Reduction of Hexavalent Chromium Using Sorbaria sorbifolia Aqueous Leaf Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of PLE
2.3. Reduction and Analysis of Cr(VI)
3. Results
3.1. Reduction Efficacies of PLE
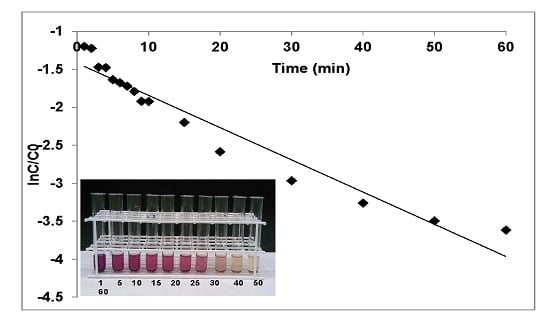
3.1.1. Effects of Contact Time and Rate Kinetics
3.1.2. Effects of pH
3.1.3. Effects of PLE Quantity
3.1.4. Effects of Ionic Strength
3.1.5. Effects of Hardness
3.1.6. Effects of Temperature and Initial Cr(VI) Concentration
3.2. Comparison of the Present Study with Other Reductants
3.3. Possible Mechanism for Reduction Efficiency of PLE
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Xu, H.J.; Huang, R.L.; Li, T.J.; Kong, X.F.; Yin, Y.L. Nutritional and physiological functions of chromium. Nat. Prod. Res. Dev. 2010, 3, 531–534. [Google Scholar]
- Faybishenko, B.; Hazen, T.C.; Long, P.E.; Brodie, E.L.; Conrad, M.E.; Hubbard, S.S.; Christensen, J.N.; Joyner, D.; Borglin, S.E.; Chakraborty, R.; et al. Tokunaga, long-term reductive bioimmobilization of Cr(VI) in groundwater using hydrogen release compound. Environ. Sci. Technol. 2008, 42, 8478–8485. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Sonntag, D.M.; Boer, J.D.; Dixon, K. Chromium (VI)-induced mutagenesis in the lungs of big blue TM transgenic mice. J. Environ. Pathol. Toxicol. Oncol. 2000, 19, 239–249. [Google Scholar] [PubMed]
- Cohen, M.D.; Kargacin, B.; Klein, C.B.; Costa, M. Mechanisms of chromium carcinogenicity and toxicity. Crit. Rev. Toxicol. 1993, 23, 255–281. [Google Scholar] [CrossRef] [PubMed]
- Altundogan, H.S. Cr(VI) removal from aqueous solution by iron(III) hydroxide-loaded sugar beet pulp. Process Biochem. 2005, 40, 1443–1452. [Google Scholar] [CrossRef]
- Dubey, S.P.; Gopal, K. Adsorption of chromium (VI) on low cost adsorbents derived from agricultural waste material: A comparative study. J. Hazard. Mater. 2007, 145, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Dharnaik, A.S.; Ghosh, P.K. Hexavalent chromium [Cr(VI)] removal by the electrochemical ion-exchange process. Environ. Technol. 2014, 35, 2272–2279. [Google Scholar] [CrossRef] [PubMed]
- Karale, R.S.; Wadkar, D.V.; Nangare, P.B. Removal and recovery of hexavalent chromium from industrial waste water by precipitation with due consideration to cost optimization. J. Environ. Res. Dev. 2007, 2, 209–216. [Google Scholar]
- Muthumareeswaran, M.R.; Alhoshan, M.; Agarwal, G.P. Ultrafiltration membrane for effective removal of chromium ions from potable water. Sci. Rep. 2017, 7, 41423. [Google Scholar] [CrossRef] [PubMed]
- Bishop, M.E.; Glasser, P.; Dong, H.; Arey, B.; Kovarik, L. Reduction and immobilization of hexavalent chromium by microbially reduced Fe-bearing clay minerals. Geochim. Cosmochim. Acta 2014, 133, 186–203. [Google Scholar] [CrossRef]
- Treviño, P.; Ibanez, J.G.; Vasquez-Medrano, R. Chromium(VI) reduction kinetics by zero-valent aluminum. Int. J. Electrochem. Sci. 2014, 9, 2556–2564. [Google Scholar]
- Sarkar, B.; Naidu, R.; Krishnamurti, G.S.R.; Meghara, M. Manganese(II)-catalyzed and clay-minerals-mediated reduction of chromium(VI) by citrate. Environ. Sci. Technol. 2013, 47, 13629–13636. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Bai, S.; Liang, J.; Zhou, L.; Lan, Y. Photocatalytic reduction of Cr(VI) by citric and oxalic acids over biogenetic jarosite. Mater. Sci. Eng. C 2013, 33, 2192–2196. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.R.; Li, H.B.; Li, X.Y.; Gu, J.D. Reduction of hexavalent chromium by ascorbic acid in aqueous solutions. Chemosphere 2004, 57, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Brose, D.A.; James, B.R. Hexavalent chromium reduction by tartaric acid and isopropyl alcohol in mid-atlantic soils and the role of Mn(III, IV)(hydr)oxides. Environ. Sci. Technol. 2013, 47, 12985–12991. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Xu, Q. Catalytic chromium reduction using formic acid and metal nanoparticles immobilized in a metal-organic framework. Chem. Commun. 2013, 49, 3327–3329. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Saha, R.; Mandal, J.; Ghosh, S.; Saha, B. Removal of hexavalent chromium by an aromatic alcohol. J. Biomed. Sci. Eng. 2010, 3, 735–741. [Google Scholar] [CrossRef]
