Effect of Seasonal Temperature on the Performance and on the Microbial Community of a Novel AWFR for Decentralized Domestic Wastewater Pretreatment
Abstract
:1. Introduction
2. Materials and Methods
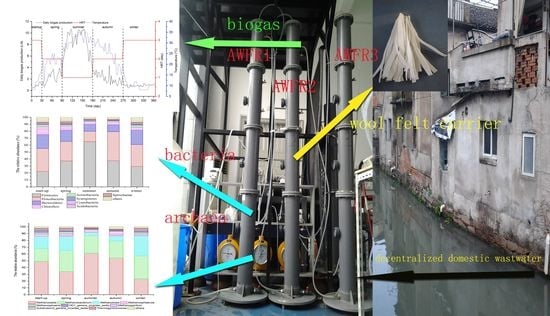
2.1. Experimental Setup and Operation
2.2. Decentralized Domestic Wastewater and Seed Sludge
2.3. Analysis Methods
2.3.1. Chemical Analysis
2.3.2. COD Mass Balance Calculation
2.3.3. Scanning Electron Microscopy (SEM)
2.3.4. Microbial Community Analysis by Illumina MiSeq Sequencing
3. Results and Discussion
3.1. Bioreactor Performance
3.1.1. COD Removal
3.1.2. VFA Accumulation and Biogas Production
3.2. COD Mass Balance
3.3. Nutrient Removal
3.4. Morphology and Structure of Anaerobic Biofilm Development
3.5. Shifts in Microbial Community Structures with Seasonal Temperature
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yu, R.; Wu, Q.; Lu, X. Constructed wetland in a compact rural domestic wastewater treatment system for nutrient removal. Environ. Eng. Sci. 2012, 29, 751–757. [Google Scholar] [CrossRef]
- Dong, H.-Y.; Qiang, Z.-M.; Wang, W.-D.; Jin, H. Evaluation of rural wastewater treatment processes in a county of eastern China. J. Environ. Monit. 2012, 14, 1906–1913. [Google Scholar] [CrossRef] [PubMed]
- McCarty, P.L.; Bae, J.; Kim, J. Domestic wastewater treatment as a net energy producer—Can this be achieved? Environ. Sci. Technol. 2011, 45, 7100–7106. [Google Scholar] [CrossRef] [PubMed]
- Chernicharo, C.; Van Lier, J.; Noyola, A.; Ribeiro, T.B. Anaerobic sewage treatment: State of the art, constraints and challenges. Rev. Environ. Sci. Bio/Technol. 2015, 14, 649–679. [Google Scholar] [CrossRef]
- López-López, A.; Albarrán-Rivas, M.G.; Hernández-Mena, L.; León-Becerril, E. An assessment of an anaerobic filter packed with a low-cost material for treating domestic wastewater. Environ. Technol. 2013, 34, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.L.; Skerlos, S.J.; Raskin, L. Membrane biofilm development improves COD removal in anaerobic membrane bioreactor wastewater treatment. Microb. Biotechnol. 2015, 8, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Wang, Z.; Miao, Y.; Wu, Z. Recover energy from domestic wastewater using anaerobic membrane bioreactor: Operating parameters optimization and energy balance analysis. Energy 2016, 98, 146–154. [Google Scholar] [CrossRef]
- Bandara, W.M.; Kindaichi, T.; Satoh, H.; Sasakawa, M.; Nakahara, Y.; Takahashi, M.; Okabe, S. Anaerobic treatment of municipal wastewater at ambient temperature: Analysis of archaeal community structure and recovery of dissolved methane. Water Res. 2012, 46, 5756–5764. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.; Sen, T.K.; Kayaalp, A.; Ang, H.M. The performance enhancements of upflow anaerobic sludge blanket (UASB) reactors for domestic sludge treatment–a state-of-the-art review. Water Res. 2012, 46, 3434–3470. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Wang, Y.; Ji, X.; Yuan, D.; Li, H. Performance and bioparticle growth of anaerobic baffled reactor (ABR) fed with low-strength domestic sewage. Front. Environ. Sci. Eng. 2014, 9, 352–364. [Google Scholar] [CrossRef]
