Optical and Transport Properties of Ni-MoS2
Abstract
:1. Introduction
2. Experimental Details
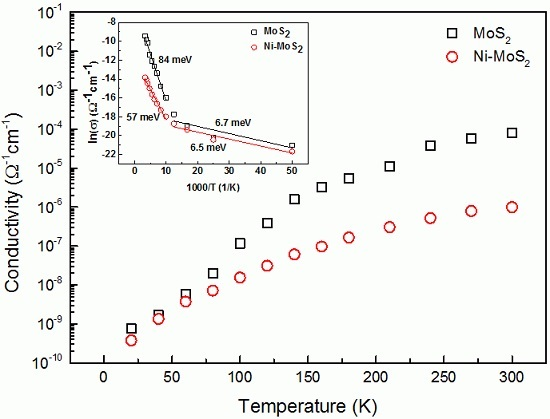
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Deoxygenation of exfoliated graphite oxide under alkaline conditions: A green route to graphene preparation. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Mayorov, A.S.; Gorbachev, R.V.; Morozov, S.V.; Britnell, L.; Jalil, R.; Ponomarenko, L.A.; Blake, P.; Novoselov, K.S.; Watanabe, K.; Taniguchi, T.; et al. Micrometer-scale ballistic transport in encapsulated graphene at room temperature. Nano Lett. 2011, 11, 2396–2399. [Google Scholar] [CrossRef] [PubMed]
- Gourmelon, E.; Lignier, O.; Hadouda, H.; Couturier, G.; Bernede, J.C.; Tedd, J.; Pouzed, J.; Salardenne, J. Solar Energy materials and solar cells. Sol. Energy Mater. Sol. Cells 1997, 46, 115–121. [Google Scholar] [CrossRef]
- Ho, W.K.; Yu, J.C.; Lin, J.; Yu, J.G.; Li, P.S. Preparation and photocatalytic behavior of MoS2 and WS2 nanocluster sensitized TiO2. Langmuir 2004, 20, 5865–5869. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Jariwala, D.; Sangwan, V.K.; Marks, T.J.; Hersam, M.C.; Lauhon, L.J. Elucidating the photoresponse of ultrathin MoS2 field-effect transistors by scanning photocurrent microscopy. J. Phys. Chem. Lett. 2013, 4, 2508–2513. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Blancon, J.C.; Kappera, R.; Lei, S.; Najmaei, S.; Mangum, B.D.; Gupta, G.; Ajayan, P.M.; Lou, J.; Chhowalla, M.; et al. Spatially Resolved photoexcited charge-carrier dynamics in phase-engineered monolayer MoS2. ACS Nano 2015, 9, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsneison, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Tan, Y.W.; Stormer, H.L.; Kim, P. Experimental observation of the quantum Hall effect and Berry’s phase in graphene. Nature 2005, 438, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Pan, L.; Yao, Z.; Li, J.; Shi, Y.; Wang, X. Electrical characterization of back-gated bi-layer MoS2 field-effect transistors and the effect of ambient on their performances. Appl. Phys. Lett. 2012, 100, 123104–123106. [Google Scholar] [CrossRef]
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. Single-layer MoS2 transistors. Nat. Nano Technol. 2011, 6, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Jariwala, D.; Sangwan, V.K.; Lauhon, L.J.; Marks, T.J.; Hersam, M.C. Emerging device applications for semiconducting two-dimensional transition metal dichalcogenides. ACS Nano 2014, 8, 1102–1120. [Google Scholar] [CrossRef] [PubMed]
- Ayari, A.; Cobas, E.; Ogundadegbe, O.; Fuhrer, M.S. Two-dimensional nanosheets produced by liquid exfoliation of layered materials. J. Appl. Phys. 2007, 101, 014507–014511. [Google Scholar] [CrossRef]
- Kadantsev, E.S.; Hawrylak, P. Electronic structure of a single MoS2 monolayer. Solid State Commun. 2012, 153, 909–913. [Google Scholar] [CrossRef]
- Howell, S.L.; Jariwala, D.; Wu, C.C.; Chen, K.S.; Sangwan, V.K.; Kang, J.; Marks, T.J.; Hersam, M.C.; Lauhon, L.J. Investigation of band-offsets at monolayer–multilayer MoS2 junctions by scanning photocurrent microscopy. Nano Lett. 2015, 15, 2278–2284. [Google Scholar] [CrossRef] [PubMed]
- Matthew, M.A.; Chin, L.; Birdwell, A.G.; O’Regan, T.P.; Najmaei, S.; Liu, Z.; Ajayan, P.M.; Lou, J.; Dubey, M. Electrical characterization of back-gated bi-layer MoS2 field-effect transistors and the effect of ambient on their performances. Appl. Phys. Lett. 2013, 102, 193107–193109. [Google Scholar]
- Goodfellow, K.M.; Chakraborty, C.; Beams, R.; Novotny, L.; Vamivakas, A.N. Direct on-chip optical plasmon detection with an atomically thin semiconductor. Nano Lett. 2015, 15, 5477–5481. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.; Park, T.E.; Lin, D.Y.; Fu, D.; Park, J.; Jung, H.J.; Chen, Y.; Ko, C.; Jang, C.; Sun, Y.; et al. Doping against the native propensity of MoS2: Degenerate hole doping by cation substitution. Nano Lett. 2014, 14, 6976–6982. [Google Scholar] [CrossRef] [PubMed]
