Review of Laser-Generated Ultrasound Transmitters and Their Applications to All-Optical Ultrasound Transducers and Imaging
Abstract
:1. Introduction
2. Laser-Generated Ultrasound
2.1. Absorptive Materials
2.1.1. Metallic Absorbers
2.1.2. Carbon-Based Absorbers
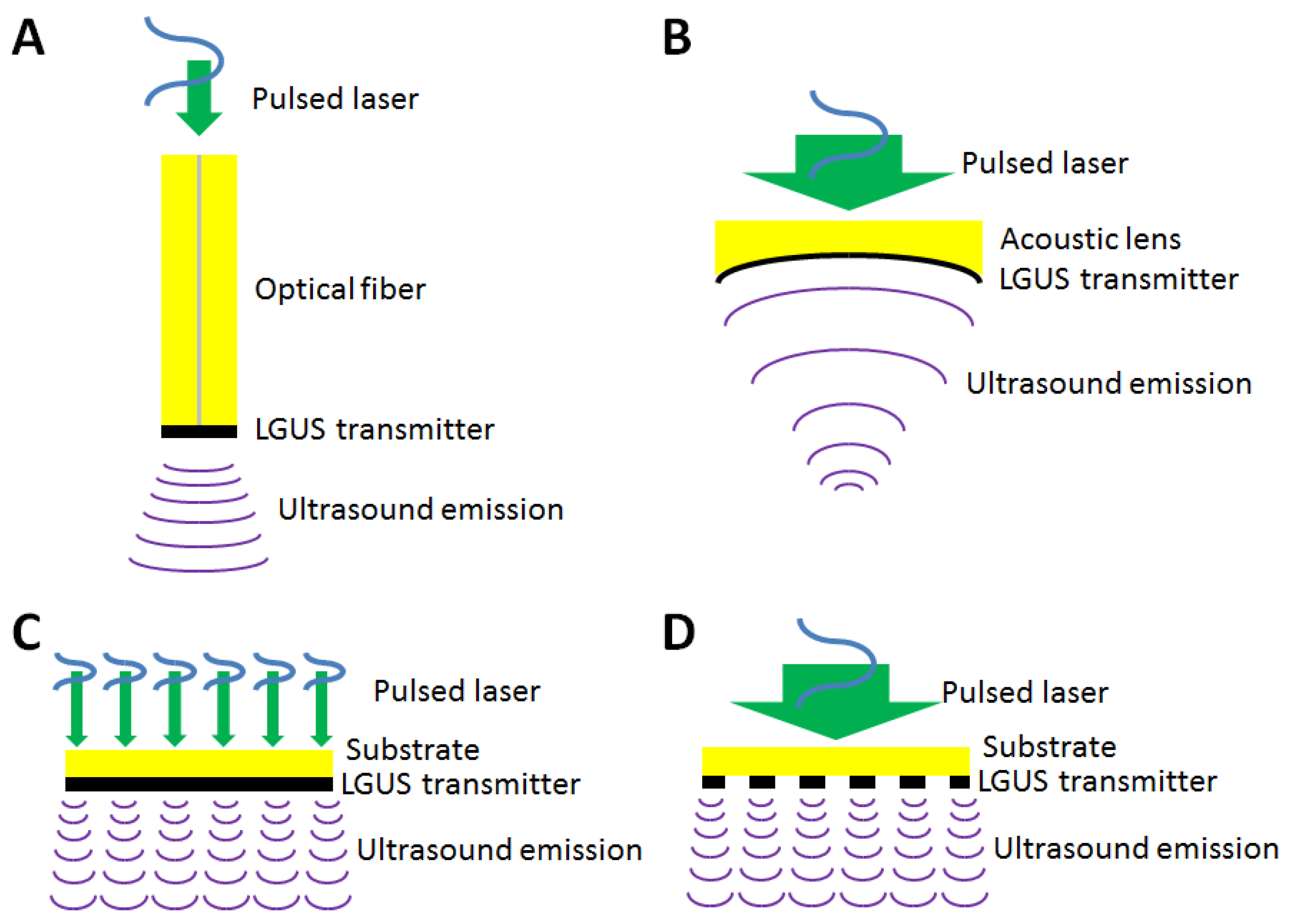
2.2. Design and Structures
2.2.1. Fiber-Optic Transmitters
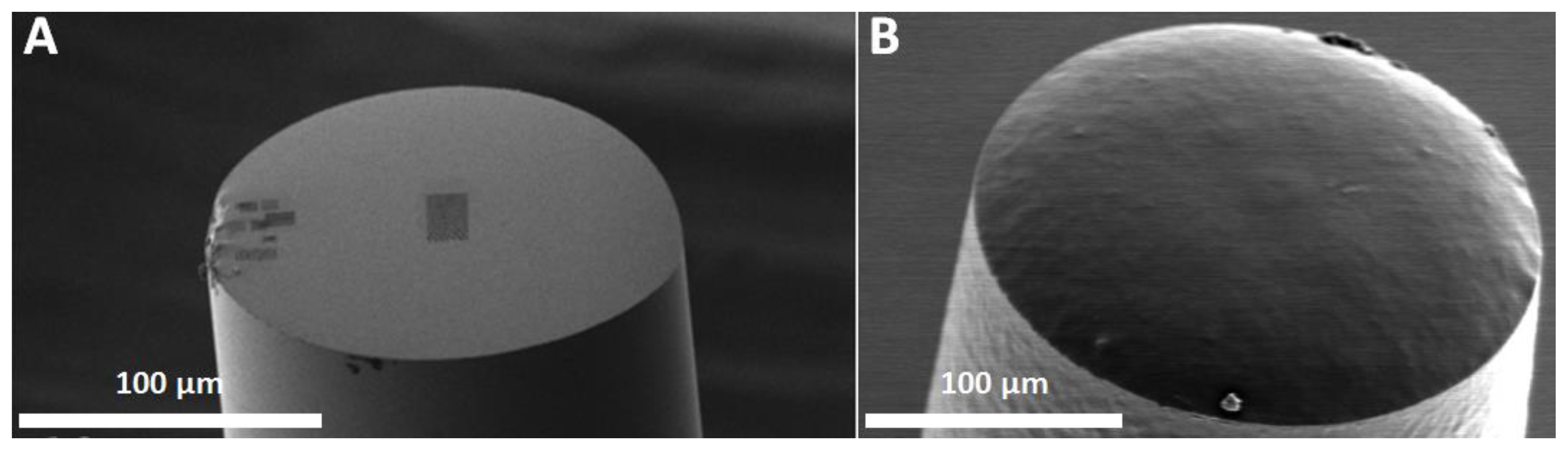
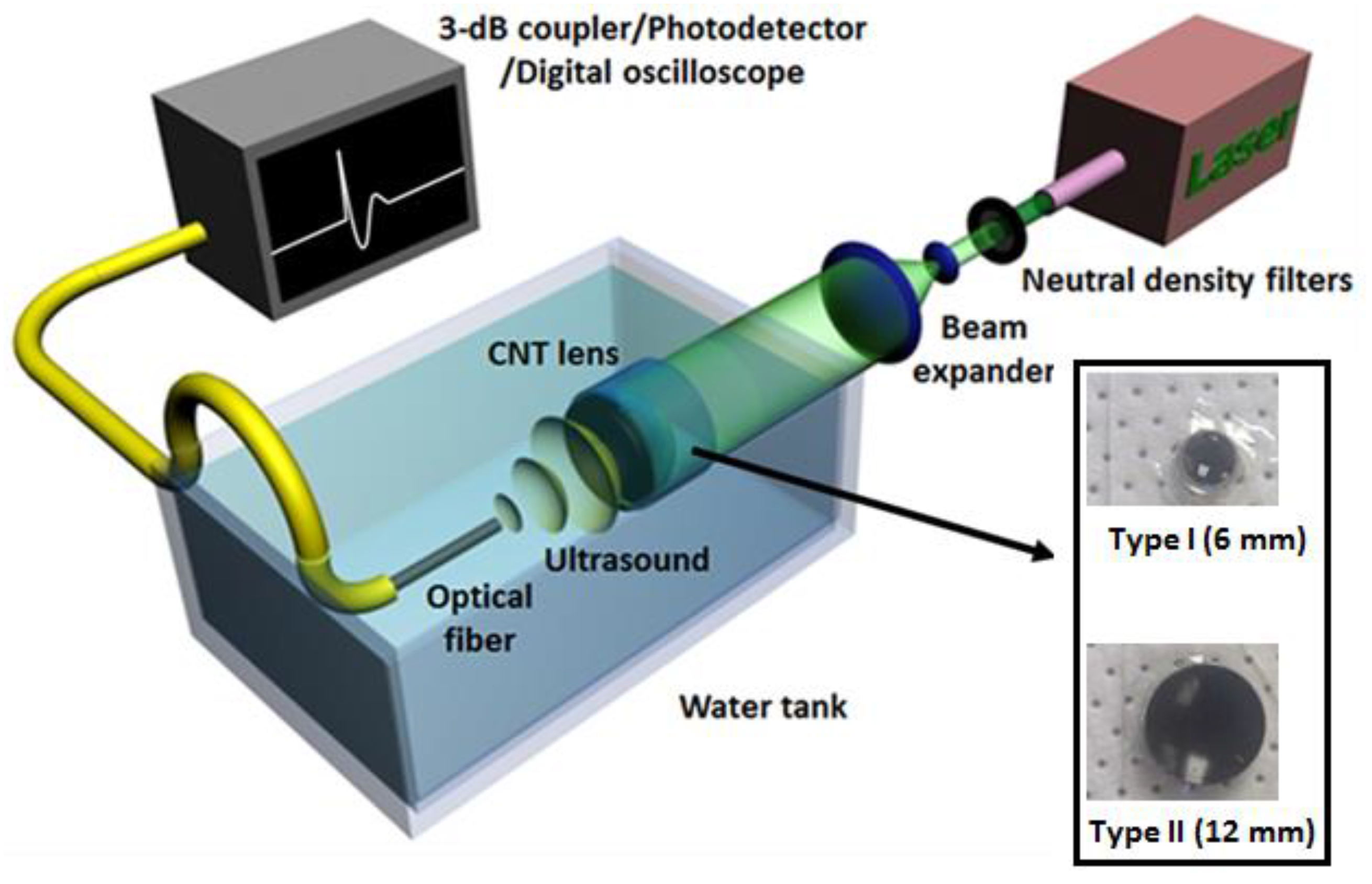
2.2.2. Focused Transmitters
2.2.3. Array Transmitters
2.3. Laser-Generated Ultrasound Applications
3. All-Optical Ultrasound Transducers and Imaging
3.1. Development of Transducers and Imaging
3.1.1. Carbon-Based Transducers
3.1.2. Fiber-Optic Transducers
3.1.3. Performance Enhancement
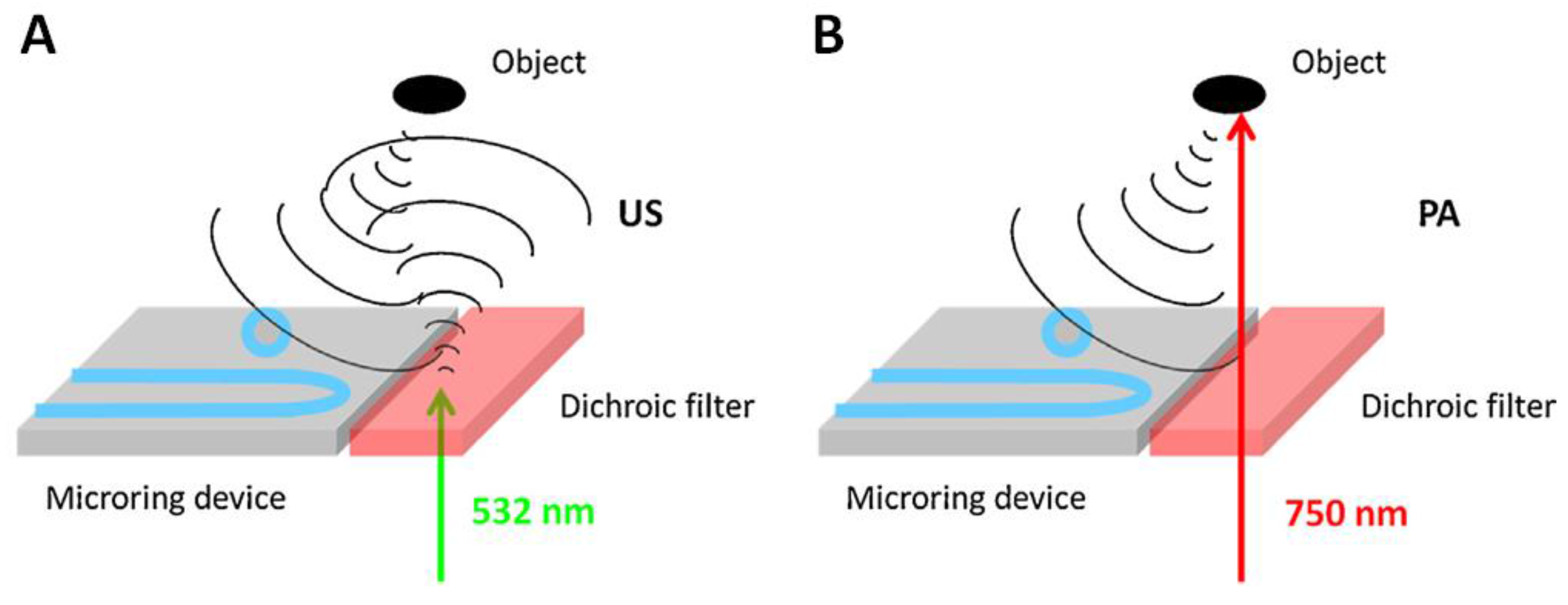
3.1.4. Multi-Modality Imaging
3.2. Imaging Applications
4. Summary and Prospects
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix
| Absorptive Material-Elastomer | Design and Structure | Specification | Peak Pressure (MPa) | Laser Density (mJ/cm2) | Band-Width (MHz) | Application | Reference |
|---|---|---|---|---|---|---|---|
| AuNPs–PDMS | Planar | - | 0.0027 | ~20 | - | - | [43] |
| AuNPs–PDMS | Planar | - | 0.19 | 13 | 3.1 | - | [39] |
| AuNPs–PDMS | Fiber | Core size: 400 μm | 0.0075 | - | - | - | [60] |
| AuNPs–PDMS | Fiber | Core size: 400 μm | 0.037 | 126 | 2.1 | - | [62] |
| AuNPs–PDMS | Fiber | Core size: 400 μm | 0.64 | 8.75 | >20 | Tissue imaging | [46] |
| AuNPs | Fiber | - | 0.0024 | ~4200 | - | - | [61] |