- Smith, A.L.; Stadler, L.B.; Love, N.G.; Skerlos, S.J.; Raskin, L. Perspectives on anaerobic membrane bioreactor treatment of domestic wastewater: A critical review. Bioresour. Technol. 2012, 122, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, J.; Plaza, F.; Garralon, G.; Fdzpolanco, F.; Pena, M. Long-term operation of a pilot scale anaerobic membrane bioreactor (AnMBR) for the treatment of municipal wastewater under psychrophilic conditions. Bioresour. Technol. 2015, 185, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.; Kim, J.; Hwang, S.; Lee, C. Anaerobic treatment of rice winery wastewater in an upflow filter packed with steel slag under different hydraulic loading conditions. Bioresour. Technol. 2015, 193, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Gannoun, H.; Khelifi, E.; Omri, I.; Jabari, L.; Fardeau, M.-L.; Bouallagui, H.; Godon, J.-J.; Hamdi, M. Microbial monitoring by molecular tools of an upflow anaerobic filter treating abattoir wastewaters. Bioresour. Technol. 2013, 142, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Gannoun, H.; Othman, N.B.; Bouallagui, H.; Moktar, H. Mesophilic and thermophilic anaerobic co-digestion of olive mill wastewaters and abattoir wastewaters in an upflow anaerobic filter. Ind. Eng. Chem. Res. 2007, 46, 6737–6743. [Google Scholar] [CrossRef]
- Couto, E.D.A.D.; Calijuri, M.L.; Assemany, P.P.; Santiago, A.D.F.; Lopes, L.S. Greywater treatment in airports using anaerobic filter followed by uv disinfection: An efficient and low cost alternative. J. Clean. Prod. 2015, 106, 372–379. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, W.; Lu, X. Identifying key parameters in a novel multistep bio-ecological wastewater treatment process for rural areas. Ecol. Eng. 2013, 61, 166–173. [Google Scholar] [CrossRef]
- Tonon, D.; Tonetti, A.L.; Coraucci Filho, B.; Bueno, D.A.C. Wastewater treatment by anaerobic filter and sand filter: Hydraulic loading rates for removing organic matter, phosphorus, pathogens and nitrogen in tropical countries. Ecol. Eng. 2015, 82, 583–589. [Google Scholar] [CrossRef]
- Lew, B.; Tarre, S.; Beliavski, M.; Green, M. Anaerobic degradation pathway and kinetics of domestic wastewater at low temperatures. Bioresour. Technol. 2009, 100, 6155–6162. [Google Scholar] [CrossRef] [PubMed]
- Dutta, K.; Tsai, C.-Y.; Chen, W.-H.; Lin, J.-G. Effect of carriers on the performance of anaerobic sequencing batch biofilm reactor treating synthetic municipal wastewater. Int. Biodeterior. Biodegrad. 2014, 95, 84–88. [Google Scholar] [CrossRef]
- Han, Z.; Chen, F.; Zhong, C.; Zhou, J.; Wu, X.; Yong, X.; Zhou, H.; Jiang, M.; Jia, H.; Wei, P. Effects of different carriers on biogas production and microbial community structure during anaerobic digestion of cassava ethanol wastewater. Environ. Technol. 2016, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bodkhe, S. Development of an improved anaerobic filter for municipal wastewater treatment. Bioresour.Technol. 2008, 99, 222–226. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Cruz, L.M.; Stefanutti, R.; Coraucci Filho, B.; Tonetti, A.L. Coconut shells as filling material for anaerobic filters. SpringerPlus 2013, 2, 655. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, A.; Beni, A.A. Novel membrane reactor design for heavy-metal removal by alginate nanoparticles. J. Ind. Eng. Chem. 2015, 26, 122–128. [Google Scholar] [CrossRef]
