Prognostic Assessment of the Viability of Hydrothermal Liquefaction as a Post-Resource Recovery Step after Enhanced Biomethane Generation Using Co-Digestion Technologies
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analytical Methods
2.3. Experimental Design
2.4. Kinetic Assessments
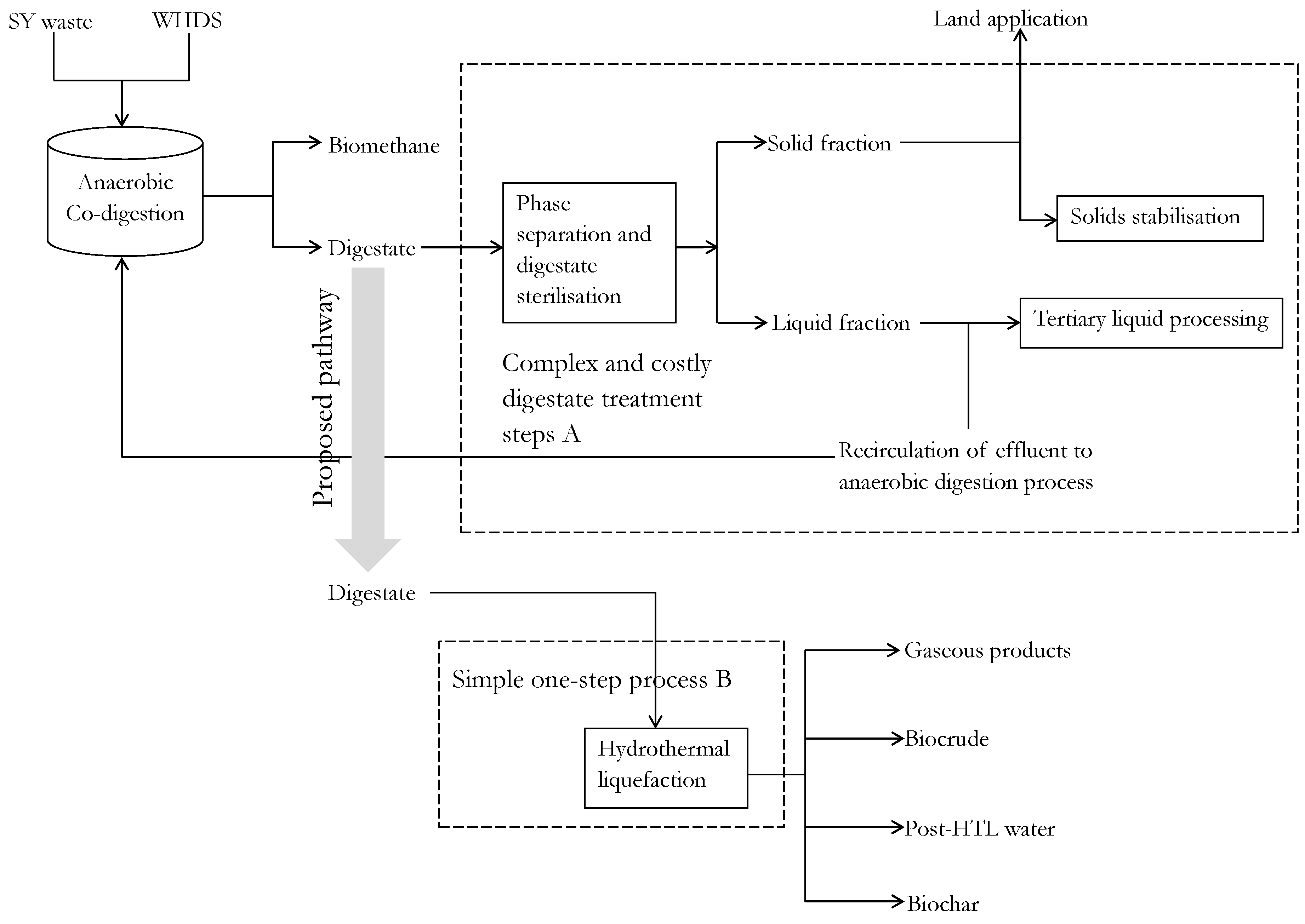
2.5. Preliminary Assessment of the Alternative One-Step Digestate Processing Approach
3. Results and Discussions
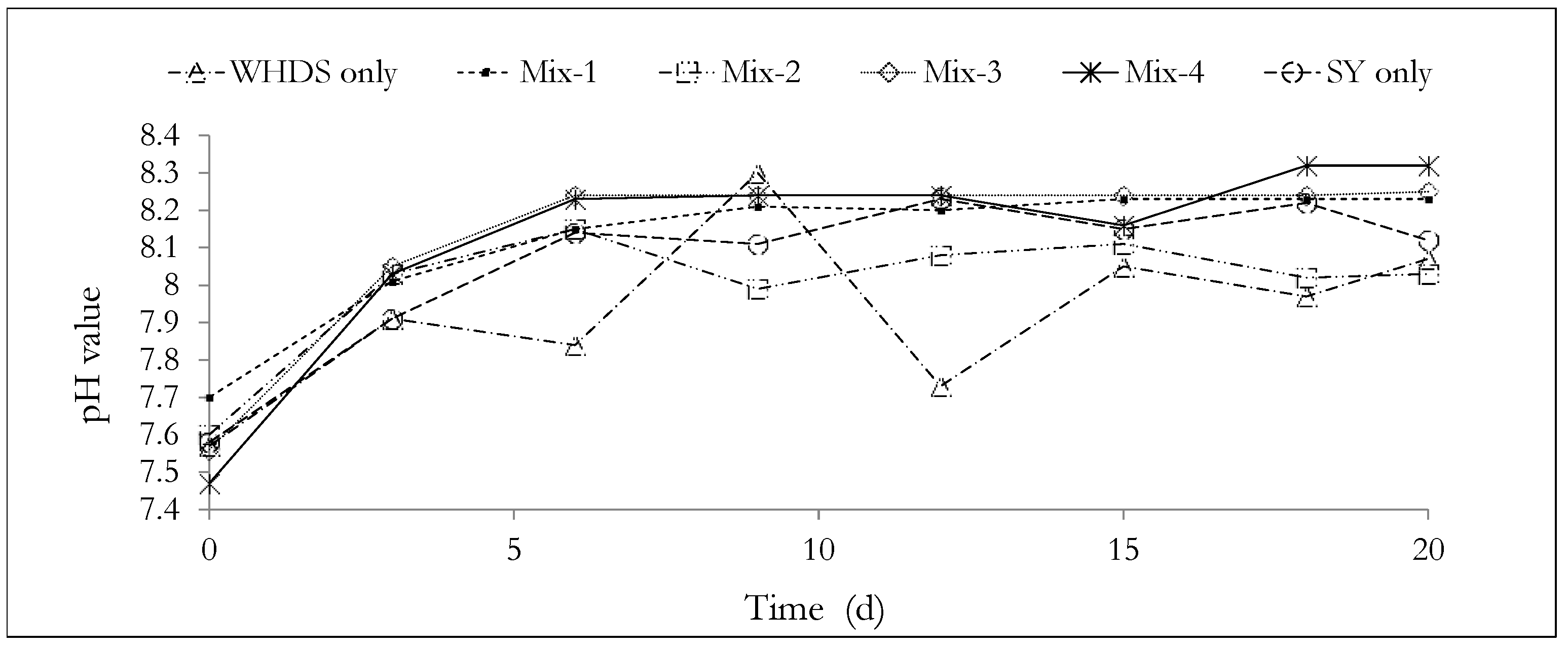
3.1. Anaerobic Digestion Experiments
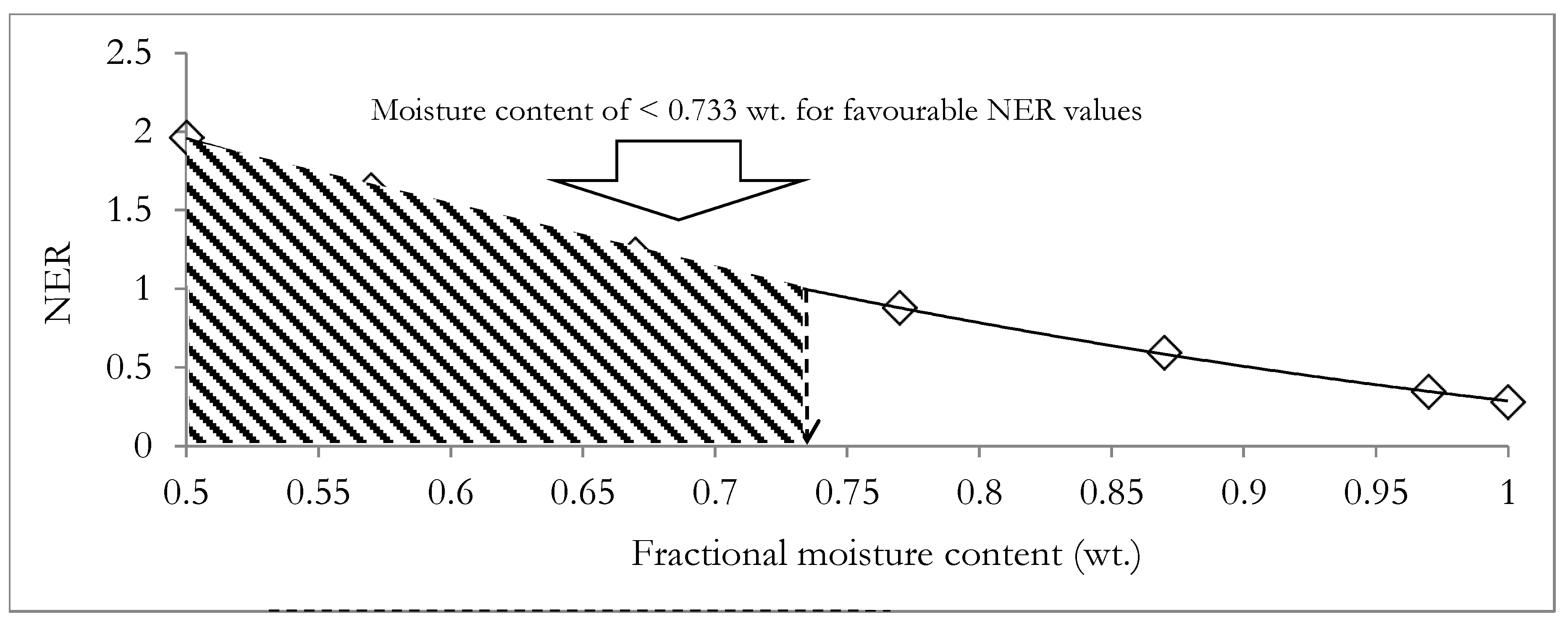
3.2. Productivity and Energetic Assessment of the Integrated System of HTL and ACD Subsystems
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Okoro, O.V.; Sun, Z.; Birch, J. Meat processing waste as a potential feedstock for biochemicals and biofuels—A review of possible conversion technologies. J. Clean. Prod. 2017, 142, 1583–1608. [Google Scholar] [CrossRef]
- Okoro, O.V.; Sun, Z.; Birch, J. Meat processing dissolved air flotation sludge as a potential biodiesel feedstock in New Zealand: A predictive analysis of the biodiesel product properties. J. Clean. Prod. 2017, 168, 1436–1447. [Google Scholar] [CrossRef]
- Okoro, O.V.; Sun, Z.; Birch, J. Experimental evaluation of a polystyrene sulphonic acid resin catalyst in the hydrolysis of low-grade lipids from the meat processing industry. Biomass Bioenergy 2018, 116, 49–59. [Google Scholar] [CrossRef]
- Okoro, O.V. Scaled-up biodiesel production from meat processing dissolved air flotation sludge: A simulation study. AgriEngineering 2018, 1, 3. [Google Scholar] [CrossRef]
- Wang, M.; Park, C. Investigation of anaerobic digestion of Chlorella sp. and Micractinium sp. grown in high-nitrogen wastewater and their co-digestion with waste activated sludge. Biomass Bioenergy 2015, 80, 30–37. [Google Scholar] [CrossRef]
- Massaro, V.; Digiesi, S.; Mossa, G.; Ranieri, L. The sustainability of anaerobic digestion plants: A win–win strategy for public and private bodies. J. Clean. Prod. 2015, 104, 445–459. [Google Scholar] [CrossRef]
- Domingues, R.F.; Sanches, T.; Silva, G.S.; Bueno, B.E.; Ribeiro, R.; Kamimura, E.S.; Franzolin Neto, R.; Tommaso, G. Effect of enzymatic pretreatment on the anaerobic digestion of milk fat for biogas production: Byproducts from agri-food industry: New strategies for their revalorisation. Food Res. Int. 2015, 73, 26–30. [Google Scholar] [CrossRef]
- Cesaro, A.; Belgiorno, V. Pretreatment methods to improve anaerobic biodegradability of organic municipal solid waste fractions. Chem. Eng. J. 2014, 240, 24–37. [Google Scholar] [CrossRef]
- Li, C.; Strömberg, S.; Liu, G.; Nges, I.A.; Liu, J. Assessment of regional biomass as co-substrate in the anaerobic digestion of chicken manure: Impact of co-digestion with chicken processing waste, seagrass and Miscanthus. Biochem. Eng. J. 2017, 118, 1–10. [Google Scholar] [CrossRef]
- Huang, X.; Yun, S.; Zhu, J.; Du, T.; Zhang, C.; Li, X. Mesophilic anaerobic co-digestion of aloe peel waste with dairy manure in the batch digester: Focusing on mixing ratios and digestate stability. Bioresour. Technol. 2016, 218, 62–68. [Google Scholar] [CrossRef] [PubMed]