| AuNPs | Fiber | Array element 1 | 0.0016 | 1770 | - | - | [74] |
| Au nanopores | Fiber | Core size: 62.5 μm | 0.0027 | ~100 | 7 | - | [59] |
| Au | Planar | - | 0.24 | 23 | 78 | - | [40] |
| Al with rGO | Planar | - | ~9 | 56 | - | - | [49] |
| Al | Array | Array element 2 | - | - | - | - | [75] |
| Steel | Focused | Aperture: 28 mm | 1 | ~10 | - | NDT of cracks | [79] |
| Cr | Focused | Aperture: 6.35 mm | 0.02–0.03 | ~0.5 | - | - | [68] |
| CNFs–PDMS | Planar | - | 12 | 3.7 | 8 | - | [56] |
| CSNPs–PDMS | Planar | - | 4.8 | 3.6 | 21 | - | [57] |
| CNTs–PDMS | Fiber | Core size: 200 μm | 4.5 | 36 | 15 | - | [54] |
| CNTs–PDMS | Fiber | Core size: 200 μm | 4 | 96 | 20 | - | [77] |
| CNTs–PDMS | Focused | Aperture: 6 mm | 57 | 260–270 | - | High-precision therapy | [30] |
| CNTs–PDMS | Focused | Aperture: 15 mm | 30 | 20 | - | - | [72] |
| CB–PDMS | Planar | - | 0.8 | ~10,000 | - | - | [52] |
| CB–PDMS | Array | Array element 3 | - | - | ~100 | - | [27] |
| CB–PDMS | Array | Array element 4 | - | - | ~100 | - | [73] |
| Graphite powder | Fiber | Core size: 600 μm | 0.15 | ~3.5 | 50 | - | [76] |
| Black acrylic dye | Focused | - | ~0.02 | - | - | - | [67] |
| Laser-Generated Ultrasound | Optical Ultrasound Detection | Performance: Bandwidth (MHz); Lateral Resolution (μm); Axial Resolution (μm) | Imaging or Application | Reference | ||
|---|---|---|---|---|---|---|
| Absorptive Material-Elastomer | Design and Structure | Specification | ||||
