Partition Behavior in Aqueous Two-Phase System and Antioxidant Activity of Flavonoids from Ginkgo biloba
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
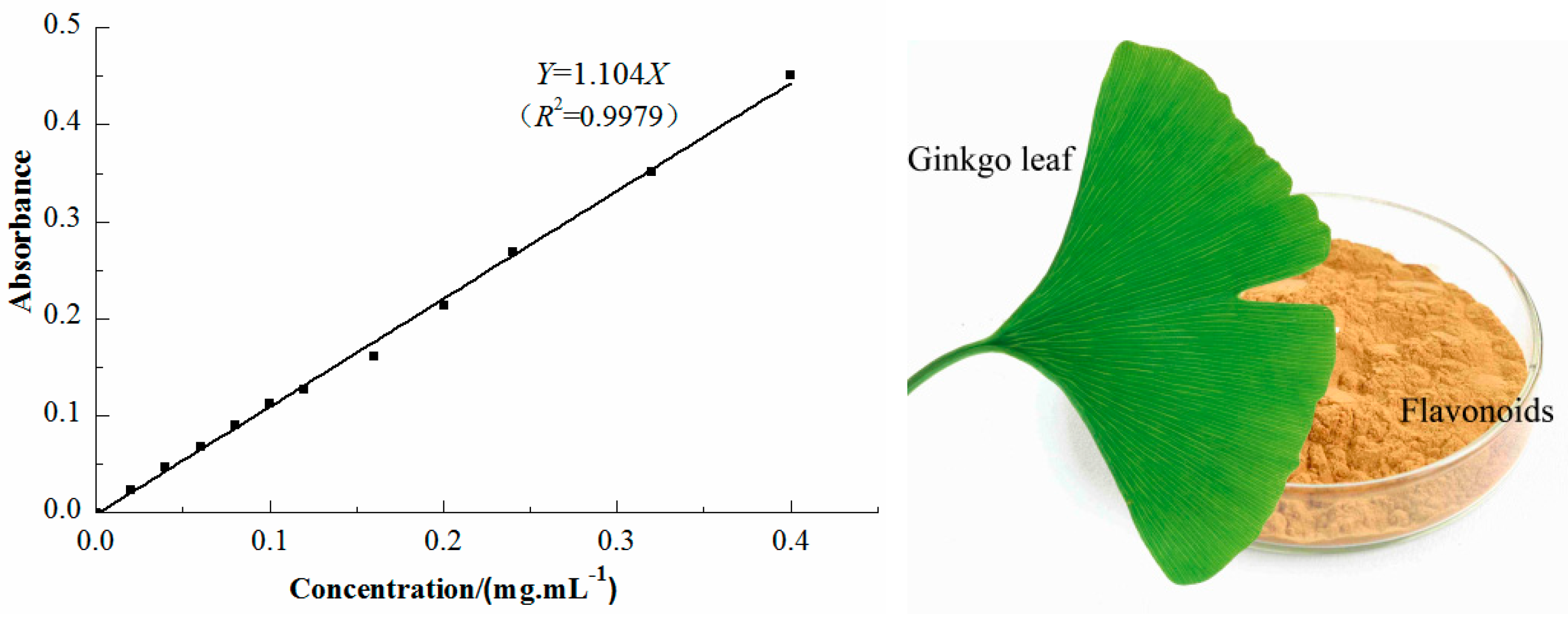
2.2. Determination of Total Flavonoid Content
2.3. Phase Diagram of PEG/(NH4)2SO4-Containing Aqueous Two-Phase Systems
2.4. Aqueous Two-Phase Extraction and Experimental Design
2.5. Determination of Free Radical Scavenging Ability
2.6. Determination of Total Reduction Capacity
3. Results and Discussion
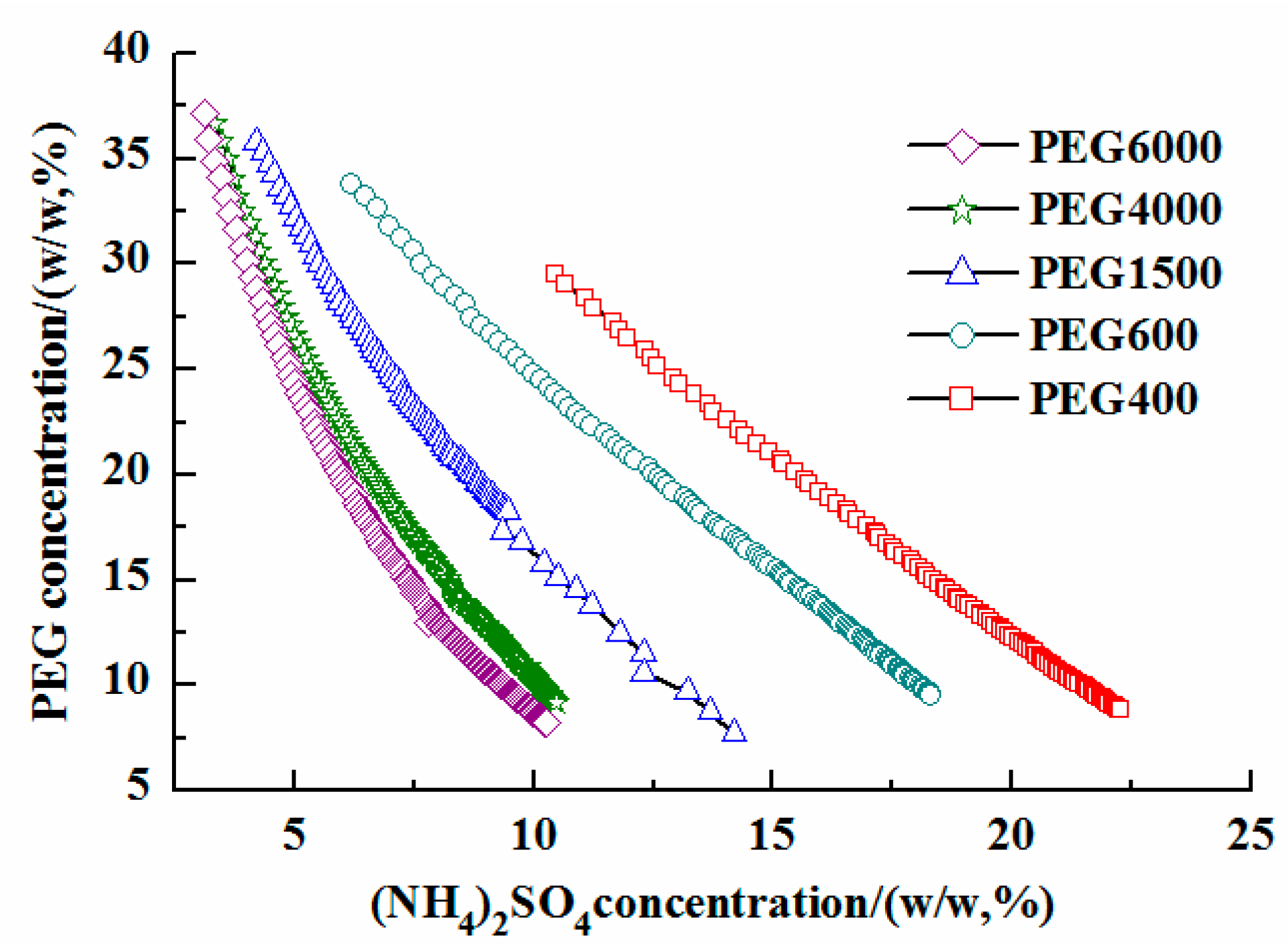
3.1. Phase Diagram of PEG/(NH4)2SO4
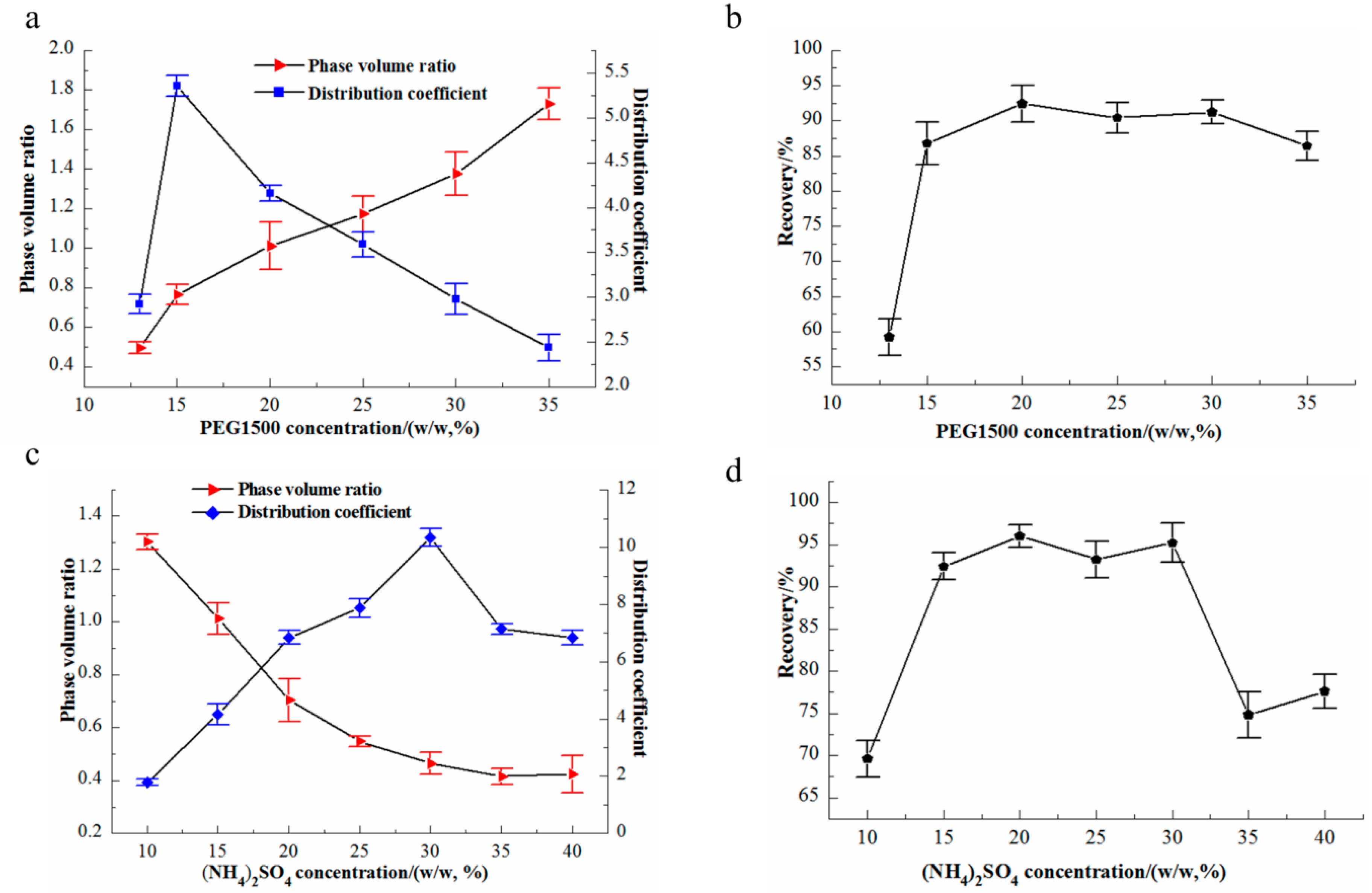
3.2. Effects of PEG1500 and (NH4)2SO4 on Partition Coefficient and Recovery in ATPS
3.3. Response Surface Methodology Optimization for Flavonoid Extraction
3.4. Regression Analysis
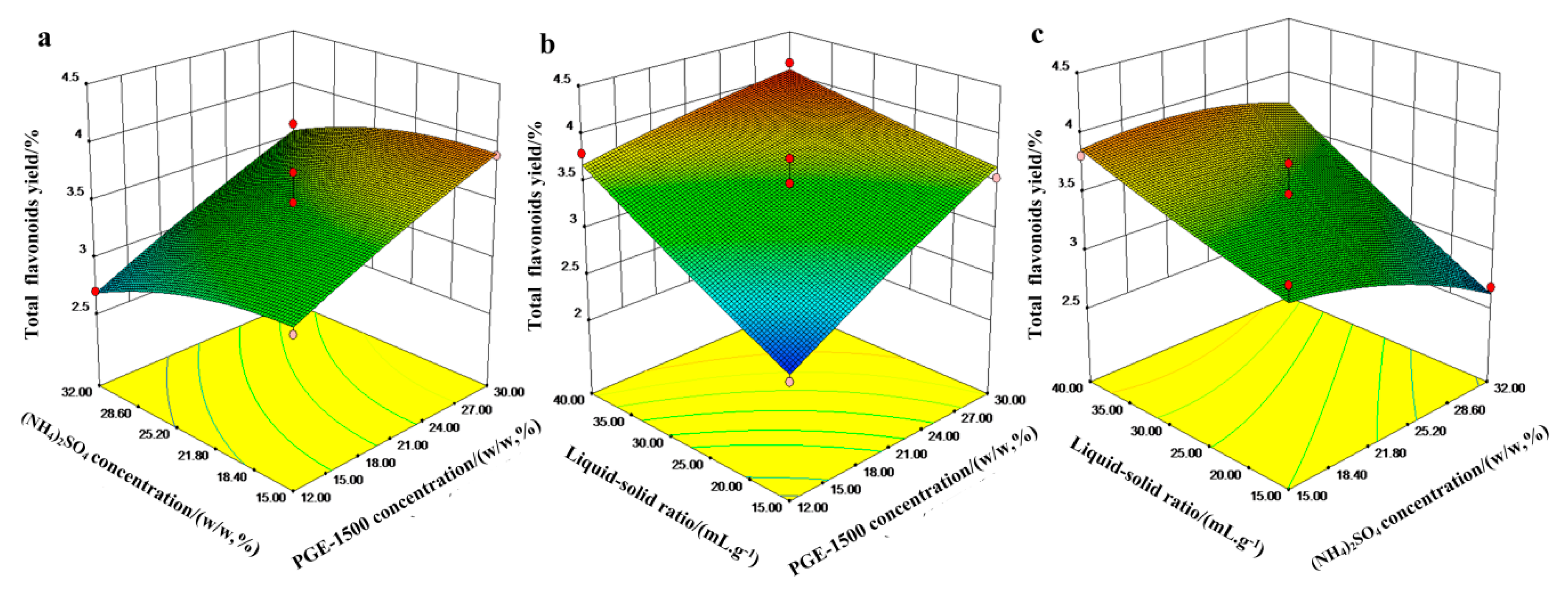
3.5. Response Surface Plot
3.6. Validation of Model
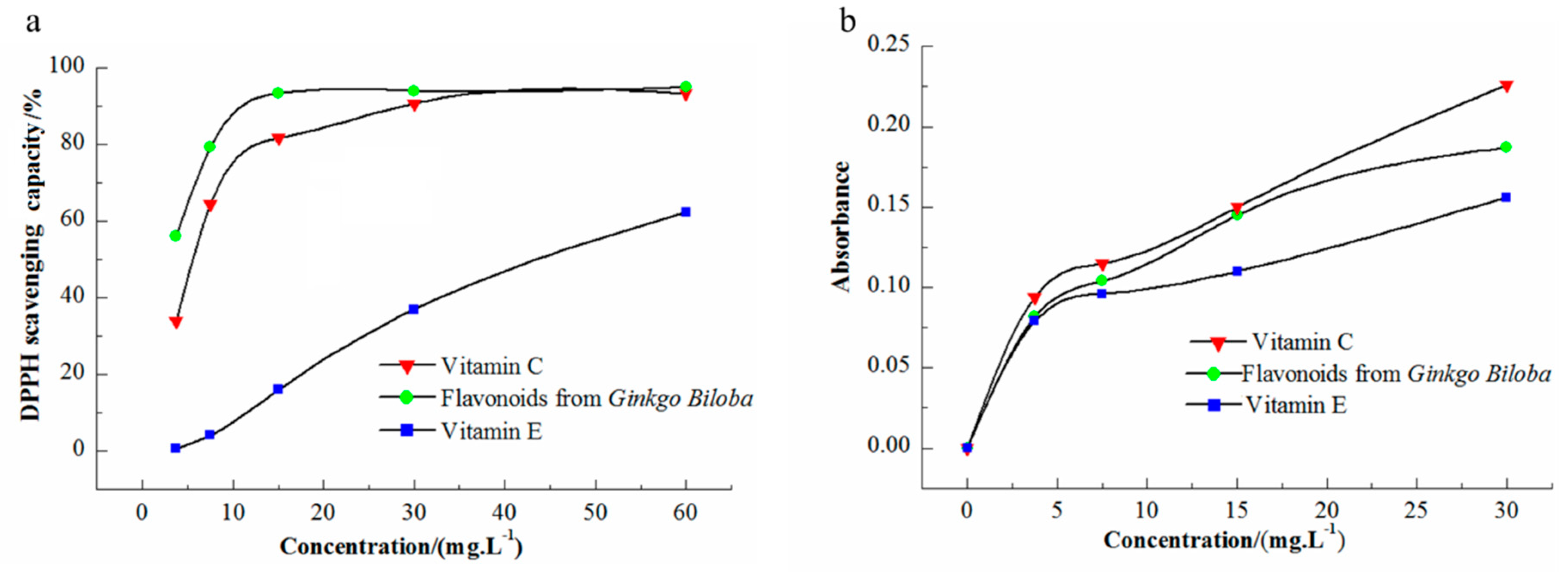
3.7. Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chen, L.Q.; Li, C.S.; Chaloner, W.G.; Beerling, D.J.; Sun, Q.G.; Collinson, M.E.; Mitchell, P.L. Assessing the potential for the stomatal characters of extant and fossil ginkgo leaves to signal atmospheric CO2 change. Am. J. Bot. 2001, 88, 1309–1315. [Google Scholar] [CrossRef] [PubMed]
- Kleijnen, J.; Knipschild, P. Ginkgo biloba. Lancet 1992, 340, 1136–1139. [Google Scholar] [CrossRef]