- Yeqing, L.; Chen, L.; Jingdong, M.; Jun, S. Influence of clay minerals on the reduction of Cr6+ by citric acid. Chemosphere 2008, 71, 781–787. [Google Scholar]
- Dubey, S.P.; Dwivedi, A.D.; Lahtinen, M.; Lee, C.; Kwon, Y.N.; Sillanpää, M. Protocol for development of various plants leaves extract in single-pot synthesis of metal nanoparticles. Spectrochim. Acta A 2013, 103, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.P.; Lahtinen, M.; Sillanpää, M. Tansy fruit mediated greener synthesis of silver and gold nanoparticles. Process Biochem. 2010, 45, 1065–1071. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Varma, R.S. Green synthesis of silver and palladium nanoparticles at room temperature using coffee and tea extract. Green Chem. 2008, 10, 859–862. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Hoag, G.E.; Collins, J.B.; Varma, R.S. Green synthesis of Au nanostructures at room temperature using biodegradable plant surfactants. Cryst. Growth Des. 2009, 9, 4979–4983. [Google Scholar] [CrossRef]
- Zaitsev, V.G.; Makarova, G.V.; Komissarenko, N.F. Phenolic compounds of Sorbaria sorbifolia. Chem. Nat. Compd. 1969, 5, 598. [Google Scholar] [CrossRef]
- Zaitsev, V.G.; Makarova, G.V.; Komissarenko, N.F. Sorbifolin—A new flavone glycoside from Sorbaria sorbifolia. Chem. Nat. Compd. 1969, 5, 504–507. [Google Scholar] [CrossRef]
- Kim, D.K.; Zee, O.P. A new cyanogenic glycoside from Sorbaria sorbifolia var. stellipila. Chem. Pharm. Bull. 2000, 48, 1766–1767. [Google Scholar] [CrossRef] [PubMed]
- Eaton, A.D.; Clesceri, L.S.; Greenberg, A.E. Standard Methods for the Examination of Water and Wastewater, 19th ed.; APHA: Washington, DC, USA, 1995. [Google Scholar]
- Pettine, M.; Campanella, L.; Millero, F.J. Reduction of hexavalent chromium by H2O2 in acidic solutions. Environ. Sci. Technol. 2002, 36, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Yurkow, E.J.; Hong, J.; Min, S.; Wang, S. Photochemical reduction of hexavalent chromium in glycerol-containing solutions. Environ. Pollut. 2002, 117, 1–3. [Google Scholar] [CrossRef]
- Lo, I.M.C.; Lam, C.S.C.; Lai, K.C.K. Hardness and carbonate effects on the reactivity of zero-valent iron for Cr(VI) removal. Water Res. 2006, 40, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Karvonen, A. Cation effects on chromium removal in permeable reactive walls. J. Environ. Eng. 2004, 130, 863–866. [Google Scholar] [CrossRef]
- Mystrioti, C.; Sparis, D.; Papassiopi, N.; Xenidis, A.; Dermatas, D.; Chrysochoou, M. Assessment of polyphenol coated nano zero valent iron for hexavalent chromium removal from contaminated waters. Bull. Environ. Contam. Toxicol. 2015, 94, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Yar, M.S.; Siddiqui, A.A.; Ali, M.A. Synthesis and antimycobacterial activity of novel heterocycles. J. Serb. Chem. Soc. 2007, 72, 5–11. [Google Scholar] [CrossRef]
- Rajarajan, K.; Anbarasan, K.; Solomon, J.S.; Madhurambal, G. XRD and FT-IR studies on lead (II) nitrate doped histidine picrate crystal: A nonlinear optical material. J. Chem. Pharm. Res. 2012, 4, 4060–4065. [Google Scholar]
- Oh, T.; Lee, K.-M.; Kim, K.S.; Choi, C.K. Comparison of the Nano-Structure due to C=O and C=C double bond. J. Korean Phys. Soc. 2004, 45, 705–708. [Google Scholar]
