Chemical Constituents and Biological Activities of Essential Oils of Hydnora africana Thumb Used to Treat Associated Infections and Diseases in South Africa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Collection and Preparation
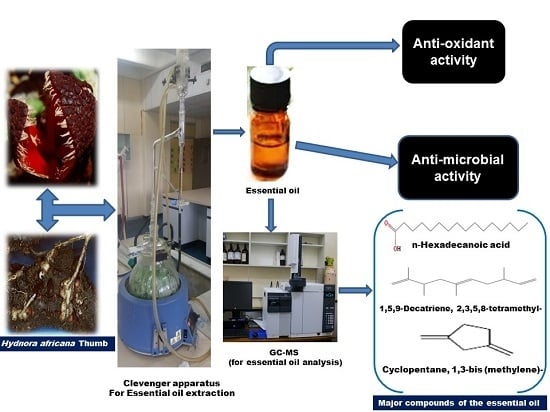
2.2. Extraction of the Essential Oil
2.3. Chemicals and Reagents Used
2.4. Antioxidant Assay
2.4.1. 1,1-diphenyl-2-picrylhydrazyl DPPH Radical Scavenging Activity Assay
2.4.2. 2,2′-Aazino-bis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) Radical Scavenging Assay
2.4.3. Nitric Oxide Scavenging Activity Assay
2.4.4. Hydrogen Peroxide (H2O2) Scavenging Activity Assay
2.5. Antimicrobial Activity
2.5.1. Microorganisms and Media
2.5.2. Antibacterial and Antifungal Susceptibility Test
2.5.3. Minimum Inhibitory Concentration (MIC) Assay
2.6. Chemical Composition Evaluation
2.6.1. Gas Chromatography-Mass Spectrometry (GC-MS)
2.6.2. Identification of Components
2.6.3. Statistical Analysis
3. Results and Discussion
3.1. Antioxidant Activities
3.2. Antimicrobial Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shaba, E.Y.; Mann, A.; Yisa, J. Antimicrobial and cytotoxic activities and GC-MS analysis of phytocomponents of methanolic extract of Curculigo pilosa (Schum and Thonn) Engl. (Hypoxidaceae) rhizomes. Br. J. Pharm. Res. 2014, 4, 1552–1567. [Google Scholar] [CrossRef]
- Prabuseenivasan, S.; Jayakumar, M.; Ignacimuthu, S. In Vitro antibacterial activity of some plant essential oils. BMC Complement. Altern. Med. 2006, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Casqueiro, J.; Casqueiro, J.; Alves, C. Infections in patients with diabetes mellitus: A review of pathogenesis. Indian J. Endocrinol. Metab. 2012, 16, S27–S36. [Google Scholar] [PubMed]
- Longdoh, N.A.; Assob, J.C.N.; Nsagha, S.D.; Nde, P.F.; Kamga, H.L.F.; Nkume, A.F.; Emmanuel, T.K. Uropathogens from diabetic patients with asymptomatic bacteriuria and urinary tract infections. West Lond. Med. J. 2013, 5, 7–14. [Google Scholar]
- Al-Salihi, S.H.; Jumaah, I.A.M. Bacterial infection of diabetic foot ulcer. Diyala J. 2013, 9, 15–21. [Google Scholar]
- Sharma, N.; Samarakoon, K.W.; Gyawali, R.; Park, Y.; Lee, S.; Jong Oh, S.; Lee, T.H.; Jeong, D.K. Evaluation of the antioxidant, anti-inflammatory and anticancer activities of Euphorbia hirta ethanolic extract. Molecules 2014, 19, 14567–14581. [Google Scholar] [CrossRef] [PubMed]
- Nickrent, D.L.; Blarer, A.; Qiu, Y.L.; Soltis, D.E.; Soltis, P.S.; Zanis, M.J. Molecular data place Hydnoraceae with Aristolochiaceae. Am. J. Bot. 2002, 89, 1809–1817. [Google Scholar] [CrossRef] [PubMed]
- Masika, P.J.; Afolayan, A.J. An ethnobotanical study of plants used for the treatment of livestock diseases in the Eastern Cape Province, South Africa. Pharm. Biol. 2003, 41, 16–21. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopoeia Commission European Directorate for the Quality of Medicines & Healthcare, 4th ed.; European Pharmacopoeia; Council of Europe: Strasbourg, France, 2002. [Google Scholar]
