Molecular Science of Lubricant Additives
Abstract
:1. Introduction
1.1. Tribology and Lubrication Engineering
1.2. The Functions of Lubricants
- Controlling friction—This is the primary role of lubricants. Reducing friction and preventing wear and seizure (or failure) are necessary in most applications. Well-controlled, high friction is required for clutches, breaks, etc. Since this is the central objective of the lubricant, we discuss the phenomena with a lubrication model (Stribeck curve, see Figure 1). Optimized performances with minimized side effects could be achieved by proper selection of lubricants for specific machine elements. As modern machines are required to work in a more energy efficient way, the roles of lubricants are increasing. The importance of additives is emphasized (see Section 2.1 tribo-improvers).
- Cooling the contact—Heat generated by rubbing motion can have many negative influences on surface materials, such as transformation of microstructure or thermal failure. Ageing of lubricants is accelerated at higher temperatures. Heat accumulation could be prevented by circulating a liquid lubricant. The heat capacity of a lubricant is the controlling factor for this function. This function is mainly supported by the properties of base fluids. Some additives contribute to the heat conductivity of primary (see Section 2.5.2) or secondary (see Section 2.3.5) base fluids.
- Cleaning the contact—Wear particles, external dusts, or deposits by aged lubricants could appear during machine operation. These contaminants negatively influence lubrication performances. Circulating a lubricant can wash out these nuisances physically. Advanced lubricants contain some substances to help the cleaning process (see Section 2.3.2 and Section 2.3.3).
1.3. The Stribeck Lubrication Model
1.4. Tribo-Chemical Reactions
- Heat induces various chemical reactions. It was estimated that rubbing surface could reach as high as 250–450 °C [19]. Friction generates heat and this is considered as the major cause of tribo-chemical reactions.
- Nascent surfaces could be exposed by wear of solid surfaces under mixed and boundary conditions. Similarly, a mechanical stress to crystalline materials can sometimes cause lattice defects. These produce a chemically active site on the rubbing surfaces [20]. The chemical activity of transition metals is induced by vacant d-atomic orbital.
- Exo-electrons could be emitted from rubbing surfaces. Those electrons have low energy to promote chemical reactions but can produce radical intermediates for further chemical reactions [21].
- Elevated pressure up to Giga-Pascal (>104 bar) could be generated at tribo-contact [22]. Since chemical reactions are initiated by a collision of molecules, compression of reactant raises the probability of the collision. Chemical reactions at high pressure under static conditions are well known [23].
- Orientation of molecules may occur if they were flown through a narrow area [24]. This means the reacting functional groups are close to each other and increases the probability of reaction. This, together with elevated pressure, contributes to the entropy factor of the reaction.
- Shearing can dissociate chemical bonds in a molecule directly. It is a well-known phenomenon that molecular mass of polymers can decrease under shearing conditions. The formation of radical species by the dissociation of a carbon–carbon bond had been reported [25]. Typical shear rate at tribological contact is in the range of 105–107 s−1 [19].
1.5. Components in Liquid Lubricants
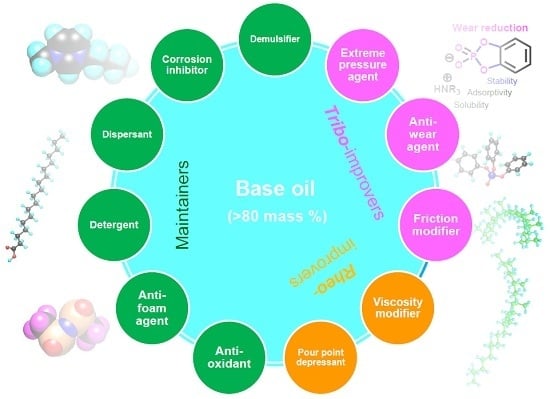
1.6. Category of Lubricant Additives
1.6.1. Working Function Category
- Tribo-improvers (tribology-improving additives) directly contribute to improve the tribological performances of the lubricant. In short, they are responsible for the primary role of lubricants (see Section 1.2). Friction modifiers (FM), anti-wear agents (AW), extreme pressure additives (EP) are representative ones. They stand at a central position in the technology of lubricant additives.
- Rheo-improvers (rheology-improving additives) concern the fluidity of the base oil. Viscosity modifiers (VM) are the main additives in this group. Pour point depressants (PPD) make the base oil applicable in a chilly environment. They indirectly contribute to the lubrication performances, mainly in hydrodynamic regime.
- Maintainers help to keep the substances (both lubricant and materials of machine elements) in good condition through preventing the degradation of substances participating in the lubrication system. They mainly contribute to prolonging the lifetime of the lubrication system and partly contribute to the lubrication performances in some cases. Antioxidants (AO) play a decisive role in preventing the ageing process of lubricants. Detergents and dispersants can mitigate the negative influences of contaminants on lubrication. Corrosion inhibitors (rust preventives) can protect the tribo-materials from corrosion. Air bubbles may be incorporated in lubricants during machine operation. They cause lubricant starvation at the contact and promote the autoxidation processes. Anti-foam agents (foam breaking agents, defoamers) can break bubbles. Water is a ubiquitous contaminant in most applications. It drops the viscosity of the lubricant and causes ageing of both lubricants and materials. Demulsifiers (emulsion breaking agents) are beneficial for separating contaminated water in a lubricant.