- Dongen, M.V.; Rossum, E.V.; Kessels, A.; Sielhorst, H.; Knipschild, P. Ginkgo for elderly people with dementia and age-associated memory impairment: A randomized clinical trial. J. Clin. Epidemiol. 2003, 56, 367–376. [Google Scholar] [CrossRef]
- Le Bars, P.L.; Katz, M.M.; Berman, N.; Itil, T.M.; Freedman, A.M.; Schatzberg, A.F. A placebo-controlled, double-blind, randomized trial of an extract of ginkgo biloba for dementia. JAMA 1997, 278, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Dekosky, S.T.; Williamson, J.D.; Fitzpatrick, A.L.; Kronmal, R.A.; Ives, D.G.; Saxton, J.A.; Lopez, O.L.; Burke, G.; Carlson, M.C.; Fried, L.P.; et al. Ginkgo biloba for prevention of dementia: A randomized controlled trial. JAMA 2008, 300, 2253–2262. [Google Scholar] [CrossRef] [PubMed]
- Beek, T.A.V. Chemical analysis of ginkgo biloba leaves and extracts. J. Chromatogr. A 2002, 967, 21–25. [Google Scholar] [CrossRef]
- Marcocci, L.; Maguire, J.J.; Droylefaix, M.T.; Packer, L. The nitric oxide-scavenging properties of ginkgo biloba extract egb 761. Biochem. Biophs. Res. Commun. 1994, 201, 748–755. [Google Scholar] [CrossRef]
- Marcocci, L.; Packer, L.; Droy-Lefaix, M.T.; Sekaki, A.; Gardès-Albert, M. Antioxidant action of ginkgo biloba extract egb 761. Methods Enzymol. 1994, 234, 462–475. [Google Scholar] [PubMed]