- Liyana-Pathiranan, C.M.; Shahidi, F. Antioxidant activity of commercial soft and hard wheat (Triticum aestivum L.) as affected by gastric pH conditions. J. Agric. Food Chem. 2005, 53, 2433–2440. [Google Scholar] [CrossRef] [PubMed]
- Oyedemi, S.O.; Bradley, G.; Afolayan, A.J. In vitro and in vivo antioxidant activities of aqueous extract of Strychonos henningsii Gilg. Afr. J. Pharm. Pharacol. 2010, 4, 70–78. [Google Scholar]
- Otang, W.M.; Grierson, D.S.; Ndip, R.N. Antifungal activity of Arctotis arctotoides (L.f.) O. Hoffm. and Gasteria bicolor Haw. against opportunistic fungi associated with human immuno deficiency virus/acquired immunodeficiency syndrome. Pharmacogn. Mag. 2012, 8, 135–140. [Google Scholar] [PubMed]
- Shahat, A.A.; Ibrahim, A.Y.; Hendawy, S.F.; Omer, E.A.; Hammouda, F.M.; Abdel-Rahman, F.H.; Saleh, M.A. Chemical composition, antimicrobial and antioxidant activities of essential oils from organically cultivated fennel cultivars. Molecules 2011, 16, 1366–1377. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, A.; Sacchetti, G.; Rossi, D.; Paganetto, G.; Muzzoli, M.; Andreotti, E. Bioactivities of Piper aduncum and Piper obliquum essential oils from Eastern Ecuador. Environ. Toxicol. Pharmacol. 2009, 27, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Nyegue, M.A.; Ndoyé, F.; AmvamZollo, H.; Etoa, F.X.; Agnaniet, H.; Menut, C. Chemical and biological evaluation of essential oil of Pentadiplandra brazzeana (Baill) roots from Cameroon. Adv. Phytother. Res. 2002, 43, 91–107. [Google Scholar]
- Ndoye Foe, F.M.; Tchinang, T.F.K.; Nyegue, A.M.; Abdou, J.; Yaya, A.J.G.; Alembert, T.T.; Essame, J.O.; Etoa, F.X. Chemical composition, in vitro antioxidant and anti-inflammatory properties of essential oils of four dietary and medicinal plants from Cameroon. BMC Complement. Altern. Med. 2016, 16, 117. [Google Scholar] [CrossRef] [PubMed]
- Mamadalievaa, N.Z.; Sharopovb, F.; Satyalc, P.; Azimovaa, S.S.; Winkb, M. Composition of the essential oils of three Uzbek Scutellaria species (Lamiaceae) and their antioxidant activities. Nat. Prod. Res. 2016, 30, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite: In health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed]
- Mondello, F.; De Bernardis, F.; Girolamo, A.; Cassone, A.; Salvatore, G. In vivo activity of terpinen-4-ol, the main bioactive component of Melaleuca alternifolia Cheel (tea tree) oil against azole-susceptible and resistant human pathogenic Candida species. BMC Infect. Dis. 2006, 6, 158. [Google Scholar] [CrossRef] [PubMed]
- Zengin, H.; Baysal, A.H. Antibacterial and antioxidant activity of essential oil terpenes against pathogenic and spoilage-forming bacteria and cell structure-activity relationships evaluated by SEM microscopy. Molecules 2014, 19, 17773–17798. [Google Scholar] [CrossRef] [PubMed]
- Salae, A.; Karalai, C.; Ponglimanont, C.; Kanjana-Opas, A.; Yuenyongsawad, S. Naphthalene derivatives from Diospyros wallichii. Can. J. Chem. 2010, 88, 922–927. [Google Scholar] [CrossRef]
- Tang, X.; Xiea, M.; Sunb, Y.X.; Liu, J.H.; Zhong, Z.C.; Wang, Y.L. Synthesis and antibacterial activity of brominated 2′(4′)-nitro-3-hydroxy diphenyl ethers. Chin. Chem. Lett. 2009, 20, 435–438. [Google Scholar] [CrossRef]