- Nadagouda, M.N.; Varma, R.S. Microwave-assisted shape-controlled bulk synthesis of noble nanocrystals and their catalytic properties. Cryst. Growth Des. 2007, 7, 686–690. [Google Scholar]
- Markova, Z.; Novak, P.; Kaslik, J.; Plachtova, P.; Brazdova, M.; Jancula, D.; Siskova, K.M.; Machala, L.; Marsalek, B.; Zboril, R.; et al. Iron(II,III)–polyphenol complex nanoparticles derived from green tea with remarkable ecotoxicological impact. ACS Sustain. Chem. Eng. 2014, 2, 1674–1680. [Google Scholar] [CrossRef]
- Stephen, A.; Seethalakshmi, S. Phytochemical synthesis and preliminary characterization of silver nanoparticles using hesperidin. J. Nanosci. 2013, 2013, 126564. [Google Scholar] [CrossRef]
- Hoag, G.E.; Collins, J.B.; Holcomb, J.L.; Hoag, J.R.; Nadagouda, M.N.; Varma, R.S. Degradation of bromothymol blue by ‘greener’ nano-scale zerovalent iron synthesized using tea polyphenols. J. Mater. Chem. 2009, 19, 8671–8677. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Castle, A.; Murdock, R.C.; Hussain, S.M.; Varma, R.S. In vitro biocompatibility of nanoscale zerovalent iron particles (nzvi) synthesized using tea polyphenols. Green Chem. 2010, 12, 114–122. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Varma, R.S. Green and controlled synthesis of gold and platinum nanomaterials using vitamin B2: Density-assisted self-assembly of nanospheres, wires and rods. Green Chem. 2006, 8, 516–518. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Varma, R.S. A greener synthesis of core (Fe, Cu)-shell (Au, Pt, Pd and Ag) nanocrystals using aqueous vitamin C. Cryst. Growth Des. 2007, 7, 2582–2587. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Polshettiwar, V.; Varma, R.S. Self-assembly of palladium nanoparticles: Synthesis of nanobelts, nanoplates and nanotrees using vitamin B1 and their application in carbon-carbon coupling reactions. J. Mater. Chem. 2009, 19, 2026–2031. [Google Scholar] [CrossRef]








| Reductants | Initial Cr(VI) Concentration | pH | Contact Time | Reduction Efficiency | References |
|---|---|---|---|---|---|
| Ferrous chloride (300 μM) | 50 μM | 7.2 | 120 min | 100% | [10] |
| Citrate (10 mM) | 192.32 μM | 2.25 | 24 h | 12% | [12] |
| Citrate (10 mM) + Mn(II) (182.02 μM) | 192.32 μM | 2.25 | 24 h | 52% | |
| Citrate (10 mM) + clay (3% (w/v)) | 192.32 μM | 2.25 | 24 h | 84% | |
| Citrate (10 mM) + clay (3% (w/v)) + Mn(II) (182.02 μM) | 192.32 μM | 2.25 | 6 h | 100% | |
| Oxalic acid (300 μM) | 50 μM | 3 | 20 min | 100% | [13] |
| Ascorbic acid (300 μM) | 100 μM | 7 | 120 min | 100% | [14] |
| Tartaric acid (12 mM) + Isopropyl alcohol (0.29 M) | 2.0 mM | 4 | 99 h | 19% | [15] |
| Russett soil (5 g) | 2.0 mM | 4 | 99 h | 25% | |
| Russett soil (5 g) + Tartaric acid (12 mM) + Isopropyl alcohol (0.29 M) | 2.0 mM | 4 | 99 h | 99% | |
| Punica granatum-nZVI (Fe(0) (1.53 mM)) | 50 mg/L | 2.73 | 2 days | 96% | [31] |
| PLE (1.5 mL) | 10 mg/L | 2–10 | 60 min | 99–66% | Present study |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubey, S.P.; Sillanpaa, M.; Varma, R.S. Reduction of Hexavalent Chromium Using Sorbaria sorbifolia Aqueous Leaf Extract. Appl. Sci. 2017, 7, 715. https://doi.org/10.3390/app7070715
Dubey SP, Sillanpaa M, Varma RS. Reduction of Hexavalent Chromium Using Sorbaria sorbifolia Aqueous Leaf Extract. Applied Sciences. 2017; 7(7):715. https://doi.org/10.3390/app7070715
Chicago/Turabian StyleDubey, Shashi Prabha, Mika Sillanpaa, and Rajender S. Varma. 2017. "Reduction of Hexavalent Chromium Using Sorbaria sorbifolia Aqueous Leaf Extract" Applied Sciences 7, no. 7: 715. https://doi.org/10.3390/app7070715
APA StyleDubey, S. P., Sillanpaa, M., & Varma, R. S. (2017). Reduction of Hexavalent Chromium Using Sorbaria sorbifolia Aqueous Leaf Extract. Applied Sciences, 7(7), 715. https://doi.org/10.3390/app7070715