- Oyama, Y.; Fuchs, P.A.; Katayama, N.; Noda, K. Myricetin and quercetin, the flavonoid constituents of ginkgo biloba extract, greatly reduce oxidative metabolism in both resting and ca(2+)-loaded brain neurons. Brain Res. 1994, 635, 125–129. [Google Scholar] [CrossRef]
- Abdel-Kader, R.; Hauptmann, S.; Keil, U.; Scherping, I.; Leuner, K.; Eckert, A.; Müller, W.E. Stabilization of mitochondrial function by ginkgo biloba extract (egb 761). Pharmacol. Res. 2007, 56, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Kleijnen, J.; Knipschild, P. Ginkgo biloba for cerebral insufficiency. Br. J. Clin. Pharmacol. 2012, 34, 352–358. [Google Scholar] [CrossRef]
- Cull, S.G.; Holbrey, J.D.; Vargasmora, V.; Seddon, K.R.; Lye, G.J. Room-temperature ionic liquids as replacements for organic solvents in multiphase bioprocess operations. Biotechnol. Bioeng. 2015, 69, 227–233. [Google Scholar] [CrossRef]
- Li, N.; Wang, Y.; Xu, K.; Huang, Y.; Wen, Q.; Ding, X. Development of green betaine-based deep eutectic solvent aqueous two-phase system for the extraction of protein. Talanta 2016, 152, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.Y.; Gu, C.B.; Li, C.Y.; Luo, M.; Wang, W.; Zu, Y.G.; Li, J.; Fu, Y.J. Microwave-assisted aqueous two-phase extraction of isoflavonoids from dalbergia odorifera t. Chen leaves. Sep. Purif. Technol. 2013, 115, 136–144. [Google Scholar] [CrossRef]
- Đorđević, T.; Antov, M. Ultrasound assisted extraction in aqueous two-phase system for the integrated extraction and separation of antioxidants from wheat chaff. Sep. Purif. Technol. 2017, 182, 52–58. [Google Scholar] [CrossRef]