- Perez-Hernandez, N.; Ponce-Monter, H.; Ortiz, M.I.; Cariño-Cortes, R.; Joseph-Nathan, P. Structure-activity relationships of Aromandendrene in uterus-relaxant activity. Z. Naturforsch. C 2009, 64, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Ashafa, A.O.T.; Grierson, D.S.; Afolayan, A.J. In vitro antioxidant activity of extracts from the leaves of Felicia muricata Thunb. an underutilized medicinal plant in the Eastern Cape Province, South Africa. Afr. J. Tradit. Complement. Altern. Med. 2010, 7, 315–321. [Google Scholar] [CrossRef]
- Udourioh, G.A.; Etokudoh, M.F. Essential oils and fatty acids composition of dry fruits of Tetrapleura tetraptera. J. Appl. Sci. Environ. Manag. 2014, 18, 419–424. [Google Scholar]





| Organism | Essential Oil | Positive Control (Amoxicillin) |
|---|---|---|
| Salmonella typhimurium − | NA | 36 ± 1.1 a |
| Enterococcus faecalis − | 17 ± 0.2 a | 34 ± 1.2 b |
| Escherichia coli − | 15 ± 1.3 a | 35 ± 0.2 b |
| Pseudomonas aeruginosa − | NA | NA |
| Bacillus cereus + | 10 ± 1.6 a | 32 ± 1.2 b |
| Shigella sonnei − | 15 ± 0.3 a | 35 ± 2.2 b |
| Streptococcus pyogens + | 22 ± 0.2 a | 35 ± 2.1 b |
| Bacillus subtilis + | 22 ± 0.2 a | 40 ± 0.2 b |
| Shigella flexneri − | 19 ± 0.2 a | 40 ± 1.1 b |
| Klebpneumoniae − | 23 ± 2.1 a | 33 ± 2.2 b |
| Staphylococcus aureus + | 25 ± 3.1 a | 37 ± 0.2 b |
| Proteus vulgaris − | NA | 38 ± 1.1 a |
| Serretiamercenscens − | 25 ± 3.1 a | 35 ± 2.2 b |
| Solvent | Inhibition Zone Diameter (mm) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| St | Ef | Ec | Pa | Bc | Ss | Sp | Bs | Sf | Kp | Sa | Pv | Sm | |
| Essential oil | NA | 2.5 a | >5 a | NA | >5 a | >5 a | 0.02 a | 0.02 a | 0.02 a | 2.5 a | 0.02 a | NA | 0.02 a |
| Positive control | 0.01 | 0.01 b | 0.01 b | NA | 0.01 b | 0.01 b | <0.01 b | 0.01 b | 0.01 b | <0.01 b | 0.01 b | 0.01 b | 0.01 b |
| Organism | Essential Oil | Positive Control (Nystatin) |
|---|---|---|
| Aspergillus fumigates | NA | 30 ± 1.1 |
| Aspergillus niger | 20 ± 1.2 a | 20 ± 1.2 a |
| Microsporium gypsum | NA | 25 ± 0.2 |
| Triphophytonrubrum | NA | 25 ± 0.2 |
| Triphophytontonsurans | NA | 30 ± 1.2 |
| Triphophytonmucoides | NA | 25 ± 2.2 |
| Penicillium aurantrognereum | NA | 30 ± 2.1 |
| Penicillium chrysogenum | NA | 25 ± 0.2 |
| Microsporum canis | NA | 35 ± 1.1 |
| Solvent | Inhibition Zone Diameter (mm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Af | An | Mg | Tr | Tt | Tm | Pa | Pc | Mc | |
| Essential oil | NA | 2.5 a | NA | NA | NA | NA | NA | NA | NA |
| Positive control | 0.01 | 2.5 a | 0.02 | 2.5 | <0.01 | 0.02 | 0.01 | 0.02 | 0.01 |
| # | RT | Name | Area (%) | Class of Compound | Molecular Formula | Molecular Weight |
|---|---|---|---|---|---|---|
| 1 | 3.585 | 2-Heptanone | 1.07 | Ketone | C7H14O | 114 |
| 2 | 3.674 | Heptanal | 0.97 | Aldehyde | C7H14O | 114 |
| 3 | 3.714 | 2H-Isoindole-1,3(1H,3H)-dione, 5-amino-2-(2-methoxyphenyl)- | 0.52 | n/a | n/a | n/a |
| 4 | 4.215 | Benzaldehyde | 0.45 | Aldehyde | C7H6O | 106 |
| 5 | 4.351 | 2-Hepten-2-one, 6-methyl- | 0.21 | n/a | n/a | n/a |