- Auxiliaries are sometimes incorporated with specific purpose in addition to above additives.
1.6.2. Working Site Category
- Interface agents work at the interfaces between different phases. Lubricated contacts involve solid (tribological material)—liquid (lubricant) interfaces. Examples are tribo-improvers as the main actors in this category. Corrosion inhibitors deactivate the surfaces to be attacked by corrosive matters. A lubricant may intake air bubbles while circulating, and thereby develops various forms. The interaction of foam decomposing additives at the border between gas–liquid phases can destroy the bubbles.
- Bulk agents concern the properties and/or stability of liquid as uni-phase matter. Rheo-improvers are representative examples of this group. Antioxidants (except metal deactivators), and other auxiliaries are in this group.
1.6.3. Working Mechanism Category
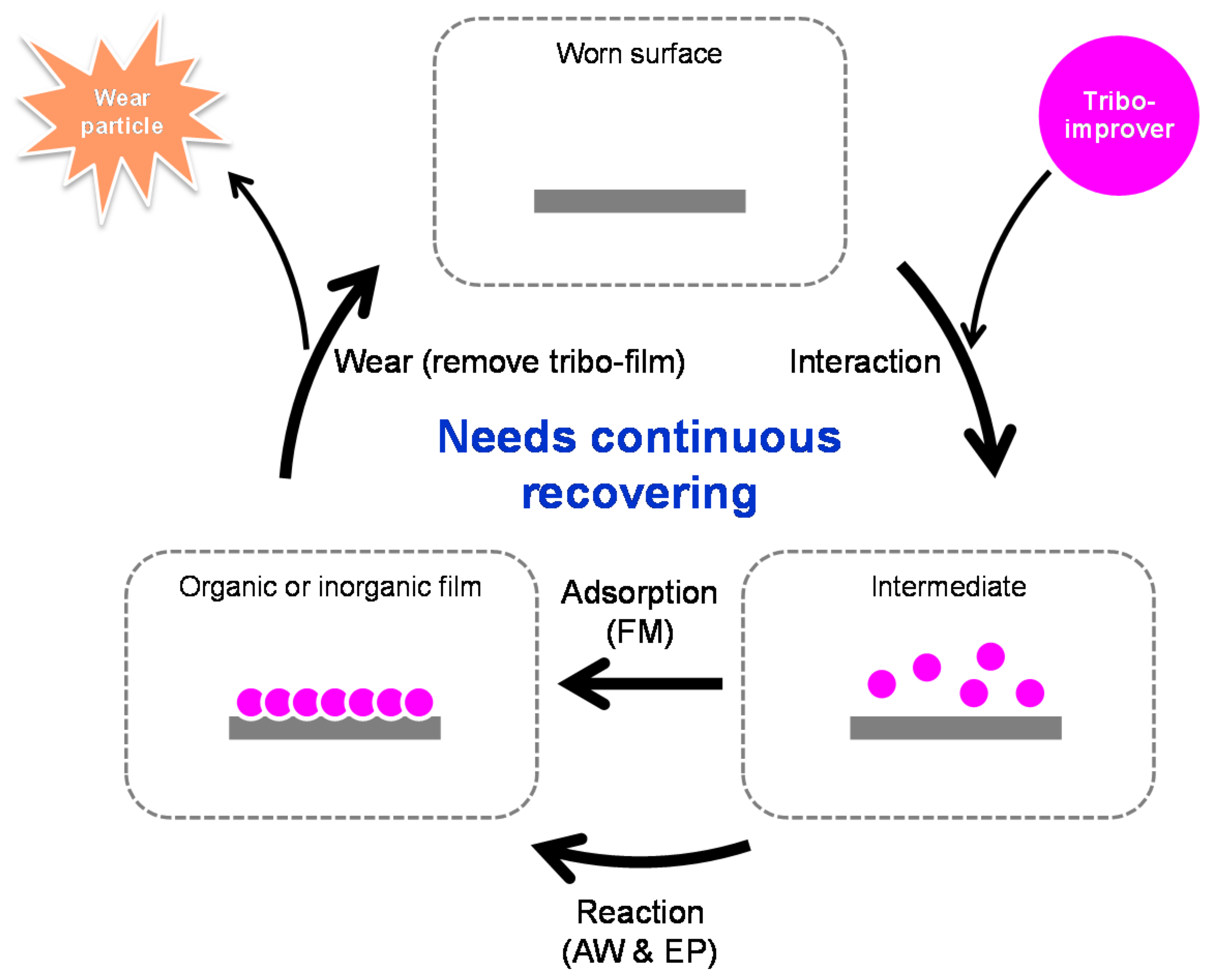
- Chemical additives are those additives that undergo chemical reaction(s) while working. A chemical reaction is defined as a rearrangement of electrons that bind atoms in a molecule. Chemical changes at interfacial phenomena in tribology are mostly irreversible, although there is a certain possibility of reversible reactions in the liquid phase. The initiation of chemical reaction needs much more energy than physical phenomena. Examples are anti-wear agents that provide boundary film on rubbing surfaces through tribo-chemical reactions. Antioxidants also belong to this group.