- Zhang, Y.; Sui, D.Y.; Zhou, J.S.; Zhou, H.L. Microwave-assisted extraction and antihyperlipidemic effect of total flavonoids from corn silk. Afr. J. Biotechnol. 2011, 10, 14583–14586. [Google Scholar] [CrossRef]
- Chen, Y.J.; Wang, J.; Wan, D.R. Determination of total flavonoids in three sedum crude drugs by uv-vis spectrophotometry. Pharmacogn. Mag. 2010, 6, 259–263. [Google Scholar] [PubMed]
- Bauer, K.W.; Parnell, G.S.; Meyers, D.A. Response surface methodology as a sensitivity analysis tool in decision analysis. J. Multi-Crit. Decis. Anal. 2015, 8, 162–180. [Google Scholar] [CrossRef]
- Liu, X.; Ardo, S.; Bunning, M.; Parry, J.; Zhou, K.; Stushnoff, C.; Stoniker, F.; Yu, L.; Kendall, P. Total phenolic content and dpph radical scavenging activity of lettuce (lactuca sativa L.) grown in colorado. LWT Food Sci. Technol. 2007, 40, 552–557. [Google Scholar] [CrossRef]
- Mraihi, F.; Journi, M.; Chérif, J.K.; Sokmen, M.; Sokmen, A.; Trabelsiayadi, M. Phenolic contents and antioxidant potential of crataegus fruits grown in tunisia as determined by dpph, frap, and β-carotene/linoleic acid assay. J. Chem. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Silvério, S.C.; Wegrzyn, A.; Lladosa, E.; Rodríguez, O.; Macedo, E.A. Effect of aqueous two-phase system constituents in different poly(ethylene glycol)–salt phase diagrams. J. Chem. Eng. Data 2012, 57, 1203–1208. [Google Scholar] [CrossRef]