- Seib, M.D.; Berg, K.J.; Zitomer, D.H. Influent wastewater microbiota and temperature influence anaerobic membrane bioreactor microbial community. Bioresour. Technol. 2016, 216, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Mckeown, R.M.; Hughes, D.; Collins, G.; Mahony, T.; Flaherty, V.O. Low-temperature anaerobic digestion for wastewater treatment. Curr. Opin. Biotechnol. 2012, 23, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Enright, A.-M.; Collins, G.; O’Flaherty, V. Temporal microbial diversity changes in solvent-degrading anaerobic granular sludge from low-temperature (15 °C) wastewater treatment bioreactors. Syst. Appl. Microbiol. 2007, 30, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Gomec, C.Y.; Letsiou, I.; Ozturk, I.; Eroglu, V.; Wilderer, P.A. Identification of archaeal population in the granular sludge of an uasb reactor treating sewage at low temperatures. J. Environ. Sci. Heal. A. 2008, 43, 1504–1510. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Leung, K.; Qin, W.; Liao, B. Effects of temperature and temperature shock on the performance and microbial community structure of a submerged anaerobic membrane bioreactor. Bioresour. Technol. 2011, 102, 8733–8740. [Google Scholar] [CrossRef] [PubMed]
- Regueiro, L.; Carballa, M.; Lema, J.M. Outlining microbial community dynamics during temperature drop and subsequent recovery period in anaerobic co-digestion systems. J. Biotechnol. 2014, 192, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Abubakkar, S.; Kundu, K.; Sreekrishnan, T.R. Comparative study of the performance of an anaerobic rotating biological contactor and its potential to enrich hydrogenotrophic methanogens. J. Chem. Technol. Biotechnol. 2015, 90, 398–406. [Google Scholar] [CrossRef]
- Takai, K.; Horikoshi, K. Rapid detection and quantification of members of the archaeal community by quantitative pcr using fluorogenic probes. Appl. Environ. Microbiol. 2000, 66, 5066–5072. [Google Scholar] [CrossRef] [PubMed]
- McElhoe, J.A.; Holland, M.M.; Makova, K.D.; Su, M.S.-W.; Paul, I.M.; Baker, C.H.; Faith, S.A.; Young, B. Development and assessment of an optimized next-generation DNA sequencing approach for the mtgenome using the Illumina Miseq. Forensic Sci. Int. Genet. 2014, 13, 20–29. [Google Scholar] [CrossRef] [PubMed]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Krishna, G.V.T.G.; Kumar, P.; Kumar, P. Treatment of low strength complex wastewater using an anaerobic baffled reactor (ABR). Bioresour. Technol. 2008, 99, 8193–8200. [Google Scholar] [CrossRef] [PubMed]
- Shin, C.; Mccarty, P.L.; Kim, J.; Bae, J. Pilot-scale temperate-climate treatment of domestic wastewater with a staged anaerobic fluidized membrane bioreactor (SAF-MBR). Bioresour. Technol. 2014, 159, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.K.; Khursheed, A.; Kazmi, A.A. Modified septic tank-anaerobic filter unit as a two-stage onsite domestic wastewater treatment system. Environ. Technol. 2014, 35, 2183–2193. [Google Scholar] [CrossRef] [PubMed]
- Hermansson, M. The DLVO theory in microbial adhesion. Colloids Surf. B: Biointerfaces 1999, 14, 105–119. [Google Scholar] [CrossRef]
- Fang, H.H.; Chan, K.-Y.; Xu, L.-C. Quantification of bacterial adhesion forces using atomic force microscopy (AFM). J. Microbiol. Methods 2000, 40, 89–97. [Google Scholar] [CrossRef]
- Fernández, N.; Díaz, E.E.; Amils, R.; Sanz, J.L. Analysis of microbial community during biofilm development in an anaerobic wastewater treatment reactor. Microb. Ecol. 2008, 56, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Lettinga, G.; Rebac, S.; Zeeman, G. Challenge of psychrophilic anaerobic wastewater treatment. Trends Biotechnol. 2001, 19, 363–370. [Google Scholar] [CrossRef]