- Physical additives are those substances that work without any chemical changes. Examples are nano-particle additives such as tribo-improvers and viscosity modifiers. Physical changes need less activation energy than chemical changes and are usually reversible processes. They last longer than the chemical ones.
2. Individual Lubricant Additives and Their Working Mechanism
2.1. Tribo-Improvers
2.1.1. Friction Modifiers (FM)
2.1.2. Anti-Wear Agents (AW)
2.1.3. Extreme Pressure Additives (EP)
2.2. Rheo-Improvers (Rheological Properties Improvers)
2.2.1. Viscosity Modifiers (VM)
2.2.2. Pour Point Depressant (PPD)
2.3. Maintainers
2.3.1. Antioxidants (AO)
- Thermal dissociation of a carbon–hydrogen bond to yield hydrogen radical and carbon radical.
- Shear stress can dissociate a carbon–carbon bond though direct mechanical forces, yielding two carbon radical intermediates. Polymers can decompose through this mechanism.
- Wear of tribo-materials exposes nascent surfaces that may catalyze the dissociation of a carbon–hydrogen bond.
2.3.2. Detergent
2.3.3. Dispersant
2.3.4. Corrosion Inhibitor, Rust Preventive
2.3.5. Anti-Foam Agent (Defoamer)
2.3.6. Demulsifiers (Emulsion Breaking Agents)
2.4. Multi-Functional Additives
2.5. Auxiliaries
2.5.1. Antibiotics
2.5.2. Conductivity Improver
2.5.3. Colorings
3. Evaluation of Tribo-Improvers
- Configuration of tribo-contact: a point contact generates high contact stress while line and square contact generate low contact stress. A careful setup of the test specimen is needed to ensure the alignment of contact. This influences the repeatability of the test considerably. Ball-on-flat type point contact provides a comparatively easy setup.
- Type of relative motion: Reciprocation sliding, unidirectional sliding, rolling, and a combination of rolling and sliding are most commonly employed.
- Tribo-materials: Various materials are used in different machine elements. Simple tribo-tests are beneficial for evaluating the compatibility of tribo-improvers with specific materials in machine elements. Surface roughness of the materials should be considered.
- Operating conditions: Load, velocity, and temperature of lubricant should be controlled in a proper way.
- Testing environment: Should be kept away from contaminants as much as possible to obtain the results with good repeatability. Humidity of room air can influence the tribological properties.
4. Discussion as Multi-Component Systems
4.1. Formulation of a Lubricant
4.2. The Quality of Each Ingredient
4.3. The Interaction of Ingredients
- Synergy is defined as the combination improves BOTH friction and wear compared to each ingredient alone.
- Enhanced performance of one while scarifying the other seems to have an improved effect but should not be defined as synergism.
- Antagonism is any other phenomena except the above two.
4.4. Additive Technology for Synthetic Fluids
4.5. Tribo-Improvers for Non-Ferrous Materials
4.6. Additive Technology for Environmentally Acceptable Lubricants
5. Summary
Acknowledgments
Conflicts of Interest
Nomenclature
| Abbreviation | Description |
| AO | Antioxidant |
| API | The American petroleum Institute, http://www.api.org/ |
| ASTM | The American Society for Testing and Materials, http://www.astm.org/ |
| AW | Anti-wear, anti-wear additive |
| BF | Base fluid (base oil) |
| EP | Experme pressure, extreme pressure additive |
| FM | Friction modifying, fricition modifier |
| ICE | Internal conbustion engine |
| MO | Mineral oil |
| PPD | Pour pont depressant |
| SF | Synthetic fluids |
| STLE | The Society of Tribologists and lubricationEngineers, http://www.stle.org/ |
| TG | Triglyceride |
| VI | Viscosity index |
| VM | Viscosity modifying, Viscosity modifier |
| ZnDTP | Zinc bis(dialkyldhitiophosphate) |
References
- Stevenson, A. “Tribology” in Oxford Dictionary of English, 3rd ed.; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Jost, H.P. “Tribology or Lubrication” in Lubrication (Tribology) Education and Research; Department of Education and Science, Her Majesty’s Stationery Office London: London, UK, 1966. [Google Scholar]