- Glyk, A.; Scheper, T.; Beutel, S. Determination of aqueous two-phase system phase-forming components in the presence of bovine serum albumin. Anal. Biochem. 2014, 455, 10–12. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wysoczanska, K.; Macedo, E.A. Influence of the molecular weight of peg on the polymer/salt phase diagrams of aqueous two-phase systems. J. Chem. Eng. Data 2016, 61, 4229–4235. [Google Scholar] [CrossRef]
- Glyk, A.; Scheper, T.; Beutel, S. Influence of different phase-forming parameters on the phase diagram of several peg–salt aqueous two-phase systems. J. Chem. Eng. Data 2014, 59, 850–859. [Google Scholar] [CrossRef]
- Liu, J.; Liu, C.; Rong, Y.; Huang, G.; Rong, L. Extraction of limonin from orange (citrus reticulata blanco) seeds by the flash extraction method. Solvent Extr. Res. Dev. 2012, 19, 137–145. [Google Scholar] [CrossRef]
- Zhong, K.; Wang, Q. Optimization of ultrasonic extraction of polysaccharides from dried longan pulp using response surface methodology. Carbohyd. Polym. 2010, 80, 19–25. [Google Scholar] [CrossRef]
- Ghaedi, M.; Khafri, H.Z.; Asfaram, A.; Goudarzi, A. Response surface methodology approach for optimization of adsorption of janus green b from aqueous solution onto zno/zn(oh) 2 -np-ac: Kinetic and isotherm study. Spectrochim. Acta A 2016, 152, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Szeto, Y.T. Total antioxidant capacity of teas by the ferric reducing/antioxidant power assay. J. Agric. Food Chem. 1999, 47, 633–636. [Google Scholar] [CrossRef] [PubMed]
| Symbol | Variable | Range and Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| X1 | PEG1500 concentration (w/w %) | 12 | 21 | 30 |
| X2 | (NH4)2SO4 concentration (w/w %) | 15 | 23.5 | 32 |
| X3 | ATPS solvent volume/leaf mass ratio (v/w mL/g) | 15 | 27.5 | 40 |
| RUN | X1 (w/w %) | X2 (w/w %) | X3 (mL g−1) | Y (mg g−1) |
|---|---|---|---|---|
| 1 | −1 | 0 | −1 | 2.37 |
| 2 | 0 | −1 | −1 | 3.46 |
| 3 | −1 | 0 | 1 | 3.80 |
| 4 | 0 | −1 | 1 | 3.81 |
| 5 | −1 | 1 | 0 | 2.70 |
| 6 | 0 | 1 | 1 | 3.59 |
| 7 | 1 | 0 | 1 | 4.14 |
| 8 | 1 | 0 | −1 | 3.54 |
| 9 | 1 | 1 | 0 | 3.62 |
| 10 | 0 | 1 | −1 | 2.68 |
| 11 | 0 | 0 | 0 | 3.75 |
| 12 | 0 | 0 | 0 | 3.48 |
| 13 | 0 | 0 | 0 | 3.37 |
| 14 | 1 | −1 | 0 | 3.89 |
| 15 | 0 | 0 | 0 | 3.38 |
| 16 | −1 | −1 | 0 | 3.11 |
| 17 | 0 | 0 | 0 | 3.38 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value Prob > F | Significance |
|---|---|---|---|---|---|---|
| Model | 3.31 | 9 | 0.37 | 13.3 | 0.0013 | ** |
| X1 | 1.29 | 1 | 1.29 | 46.65 | 0.0002 | ** |
| X2 | 0.35 | 1 | 0.35 | 12.81 | 0.009 | ** |
| X3 | 1.35 | 1 | 1.35 | 49.01 | 0.0002 | ** |
| X1X2 | 4.70 × 10−3 | 1 | 4.70 × 10−3 | 0.17 | 0.6925 | |
| X1X3 | 0.17 | 1 | 0.17 | 6.21 | 0.0414 | * |