| AuNPs | Planar | - | Thin-film FP etalon | 57; 38; 19 | - | [28,29] |
| CB–PDMS | Planar | - | Thin-film FP etalon | 40; NA; NA | - | [89] |
| CNTs–PDMS | Planar | - | Thin-film FP etalon | 27; NA; NA | - | [88] |
| Polyimide | Planar | - | Thin-film FP etalon | 29; 71; 35 | - | [95] |
| Polyimide | Planar | - | Thin-film FP etalon | ~48; 70; 35 | - | [96] |
| Silicon | Planar | - | Microring resonator | 28; 305; 169 | US/PA dual modality | [100] |
| Dichroic filter | Planar | - | Microring resonator | ~17; 2.52° 1; 125 | US/PA dual modality | [102] |
| Ti | Planar | - | Pump-probe detection | ~105 ; 2; NA | Cell mechanics | [104] |
| CNTs–PDMS | Fiber | Core size: 200 μm 2 | Fiber FP etalon | ~20; 88; 64 | AOUSI of swine aorta | [77] |
| CNTs–PDMS | Fiber | - | Thin-film FP etalon | ~40; NA; NA | AOUSI of swine aorta | [90] |
| CNTs–PDMS | Fiber | Core size: 200 μm | Fiber FP etalon | NA | Real-time guidance | [103] |
| CNTs–PDMS | Fiber | - | Fiber FP etalon | NA; NA; 60 | IVUS | [105] |
| Carbon | Fiber | - | Fiber FP etalon | 40–50; NA; NA | - | [94] |
| AuNPs | Fiber, array | - | Fiber FP etalon | NA | - | [74] |
| CB | Fiber, focused | Lens aperture: 2 mm | Fiber FP etalon | - | AOUSI of aorta | [93] |
| Cr | Focused | Cylindrical focus | Integrating detector | ~18; ~60 3 | US/PA dual modality | [101] |
References
- Learning Ultrasound Imaging, 1st ed.; del Cura, J.L.; Seguí, P.; Nicolau, C. (Eds.) Springer: Berlin/Heidelberg, Germany, 2012.