- Dowson, D. “The Early Civilizations” (Chapter 4) in History of Tribology, 2nd ed.; Professional Engineering Publishing: London, UK, 1998. [Google Scholar]
- Wang, Y.; Wang, Q.J. “Stribeck Curves” in Encyclopedia of Tribology; Wang, Q.J., Chung, Y.W., Eds.; Springer: New York, NY, USA, 2013; Volume 5. [Google Scholar]
- Zhu, D.; Wang, Q.J. “EHL History (Elastohydrodynamic Lubrication)” in Encyclopedia of Tribology; Wang, Q.J., Chung, Y.W., Eds.; Springer: New York, NY, USA, 2013; Volume 2. [Google Scholar]
- Rennie, R. “Viscosity” in Oxford Dictionary of Chemistry, 7th ed.; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Kaupp, G. Mechanochemistry: The varied applications of mechanical bond-breaking. CrystEngComm 2009, 11, 388–403. [Google Scholar] [CrossRef]
- Takacs, L. The historical development of mechanochemistry. Chem. Soc. Rev. 2013, 42, 7649–7659. [Google Scholar] [CrossRef] [PubMed]
- Cravotto, G.; Gaudino, E.C.; Cintas, P. On the mechanochemical activation by ultrasound. Chem. Soc. Rev. 2013, 42, 7521–7534. [Google Scholar] [CrossRef] [PubMed]
- Šepelák, V.; Düvel, A.; Wilkening, M.; Becker, K.-D.; Heitjans, P. Mechanochemical reactions and syntheses of oxides. Chem. Soc. Rev. 2013, 42, 7507–7520. [Google Scholar] [CrossRef] [PubMed]
- Ribas-Arino, J.; Marx, D. Covalent Mechanochemistry: Theoretical Concepts and Computational Tools with Applications to Molecular Nanomechanics. Chem. Rev. 2012, 112, 5412–5487. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Nagamani, C.; Moore, J.S. Polymer Mechanochemistry: From Destructive to Productive. Acc. Chem. Res. 2015, 48, 2181–2190. [Google Scholar] [CrossRef] [PubMed]
- Todres, Z.V. Organic Mechanochemistry and Its Practical Applications; CRC Press Inc.: Boca Raton, FL, USA, 2006. [Google Scholar]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friščić, T.; Grepioni, F.; Harris, K.D.M.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef] [PubMed]
- Birke, V.; Mattik, J.; Runne, D. Mechanochemical reductive dehalogenation of hazardous polyhalogenated contaminants. J. Mater. Sci. 2004, 39, 5111–5116. [Google Scholar] [CrossRef]
- Nah, W.; Hwang, K.-Y.; Shul, Y.-G. Effect of metal and glycol on mechanochemical dechlorination of polychlorinated biphenyls (PCBs). Chemosphere 2008, 73, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Bruere, P. Alterations by light, heat and agitation of the heavy petroleum oils refined for therapeutic use. Bull. Travaux Societe de Pharmacie Bordeaux 1928, 9, 142–144. [Google Scholar]
- Minami, I. Ionic liquids in tribology. Molecules 2009, 14, 2286–2305. [Google Scholar] [CrossRef] [PubMed]
- Chao, K.K.; Saba, C.S. Tribo-Evaluation of High Temperature Candidate Fluids in a Sliding “TBOD” Bench Tester. Tribol. Trans. 1995, 38, 63–68. [Google Scholar] [CrossRef]
- Philippon, D.; De Barros-Bouchet, M.-I.; Le Mognea, T.; Lerasle, O.; Bouffet, A.; Martin, J.-M. Role of nascent metallic surfaces on the tribochemistry of phosphite lubricant additives. Tribol. Int. 2011, 44, 684–691. [Google Scholar] [CrossRef]
- Warsaw, C.K.; Furey, M.J.; Ritter, A.L.; Molina, G.J. Triboemission as a basic part of the boundary friction regime: A review. Lubr. Sci. 2002, 14, 223–254. [Google Scholar] [CrossRef]
- Wang, Q.J.; Zhu, D. “Hertz Theory: Contact of Spherical Surfaces” in Encyclopedia of Tribology; Wang, Q.J., Chung, Y.W., Eds.; Springer: New York, NY, USA, 2013; Volume 5. [Google Scholar]
- Schettino, V.; Bini, R. Molecules under extreme conditions: Chemical reactions at high pressure. Phys. Chem. Chem. Phys. 2003, 5, 1951–1965. [Google Scholar] [CrossRef]
- Cann, P.M.; Spikes, H.A. In Lubro Studies of Lubricants in EHD Contact Using FTIR Absorption Spectroscopy. Tribol. Trans. 1991, 34, 248–256. [Google Scholar] [CrossRef]
- Kauzmann, W.; Eyring, H. The Viscous Flow of Large Molecules. J. Am. Chem. Soc. 1940, 62, 3113–3125. [Google Scholar] [CrossRef]
- Carnes, C. The ten greatest events in tribology history. Tribol. Lubr. Technol. 2005, 61, 38–47. [Google Scholar]
- Papke, B.L. “Mineral Oil Base Fluids” in Encyclopedia of Tribology; Wang, Q.J., Chung, Y.W., Eds.; Springer: New York, NY, USA, 2013; Volume 4. [Google Scholar]
