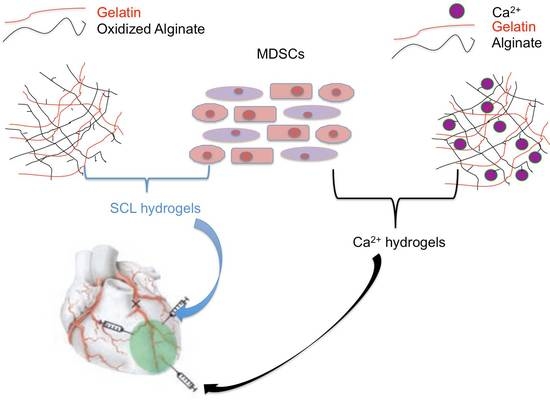
Synthesis of Injectable Alginate Hydrogels with Muscle-Derived Stem Cells for Potential Myocardial Infarction Repair
Abstract
:1. Introduction
2. Materials and Methods
2.1. Gelatin Extraction from Rat Tails
2.2. Cell Cultivation
2.3. SCL Hydrogel Synthesis
2.4. Ca2+ Hydrogel Synthesis
2.5. SEM Examination of Hydrogel Morphology
2.6. Mechanical Strength Measurement
2.7. Hydrogel Degradation
2.8. MTT Assay for Cell Proliferation
2.9. Immunofluorescence for Live/Dead Cells
3. Results and Discussion
3.1. Morphology
3.2. Mechanical Strength
3.3. Degradation
3.4. MTT
3.5. Immunofluorescence
4. Limitations and Future Work
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Izadifar, M.; Kelly, M.E.; Chen, X. Engineering Angiogenesis for Myocardial Infarction Repair: Recent Developments, Challenges, and Future Directions. Cardiovasc. Eng. Technol. 2014, 5, 281–307. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Despres, J.P.; Fullerton, H.J. On behalf of the American Heart Association Statistics committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2016 Update: A Report from the American Heart Association. Circulation 2016, 133, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Zwetsloot, P.P.; Vegh, A.M.; Lorkeers, S.J.J.; van Hout, G.P.; Currie, G.L.; Sena, E.S.; Gremmels, H.; Buikema, J.W.; Goumans, M.J.; Macleod, M.R.; et al. Cardiac Stem Cell Treatment in Myocardial Infarction: A Systematic Review and Meta-Analysis of Preclinical Studies. Circ. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Beltrami, A.P.; Urbanek, K.; Kajstura, J.; Yan, S.M.; Finato, N.; Bussani, R.; Nadal-Ginard, B.; Silvestri, F.; Leri, A.; Beltrami, C.A.; et al. Evidence that Human Cardiac Myocytes Divide after Myocardial Infarction. N Engl. J. Med. 2001, 344, 1750–1757. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Kim, J.J.; Woo, Y.J.; Huang, N.F. Stem Cell-Based Therapies to Promote Angiogenesis in Ischemic Cardiovascular Disease. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H455–H465. [Google Scholar] [CrossRef] [PubMed]
- Jing, D.; Parikh, A.; Canty, J.M., Jr.; Tzanakakis, E.S. Stem Cells for Heart Cell Therapies. Tissue Eng. B Rev. 2008, 14, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, W.H.; Schneiderbanger, K.; Schubert, P.; Didie, M.; Munzel, F.; Heubach, J.F.; Kostin, S.; Neuhuber, W.L.; Eschenhagen, T. Tissue Engineering of a Differentiated Cardiac Muscle Construct. Circ. Res. 2002, 90, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Black, L.D., 3rd; Meyers, J.D.; Weinbaum, J.S.; Shvelidze, Y.A.; Tranquillo, R.T. Cell-Induced Alignment Augments Twitch Force in Fibrin Gel-Based Engineered Myocardium via Gap Junction Modification. Tissue Eng. A 2009, 15, 3099–3108. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Liao, C.K.; Yang, M.J.; Chen, C.H.; Hwang, S.M.; Hung, Y.W.; Chang, Y.; Sung, H.W. A Strategy for Fabrication of a Three-Dimensional Tissue Construct Containing Uniformly Distributed Embryoid Body-Derived Cells as a Cardiac Patch. Biomaterials 2010, 31, 6218–6227. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.N.; Lu, S.H.; Wang, H.B.; Li, D.X.; Duan, C.M.; Liu, Z.Q.; Hao, T.; He, W.J.; Xu, B.; Fu, Q.; et al. Functional Improvement of Infarcted Heart by Co-Injection of Embryonic Stem Cells with Temperature-Responsive Chitosan Hydrogel. Tissue Eng. A 2009, 15, 1437–1447. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.J.; Chen, C.H.; Lee, W.Y.; Chiu, I.; Hwang, S.M.; Lin, W.W.; Huang, C.C.; Yeh, Y.C.; Chang, Y.; Sung, H.W. Bioengineered Cardiac Patch Constructed from Multilayered Mesenchymal Stem Cells for Myocardial Repair. Biomaterials 2008, 29, 3547–3556. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Ooi, T.H.; Tan, G.; Lim, S.Y.; Qian, L.; Wong, P.; Shim, W. Cell Delivery and Tracking in Post-Myocardial Infarction Cardiac Stem Cell Therapy: An Introduction for Clinical Researchers. Heart Fail. Rev. 2010, 15, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, T.; Akatsuka, A.; Okada, Y.; Uchiyama, Y.; Tono, K.; Wada, M.; Hoshi, A.; Iwaguro, H.; Iwasaki, H.; Oyamada, A.; et al. Cardiomyocyte Formation by Skeletal Muscle-Derived Multi-Myogenic Stem Cells after Transplantation into Infarcted Myocardium. PLoS ONE 2008, 3, e1789. [Google Scholar] [CrossRef] [PubMed]
- Oshima, H.; Payne, T.R.; Urish, K.L.; Sakai, T.; Ling, Y.; Gharaibeh, B.; Tobita, K.; Keller, B.B.; Cummins, J.H.; Huard, J. Differential Myocardial Infarct Repair with Muscle Stem Cells Compared to Myoblasts. Mol. Ther. 2005, 12, 1130–1141. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Kovanecz, I.; Vernet, D.; Nolazco, G.; Kopchok, G.E.; Chow, S.L.; White, R.A.; Gonzalez-Cadavid, N.F. Effects of Sildenafil and/or Muscle Derived Stem Cells on Myocardial Infarction. J. Transl. Med. 2012, 10, 159. [Google Scholar] [CrossRef] [PubMed]
- Payne, T.R.; Oshima, H.; Okada, M.; Momoi, N.; Tobita, K.; Keller, B.B.; Peng, H.; Huard, J. A Relationship between Vascular Endothelial Growth Factor, Angiogenesis, and Cardiac Repair after Muscle Stem Cell Transplantation into Ischemic Hearts. J. Am. Coll. Cardiol. 2007, 50, 1677–1684. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Payne, T.R.; Zheng, B.; Oshima, H.; Momoi, N.; Tobita, K.; Keller, B.B.; Phillippi, J.A.; Peault, B.; Huard, J. Myogenic Endothelial Cells Purified from Human Skeletal Muscle Improve Cardiac Function after Transplantation into Infarcted Myocardium. J. Am. Coll. Cardiol. 2008, 52, 1869–1880. [Google Scholar] [CrossRef] [PubMed]
- Izadifar, M.; Haddadi, A.; Chen, X.; Kelly, M.E. Rate-Programming of Nano-Particulate Delivery Systems for Smart Bioactive Scaffolds in Tissue Engineering. Nanotechnology 2015, 26, 012001. [Google Scholar] [CrossRef] [PubMed]
- Izadifar, M.; Kelly, M.E.; Haddadi, A.; Chen, X. Optimization of Nanoparticles for Cardiovascular Tissue Engineering. Nanotechnology 2015, 26, 235301. [Google Scholar] [CrossRef] [PubMed]
- Izadifar, M.; Kelly, M.E.; Chen, X. Regulation of Sequential Release of Growth Factors Using Bilayer Polymeric Nanoparticles for Cardiac Tissue Engineering. Nanomedicine 2016, 11, 3237–3259. [Google Scholar] [CrossRef] [PubMed]
- Nicodemus, G.D.; Bryant, S.J. Cell Encapsulation in Biodegradable Hydrogels for Tissue Engineering Applications. Tissue Eng. B Rev. 2008, 14, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tian, X.; Zhu, N.; Schreyer, D.J.; Chen, X. Modeling Process-Induced Cell Damage in the Biodispensing Process. Tissue Eng. C Methods 2010, 16, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.D.; Christman, K.L. Injectable Hydrogel Therapies and their Delivery Strategies for Treating Myocardial Infarction. Expert Opin. Drug Deliv. 2013, 10, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Wheeldon, I.; Farhadi, A.; Bick, A.G.; Jabbari, E.; Khademhosseini, A. Nanoscale Tissue Engineering: Spatial Control over Cell-Materials Interactions. Nanotechnology 2011, 22, 212001. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Ahn, S.; Chun, W.; Kim, G. Enhancement of Cell Viability by Fabrication of Macroscopic 3D Hydrogel Scaffolds using an Innovative Cell-Dispensing Technique Supplemented by Preosteoblast-Laden Micro-Beads. Carbohydr. Polym. 