- Das, A.; Sivak, M.V.; Chak, A.; Wong, R.C.; Westphal, V.; Rollins, A.M.; Willis, J.; Isenberg, G.; Izatt, J.A. High-resolution endoscopic imaging of the GI tract: A comparative study of optical coherence tomography versus high-frequency catheter probe EUS. Gastrointest. Endosc. 2001, 54, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Fledelius, H.C. Ultrasound in ophthalmology. Ultrasound Med. Biol. 1997, 23, 365–375. [Google Scholar] [CrossRef]
- Nair, A.; Kuban, B.D.; Tuzcu, E.M.; Schoenhagen, P.; Nissen, S.E.; Vince, D.G. Coronary plaque classification with intravascular ultrasound radiofrequency data analysis. Circulation 2002, 106, 2200–2206. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Wendtner, M.H.; Burgdorf, W. Ultrasound scanning in dermatology. Arch. Dermatol. 2005, 141, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Van Wijk, D.F.; Strang, A.C.; Duivenvoorden, R.; Enklaar, D.J.; van der Geest, R.J.; Kastelein, J.J.P.; de Groot, E.; Stroes, E.S.G.; Nederveen, A.J. Increasing spatial resolution of 3T MRI scanning improves reproducibility of carotid arterial wall dimension measurements. Magn. Reson. Mater. Phys. 2014, 27, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Brun, E.; Coan, P.; Huang, Z.; Sztrókayd, A.; Diemozc, P.C.; Liebhardtd, S.; Mittonec, A.; Gasilovc, S.; Miaoa, J.; et al. High-resolution, low-dose phase contrast X-ray tomography for 3D diagnosis of human breast cancers. Proc. Natl. Acad. Sci. USA 2012, 109, 18290–18294. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.V.; Wu, H.I. Biomedical Optics: Principles and Imaging, 1st ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007. [Google Scholar]
- Beaurepaire, E.; Boccara, A.C.; Lebec, M.; Blanchot, L.; Saint-Jalmes, H. Full-field optical coherence microscopy. Opt. Lett. 1998, 23, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.V. Multiscale photoacoustic microscopy and computed tomography. Nat. Photonics 2009, 3, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.V.; Hu, S. Photoacoustic tomography: In vivo imaging from organelles to organs. Science 2012, 335, 1458–1462. [Google Scholar] [CrossRef] [PubMed]
- Bossy, E.; Gigan, S. Photoacoustics with coherent light. Photoacoustics 2016, 4, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Tanter, M.; Fink, M. Ultrafast imaging in biomedical ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2014, 61, 102–119. [Google Scholar] [CrossRef] [PubMed]
- Correia, M.; Provost, J.; Tanter, M.; Pernot, M. 4D ultrafast ultrasound flow imaging: In vivo quantification of arterial volumetric flow rate in a single heartbeat. Phys. Med. Biol. 2016, 61, L48–L61. [Google Scholar] [CrossRef] [PubMed]
- Dubinsky, T.J.; Cuevas, C.; Dighe, M.K.; Kolokythas, O.; Hwang, J.H. High-intensity focused ultrasound: Current potential and oncologic applications. AJR Am. J. Roentgenol. 2008, 190, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Larrat, B.; Pernot, M.; Aubry, J.F.; Dervishi, E.; Sinkus, R.; Seilhean, D.; Marie, Y.; Boch, A.L.; Fink, M.; Tanter, M. MR-guided transcranial brain HIFU in small animal models. Phys. Med. Biol. 2010, 55, 365–388. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Kim, H.H.; Cannata, J.M.; Chen, G.S.; Shung, K.K. 10F-4 Self-focused 1–3 composite LiNbO3 single element transducers for high frequency HIFU applications. In Proceedings of the 2007 IEEE International Ultrasonics Symposium, New York, NY, USA, 28–31 October 2007; pp. 949–952.