- González, L.M.L.; Reyes, I.P.; Romero, O.R. Anaerobic co-digestion of sugarcane press mud with vinasse on methane yield. Waste Manag. 2017, 68, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Sosnowski, P.; Wieczorek, A.; Ledakowicz, S. Anaerobic co-digestion of sewage sludge and organic fraction ofmunicipal solid wastes. Adv. Environ. Res. 2003, 7, 609–616. [Google Scholar] [CrossRef]
- Tanimu, M.I.; Ghazi, T.I.M.; Harun, R.M.; Idris, A. Effect of carbon to nitrogen ratio of food waste on biogas methane production in a batch mesophilic. Int. J. Innov. Manage. Technol. 2014, 5, 116–119. [Google Scholar]
- Hall, P.W.; Gifford, J.; Richardson, M. Bioenergy Options for New Zealand: A Situation Analysis of Biomass Resources and Conversion Technologies; Scion, Energy Group: Wellington, New Zealand, 2007. [Google Scholar]
- Baere, D. Anaerobic digestion of solid waste: State of the art. Water Sci. Technol. 2000, 41, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Drosg, B.; Fuchs, W.; Al seadi, T.; Madsen, M.; Linke, B. Nutrient recovery by biogas digestate processing. IEA Bioenergy 2015, 2015, 711. [Google Scholar]
- Baggesen, D.L. Veterinary Safety in Relation to Handling of Manure and Animal by Products and the Use of Biogas Technologies; Presentation National Food Institute Denmark: Kgs Lyngby, Denmark, 2007. [Google Scholar]
- Risberg, K.; Cederlund, H.; Pell, M.; Arthurson, V.; Schnurer, A. Comparative characterization of digestate versus pig slurry and cow manure—Chemical composition and effects on soil microbial activity. Waste Manag. 2017, 61, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Camilleri-Rumbau, M.S.; Norddahl, B.; Wei, J.; Christensen, K.V.; Søtoft, L.F. Microfiltration and ultrafiltration as a post-treatment of biogas plant digestates for producing concentrated fertilizers. Desalin. Water Treat. 2014, 55, 1639–1653. [Google Scholar] [CrossRef]
- Jones, S.B.; Zhu, Y.; Anderson, D.B.; Hallen, R.T.; Elliott, D.C.; Schmidt, A.J.; Albrecht, K.O.; Hart, T.R.; Butcher, M.G.; Drennan, C.; et al. Process Design and Economics for the Conversion of Algal Biomass to Hydrocarbons: Whole Algae Hydrothermal Liquefaction and Upgrading; Springfield: Sangamon, IL, USA, 2014. [Google Scholar]
- Okoro, O.V.; Sun, Z.; Birch, J. Enhanced Fatty Acid Generation from Meat Processing Dissolved Air Flotation Sludge. In Proceedings of the 25th European Biomass Conference and Exhibition, Stockholm, Sweden, 12–15 June 2017. [Google Scholar]
- Batstone, D.J.; Tait, S.; Starrenburg, D. Estimation of hydrolysis parameters in full-scale anerobic digesters. Biotechnol. Bioeng. 2009, 102, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- ASTM. Standard Test Method for Determination of Total Solids in Biomass; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- ASTM. Standard Test Method for Volatile Matter in the Analysis Sample of Coal and Coke; American Society for Testing and materials International: West Conshohocken, PA, USA, 2011. [Google Scholar]