| 6 | 4.393 | Beta-Myrcene | 0.64 | Monoterpene | C10H16 | 136 |
| 7 | 4.487 | Octanal | 0.27 | Aldehyde | C8H16O | 128 |
| 8 | 4.675 | Benzene, 1-methoxy-3-methyl | 0.47 | n/a | C8H10O | 122 |
| 9 | 4.725 | p-Cymene | 0.58 | Alkylbenzene | C10H14 | 134 |
| 10 | 4.765 | o-Limonene | 3.05 | Monoterpene | C10H16 | 136 |
| 11 | 5.008 | Octyl chloroformate | 0.37 | n/a | C9H17ClO2 | 193 |
| 12 | 5.199 | 8-Nonen-2-one | 1.31 | Ketone | C9H16O | 140 |
| 13 | 5.250 | 2-Nonanol | 0.28 | Alcohol | C9H20O | 144 |
| 14 | 5.308 | Nonanal | 0.84 | Aldehyde | C9H18O | 142 |
| 15 | 5.527 | 2-Cyclohexen-1-ol, 1-methyl-4-(1-methylethyl)-, trans- | 0.18 | n/a | C10H18O | 154 |
| 16 | 5.617 | Cyclopentane, 1,3-bis (methylene)- | 6.38 | n/a | C7H10 | 94 |
| 17 | 5.756 | Bicyclo [2.2.1] heptan-2-one, 1,7,7-trimethyl-, (1S)- | 0.42 | Monoterpenoid | C10H16O | 152 |
| 18 | 5.808 | Nona-3,5-dien-2-one | 0.47 | Ketone | C9H14O | 138 |
| 19 | 5.916 | Cyclohexanol, 5-methyl-2-(1-methylethyl)-, (1. alpha, 2. beta, 5. alpha.)-(.+/−.)-(Menthacamphor) | 0.30 | n/a | C10H20O | 156 |
| 20 | 5.976 | Terpinen-4-ol | 0.22 | Monoterpenoid | C10H18O | 154 |
| 21 | 6.012 | Sorbic acid vinyl ester | 0.63 | n/a | C8H10O2 | |
| 22 | 6.089 | Naphthalene | 0.81 | n/a | C10H8 | 128 |
| 23 | 6.184 | 2(3H)-Naphthalenone, 4,4a,5,6,7,8-hexahydro- | 0.28 | n/a | C10H14O | 150 |
| 24 | 6.452 | Pulegone | 0.16 | Monterpenoid | C10H16O | 152 |
| 25 | 6.520 | 2-Decanal, (E) − | 0.17 | Aldehyde | ||
| 26 | 6.588 | 3,5-Dimethoxytoluene | 0.23 | n/a | C9H12O2 | 152 |
| 27 | 6.696 | Phenol, 4-ethyl-2-methoxy- | 0.50 | n/a | C9H12O2 | 152 |
| 28 | 6.735 | Phenol, p-tert-butyl | 0.38 | n/a | C10H14O | 150 |
| 29 | 6.927 | 2,4-Decadienal, (E,E) − | 0.17 | Aldehyde | C10H16O | 152 |
| 30 | 6.959 | Benzene, 4-ethyl-1,2-dimethoxy- | 0.26 | n/a | C10H14O2 | 166 |
| 31 | 7.243 | Cyclotetradecane | 0.24 | Cycloalkane | C14H28 | 196 |
| 32 | 7.302 | 6-Bromomethyl-5-methyl-bicyclo[3.1.0]hexan-2-one | 0.51 | n/a | n/a | n/a |
| 33 | 7.362 | Butanoic acid, butyl ester | 0.20 | Fatty acid ester | C8H16O2 | 144 |
| 34 | 7.610 | Diphenyl ether | 0.77 | n/a | C12H10O | 170 |
| 35 | 7.833 | 5,9-Undecadien-2-one, 6,10-dimethyl-,(Z)-(Nerylacetone) | 0.37 | n/a | C13H22O | 194 |
| 36 | 8.008 | 2-Undecanone, 6,10-dimethyl- | 0.18 | n/a | C13H26O | 198 |
| 37 | 8.142 | Methyl (Z)-dec-2-en-4,6-diynoate | 0.24 | n/a | C11H12O2 | 176 |
| 38 | 8.244 | Beta-Bisabolene | 1.49 | Sesquiterpene | C15H24 | 204 |
| 39 | 8.682 | 1,7-Dimethyl-4-oxa-tricyclo[5.2.1. 0(2,6)]decane-3,5,8-trione | 0.52 | n/a | n/a | n/a |
| 40 | 8.787 | (−)-Spathulenol | 0.87 | Sesquiterpenoid | C15H24O | 220 |
| 41 | 8.848 | Lanceol, cis | 0.68 | Sesquiterpenoid | C15H24O | 220 |
| 42 | 8.898 | 3-Pyridinecarboxylic acid, 1,6-dihydro-6-oxo- | 2.29 | Carboxylic acid | C6H5NO3 | 139 |
| 43 | 8.965 | Alloaromadendrene | 0.43 | Sesquiterpene | C15H24 | 204 |
| 44 | 9.054 | Longifolene | 0.23 | Sesquiterpene | C15H24 | 204 |
| 45 | 9.098 | 3-Cyclohexene-1-acetaldehyde, alpha.,4-dimethyl-(Carvomenthenal) | 1.99 | Aldehyde | C10H16O | 152 |