- Ladabaum, I.; Jin, X.; Soh, H.Y.; Atalar, A.; Khuri-Yakub, B.T. Surface micromachined capacitive ultrasonic transducers. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1998, 45, 678–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eccardt, P.C.; Niederer, K. Micromachined ultrasound transducers with improved coupling factors from a CMOS compatible process. Ultrasonics 2000, 38, 774–780. [Google Scholar] [CrossRef]
- Park, K.K.; Oralkan, O.; Khuri-Yakub, B.T. A comparison between conventional and collapse-mode capacitive micromachined ultrasonic transducers in 10-MHz 1-D arrays. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2013, 60, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- Gurun, G.; Tekes, C.; Zahorian, J.; Xu, T.; Satir, S.; Karaman, M.; Hasler, J.; Degertekin, F.L. Single-chip CMUT-on-CMOS front-end system for real-time volumetric IVUS and ICE imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2014, 61, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y. Broadband All-Optical Ultrasound Transducers for High-Resolution Ultrasound Imaging. Ph.D. Thesis, University of Michigan, Ann Arbor, MI, USA, 2008. [Google Scholar]
- Scruby, C.B.; Drain, L.E. Laser Ultrasonics Techniques and Applications; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Gusev, V.E.; Karabutov, A.A. Laser Optoacoustics; American Institute of Physics: College Park, MD, USA, 1993. [Google Scholar]
- Davies, S.J.; Edwards, C.; Taylor, G.S.; Palmer, S.B. Laser-generated ultrasound: Its properties, mechanisms and multifarious applications. J. Phys. D 1993, 26, 329–348. [Google Scholar] [CrossRef]
- Wang, L.V. Tutorial on photoacoustic microscopy and computed tomography. IEEE J. Sel. Top. Quantum Electron. 2008, 14, 171–179. [Google Scholar] [CrossRef]
- Buma, T.; Spisar, M.; O’Donnell, M. High-frequency ultrasound array element using thermoelastic expansion in an elastomeric film. Appl. Phys. Lett. 2001, 79, 548–550. [Google Scholar] [CrossRef]
- Hou, Y.; Kim, J.S.; Ashkenazi, S.; Huang, S.W.; Guo, L.J.; O’Donnell, M. Broadband all-optical ultrasound transducers. Appl. Phys. Lett. 2007, 91, 073507. [Google Scholar] [CrossRef]
- Hou, Y.; Kim, J.S.; Huang, S.W.; Ashkenazi, S.; Guo, L.J.; O’Donnell, M. Characterization of a broadband all-optical ultrasound transducer from optical and acoustical properties to imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2008, 55, 1867–1877. [Google Scholar] [PubMed]
- Baac, H.W.; Ok, J.G.; Maxwell, A.; Lee, K.T.; Chen, Y.C.; Hart, A.J.; Xu, X.; Yoon, E.; Guo, L.J. Carbon-nanotube optoacoustic lens for focused ultrasound generation and high-precision targeted therapy. Sci. Rep. 2012, 2, 989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, T.; Baac, H.W.; Ok, J.G.; Youn, H.S.; Guo, L.J. Nozzle-free liquid microjetting via homogeneous bubble nucleation. Phys. Rev. Appl. 2015, 3, 044007. [Google Scholar] [CrossRef]
- Zhang, C.; Ling, T.; Chen, S.L.; Guo, L.J. Ultrabroad bandwidth and highly sensitive optical ultrasonic detector for photoacoustic imaging. ACS Photonics 2014, 1, 1093–1098. [Google Scholar] [CrossRef]
- Hamilton, J.D.; Buma, T.; Spisar, M.; O’Donnell, M. High frequency optoacoustic arrays using etalon detection. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2000, 47, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Paltauf, G.; Nuster, R.; Haltmeier, M.; Burgholzer, P. Photoacoustic tomography using a Mach-Zehnder interferometer as an acoustic line detector. Appl. Opt. 2007, 46, 3352–3358. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Chen, S.L.; Ling, T.; Guo, L.J.; Carson, P.L.; Wang, X. Pure optical photoacoustic microscopy. Opt. Express 2011, 19, 9027–9034. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Huang, S.W.; Ashkenazi, S.; Witte, R.; O’Donnell, M. Thin polymer etalon arrays for high-resolution photoacoustic imaging. J. Biomed. Opt. 2008, 13, 064033. [Google Scholar] [CrossRef] [PubMed]
- Ling, T.; Chen, S.L.; Guo, L.J. High-sensitivity and wide-directivity ultrasound detection using high Q polymer micro-ring resonators. Appl. Phys. Lett. 2011, 98, 204103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, S.L.; Ling, T.; Guo, L.J. Review of imprinted polymer microring as ultrasound detector: Design, fabrication, and characterization. IEEE Sens. J. 2015, 15, 3241–3248. [Google Scholar] [CrossRef]
- Wu, N.; Tian, Y.; Zou, X.; Silva, V.; Chery, A.; Wang, X. High-efficiency optical ultrasound generation using one-pot synthesized polydimethylsiloxane-gold nanoparticle nanocomposite. J. Opt. Soc. Am. B 2012, 29, 2016–2020. [Google Scholar] [CrossRef]
- Guo, Y.; Baac, H.W.; Chen, S.L.; Norris, T.B.; Guo, L.J. Broad-band high-efficiency optoacoustic generation using a novel photonic crystal-metallic structure. Proc. SPIE 2011, 7899, 78992C–78992C-8. [Google Scholar]
- White, R.M. Generation of elastic waves by transient surface heating. J. Appl. Phys. 1963, 34, 3559–3567. [Google Scholar] [CrossRef]
- Kang, S.; Yoon, Y.; Kim, J.; Kim, W. Thermoelastic response of thin metal films and their adjacent materials. Appl. Phys. Lett. 2013, 102, 021908. [Google Scholar] [CrossRef]
- Hou, Y.; Kim, J.S.; Ashkenazi, S.; O’Donnell, M.; Guo, L.J. Optical generation of high frequency ultrasound using two-dimensional gold nanostructure. Appl. Phys. Lett. 2006, 89, 093901. [Google Scholar] [CrossRef] [Green Version]