- ASTM. D2017-98 Standard Test Method of Accelerated Laboratory Test of Natural Decay Resistance of Woods, Decay, Evaluation, Laboratory, Natural, Resistance and Subjected to Termite Bioassay According to No-Choice Test Procedure Based upon AWPA E1-97; American Society for Testing and Materials: West Conshohocken, PA, USA, 1998. [Google Scholar]
- ASTM. Standard Test Method for Specific Gravity and Density of Semi-Solid Bituminous Materials (Pycnometer Method); American Society for Testing and Materials International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Jensen, P.D.; Ge, H.; Batstone, D.J. Assessing the role of biochemical methane potential tests in determining anaerobic degradability rate and extent. Water Sci. Technol. 2011, 64, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, N.R.; Alves, M.M. Influence of inoculum activity on the bio-methanization of a kitchen waste under different waste/inoculum ratios. Process Biochem. 2004, 39, 2019–2024. [Google Scholar] [Green Version]
- Ehimen, E.; Connaughtonw, S.; Sun, Z.; Carrington, G.C. Energy recovery from lipid extracted, transesterified and glycerol co digested microalgae biomass. GCB Bioenergy 2009, 88, 371–381. [Google Scholar] [CrossRef]
- Demirel, B.; Scherera, P. Trace element requirements of agricultural biogas digesters during biological conversion of renewable biomass to methane. Biomass Bioenergy. 2011, 35, 992–998. [Google Scholar] [CrossRef]
- Li, J.; Jhaa, A.K.; He, J.; Ban, Q.; Chang, S.; Wang, P. Assessment of the effects of dry anaerobic codigestion codigestion of cow dung with waste water sludge on biogas yield and biodegradability. Int. J. Phys. Sci. 2011, 6, 3723–3732. [Google Scholar]
- Hinds, G.R.; Mussoline, W.; Casimir, L.; Dick, G.; Yeh, D.H.; Ergas, S.J. Enhanced methane yields in high-solids anaerobic digestion through inoculation with pulp and paper mill sludge. Environ. Eng. Sci. 2016, 33, 907–917. [Google Scholar] [CrossRef]
- Guwy, A.J. Equipment used for testing anaerobic biodegradability and activity. Rev. Environ. Sci. Biotechnol. 2004, 3, 131–139. [Google Scholar] [CrossRef]
- Zhang, W.; Wei, Q.; Wu, S.; Qi, D.; Li, W.; Zuo, Z.; Dong, D. Batch anaerobic co-digestion of pig manure with dewatered sewage sludge under mesophilic conditions. Appl. Energy 2014, 128, 175–183. [Google Scholar] [CrossRef]
- Gamble, K.J. Anaerobic Digestion from the Laboratory to the Field: An Experimental Study into the Scalability of Anaerobic Digestion. Ph.D. Thesis, Appalachian State University, Boone, NC, USA, 2014. [Google Scholar]
- Walker, M.; Zhang, Y.; Heaven, S.; Banks, C. Potential errors in the quantitative evaluation of biogas production in anaerobic digestion processes. Bioresour. Technol. 2009, 100, 6339–6346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Li, G.; Chi, M.; Sun, Y.; Zhang, J.; Jiang, S.; Cui, Z. Effects of co-digestion of cucumber residues to corn stover and pig manure ratio on methane production in solid state anaerobic digestion. Bioresour. Technol. 2018, 250, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Ebner, J.H.; Labatut, R.A.; Lodge, J.S.; Williamson, A.A.; Trabold, T.A. Anaerobic co-digestion of commercial food waste and dairy manure: Characterizing biochemical parameters and synergistic effects. Waste Manag. 