- Mckeown, R.M.; Scully, C.; Enright, A.; Chinalia, F.A.; Lee, C.; Mahony, T.; Collins, G.; Oflaherty, V. Psychrophilic methanogenic community development during long-term cultivation of anaerobic granular biofilms. ISME J. 2009, 3, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Bialek, K.; Kumar, A.; Mahony, T.; Lens, P.N.L.; Flaherty, V.O. Microbial community structure and dynamics in anaerobic fluidized-bed and granular sludge-bed reactors: Influence of operational temperature and reactor configuration. Microb. Biotechnol. 2012, 5, 738–752. [Google Scholar] [CrossRef] [PubMed]
- Fey, A.; Conrad, R. Effect of temperature on carbon and electron flow and on the archaeal community in methanogenic rice field soil. Appl. Environ. Microbiol. 2000, 66, 4790–4797. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.L.; Skerlos, S.J.; Raskin, L. Psychrophilic anaerobic membrane bioreactor treatment of domestic wastewater. Water Res. 2013, 47, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Mchugh, S.; Carton, M.W.; Collins, G.; Oflaherty, V. Reactor performance and microbial community dynamics during anaerobic biological treatment of wastewaters at 16–37 °C. FEMS Microbiol. Ecol. 2004, 48, 369–378. [Google Scholar] [CrossRef]
- Sundberg, C.; Al-Soud, W.A.; Larsson, M.; Alm, E.; Yekta, S.S.; Svensson, B.H.; Sørensen, S.J.; Karlsson, A. 454 pyrosequencing analyses of bacterial and archaeal richness in 21 full-scale biogas digesters. FEMS Microbiol. Ecol. 2013, 85, 612–626. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kang, H.J.; Lee, Y.H.; Lee, T.J.; Han, K.; Choi, Y.; Park, H.D. Monitoring bacterial community structure and variability in time scale in full-scale anaerobic digesters. J. Environ. Monit. 2012, 14, 1893–1905. [Google Scholar] [CrossRef] [PubMed]
- Goux, X.; Calusinska, M.; Lemaigre, S.; Marynowska, M.; Klocke, M.; Udelhoven, T.; Benizri, E.; Delfosse, P. Microbial community dynamics in replicate anaerobic digesters exposed sequentially to increasing organic loading rate, acidosis, and process recovery. Biotechnol. Biofuels 2015, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Chebbi, A.; Mnif, S.; Mhiri, N.; Jlaiel, L.; Sayadi, S.; Chamkha, M. A moderately thermophilic and mercaptan-degrading bacillus licheniformis strain can55 isolated from gas-washing wastewaters of the phosphate industry, Tunisia. Int. Biodeterior. Biodegrad. 2014, 94, 207–213. [Google Scholar] [CrossRef]
- Nakkabi, A.; Sadiki, M.; Fahim, M.; Ittobane, N.; Ibnsoudakoraichi, S.; Barkai, H.; Abed, S.E. Biodegradation of Poly(ester urethane)s by Bacillus subtilis. Int. J. Environ. Res. 2015, 9, 157–162. [Google Scholar]
- Patowary, K.; Saikia, R.R.; Kalita, M.C.; Deka, S. Degradation of polyaromatic hydrocarbons employing biosurfactant-producing Bacillus pumilus KS2. Ann. Microbiol. 2014, 65, 225–234. [Google Scholar] [CrossRef]
- Xiao, Y.; Chen, S.; Gao, Y.; Hu, W.; Hu, M.; Zhong, G. Isolation of a novel beta-cypermethrin degrading strain Bacillus subtilis BSF01 and its biodegradation pathway. Appl. Microbiol. Biotechnol. 2014, 99, 2849–2859. [Google Scholar] [CrossRef] [PubMed]
- Dhall, P.; Kumar, R.; Kumar, A. Biodegradation of sewage wastewater using autochthonous bacteria. Sci. World J. 2012, 2012, 861903. [Google Scholar] [CrossRef] [PubMed]
