnO2(s) + H+ (5)
3Fe (OH) 3(s) + MnO2(s) + H+ (5)3.6. Kinetics of KMnO4 Decomposition

Conclusion
References
- Banerjee, K.; Amy, G.L.; Prevost, M.; Nour, S.; Jekel, M.; Gallagher, P.M.; Blumenschein, C.D. Kinetic and thermodynamic aspects of adsorption of arsenic onto granular ferric hydroxide (GFH). Water Res. 2008, 42, 3371–3378. [Google Scholar]
- Chen, R.; Zhi, C.; Yang, H.; Bando, Y.; Zhang, Z.; Sugiur, N.; Golberg, D. Arsenic (V) adsorption on Fe3O4 nanoparticle-coated boron nitride nanotubes. J. Colloid Interface Sci. 2011, 359, 261–268. [Google Scholar]
- Simeonidis, K.; Gkinis, T.; Tresintsi, S.; Martinez-Boubeta, C.; Vourlias, G.; Tsiaoussis, I.; Stavropoulos, G.; Mitrakas, M.; Angelakeris, M. Magnetic separation of hematite-coated Fe3O4 particles used as arsenic adsorbents. Chem. Eng. J. 2011, 168, 1008–1015. [Google Scholar]
- Tang, Y.; Wang, J.; Gao, N. Characteristics and model studies for fluoride and arsenic adsorption on goethite. J. Environ. Sci. 2010, 22, 1689–1694. [Google Scholar]
- Yang, W.; Kan, A.T.; Chen, W.; Tomson, M.B. pH-dependent effect of zinc on arsenic adsorption to magnetite nanoparticles. Water Res. 2010, 44, 5693–5701. [Google Scholar]
- Youngran, J.; Fan, M.; van Leeuwen, J.; Belczyk, J.F. Effect of competing solutes on arsenic(V) adsorption using iron and aluminum oxides. J. Environ. Sci. 2007, 19, 910–919. [Google Scholar]
- Zhang, J.S.; Stanforth, R.S.; Pehkonen, S.O. Effect of replacing a hydroxyl group with a methyl group on arsenic (V) species adsorption on goethite ([alpha]-FeOOH). J. Colloid Interface Sci. 2007, 306, 16–21. [Google Scholar]
- Manning, B.A.; Fendorf, S.E.; Bostick, B.; Suarez, D.L. Arsenic(III) oxidation and Arsenic(V) adsorption reactions on synthetic birnessite. Environ. Sci. Technol. 2002, 36, 976–981. [Google Scholar]
- Nesbitt, H.W.; Canning, G.W.; Bancroft, G.M. XPS study of reductive dissolution of 7Å-birnessite by H3AsO3, with constraints on reaction mechanism. Geochim. Gosmochim. Acta 1998, 62, 2097–2110. [Google Scholar]
- Scott, M.J.; Morgan, J.J. Reactions at oxide surfaces. 1. Oxidation of As(III) by synthetic birnessite. Environ. Sci. Technol. 1995, 29, 1898–1905. [Google Scholar] [CrossRef]
- Zhang, G.S.; Qu, J.H.; Liu, H.J.; Liu, R.P.; Li, G.T. Removal mechanism of As(III) by a novel Fe-Mn binary oxide adsorbent: Oxidation and sorption. Environ. Sci. Technol. 2007, 41, 4613–4619. [Google Scholar]
- Zhang, G.; Qu, J.; Liu, H.; Liu, R.; Wu, R. Preparation and evaluation of a novel Fe-Mn binary oxide adsorbent for effective arsenite removal. Water Res. 2007, 41, 1921–1928. [Google Scholar]
- Wu, K.; Wang, H.; Liu, R.; Zhao, X.; Liu, H.; Qu, J. Arsenic removal from a high-arsenic wastewater using in situ formed Fe-Mn binary oxide combined with coagulation by poly-aluminum chloride. J. Hazard. Mater. 2011, 185, 990–995. [Google Scholar] [CrossRef]
- Huang, Y.H.; Shih, Y.J.; Chang, C.C. Adsorption of fluoride by waste iron oxide: The effects of solution pH, major coexisting anions, and adsorbent calcination temperature. J. Hazard. Mater. 2011, 186, 1355–1359. [Google Scholar] [CrossRef]
- Huang, Y.H.; Hsueh, C.L.; Cheng, H.P.; Su, L.C.; Chen, C.Y. Thermodynamics and kinetics of adsorption of Cu(II) onto waste iron oxide. J. Hazard. Mater. 2007, 144, 406–411. [Google Scholar]
- Huang, Y.H.; Hsueh, C.L.; Huang, C.P.; Su, L.C.; Chen, C.Y. Adsorption thermodynamic and kinetic studies of Pb(II) removal from water onto a versatile Al2O3-supported iron oxide. Sep. Purif. Technol. 2007, 55, 23–29. [Google Scholar]
- Chen, Y.; Liu, C.; Li, F.; Cheng, H.M. Preparation of single-crystal [alpha]-MnO2 nanorods and nanoneedles from aqueous solution. J. Alloy. Compd. 2005, 397, 282–285. [Google Scholar]
- Ma, S.B.; Ahn, K.Y.; Lee, E.S.; Oh, K.H.; Kim, K.B. Synthesis and characterization of manganese dioxide spontaneously coated on carbon nanotubes. Carbon 2007, 45, 375–382. [Google Scholar]
- Lin, S.S.; Gurol, M.D. Catalytic decomposition of hydrogen peroxide on iron oxide: Kinetics, mechanism, and implications. Environ. Sci. Technol. 1998, 32, 1417–1423. [Google Scholar] [CrossRef]
- Chou, S.; Huang, C.; Huang, Y.H. Heterogeneous and homogeneous catalytic oxidation by supported γ-feooh in a fluidized-bed reactor: Kinetic approach. Environ. Sci. Technol. 2001, 35, 1247–1251. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, G.-X.; Huaug, Y.-H.; Chen, T.-C.; Shih, Y.-J.; Zhang, H. Reduction and Immobilization of Potassium Permanganate on Iron Oxide Catalyst by Fluidized-Bed Crystallization Technology. Appl. Sci. 2012, 2, 166-174. https://doi.org/10.3390/app2010166
Li G-X, Huaug Y-H, Chen T-C, Shih Y-J, Zhang H. Reduction and Immobilization of Potassium Permanganate on Iron Oxide Catalyst by Fluidized-Bed Crystallization Technology. Applied Sciences. 2012; 2(1):166-174. https://doi.org/10.3390/app2010166
Chicago/Turabian StyleLi, Guang-Xia, Yao-Hui Huaug, Teng-Chien Chen, Yu-Jen Shih, and Hui Zhang. 2012. "Reduction and Immobilization of Potassium Permanganate on Iron Oxide Catalyst by Fluidized-Bed Crystallization Technology" Applied Sciences 2, no. 1: 166-174. https://doi.org/10.3390/app2010166
APA StyleLi, G.-X., Huaug, Y.-H., Chen, T.-C., Shih, Y.-J., & Zhang, H. (2012). Reduction and Immobilization of Potassium Permanganate on Iron Oxide Catalyst by Fluidized-Bed Crystallization Technology. Applied Sciences, 2(1), 166-174. https://doi.org/10.3390/app2010166