- Sassaroli, E.; Li, K.C.P.; O’Neill, B.E. Numerical investigation of heating of a gold nanoparticle and the surrounding microenvironment by nanosecond laser pulses for nanomedicine applications. Phys. Med. Biol. 2009, 54, 5541–5560. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Jaradat, H.; Wu, N.; Zou, X.; Zhang, Y.; Liu, Y.; Akyurtlu, A.; Cao, C.; Wang, X. Numerical simulation of gold nanostructure absorption efficiency for fiber-optic photoacoustic generation. Prog. Electromagn. Res. Lett. 2013, 42, 209–223. [Google Scholar] [CrossRef]
- Zou, X.; Wu, N.; Tian, Y.; Wang, X. Broadband miniature fiber optic ultrasound generator. Opt. Express 2014, 22, 18119–18127. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wu, N.; Bi, S.; Wang, X. Ultrasound generation from an optical fiber sidewall. Proc. SPIE 2016, 98031U. [Google Scholar] [CrossRef]
- SoltaniI, P.; Akbareian, N. Finite element simulation of laser generated ultrasound waves in aluminum plates. Lat. Am. J. Solids Struct. 2014, 11, 1761–1776. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, M.A.; Yoh, J.J.; Song, H.; Jang, E.Y.; Kim, Y.H.; Kang, S.; Yoon, Y.S. Reduced graphene oxide coated thin aluminum film as an optoacoustic transmitter for high pressure and high frequency ultrasound generation. Appl. Phys. Lett. 2012, 101, 241909. [Google Scholar]
- Karabutov, A.A.; Kaptilniy, A.G.; Ivochkin, A.Y.; Ksenofontov, D.M.; Trofimov, A.D. Optoacoustic study of laser-induced near-critical states of thin aluminum films. Moscow Univ. Phys. 2013, 68, 383–386. [Google Scholar] [CrossRef]
- Yoo, G.; Park, Y.; Sang, P.; Baac, H.W.; Heo, J. High-frequency optoacoustic transmitter based on nanostructured germanium via metal-assisted chemical etching. Opt. Mater. Express 2016, 6, 2567–2572. [Google Scholar] [CrossRef]
- Hou, Y.; Ashkenazi, S.; Huang, S.W.; O’Donnell, M. Improvements in optical generation of high-frequency ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2007, 54, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Baac, H.W.; Ok, J.G.; Park, H.J.; Ling, T.; Chen, S.L.; Hart, A.J.; Guo, L.J. Carbon nanotube composite optoacoustic transmitters for strong and high frequency ultrasound generation. Appl. Phys. Lett. 2010, 97, 234104. [Google Scholar] [CrossRef] [PubMed]
- Colchester, R.J.; Mosse, C.A.; Bhachu, D.S.; Bear, J.C.; Carmalt, C.J.; Parkin, I.P.; Treeby, B.E.; Papakonstantinou, I.; Desjardins, A.E. Laser-generated ultrasound with optical fibres using functionalised carbon nanotube composite coatings. Appl. Phys. Lett. 2014, 104, 173502. [Google Scholar] [CrossRef]
- Baac, H.W.; Ok, J.G.; Lee, T.; Guo, L.J. Nano-structural characteristics of carbon nanotube–polymer composite films for high-amplitude optoacoustic generation. Nanoscale 2015, 7, 14460–14468. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, B.Y.; Kim, J.; Zhu, J.; Li, S.; Zhang, X.; Jiang, X. A laser ultrasound transducer using carbon nanofibers–polydimethylsiloxane composite thin film. Appl. Phys. Lett. 2015, 106, 021902. [Google Scholar] [CrossRef]
- Chang, W.Y.; Huang, W.; Kim, J.; Li, S.; Jiang, X. Candle soot nanoparticles-polydimethylsiloxane composites for laser ultrasound transducers. Appl. Phys. Lett. 2015, 107, 161903. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, Q.; Han, M. Distributed fiber-optic laser-ultrasound generation based on ghost-mode of tilted fiber Bragg gratings. Opt. Express 2013, 21, 6109–6114. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wu, N.; Zou, X.; Felemban, H.; Cao, C.; Wang, X. Fiber-optic ultrasound generator using periodic gold nanopores fabricated by a focused ion beam. Opt. Eng. 2013, 52, 065005. [Google Scholar] [CrossRef]
- Tian, Y.; Wu, N.; Sun, K.; Zou, X.; Wang, X. Numerical simulation of fiber-optic photoacoustic generator using nanocomposite material. J. Comput. Acoust. 2013, 21, 1350002. [Google Scholar] [CrossRef]
- Wu, N.; Sun, K.; Wang, X. Fiber optics photoacoustic generation using gold nanoparticles as target. Proc. SPIE 2011, 798, 765–768. [Google Scholar]
- Wu, N.; Tian, Y.; Zou, X.; Wang, X. Fiber optic photoacoustic ultrasound generator based on gold nanocomposite. Proc. SPIE 2013, 86940Q–86940Q-6. [Google Scholar]
- Biagi, E.; Margheri, F.; Menichelli, D. Efficient laser-ultrasound generation by using heavily absorbing films as targets. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2001, 48, 1669–1680. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Schmittb, T.; Perloffc, D.; Wu, N.; Yu, T.Y.; Wang, X. Nondestructive corrosion detection using fiber optic photoacoustic ultrasound generator. Measurement 2015, 62, 74–80. [Google Scholar] [CrossRef]
- Roome, K.A.; Payne, P.A.; Dewhurst, R.J. Towards a sideways looking intravascular laser-ultrasound probe. Sens. Actuators 1999, 76, 197–202. [Google Scholar] [CrossRef]
- Belsito, L. Design and Fabrication of MOMS-Based Ultrasonic Probes for Minimally Invasive Endoscopic Applications. Ph.D. Thesis, University of Bologna, Bologna, Italy, 2010. [Google Scholar]
- Passler, K.; Nuster, R.; Gratt, S.; Burgholzer, P.; Paltauf, G. Laser-generation of ultrasonic X-waves using axicon transducers. Appl. Phys. Lett. 2009, 94, 064108. [Google Scholar] [CrossRef]
- Baac, H.W.; Ling, T.; Ashkenazi, S.; Huang, S.W.; Guo, L.J. Photoacoustic concave transmitter for generating high frequency focused ultrasound. Proc. SPIE 2010, 7564, 116. [Google Scholar]
- Baac, H.W.; Ok, J.G.; Guo, L.J. Design of high-intensity focused ultrasound transmitters based on optoacoustic generation. In Proceedings of the 2011 IEEE International Ultrasonics Symposium, Orlando, FL, USA, 18–21 October 2011; pp. 2353–2356.