- Michael, J. Covitch Viscosity Index Additives in Encyclopedia of Tribology; Wang, Q.J., Chung, Y.W., Eds.; Springer: New York, NY, USA, 2013; Volume 2. [Google Scholar]
- Gresham, R.M. Viscosity index’s new importance. Tribol. Lubr. Technol. 2017, 73, 18–19. [Google Scholar]
- Nyberg, E.; Respatiningsih, C.Y.; Minami, I. Molecular design of advanced lubricant base fluids: Hydrocarbon-mimicking ionic liquids. RSC Adv. 2017, 7, 6364–6373. [Google Scholar] [CrossRef]
- Hardy, W.B.; Doubleday, I. Boundary Lubrication. The Paraffin Series. Proc. R. Soc. Lond. Ser. A 1922, 100, 550–574. [Google Scholar] [CrossRef]
- Papay, A.G. Antiwear and Extreme-Pressure Additives in Lubricants. Lubr. Sci. 1998, 10, 209–224. [Google Scholar] [CrossRef]
- Rizvi, S.Q.A. Additives for Automotive Fuels and Lubricants. Lubr. Eng. 1999, 55, 33–39. [Google Scholar]
- Taylor, R.I. Tribology and energy efficiency: From molecules to lubricated contacts to complete machines. Faraday Discuss. 2012, 156, 361–382. [Google Scholar] [CrossRef] [PubMed]
- Bowden, F.P.; Gregory, J.N.; Tabor, D. Lubrication of metal Surfaces by Fatty Acids. Nature 1945, 156, 97–101. [Google Scholar] [CrossRef]
- Spikes, H.A. Friction Modifier Additives. Tribol. Lett. 2015, 60, 1–26. [Google Scholar] [CrossRef]
- Rowe, G.W. The chemistry of tribology, friction, lubrication and wear. R. Inst. Chem. Rev. 1968, 1, 135–204. [Google Scholar] [CrossRef]
- Loehlé, S.; Matta, C.; Minfray, C.; le Mogne, T.; Iovine, R.; Obara, Y.; Miyamoto, A.; Martin, J.M. Mixed lubrication of steel by C18 fatty acids revisited. Part I: Toward the formation of carboxylate. Tribol. Int. 2015, 82, 218–227. [Google Scholar] [CrossRef]
- Jahanmir, S. Chain Length Effects in Boundary Lubrication. Wear 1985, 102, 331–349. [Google Scholar] [CrossRef]
- Spikes, H.A. Film-forming additives—Direct and indirect ways to reduce friction. Lubr. Sci. 2002, 14, 147–167. [Google Scholar] [CrossRef]
- Campen, S.; Green, J.H.; Lamb, G.D.; Spikes, H.A. In Situ Study of Model Organic Friction Modifiers Using Liquid Cell AFM; Saturated and Mono-unsaturated Carboxylic Acids. Tribol. Lett. 2015, 57, 18. [Google Scholar] [CrossRef]
- Minami, I.; Kubo, T.; Nanao, H.; Mori, S.; Okuda, S.; Sagawa, T. Investigation of Tribo-Chemistry by Means of Stable Isotopic Tracers, Part 2: Lubrication Mechanism of Friction Modifiers on Diamond-Like Carbon. Tribol. Trans. 2007, 50, 477–487. [Google Scholar] [CrossRef]
- Gellman, A.J.; Spencer, N.D. Surface chemistry in tribology, Proceedings of the Institution of Mechanical Engineers, Part J. J. Eng. Tribol. 2002, 216, 443–461. [Google Scholar]
- Haider, J. “MoSx Coatings by Closed-Field Magnetron Sputtering” in Encyclopedia of Tribology; Wang, Q.J., Chung, Y.W., Eds.; Springer: New York, NY, USA, 2013; Volume 2. [Google Scholar]
- Mitchell, P.C.H. Oil-Soluble Mo-S Compounds as Lubricant Additives. Wear 1984, 100, 281–300. [Google Scholar] [CrossRef]
- Graham, J.; Spikes, H.; Korcek, S. The Friction Reducing Properties of Molybdenum Dialkyldithiocarbamate Additives: Part I—Factors Influencing Friction Reduction. Tribol. Trans. 2001, 44, 626–636. [Google Scholar] [CrossRef]
- Graham, J.; Spikes, H.; Korcek, S. The Friction Reducing Properties of Molybdenum Dialkyldithiocarbamate Additives: Part II—Durability of Friction Reducing Capability. Tribol. Trans. 2001, 44, 637–647. [Google Scholar] [CrossRef]
- Sunqing, Q.; Junxiu, D.; Guoxu, C. A Review of Ultrafine Particle as Antiwear Additives and Friction Modifiers in Lubricating Oils. Lubr. Sci. 1999, 11, 217–226. [Google Scholar] [CrossRef]
- Tevet, O.; Von-Huth, P.; Popovitz-Biro, R.; Rosentsveig, R.; Wagner, H.D.; Tenne, R. Friction mechanism of individual multilayered nanoparticles. Proc. Natl. Acad. Sci. USA 2011, 108, 19901–19906. [Google Scholar] [CrossRef] [PubMed]
- Joly-Pottuz, L. “Nanolubricants” in Encyclopedia of Tribology; Wang, Q.J., Chung, Y.W., Eds.; Springer: New York, NY, USA, 2013; Volume 4. [Google Scholar]
- Wang, X.-B.; Liu, W.-M. “Nanoparticle-Based Lubricant Additives” in Encyclopedia of Tribology; Wang, Q.J., Chung, Y.W., Eds.; Springer: New York, NY, USA, 2013; Volume 4. [Google Scholar]