2016, 52, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.P.; Jensen, P.D.; Batstone, D.J. Methanosarcinaceae and acetate-oxidizing pathways dominate in high-rate thermophilic anaerobic digestion of waste-activated sludge. Appl. Environ. Microbiol. 2013, 79, 6491–6500. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.; Davies, W.J.; Forster, C.F. Two-stage, thermophilic-mesophilic anaerobic digestion of sewage sludge. Process Saf. Environ. Prot. 1999, 77, 93–97. [Google Scholar] [CrossRef]
- Zwietering, M.H.; Jongenburger, L.; Rombouts, F.M.; Van’t Riet, K. Modeling the bacterial growth curve. Appl. Environ. Microbiol. 1990, 56, 1875–1881. [Google Scholar] [PubMed]
- Pitt, R.E.; Cross, T.L.; Pell, A.N.; Schofield, P.; Doane, P.H. Use of in vitro gas production models in ruminal kinetics. Math. Biosci. 1999, 159, 145–163. [Google Scholar] [CrossRef]
- Zhen, G.; Lu, X.; Kobayashi, T.; Kumar, G.; Xu, K. Anaerobic co-digestion on improving methane production from mixed microalgae (Scenedesmus sp., Chlorella sp.) and food waste: Kinetic modeling and synergistic impact evaluation. Biochem. Eng. J. 2016, 299, 332–341. [Google Scholar] [CrossRef]
- Leow, S.; Witter, J.R.; Vardon, D.R.; Sharma, B.K.; Guest, J.S.; Strathmann, T.J. Prediction of microalgae hydrothermal liquefaction products from feedstock biochemical composition. Green Chem. 2015, 17, 3584–3599. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Leow, S.; Fedders, A.C.; Sharma, B.K.; Guest, J.S. Quantitative multiphase model for hydrothermal liquefaction of algal biomass. Green Chem. 2017, 19, 1163–1174. [Google Scholar] [CrossRef]
- Sheng, L.; Wang, X.; Yang, X. Prediction model of biocrude yield and nitrogen heterocyclic compounds analysis by hydrothermal liquefaction of microalgae with model compounds. Bioresour. Technol. 2018, 247, 14–20. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Animal feed. In Official Methods of Analysis; Association of Official Analytical Chemists International: Arlington, VA, USA, 1995. [Google Scholar]
- AOCS. Combustion Method for Determination of Crude Protein in Animal Feeds, Oilseed Meals and Oilseeds; American Oil Chemists Society: Urbana, IL, USA, 1998. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, 19th ed.; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Hill, J.; Nelson, E.; Tilman, D.; Polasky, S.; Tiffany, D. Environmental, economic and energetic costs and benefits of biodiesel and ethanol biofuels. Proc. Natl. Acad. Sci. USA 2006, 30, 11206–11210. [Google Scholar] [CrossRef] [PubMed]
- Silalertruksa, T.; Gheewala, S.H. Environmental sustainability assessment of bio-ethanol production in Thailand. Energy 2009, 34, 1933–1946. [Google Scholar] [CrossRef]
- Freris, L.; Infield, D. Renewable Energy in Power Systems; Wiley: New York, NY, USA, 2008. [Google Scholar]
- Efficiency of Conventional Thermal Electricity and Heat Production. Available online: https://www.eea.europa.eu/data-and-maps/indicators/efficiency-of-conventional-thermal-electricity-generation-4/assessment-1 (accessed on 7 August 2018).