- Lee, T.; Ok, J.G.; Youn, H.S.; Guo, L.J.; Baac, H.W. Micro-bubbles generated by a single focused optoacoustic wave. In Proceedings of the 2014 IEEE International Ultrasonics Symposium, Chicago, IL, USA, 3–6 September 2014; pp. 1041–1044.
- Tong, L.H.; Lim, C.W.; Li, Y.C. Generation of high-intensity focused ultrasound by carbon nanotube opto-acoustic lens. J. Appl. Mech. 2014, 81, 081014. [Google Scholar] [CrossRef]
- Chan, W.; Hies, T.; Ohl, C.D. Laser-generated focused ultrasound for arbitrary waveforms. Appl. Phys. Lett. 2016, 109, 174102. [Google Scholar] [CrossRef]
- Buma, T.; Spisar, M.; O’Donnell, M. A high-frequency, 2-D array element using thermoelastic expansion in PDMS. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2003, 50, 1161–1176. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Tian, Y.; Zou, X.; Wang, X. Study of the compact fiber optic photoacoustic ultrasonic transducer. Proc. SPIE 2012, 8345, 83453Z–83453Z-10. [Google Scholar]
- Schmieder, F.; Büttner, L.; Czarske, J. Adaptive laser-induced ultrasound generation using a micro-mirror array spatial light modulator. Opt. Express 2016, 24, 22536–22543. [Google Scholar] [CrossRef] [PubMed]
- Biagi, E.; Cerbai, S.; Gambacciani, P.; Masotti, L. Fiber optic broadband ultrasonic probe. In Proceedings of the 7th IEEE Conference on Sensors, Lecce, Italy, 26–29 October 2008; pp. 363–366.
- Colchester, R.J.; Zhang, E.Z.; Mosse, C.A.; Beard, P.C.; Papakonstantinou, I.; Desjardins, A.E. Broadband miniature optical ultrasound probe for high resolution vascular tissue imaging. Biomed. Opt. Express 2015, 6, 1502–1511. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Wu, N.; Tian, Y.; Zhang, Y.; Wang, X. Polydimethylsiloxane thin film characterization using all-optical photoacoustic mechanism. Appl. Opt. 2013, 52, 6239–6244. [Google Scholar] [CrossRef] [PubMed]
- Kozhushko, V.V.; Hess, P. Nondestructive evaluation of microcracks by laser-induced focused ultrasound. Appl. Phys. Lett. 2007, 91, 224107. [Google Scholar] [CrossRef]
- Kozhushko, V.V.; Hess, P. Laser-induced focused ultrasound for nondestructive testing and evaluation. J. Appl. Phys. 2008, 103, 124902. [Google Scholar] [CrossRef]
- Karabutov, A.A.; Kaptil’nyi, A.G.; Ivochkin, A.Y. A laser optoacoustic method of inducing high-energy states and the investigation of phase transitions in metals at high pressures. High Temp. 2007, 45, 613–620. [Google Scholar] [CrossRef]
- Xia, W.; Mosse, C.A.; Colchester, R.J.; Mari, J.M.; Nikitichev, D.I.; West, S.J.; Ourselin, S.; Beard, P.C.; Desjardins, A.E. Fiber optic photoacoustic probe with ultrasonic tracking for guiding minimally invasive procedures. Proc. SPIE 2015, 13, 244–248. [Google Scholar]
- Sorazu, B.; Thursby, G.; Culshaw, B.; Dong, F.; Pierce, S.G.; Yang, Y.; Betz, D. Optical generation and detection of ultrasound. Strain 2003, 39, 111–114. [Google Scholar] [CrossRef]
- Yao, J.; Wang, L.V. Sensitivity of photoacoustic microscopy. Photoacoustics 2014, 2, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Li, Z.; Chen, S.L. In vivo deconvolution acoustic-resolution photoacoustic microscopy in three dimensions. Biomed. Opt. Express 2016, 7, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.L.; Chang, Y.C.; Zhang, C.; Ok, J.G.; Ling, T.; Mihnev, M.T.; Norris, T.B.; Guo, L.J. Efficient real-time detection of terahertz pulse radiation based on photoacoustic conversion by carbon nanotube nanocomposite. Nat. Photonics 2014, 8, 537–542. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, S.L.; Ling, T.; Guo, L.J. Imprinted polymer microrings as high-performance ultrasound detectors in photoacoustic imaging. IEEE J. Lightw. Technol. 2015, 33, 4318–4328. [Google Scholar] [CrossRef]
- Yoo, G.; Yoon, H.; Heo, J.; Thakur, U.K.; Park, H.J.; Baac, H.W.; Heo, J. All-optical ultrasound transducer using CNT-PDMS and etalon thin-film structure. IEEE Photonics J. 2015, 7, 6903708. [Google Scholar] [CrossRef]
- Hou, Y.; Ashkenazi, S.; Huang, S.W.; O’Donnell, M. An integrated optoacoustic transducer combining etalon and black PDMS structures. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2008, 55, 2719–2725. [Google Scholar] [PubMed]
- Noimark, S.; Colchester, R.J.; Blackburn, B.J.; Zhang, E.Z.; Alles, E.J.; Ourselin, S.; Beard, P.C.; Papakonstantinou, I.; Parkin, I.P.; Desjardins, A.E. Carbon-nanotube–PDMS composite coatings on optical fibers for all-optical ultrasound imaging. Adv. Funct. Mater. 2016. [Google Scholar] [CrossRef]
- Cai, D.; Li, Z.; Chen, S.L. Photoacoustic microscopy by scanning mirror-based synthetic aperture focusing technique. Chin. Opt. Lett. 2015, 13, 101101. [Google Scholar] [CrossRef]
- Miida, Y.; Matsuura, Y. All-optical photoacoustic imaging system using fiber ultrasound probe and hollow optical fiber bundle. Opt. Express 2013, 21, 22023–22033. [Google Scholar] [CrossRef] [PubMed]