| X2X3 | 0.076 | 1 | 0.076 | 2.74 | 0.1419 | |
| X12 | 4.01 × 10−3 | 1 | 4.01 × 10−3 | 0.15 | 0.7144 | |
| X22 | 0.051 | 1 | 0.051 | 1.85 | 0.2161 | |
| X32 | 2.06 × 10−3 | 1 | 2.06 × 10−3 | 0.075 | 0.7925 | |
| Residual | 0.19 | 7 | 0.028 | |||
| Lack of Fit | 0.088 | 3 | 0.029 | 1.11 | 0.442 | Not significant |
| Pure Error | 0.11 | 4 | 0.026 | |||
| Cor Total | 3.5 | 16 | ||||
| R2 | 0.9447 | |||||
| C.V. | 4.86 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Liu, S.; Zhang, L.; Wang, X.; Ma, L. Partition Behavior in Aqueous Two-Phase System and Antioxidant Activity of Flavonoids from Ginkgo biloba. Appl. Sci. 2018, 8, 2058. https://doi.org/10.3390/app8112058
Liu C, Liu S, Zhang L, Wang X, Ma L. Partition Behavior in Aqueous Two-Phase System and Antioxidant Activity of Flavonoids from Ginkgo biloba. Applied Sciences. 2018; 8(11):2058. https://doi.org/10.3390/app8112058
Chicago/Turabian StyleLiu, Can, Shuochen Liu, Lingli Zhang, Xiaoxue Wang, and Lanqing Ma. 2018. "Partition Behavior in Aqueous Two-Phase System and Antioxidant Activity of Flavonoids from Ginkgo biloba" Applied Sciences 8, no. 11: 2058. https://doi.org/10.3390/app8112058
APA StyleLiu, C., Liu, S., Zhang, L., Wang, X., & Ma, L. (2018). Partition Behavior in Aqueous Two-Phase System and Antioxidant Activity of Flavonoids from Ginkgo biloba. Applied Sciences, 8(11), 2058. https://doi.org/10.3390/app8112058