- Eurelectric. Efficiency in Electricity Generation; VGB PowerTech: Essen, Germany, 2003. [Google Scholar]
- Heat Values of Various Fuels. Available online: www.world-nuclear.org/information-library/facts-and-figures/heat-values-of-various-fuels.aspx (accessed on 27 February 2017).
- Vardona, D.R.; Sharma, B.K.; Blazina, G.V.; Rajagopalan, K.; Strathmann, T.J. Thermochemical conversion of raw and defatted algal biomass via hydrothermal liquefaction and slow pyrolysis. Bioresour. Technol. 2012, 109, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, S.; Suzuki, A.; Masanori Murakamit, T.O. Liquid fuel production from sewage sludge by catalytic conversion using sodium carbonate. Fuel 1987, 66, 1150–1155. [Google Scholar] [CrossRef]
- ToolBox, E. Water—Thermophysical Properties. Available online: https://www.engineeringtoolbox.com/water-thermal-properties-d_162.html (accessed on 12 August 2017).
- Gupta, M.; Yanga, Y.; Roy, C. Specific heat and thermal conductivity of softwood bark and softwood char particles. Fuel 2003, 82, 919–927. [Google Scholar] [CrossRef]
- Ehimen, E.A. An Investigation on the Coproduction of Biodiesel and Methane from Microalgae. Ph.D. Thesis, University of Otago, Dunedin, New Zealand, 2010. [Google Scholar]
- Budde, J.; Prochnow, A.; Plöchl, M.; Quiñones, T.S.; Heiermann, M. Energy balance, greenhouse gas emissions, and profitability of thermobarical pretreatment of cattle waste in anaerobic digestion. Waste Manag. 2016, 49, 390–410. [Google Scholar] [CrossRef] [PubMed]
- Banks, C.J.; Salter, A.M.; Heaven, S.; Riley, K. Energetic and environmental benefits of co-digestion of food waste and cattle slurry: A preliminary assessment. Resour. Conserv. Recycl. 2012, 56, 71–79. [Google Scholar] [CrossRef] [Green Version]
- GSES. Planning and Installing Bioenergy Systems: A Guide for Installers, Architects and Engineers; The German Solar Energy Society: James and James Science Publishers: London, UK, 2005; p. 359. [Google Scholar]
- Welty, J.R.; Wicks, C.E.; Wilson, C.E.; Rorrer, G.L. Fundementals of Momentum, Heat and Mass Transfer; Wiley: New York, NY, USA, 2008. [Google Scholar]
- Sustainability-Victoria. Energy Efficiency Best Practice Guide, Pumping Systems; Sustainability Victoria: Melbourne, Australia, 2009. [Google Scholar]
- Pemberton, M. Pump Efficiency Drives Cost Savings. Available online: https://www.controleng.com/single-article/pump-efficiency-drives-cost-savings (accessed on 3 April 2018).
- Deublein, D.; Steinhauser, A. Biogas from Waste and Renewable Resources: An Introduction, 2nd Revised and Expanded Edition; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Lima, D.M.F.; Rodrigues, J.A.D.; Boe, M.; Alvarado-Morales, M.; Ellegaard, L.; Angelidaki, I. anaerobic modelling for improving the synegy and robustness of manure co-digestion process. Braz. J. Chem. Eng. 2016, 33, 871–883. [Google Scholar] [CrossRef]