- Daia, W.; Kheireddinb, B.; Gaob, H.; Lianga, H. Roles of nanoparticles in oil lubrication. Tribol. Int. 2016, 102, 88–98. [Google Scholar] [CrossRef]
- Guan, B.; Pochopien, B.A.; Wright, D.S. The chemistry, mechanism and function of tricresyl phosphate (TCP) as an anti-wear lubricant additive. Lubr. Sci. 2016, 28, 257–265. [Google Scholar] [CrossRef]
- Spikes, H.A. The history and mechanisms of ZDDP. Tribol. Lett. 2004, 17, 469–489. [Google Scholar] [CrossRef]
- Nicholls, M.A.; Do, T.; Norton, P.R.; Kasrai, M.; Bancroft, G.M. Review of the lubrication of metallic surfaces by zinc dialkyl-dithiophosphates. Tribol. Int. 2005, 38, 15–39. [Google Scholar] [CrossRef]
- Cen, H.; Morina, A.; Neville, A.; Pasaribu, R.; Nedelcu, I. Effect of water on ZDDP anti-wear performance and related tribochemistry in lubricated steel/steel pure sliding contacts. Tribol. Int. 2012, 56, 47–57. [Google Scholar] [CrossRef]
- Parsaeian, P.; Ghanbarzadeh, A.; Eijk, M.C.P.V.; Nedelcu, I.; Neville, A.; Morina, A. A new insight into the interfacial mechanisms of the tribofilm formed by zinc dialkyl dithiophosphate. Appl. Surf. Sci. 2017, 403, 472–486. [Google Scholar] [CrossRef]
- Parsaeian, P.; Ghanbarzadeh, A.; Wilson, M.; Eijk, M.C.P.V.; Nedelcu, I.; Dowson, D.; Neville, A.; Morina, A. An experimental and analytical study of the effect of water and its tribochemistry on the tribocorrosive wear of boundary lubricated systems with ZDDP-containing oil. Wear 2016, 358–359, 23–31. [Google Scholar] [CrossRef]
- Parsaeian, P.; Eijk, M.C.P.V.; Nedelcu, I.; Neville, A.; Morina, A. Study of the interfacial mechanism of ZDDP tribofilm in humid environment and its effect on tribochemical wear; Part I: Experimental. Tribol. Int. 2017, 107, 135–143. [Google Scholar] [CrossRef]
- Onodera, T.; Martin, J.-M.; Minfray, C.; Dassenoy, F.; Miyamoto, A. Antiwear Chemistry of ZDDP: Coupling Classical MD and Tight-Binding Quantum Chemical MD Methods (TB-QCMD). Tribol. Lett. 2013, 50, 31–39. [Google Scholar] [CrossRef]
- Grrossiord, C.; Martin, J.-M.; Varlot, K.; Vacher, B.; Mogne, T.L.; Yamada, Y. Tribological interactions between Zndtp, Modtc and calcium borate. Tribol. Lett. 2000, 8, 203–212. [Google Scholar] [CrossRef]
- De Barros, M.I.; Bouchet, J.; Raoult, I.; Le Mogne, T.; Martin, J.M.; Kasrai, M.; Yamada, Y. Friction reduction by metal sulfides in boundary lubrication studied by XPS and XANES analyses. Wear 2003, 254, 863–870. [Google Scholar] [CrossRef]
- Khaemba, D.N.; Neville, A.; Morina, A. New insights on the decomposition mechanism of Molybdenum DialkyldiThioCarbamate (MoDTC): A Raman spectroscopic study. RSC Adv. 2016, 6, 38637–38646. [Google Scholar] [CrossRef]
- Khaemba, D.N.; Jarnias, F.; Thiebaut, B.; Neville, A.; Morina, A. The role of surface roughness and slide-roll ratio on the decomposition of MoDTC in tribological contacts. J. Phys. D Appl. Phys. 2017, 50, 085302. [Google Scholar] [CrossRef]
- Allum, K.G.; Forbes, E.S. The Load-carrying Properties of Organic Sulphur Compounds. Part II. The Influence of Chemical Structure on the Anti-wear Properies of Organic Disulphides. J. Inst. Petrol. 1967, 53, 173–185. [Google Scholar]
- Covitch, M.J.; Weiss, J.; Kreutzer, I.M. Low-temperature rheology of engine lubricants subjected to mechanical shear: Viscosity modifier effects. Lubr. Sci. 1999, 11, 337–364. [Google Scholar] [CrossRef]
- Snyder, C.E., Jr.; Gschwender, L.J.; Paciorek, K.; Kratzer, R.; Nakahra, J. Development of a Shear Stable Viscosity-Index Improver for Use in Hydrogenated Polyalphaolefin-Based Fluids. Lubr. Eng. 1986, 42, 547–557. [Google Scholar]
- Souchik, J. “Pour Point Depressants” in Lubricant Additives, 2nd ed.; Rudnick, L.R., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 2009; pp. 339–353. [Google Scholar]
- Ingold, K.U. Inhibition of the Autoxidation of Organic Substrates in the Liquid Phase. Chem. Rev. 1961, 6, 563–589. [Google Scholar] [CrossRef]
- Jensen, R.K.; Korcek, S.; Mahoney, L.R.; Zinbo, M. Liquid-Phase Autoxidation of Organic Compounds at Elevated Temperaturess. 1. The Stirred Flow Reactor Technique and Analysis of Primary Products from n-Hexadecane Autoxidation at 120–180 °C. J. Am. Chem. Soc. 1979, 101, 7574–7584. [Google Scholar] [CrossRef]