- Thakurta, S.R.G.; Dutta, A.K. Electrical conductivity, thermoelectric power and Hall effect in p-type molybdenite (MoS2) crystal. J. Phys. Chem. Solids 1983, 44, 407–416. [Google Scholar] [CrossRef]
- Fivaz, R.; Mooser, E. Mobility of charge carriers in semiconducting layer structures. Phys. Rev. 1967, 163, 743–755. [Google Scholar] [CrossRef]
- Kim, S.; Konar, A.; Hwang, W.S.; Lee, J.H.; Lee, J.; Yang, J.; Jung, C.; Kim, H.; Yoo, J.B.; Choi, J.Y.; et al. High-mobility and low-power thin-film transistors based on multilayer MoS2 crystals. Nat. Commun. 2012, 3, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Van Der Pauw, L.J. A method of measuring specific resistivity and Hall effect of discs of arbitrary shape. Philips Tech. Rev. 1958, 20, 220–224. [Google Scholar]
- Park, K.T.; Babb, M.R.; Freund, M.S.; Weiss, J.; Klier, K. Surface structure of single-crystal MoS2 (0002) and Cs/MoS2 (0002) by X-ray photoelectron diffraction. J. Phys. Chem. 1996, 100, 10739–10745. [Google Scholar] [CrossRef]
- Suzuki, R.; Sakano, M.; Zhang, Y.J.; Akashi, R.; Morikawa, D.; Harasawa, A.; Yaji, K.; Miyamoto, K.; Okuda, T.; Ishizaka, K.; et al. Valley-dependent spin polarization in bulk MoS2 with broken inversion symmetry. Nat. Nano Technol. 2014, 9, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Andersen, A.; Kathmann, S.M.; Lilga, M.A.; Albrecht, K.O.; Hallen, R.T.; Mei, D. First-principles characterization of potassium intercalation in hexagonal 2H-MoS2. J. Phys. Chem. C 2012, 116, 1826–1832. [Google Scholar] [CrossRef]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nano Technol. 2012, 7, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Korona, K.P.; Wysmolek, A.; Pakula, K.; Stepniewski, R.; Baranowski, J.M.; Grzegory, I.; Lucznik, B.; Wroblewski, M.; Porowski, S. Exciton region reflectance of homoepitaxial GaN layers. Appl. Phys. Lett. 1996, 69, 788–790. [Google Scholar] [CrossRef]
- Xiao, D.; Liu, G.B.; Feng, W.; Xu, X.; Yao, W. Coupled spin and valley physics in monolayers of MoS2 and other group-VI dichalcogenides. Phys. Rev. Lett. 2012, 108, 196802–196804. [Google Scholar] [CrossRef] [PubMed]
- Varshni, Y.P. Temperature dependence of the energy gap in semiconductors. Physica 1967, 34, 149–154. [Google Scholar] [CrossRef]
- O’Donnell, K.P.; Chen, X. Temperature dependence of semiconductor band gaps. Appl. Phys. Lett. 1991, 58, 2924–2926. [Google Scholar] [CrossRef]
- Ho, C.H.; Wu, C.S.; Huang, Y.S.; Liao, P.C.; Tiong, K.K. Temperature dependence of energies and broadening parameters of the band-edge excitons of Mo1-xWxS2 single crystals. J. Phys. Condens. Matter. 1998, 10, 9317–9328. [Google Scholar] [CrossRef]






| Material | Feature | (eV) | Α (meV/K) | Β (K) | S | <ħΩ> (meV) |
|---|---|---|---|---|---|---|
| MoS2 | A | 1.940 ± 0.005 | 0.353 ± 0.05 | 125.2 ± 100 | 2.033 ± 0.1 | 13.1 ± 3 |
| B | 2.130 ± 0.005 | 0.355 ± 0.05 | 252.2 ± 100 | 2.007 ± 0.1 | 28.5 ± 3 | |
| Ni-MoS2 | A | 1.918 ± 0.005 | 0.582 ± 0.05 | 399.7 ± 100 | 2.271 ± 0.05 | 20.38 ± 3 |
| B | 2.118 ± 0.005 | 0.517 ± 0.05 | 236.2 ± 100 | 2.215 ± 0.05 | 14.67 ± 3 |
| Sample | MoS2 | Ni-MoS2 |
|---|---|---|
| Type | n | n |
| Resistance (Ω) | 6733 | 283188 |
| Carrier density (cm−2) | 1.102 × 1013 | 1.183 × 1013 |
| Mobility(cm2/V·s) | 78.5089 | 1.86593 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, T.-S.; Huang, C.-C.; Lin, D.-Y. Optical and Transport Properties of Ni-MoS2. Appl. Sci. 2016, 6, 227. https://doi.org/10.3390/app6080227
Ko T-S, Huang C-C, Lin D-Y. Optical and Transport Properties of Ni-MoS2. Applied Sciences. 2016; 6(8):227. https://doi.org/10.3390/app6080227
Chicago/Turabian StyleKo, Tsung-Shine, Cheng-Ching Huang, and Der-Yuh Lin. 2016. "Optical and Transport Properties of Ni-MoS2" Applied Sciences 6, no. 8: 227. https://doi.org/10.3390/app6080227
APA StyleKo, T.-S., Huang, C.-C., & Lin, D.-Y. (2016). Optical and Transport Properties of Ni-MoS2. Applied Sciences, 6(8), 227. https://doi.org/10.3390/app6080227