- Procházka, J.; Dolejš, P.; MácA, J.; Dohányos, M. Stability and inhibition of anaerobic processes caused by insufficiency or excess of ammonia nitrogen. Appl. Microbiol. Biotechnol. 2012, 93, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lu, X.; Li, F.; Yang, G. Effects of temperature and carbon-nitrogen (C/N) ratio on the performance of anaerobic co-digestion of dairy manure, chicken manure and rice straw: Focusing on ammonia inhibition. PLoS ONE 2014, 9, e97265. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Møller, H.B.; Saha, C.K.; Alam, M.M.; Wahid, R.; Feng, L. Optimal ratio for anaerobic co-digestion of poultry droppings and lignocellulosic-rich substrates for enhanced biogas production. Energy Sustain. Dev. 2017, 39, 59–66. [Google Scholar] [CrossRef]
- Vardon, D.R.; Sharma, B.K.; Scott, J.; Yu, G.; Wang, Z.; Schideman, L.; Zhang, Y.; Strathmann, T.J. Chemical properties of biocrude oil from the hydrothermal liquefaction. Bioresour. Technol. 2011, 102, 8295–8303. [Google Scholar] [CrossRef] [PubMed]
- Martin-Ryals, A.D. Evaluating the Potential for Improving Anaerobic Digestion of Cellulosic Waste via Routine Bioaugmentation and Alkaline Pretreatment. Ph.D. Thesis, University of Illinois at Urbana-Champaign, Champaign, IL, USA, 2012. [Google Scholar]
- Ramirez, J.A.; Brown, R.J.; Rainey, T.J. A review of hydrothermal liquefaction bio-crude properties and prospects for upgrading to transportation fuels. Energies 2015, 8, 6765–6794. [Google Scholar] [CrossRef] [Green Version]
- Biller, P.; Ross, A.P. Potential yields and properties of oil from the hydrothermal liquefaction of microalgae with different biochemical contents. Bioresour. Technol. 2011, 102, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Maddi, B.; Panisko, E.; Wietsma, T.; Lemmon, T.; Swita, M.; Albrecht, K. Quantitative characterization of aqueous byproducts from hydrothermal liquefaction of municipal wastes, food industry wastes, and biomass grown on waste. ACS Sustain. Chem. Eng. 2017, 5, 2205–2214. [Google Scholar] [CrossRef]
- Mørup, A.J.; Becker, J.; Christensen, P.R.; Houlberg, K.; Lappa, E.; Klemmer, M.; Madsen, R.; Glasius, M.; Iversen, B. Construction and commisioning of a continuous reactor for hydrothermal liquefaction. Ind. Chem. Eng. Res. 2015, 54, 5935–5947. [Google Scholar] [CrossRef]