- Alles, E.J.; Noimark, S.; Zhang, E.; Beard, P.C.; Desjardins, A.E. Pencil beam all-optical ultrasound imaging. Biomed. Opt. Express 2016, 7, 3696–3704. [Google Scholar] [CrossRef] [PubMed]
- Biagi, E.; Cerbai, S.; Masotti, L.; Belsito, L.; Roncaglia, A.; Masetti, G.; Speciale, N. Fiber optic broadband ultrasonic probe for virtual biopsy: Technological solutions. J. Sens. 2010, 2010, 917314. [Google Scholar] [CrossRef]
- Sheaff, C.; Ashkenazi, S. A polyimide-etalon thin film structure for all-optical high-frequency ultrasound transduction. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2012, 59, 2254–2261. [Google Scholar] [CrossRef] [PubMed]
- Sheaff, C.; Ashkenazi, S. Characterization of an improved polyimide-etalon all-optical transducer for high-resolution ultrasound imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2014, 61, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Alles, E.J.; Colchester, R.J.; Desjardins, A.E. Adaptive light modulation for improved resolution and efficiency in all-optical pulse-echo ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2016, 63, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Laufer, J.; Beard, P. Backward-mode multiwavelength photoacoustic scanner using a planar Fabry-Perot polymer film ultrasound sensor for high-resolution three-dimensional imaging of biological tissues. Appl. Opt. 2008, 47, 561–577. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.L.; Guo, L.J.; Wang, X. All-optical photoacoustic microscopy. Photoacoustics 2015, 3, 143–150. [Google Scholar] [CrossRef]
- Hsieh, B.Y.; Chen, S.L.; Ling, T.; Guo, L.J.; Li, P.C. All-optical scanhead for ultrasound and photoacoustic dual-modality imaging. Opt. Express 2012, 20, 1588–1596. [Google Scholar] [CrossRef] [PubMed]
- Nuster, R.; Schmitner, N.; Wurzinger, G.; Gratt, S.; Salvenmoser, W.; Meyer, D.; Paltauf, G. Hybrid photoacoustic and ultrasound section imaging with optical ultrasound detection. J. Biophotonics 2013, 6, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, B.Y.; Chen, S.L.; Ling, T.; Guo, L.J.; Li, P.C. All-optical scanhead for ultrasound and photoacoustic imaging-Imaging mode switching by dichroic filtering. Photoacoustics 2014, 2, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Colchester, R.J.; Mosse, C.A.; Nikitichev, D.I.; Zhang, E.Z.; West, S.; Beard, P.C.; Papakonstantinou, I.; Desjardins, A.E. Real-time needle guidance with photoacoustic and laser-generated ultrasound probes. Proc. SPIE 2015, 9323. [Google Scholar] [CrossRef]
- Dehoux, T.; Abi Ghanem, M.; Zouani, O.F.; Rampnoux, J.M.; Guillet, Y.; Dilhaire, S.; Durrieu, M.C.; Audoin, B. All-optical broadband ultrasonography of single cells. Sci. Rep. 2015, 5, 8650. [Google Scholar] [CrossRef] [PubMed]
- Colchester, R.J.; Noimark, S.; Mosse, C.A.; Zhang, E.Z.; Beard, P.C.; Parkin, I.P.; Papakonstantinou, I.; Desjardins, A.E. All-optical pulse-echo ultrasound probe for intravascular imaging (Conference Presentation). Proc. SPIE 2016, 9689. [Google Scholar] [CrossRef]
- Decremps, F.; Gauthier, M.; Ayrinhac, S.; Bove, L.; Belliard, L.; Perrin, B.; Morand, M.; Marchand, G.L.; Bergame, F.; Philippe, J. Picosecond acoustics method for measuring the thermodynamical properties of solids and liquids at high pressure and high temperature. Ultrasonics 2015, 56, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Majlesi, J.; Unalan, H. High-power pain threshold ultrasound technique in the treatment of active myofascial trigger points: A randomized, double-blind, case-control study. Arch. Phys. Med. Rehabil. 2004, 85, 833–836. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Chen, S.; Zhang, Z.; Sun, C.; Zhang, H.F. Photoacoustic probe using a microring resonator ultrasonic sensor for endoscopic applications. Opt. Lett. 2014, 39, 4372–4375. [Google Scholar] [CrossRef] [PubMed]
- Stasio, N.; Shibukawa, A.; Papadopoulos, I.N.; Farahi, S.; Simandoux, O.; Huignard, J.P.; Bossy, E.; Moser, C.; Psaltis, D. Towards new applications using capillary waveguides. Biomed. Opt. Express 2015, 6, 4619–4631. [Google Scholar] [CrossRef] [PubMed]





© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.-L. Review of Laser-Generated Ultrasound Transmitters and Their Applications to All-Optical Ultrasound Transducers and Imaging. Appl. Sci. 2017, 7, 25. https://doi.org/10.3390/app7010025
Chen S-L. Review of Laser-Generated Ultrasound Transmitters and Their Applications to All-Optical Ultrasound Transducers and Imaging. Applied Sciences. 2017; 7(1):25. https://doi.org/10.3390/app7010025
Chicago/Turabian StyleChen, Sung-Liang. 2017. "Review of Laser-Generated Ultrasound Transmitters and Their Applications to All-Optical Ultrasound Transducers and Imaging" Applied Sciences 7, no. 1: 25. https://doi.org/10.3390/app7010025
APA StyleChen, S.-L. (2017). Review of Laser-Generated Ultrasound Transmitters and Their Applications to All-Optical Ultrasound Transducers and Imaging. Applied Sciences, 7(1), 25. https://doi.org/10.3390/app7010025