- Antwi, P.; Li, J.; Boadi, P.O.; Meng, J.; Shi, E.; Xue, C.; Zhang, Y.; Ayivi, F. Functional bacterial and archaeal diversity revealed by 16S rRNA gene pyrosequencing during potato starch processing wastewater treatment in an UASB. Bioresour. Technol. 2017, 235, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Wang, Z.; Wang, Q.; Zhu, C.; Wu, Z. An anaerobic dynamic membrane bioreactor (AnMBR) for landfill leachate treatment: Performance and microbial community identification. Bioresour. Technol. 2014, 161, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Yu, G.; Louie, T.S.; Liu, T.; Zhu, C.; Xue, G.; Gao, P. From mesophilic to thermophilic digestion: The transitions of anaerobic bacterial, archaeal, and fungal community structures in sludge and manure samples. Appl. Microbiol. Biotechnol. 2015, 99, 10271–10282. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Shigematsu, T.; Morimura, S.; Kida, K. Microbial community analysis of mesophilic anaerobic protein degradation process using bovine serum albumin (BSA)-fed continuous cultivation. J. Biosci. Bioeng. 2005, 99, 150–164. [Google Scholar] [CrossRef] [PubMed]
- Regueiro, L.; Lema, J.M.; Carballa, M. Key microbial communities steering the functioning of anaerobic digesters during hydraulic and organic overloading shocks. Bioresour. Technol. 2015, 197, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Resende, J.A.; Silva, V.L.D.; De Oliveira, T.L.R.; Fortunato, S.; Carneiro, J.D.C.; Otenio, M.H.; Diniz, C.G. Prevalence and persistence of potentially pathogenic and antibiotic resistant bacteria during anaerobic digestion treatment of cattle manure. Bioresour. Technol. 2014, 153, 284–291. [Google Scholar] [CrossRef] [PubMed]










| Stage | Phase (Days) | Duration (Days) | Hydraulic Retention Time (HRT) (Days) | Hydraulic Loading Rate (HLR) (m3/m2/day) | Organic Loading Rate (OLR) (mgCOD/L/day) 1 | Temperature (°C) 1 |
|---|---|---|---|---|---|---|
| start-up | 1–30 | 30 | 3 | 2.2 | 75 ± 13.6 (n = 30) | 15 ± 3.4 (n = 30) |
| spring | 31–90 | 60 | 2 | 4.4 | 143 ± 28.3 (n = 60) | 21 ± 3.0 (n = 60) |
| summer | 91–180 | 90 | 1 | 6.6 | 352 ± 61.8 (n = 90) | 31 ± 3.7 (n = 90) |
| autumn | 181–270 | 90 | 2 | 4.4 | 135 ± 32.9 (n = 90) | 25 ± 5.2 (n = 90) |
| winter | 271–364 | 94 | 3 | 2.2 | 82 ± 12.9 (n = 94) | 10 ± 2.2 (n = 94) |
| Parameter | Chemical Oxygen Demand (COD) | Total Phosphorus (TP) | Total Nitrogen (TN) | pH |
|---|---|---|---|---|
| Value 1 | 284 ± 69.2 | 2.8 ± 0.8 | 32.2 ± 7.7 | 7.1 ± 0.2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Lu, X. Effect of Seasonal Temperature on the Performance and on the Microbial Community of a Novel AWFR for Decentralized Domestic Wastewater Pretreatment. Appl. Sci. 2017, 7, 605. https://doi.org/10.3390/app7060605
Li J, Lu X. Effect of Seasonal Temperature on the Performance and on the Microbial Community of a Novel AWFR for Decentralized Domestic Wastewater Pretreatment. Applied Sciences. 2017; 7(6):605. https://doi.org/10.3390/app7060605
Chicago/Turabian StyleLi, Juanhong, and Xiwu Lu. 2017. "Effect of Seasonal Temperature on the Performance and on the Microbial Community of a Novel AWFR for Decentralized Domestic Wastewater Pretreatment" Applied Sciences 7, no. 6: 605. https://doi.org/10.3390/app7060605
APA StyleLi, J., & Lu, X. (2017). Effect of Seasonal Temperature on the Performance and on the Microbial Community of a Novel AWFR for Decentralized Domestic Wastewater Pretreatment. Applied Sciences, 7(6), 605. https://doi.org/10.3390/app7060605