- Madsen, R.; Christensen, P.; Houlberg, K.; Lappa, E.; Mørup, A.; Klemmer, M.; Olsen, E.; Jensen, M.; Becker, J.; Iversen, B.; et al. Analysis of organic gas phase compounds formed by hydrothermal liquefaction of dried distillers grains with solubles. Bioresour. Technol. 2015, 192, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Yuan, X.; Wu, G. Liquefaction of biomass for bio-oil products. In Waste Biomass Management—A Holistic Approach; Springer: Cham, Switzerland, 2017; pp. 231–251. [Google Scholar]
- Tian, C.; Zhidan, L.; Zhang, Y. Hydrothermal Liquefaction (HTL): A promising pathway for biorefinery of algae. In Algal Biofuels; Springer: Cham, Switzerland, 2017; pp. 361–391. [Google Scholar]




| Parameters | Inoculum | WHDS | SY |
|---|---|---|---|
| Total solids (%w/w, wet feedstock) | 2.33 (0.07) | 7.35 (0.65) | 16.73 (0.01) |
| Volatile solids (%w/w TS 1) | 41.89 (1.64) | 24.86 (0.42) | 42.45 (0.94) |
| Fixed carbon (%w/w TS) | 23.21 (1.94) | 49.57 (0.64) | 23.68 (1.16) |
| VS 2 to TS mass ratio | 0.42 (0.02) | 0.25 (0.03) | 0.42 (0.02) |
| Ash (%w/w TS) | 34.90 (0.07) | 25.57 (0.48) | 33.87 (0.68) |
| Carbon content (%w/w TS) | 29.66 (0.14) | 42.88 (0.13) | 33.37 (0.29) |
| Nitrogen content (%w/w TS) | 3.61 (0.05) | 2.49 (0.07) | 2.40 (0.02) |
| Sulphur content (%w/w TS) | 0.795 (0.03) | 2.40 (0.06) | 0.30 (0.00) |
| Hydrogen content (%w/w TS) | 4.87 (0.07) | 5.91 (0.07) | 4.73 (0.02) |
| Oxygen content (%w/w TS) | 26.18 (0.16) | 20.76 (0.51) | 25.33 (0.68) |
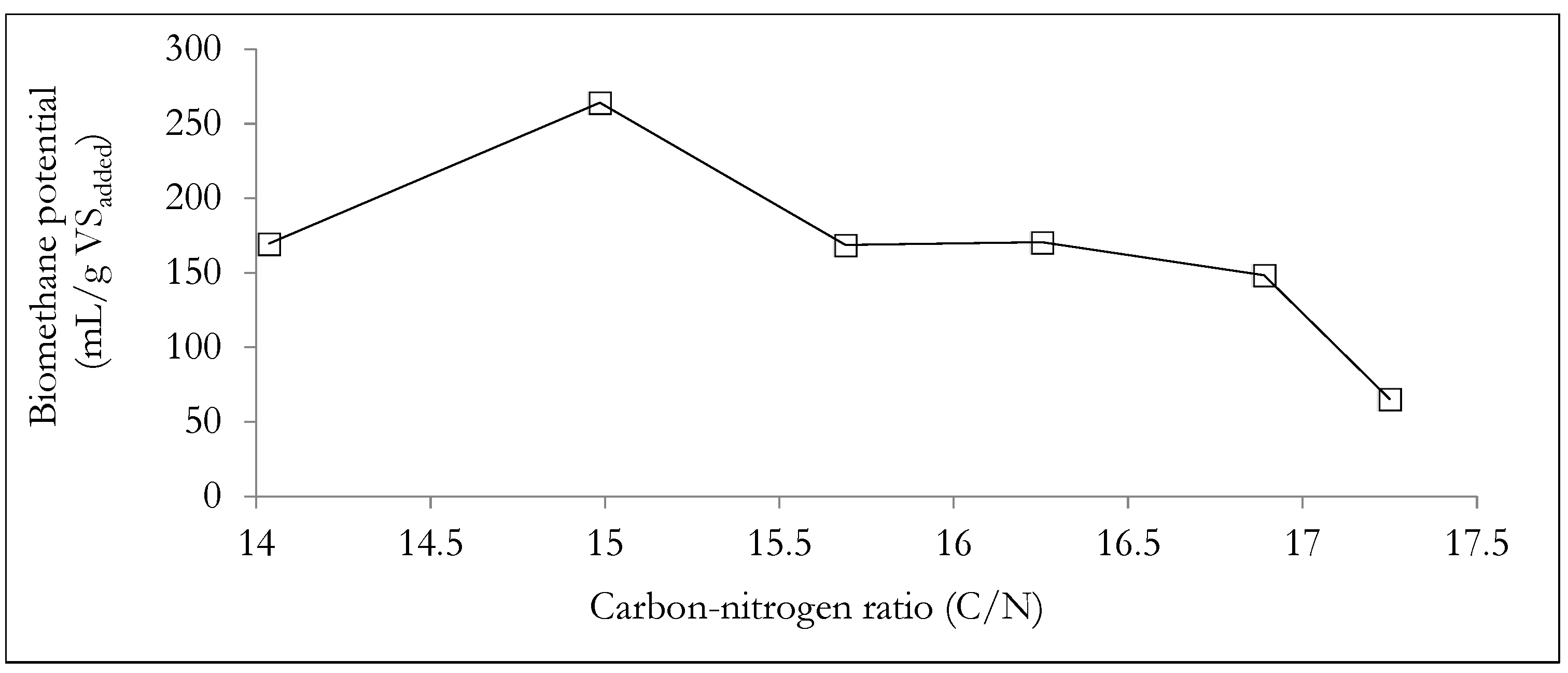
| Carbon to nitrogen mass ratio | 8.23 (0.11) | 17.22 (0.48) | 13.90 (0.167) |
| pH value | 7.46 (0.01) | 8.09 (0.00) | 7.56 (0.00) |
| Specific gravity at 20 °C | 0.97 (0.01) | 0.96 (0.01) | 1.07 (0.05) |
| Empirical formula (ash free db 3) | C100H196N10O66S | C48H79N2O17S | C297H504N18O169S |
| (VSWHDS/VSSY) | 1:0 | 4:1 | 3:2 | 2:3 | 1:4 | 0:1 |
|---|---|---|---|---|---|---|
| Bioreactor Designation | WHDS Only | Mix-1 | Mix-2 | Mix-3 | Mix-4 | SY Only |
| Parameter | ||||||
| WHDS (g wet basis) | 54.728 | 43.783 | 32.837 | 21.891 | 10.946 | 0.000 |
| SY (g wet basis) | 0.000 | 2.816 | 5.632 | 8.448 | 11.263 | 14.081 |
| Total carbon (g dry basis) | 1.725 | 1.537 | 1.349 | 1.161 | 0.974 | 0.786 |
| Total nitrogen (g dry basis) | 0.100 | 0.091 | 0.083 | 0.074 | 0.065 | 0.056 |
| carbon to nitrogen (C/N) ratio | 17.250 | 16.890 | 16.253 | 15.689 | 14.985 | 14.036 |
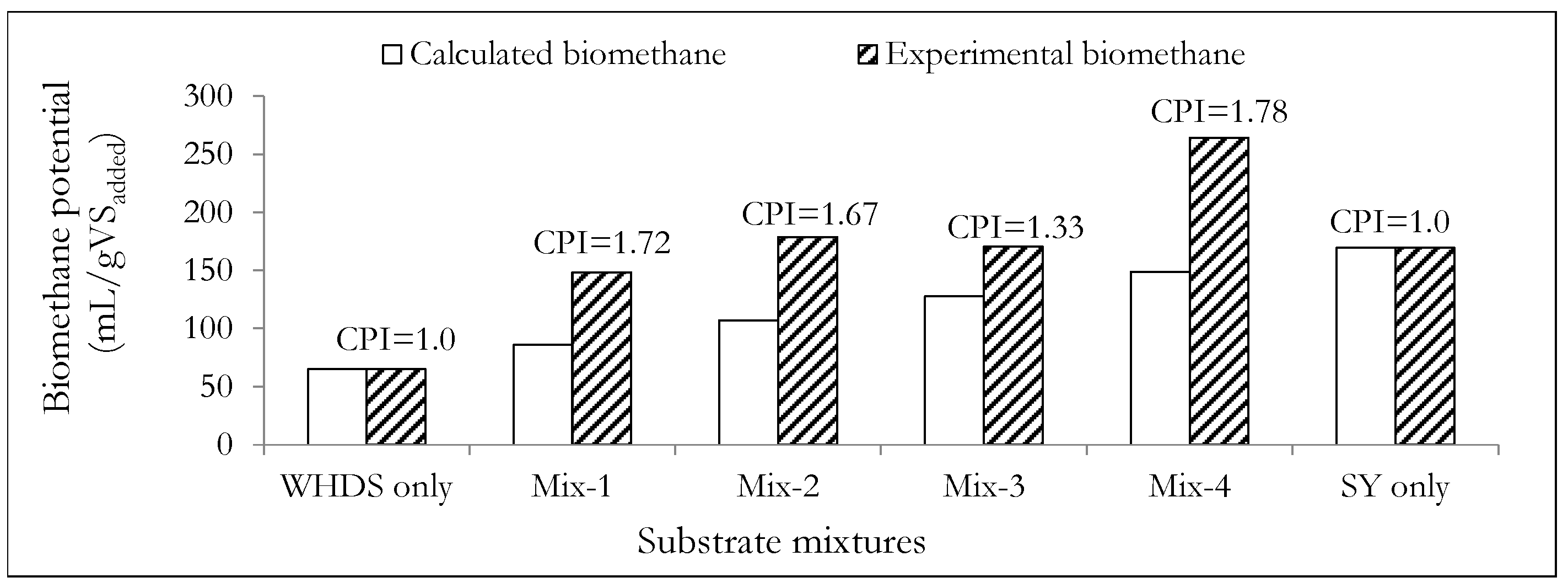
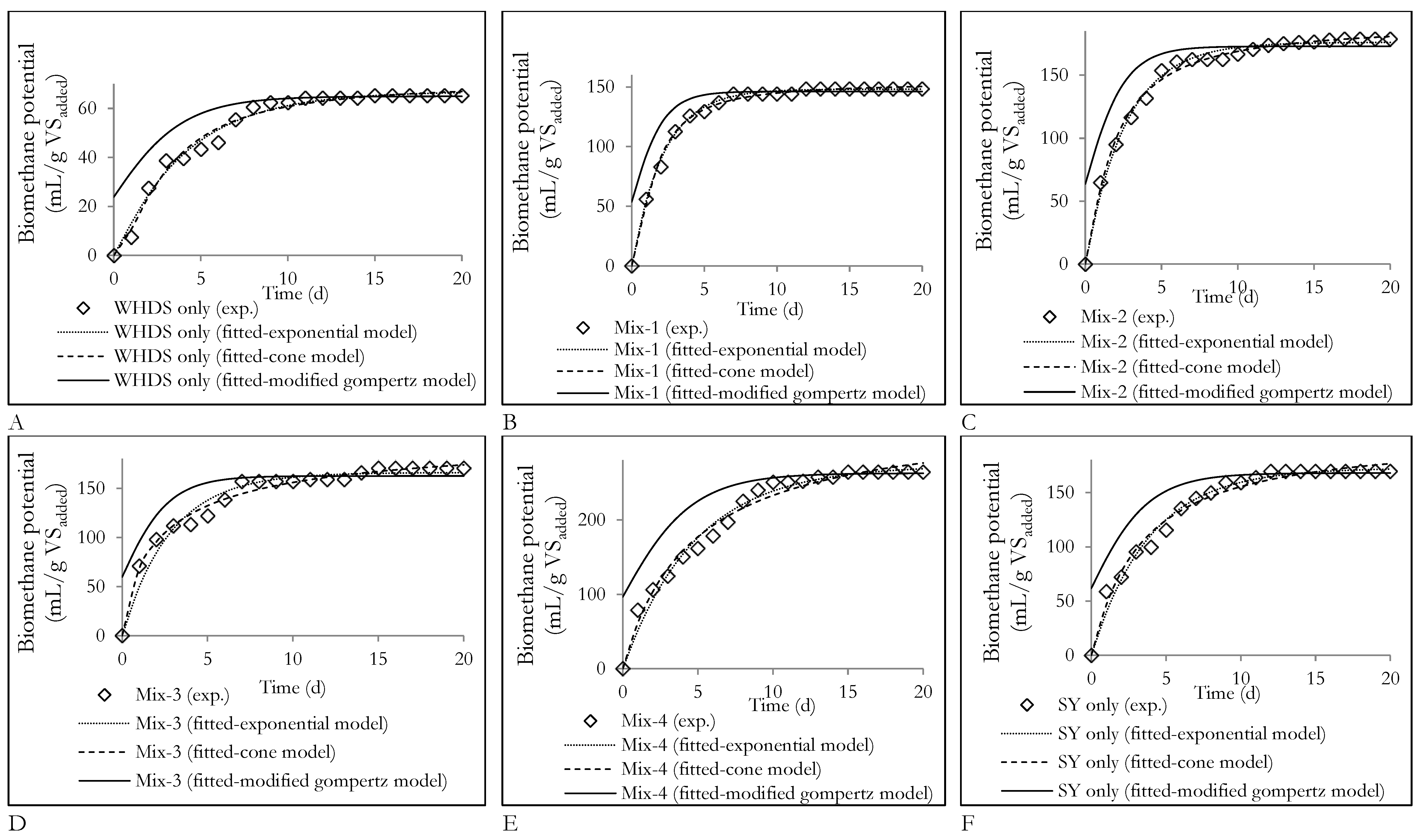
| VSWHDS: VSSY | Designation | Biomethane Potential (mL/gVSadded) 1 | Biomethane Content (vol.%) 1 |
|---|---|---|---|
| 1:0 | WHDS only | 65.43 | 56.5 |
| 4:1 | Mix-1 | 148.58 | 58.8 |
| 3:2 | Mix-2 | 179.03 | 61.1 |
| 2:3 | Mix-3 | 170.53 | 61.9 |
| 1:4 | Mix-4 | 264.13 | 61.2 |
| 0:1 | SY only | 169.64 | 61.5 |