2014, 104, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Cha, M.J.; Lim, K.S.; Kim, J.K.; Lee, S.K.; Kim, Y.H.; Hwang, K.C.; Lee, K.Y. Injectable Microsphere/Hydrogel Hybrid System Containing Heat Shock Protein as Therapy in a Murine Myocardial Infarction Model. J. Drug Target. 2013, 21, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Maureira, P.; Tran, N.; Djaballah, W.; Angioi, M.; Bensoussan, D.; Didot, N.; Fay, R.; Sadoul, N.; Villemot, J.P.; Marie, P.Y. Residual Viability is a Predictor of the Perfusion Enhancement Obtained with the Cell Therapy of Chronic Myocardial Infarction: A Pilot Multimodal Imaging Study. Clin. Nucl. Med. 2012, 37, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Ruvinov, E.; Leor, J.; Cohen, S. The Promotion of Myocardial Repair by the Sequential Delivery of IGF-1 and HGF from an Injectable Alginate Biomaterial in a Model of Acute Myocardial Infarction. Biomaterials 2011, 32, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.P.; Zheng, H.X.; Fang, R.; Wang, T.R.; Hou, X.L.; Li, Y.; Chen, X.B.; Tian, W.M. Fabrication of Engineered Heart Tissue Grafts from Alginate/Collagen Barium Composite Microbeads. Biomed. Mater. 2011, 6, 045002. [Google Scholar] [CrossRef] [PubMed]
- Rowley, J.A.; Madlambayan, G.; Mooney, D.J. Alginate Hydrogels as Synthetic Extracellular Matrix Materials. Biomaterials 1999, 20, 45–53. [Google Scholar] [CrossRef]
- Cabrales, P.; Tsai, A.G.; Intaglietta, M. Alginate Plasma Expander Maintains Perfusion and Plasma Viscosity during Extreme Hemodilution. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H1708–H1716. [Google Scholar] [CrossRef] [PubMed]
- Chevallay, B.; Herbage, D. Collagen-Based Biomaterials as 3D Scaffold for Cell Cultures: Applications for Tissue Engineering and Gene Therapy. Med. Biol. Eng. Comput. 2000, 38, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Cen, L.; Liu, W.; Cui, L.; Zhang, W.; Cao, Y. Collagen Tissue Engineering: Development of Novel Biomaterials and Applications. Pediatr. Res. 2008, 63, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.P.; Fang, R.; Zhang, S.; Shi, X.L.; Wang, Z.L.; Chen, X.B.; Yang, J.; Hou, X.L.; Nie, Y.Z.; Li, Y.; Tian, W.M. Self-Cross-Linkable Hydrogels Composed of Partially Oxidized Alginate and Gelatin for Myocardial Infarction Repair. J.Bioact. Compat. Polym. 2013, 28, 126–140. [Google Scholar] [CrossRef]
- Fang, R.; Qiao, S.; Liu, Y.; Meng, Q.; Chen, X.; Song, B.; Hou, X.; Tian, W. Sustained Co-Delivery of BIO and IGF-1 by a Novel Hybrid Hydrogel System to Stimulate Endogenous Cardiac Repair in Myocardial Infarcted Rat Hearts. Int. J. Nanomed. 2015, 10, 4691–4703. [Google Scholar] [CrossRef] [PubMed]





© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, R.; Tian, W.; Chen, X. Synthesis of Injectable Alginate Hydrogels with Muscle-Derived Stem Cells for Potential Myocardial Infarction Repair. Appl. Sci. 2017, 7, 252. https://doi.org/10.3390/app7030252
Fang R, Tian W, Chen X. Synthesis of Injectable Alginate Hydrogels with Muscle-Derived Stem Cells for Potential Myocardial Infarction Repair. Applied Sciences. 2017; 7(3):252. https://doi.org/10.3390/app7030252
Chicago/Turabian StyleFang, Rui, Weiming Tian, and Xiongbiao Chen. 2017. "Synthesis of Injectable Alginate Hydrogels with Muscle-Derived Stem Cells for Potential Myocardial Infarction Repair" Applied Sciences 7, no. 3: 252. https://doi.org/10.3390/app7030252
APA StyleFang, R., Tian, W., & Chen, X. (2017). Synthesis of Injectable Alginate Hydrogels with Muscle-Derived Stem Cells for Potential Myocardial Infarction Repair. Applied Sciences, 7(3), 252. https://doi.org/10.3390/app7030252