- Jensen, R.K.; Korcek, S.; Mahoney, L.R.; Zinbo, M. Liquid-Phase Autoxidation of Organic Compounds at ElevatedTemperaturess. 2. Kinetics and Mechanisms of the Formation of Cleavage Products n-Hexadecane. J. Am. Chem. Soc. 1981, 103, 1742–1749. [Google Scholar] [CrossRef]
- Jensen, R.K.; Korcek, S.; Zinbo, M.; Jensen, M.D. Initiation in Hydrocarbon Autoxidation at Elevated Temperatures. Int. J. Chem. Kinet. 1990, 22, 1095–1107. [Google Scholar] [CrossRef]
- Maleville, X.; Faure, D.; Legros, A.; Hipeaux, J.C. Oxidation of mineral base oils of petroleum origin: The relationship between chemical composition, thickening, and composition of degradation products. Lubr. Sci. 1996, 9, 3–60. [Google Scholar] [CrossRef]
- Horswill, E.C.; Ingold, K.U. The Oxidation of Phenols I. The Oxidation of 2,6-Di-t-butyl-4-methylphenol, 2,6-Di-t-butylphenol, and2,6-Dimethylphenol with Peroxy Radicals. Can. J. Chem. 1966, 44, 263–268. [Google Scholar] [CrossRef]
- Horswill, E.C.; Ingold, K.U. The Oxidation of Phenols II. The Oxidation of 2,4-Di-t-butylphenol with Peroxy Radicals. Can. J. Chem. 1966, 44, 269–277. [Google Scholar] [CrossRef]
- Dong, J.; Migdal, C.A. “Antioxidants” in Lubricant Additives, 2nd ed.; Rudnick, L.R., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 2009; pp. 3–50. [Google Scholar]
- Zeman, A.; Romer, R.; Roenne, V. Fate of Amine Antioxidants During Thermal Oxidative Aging of Neopentylpolyol Ester Oils: Part 1. J. Synth. Lubr. 1987, 3, 309–326. [Google Scholar] [CrossRef]
- Zeman, A.; Roenne, V.; Trebert, Y. Fate of Amine Antioxidants During Thermal Oxidation Ageing of Neopentylpolyl Ester Oils. J. Synth. Lubr. 1987, 4, 179–201. [Google Scholar] [CrossRef]
- Holdsworth, J.D.; Scott, G.; Williams, D. Mechanism of Antioxidant Action: Sulpur-containing Antioxidants. J. Chem. Soc. 1964, 1964, 4692–4699. [Google Scholar] [CrossRef]
- Burn, A.J. The Mechanism of the Antioxidant Action of Zinc Dialkyl Dithiophosphates. Tetrahedron 1966, 22, 2153–2161. [Google Scholar] [CrossRef]
- Gatto, V.J.; Moehle, W.E.; Cobb, T.W.; Schneller, E.R. The relationship between oxidation stability and antioxidant depletion in turbine oils formulated with Groups II, III and IV base stocks. J. Synth. Lubr. 2007, 24, 111–124. [Google Scholar] [CrossRef]
- O’Connor, S.P.; Crawford, J.; Cane, C. Overbased Lubricant Detergents—A Comparative Study. Lubr. Sci. 1994, 6, 297–325. [Google Scholar] [CrossRef]
- Olomolehin, Y.; Kapadia, R.; Spikes, H. Antagonistic Interaction of Antiwear Additives and Carbon Black. Tribol. Lett. 2010, 37, 49–58. [Google Scholar] [CrossRef]
- Bartha, L.; deak, G.; Baladincz, J.; Auer, J.; Kocsis, Z. Polyfunctional PIB Succinimide Type Engine Oil Additives. Lubr. Sci. 2001, 13, 313–328. [Google Scholar] [CrossRef]
- Costello, M.T. “Corrosion Inhibitors and Rust Preventatives” in Lubricant Additives, 2nd ed.; Rudnick, L.R., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 2009; pp. 421–444. [Google Scholar]
- Duncanson, M. Effects of Physical and Chemical Properties on Foam in Lubricating Oil. Lubr. Eng. 2003, 59, 9–13. [Google Scholar]
- Centers, P.W. Behavior of Silicone Antifoam Additives in Synthetic Ester Lubricants. Tribol. Trans. 1993, 36, 381–386. [Google Scholar] [CrossRef]
- Rowe, C.N.; Dickert, J.J. The Relation of Antiwear Function to Thermal Stability and Structure for Metal O,O-Dialkylphosphorodithioates. ASLE Trans. 1967, 10, 85–90. [Google Scholar] [CrossRef]
- McDonald, R.A. “Zinc Dithiophosphates” in Lubricant Additives, 2nd ed.; Rudnick, L.R., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 2009; pp. 51–62. [Google Scholar]
- Hong, H.; Riga, A.T.; Cahoon, J.M.; Vinci, J.N. Evaluation of Overbased Sulfonates as Extreme-Pressure Additives in Metalworking Fluids. Lubr. Eng. 1993, 49, 19–24. [Google Scholar]
- Keromest, C.; Durand, J.-P.; Born, M.; Gateau, P.; Tessier, M.; Marechal, E. Phosphosulphurised Antiwear and Extreme Pressure VI Improver Polymer Additives: Synthesis, Properties, and Lubricant Applications. Lubr. Sci. 1988, 10, 179–197. [Google Scholar] [CrossRef]