| Substrate | Exponential (First Order) Model | Cone Model | Modified Gompertz Model | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bmax | k | λ | RMSE | R2 | Bmax | k | n | RMSE | R2 | Bmax | Rm | λ | RMSE | R2 | |
| WHDS only | 67.1 | 0.248 | 0.132 | 2.747 | 0.982 | 70.94 | 0.324 | 1.535 | 2.784 | 0.982 | 65.1 | 10.35 | 0.0 | 3.33 | 0.974 |
| Mix-1 | 148.3 | 0.448 | 0.0 | 2.017 | 0.997 | 153.5 | 0.648 | 1.530 | 2.791 | 0.995 | 146.6 | 42.13 | 0.0 | 4.96 | 0.984 |
| Mix-2 | 176.2 | 0.377 | 0.0 | 4.100 | 0.993 | 190.7 | 0.532 | 1.227 | 3.568 | 0.995 | 173.0 | 42.50 | 0.0 | 8.23 | 0.967 |
| Mix-3 | 166.1 | 0.355 | 0.0 | 8.508 | 0.962 | 206.0 | 0.417 | 0.798 | 5.417 | 0.985 | 162.6 | 37.77 | 0.0 | 12.99 | 0.912 |
| Mix-4 | 273.0 | 0.207 | 0.0 | 10.03 | 0.983 | 341.5 | 0.215 | 0.990 | 10.88 | 0.981 | 262.7 | 36.20 | 0.0 | 14.72 | 0.962 |
| SY only | 172.7 | 0.258 | 0.0 | 5.967 | 0.985 | 202.9 | 0.311 | 1.046 | 6.613 | 0.980 | 168.2 | 27.99 | 0.0 | 9.47 | 0.962 |
| Biogas Digestate | Value |
|---|---|
| Lipid content 1 (wt.%) | 1.60 (0.2) |
| Protein content 1 (wt.%) | 17.50 (0.09) |
| Ash content 1 (wt.%) | 39.53 (0.39) |
| Carbohydrate content 1 (wt.%) | 41.40 (0.44) |
| Carbon content 1 (wt.%) | 28.64 (0.10) |
| Nitrogen content 1 (wt.%) | 2.80 (0.02) |
| Sulphur content 1 (wt.%) | Trace |
| Hydrogen content 1 (wt.%) | 4.45 (0.07) |
| Oxygen content 1 (wt.%) | 24.60 (0.40) |
| pH value of wet digestate | 8.32 (0.32) |
| Bulk specific gravity at 20 °C | 1.02 (0.02) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okoro, O.V.; Sun, Z.; Birch, J. Prognostic Assessment of the Viability of Hydrothermal Liquefaction as a Post-Resource Recovery Step after Enhanced Biomethane Generation Using Co-Digestion Technologies. Appl. Sci. 2018, 8, 2290. https://doi.org/10.3390/app8112290
Okoro OV, Sun Z, Birch J. Prognostic Assessment of the Viability of Hydrothermal Liquefaction as a Post-Resource Recovery Step after Enhanced Biomethane Generation Using Co-Digestion Technologies. Applied Sciences. 2018; 8(11):2290. https://doi.org/10.3390/app8112290
Chicago/Turabian StyleOkoro, Oseweuba Valentine, Zhifa Sun, and John Birch. 2018. "Prognostic Assessment of the Viability of Hydrothermal Liquefaction as a Post-Resource Recovery Step after Enhanced Biomethane Generation Using Co-Digestion Technologies" Applied Sciences 8, no. 11: 2290. https://doi.org/10.3390/app8112290
APA StyleOkoro, O. V., Sun, Z., & Birch, J. (2018). Prognostic Assessment of the Viability of Hydrothermal Liquefaction as a Post-Resource Recovery Step after Enhanced Biomethane Generation Using Co-Digestion Technologies. Applied Sciences, 8(11), 2290. https://doi.org/10.3390/app8112290