| 46 | 9.225 | 3-Methyl-2-butenoic acid, 2-methyloct-5-yn-4-yl ester | 1.12 | Fatty acid ester | C14H22O2 | 222 |
| 47 | 9.283 | Bicyclo[3.1.1]heptan-3-one, 2-(but-3-enyl)-6,6-dimethyl- | 0.45 | n/a | n/a | n/a |
| 48 | 9.325 | Triisopropylphosphite | 0.96 | C9H21O3P | 208 | |
| 49 | 9.360 | 2-Cyclohexen-1-one, 3,6-dimethyl-6-(1-methylethyl)- | 0.75 | n/a | n/a | n/a |
| 50 | 9.472 | 3-Methylbut-2-enoic acid, 3,5-dime | 2.23 | n/a | n/a | n/a |
| 51 | 9.567 | s-Indacene, 1,2,3,5,6,7-hexahydro-1,1,4,8-tetramethyl- | 0.27 | n/a | n/a | n/a |
| 52 | 9.714 | 4-[3-(4-Nitrophenoxy)propyl]-1H-imidazole | 3.55 | n/a | n/a | n/a |
| 53 | 9.818 | Ethyltetramethylcyclopentadiene | 2.84 | n/a | n/a | n/a |
| 54 | 9.902 | Dodecanoic acid, 10-undecen-1-yl ester | 0.37 | n/a | n/a | n/a |
| 55 | 9.971 | Neoisolongifolene, 8,9-epoxy- | 2.86 | n/a | n/a | n/a |
| 56 | 10.048 | 3-Metthoxybenzyl alcohol | 1.02 | n/a | n/a | n/a |
| 57 | 10.268 | 1,2-Benzenedicarboxylic acid, butyl octyl ester | 0.50 | Fatty acid ester | C20H30O4 | 335 |
| 58 | 10.305 | 4,4′-Bis (tetrahydrothiopyran) | 1.02 | n/a | n/a | n/a |
| 59 | 10.365 | 3-Fluorobenzoic acid, 3,5-dimethyl cyclo hexyl ester | 0.34 | n/a | n/a | n/a |
| 60 | 10.501 | 5,9,13-Pentadecatrien-2-one, 6,10,14-trimethyl- | 0.30 | n/a | C18H30O | 262 |
| 61 | 10.571 | Hexanoic acid, 2-tetradecyl ester | 0.49 | n/a | n/a | n/a |
| 62 | 10.745 | Dibutyl phthalate | 2.78 | n/a | C16H22O4 | 278 |
| 63 | 10.877 | Cis-9-hexadecenoic acid | 4.09 | Carboxylic acid | C16H30O2 | 254 |
| 64 | 11.355 | 1,5,9-Decatriene, 2,3,5,8-tetramethyl- | 10.52 | n/a | C14H24 | 192 |
| 65 | 11.396 | n-Hexadecanoic acid | 24.30 | Carboxylic acid | C16H32O2 | 256 |
| 66 | 11.421 | 7-Oxabicyclo[4.1.0]heptane, 1,5-dimethyl- | 3.18 | n/a | n/a | n/a |
| 67 | 12.218 | Phenanthrene, 7-ethenyl-1,2,3,4,4a,4b,5,6,7,8,8a,9-dodecahydro-1,1,4b,7-tetramethyl-, [4aS-(4a.alpha., 4b.beta.,7.alpha.,8a.alpha.)]- | 2.51 | Diterpene | C20H32 | 273 |
| Total | 100.05 | |||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wintola, O.A.; Afolayan, A.J. Chemical Constituents and Biological Activities of Essential Oils of Hydnora africana Thumb Used to Treat Associated Infections and Diseases in South Africa. Appl. Sci. 2017, 7, 443. https://doi.org/10.3390/app7050443
Wintola OA, Afolayan AJ. Chemical Constituents and Biological Activities of Essential Oils of Hydnora africana Thumb Used to Treat Associated Infections and Diseases in South Africa. Applied Sciences. 2017; 7(5):443. https://doi.org/10.3390/app7050443
Chicago/Turabian StyleWintola, Olubunmi Abosede, and Anthony Jide Afolayan. 2017. "Chemical Constituents and Biological Activities of Essential Oils of Hydnora africana Thumb Used to Treat Associated Infections and Diseases in South Africa" Applied Sciences 7, no. 5: 443. https://doi.org/10.3390/app7050443
APA StyleWintola, O. A., & Afolayan, A. J. (2017). Chemical Constituents and Biological Activities of Essential Oils of Hydnora africana Thumb Used to Treat Associated Infections and Diseases in South Africa. Applied Sciences, 7(5), 443. https://doi.org/10.3390/app7050443