- Ota, J.; Hait, S.K.; Sastry, M.I.S.; Ramakumar, S.S.V. Graphene dispersion in hydrocarbon medium and its application in lubricant technology. RSC Adv. 2015, 5, 53326–53332. [Google Scholar] [CrossRef]
- Minami, I.; Hong, H.S.; Mathur, N.C. Lubrication Performance of Model Organic Compounds in High Oleic Sunflower oil. J. Synth. Lubr. 1999, 16, 3–12. [Google Scholar] [CrossRef]
- Palacios, J.M. The Performance of some Antiwear Additives and Interference with Other Additives. Lubr. Sci. 1992, 4, 201–209. [Google Scholar] [CrossRef]
- Guerret-Piecourt, C.; Grossiord, C.; Mogne, T.L.; Martin, J.M.; Palermo, T. Role of Complexation in the Interaction between Antiwear and Dispersant Additives in Lubricants. Lubr. Sci. 2001, 13, 201–218. [Google Scholar] [CrossRef]
- Kuhlman, R.E. “Environmentally Friendly Lubrication Issues” in Encyclopedia of Tribology; Wang, Q.J., Chung, Y.W., Eds.; Springer: New York, NY, USA, 2013; Volume 2, pp. 985–991. [Google Scholar]
- Minami, I.; Kikuta, S.; Okabe, H. Anti-wear and friction reducing additives composed of ortho-phenylene phosphate-amine salts for polyether type base stocks. Tribol. Int. 1998, 31, 305–312. [Google Scholar] [CrossRef]
- Van der Waal, G. The Relationship between the Chemical Structure of Ester Base Fluids and their Influence on Elastomer Seals, and Wear Characteristics. J. Synth. Lubr. 1985, 1, 280–301. [Google Scholar] [CrossRef]
- Minami, I.; Mori, S. Concept of molecular design towards additive technology for advanced lubricants. Lubr. Sci. 2007, 19, 127–149. [Google Scholar] [CrossRef]
- Jessop, P.G.; Ahmadpour, F.; Buczynski, M.A.; Burns, T.J.; Green, N.B., II; Korwin, R.; Long, D.; Massad, S.K.; Manley, J.B.; Omidbakhsh, N.; et al. Opportunities for greener alternatives in chemical formulations. Green Chem. 2015, 17, 2664–2678. [Google Scholar] [CrossRef]
























| Category | Classification | Contents | Viscosity Index | Remarks | |||
|---|---|---|---|---|---|---|---|
| Saturates, Mass% | Aromatics, Mass% | Sulfur, ppm | Examples | ||||
| Group I | Solvent-refined mineral oil | 65–85 | 15–35 | 300–3000 | 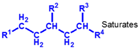 | 80–119 | Extraction of impurities by solvents |
| Group II | Hydro-processed mineral oil | ≧93 | <7 | 5–300 | 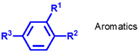 | 80–119 | Decomposition of organic sulfides by catalytic hydrogenolysis |
| Group III | Hydro-cracken mineral oil | ≧95 | <5 | 0–30 |  | ≧120 | Isomerization of hydrcarbons |
| Group IV | Oligomers of 1-alkene | - | - | - | 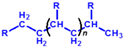 | - | Synthetic hydrocarbons,so-called PAO(poly alpha-olefin) in the market |
| Group V | All fluids not included in groups I–IV | - | - | - | 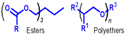 | - | Non-PAO synthetics, plant oils, and some low quality mineral oils |
| Working Mechanism | Working Site | |
|---|---|---|
| Interface | Bulk | |
| chemically | 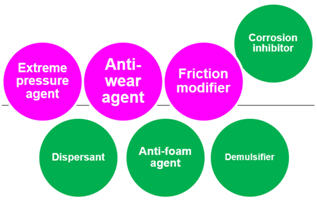 |  |
| physically |  | |
 | ||
| R&D Phase | Focus | Test Category | Sample Lubricant | Test Equipment | Outcome | Test Periods | Cost of Evaluation |
|---|---|---|---|---|---|---|---|
| Incubate-break through | Possibility | Laboratory test | Specific components | Universal tribo-tester | The role component |  |  |
| Improve | Applicability | Prototype | Tribo-tester according to industrial standards | Technical benefit | |||
| Optimize | Feasibility | Bench test | Full formulated | Machine component | Engineering benefit | ||
| Verity& tune | Productivity | Field test | Real machine | Industrial benefit |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minami, I. Molecular Science of Lubricant Additives . Appl. Sci. 2017, 7, 445. https://doi.org/10.3390/app7050445
Minami I. Molecular Science of Lubricant Additives . Applied Sciences. 2017; 7(5):445. https://doi.org/10.3390/app7050445
Chicago/Turabian StyleMinami, Ichiro. 2017. "Molecular Science of Lubricant Additives " Applied Sciences 7, no. 5: 445. https://doi.org/10.3390/app7050445
APA StyleMinami, I. (2017). Molecular Science of Lubricant Additives . Applied Sciences, 7(5), 445. https://doi.org/10.3390/app7050445