Featured Application
The right selection of plant species, mostly in the Mediterranean area, and their test on a simulated green roof is the way to improve the efficiency of green roofs. This study and the preliminary results derived will be a useful tool to improve the design of green roofs increasing the sustainability of this green infrastructure and its mitigation ability for pollution and water retention. Moreover, our preliminary data can be applied to the study of urban climate.
Abstract
Green roofs provide a number of environmental advantages like increasing urban biodiversity, reducing pollution, easing burdens on drainage systems, and lowering energy costs thanks to thermal insulation. Frankenia laevis, Dymondia margaretae and Iris lutescens were tested in a green roof installation. For all three species, we assessed two minimal irrigation treatments and one rain-fed treatment to resemble Mediterranean climate conditions analyzing the thermal and hydrological performance of all three species and their substrates through an evaluation of green cover, mortality, and biomass. The most influential factors registered for all three species are the relationship between air and water in the substrate and the interaction between green cover and substrate, respectively, for summer and winter seasons. In particular, D. margaretae preserved more water in its substrate than the other species both in summer and winter and after each rainfall event. F. laevis registered the highest level of variation in terms of substrate water content and of rainwater retention. I. lutescens achieved low hydrological performance, a limited amount of green cover, and slow growth. Our results suggest the absolute need of additional irrigation, managed in accordance with specific functional objectives, for all three species analyzed under Mediterranean conditions and different water regime.
1. Introduction
In recent years, there has been a remarkable increase in green roof use, and knowledge of the benefits that they can provide is now far more widespread. As known, green roofs can play a central role in ecological integration projects and in the spread of urban biodiversity [1,2,3,4,5,6]. Moreover, they help to regulate building temperatures, and their insulating properties help to save on energy costs. Energy savings and the positive effect on urban climate are not the only benefits provided, they have also absorbed rainwater, regulating the drainage process and its natural retention/recharge of water [7,8,9]. The amount of water that a green roof can absorb and recycle depends on a number of factors including climate, type of installation, and the composition of species present [9,10,11].
The plants, therefore, can protect the surface of buildings by absorbing a significant amount of solar radiation through photosynthesis, respiration and transpiration [12,13,14,15,16,17,18]. However, the plant structure and composition influence the performance of the green roof as heat insulation, protection by ultraviolet (UV) component, reduction of the heat island effect, and water retention [12,18,19,20]. It is important consider that a combination of different species is more effective than monocultures in favouring the thermal and hydrological properties of a green roof [20,21]. For this reason, many studies have attempted to understand how certain species influence the specific aims of a green roof, selecting and testing several species, different respect to Sedum genus, and in certain environments [4,22,23,24,25,26]. Several studies, instead, have focused the attention on the evaporation and photosynthesis activity of the species in order to define the thermal properties and energy performance of the green roof [27,28,29]. Moreover, a number of studies examined the relationship between different types of vegetation and rainwater retention in green roofs suggesting that the plants’ structural characteristics are influential in the interception and storage of rainwater [21,22]. These studies involved artificial rainfall and different types of vegetation, observing significant differences between amounts of rainwater runoff according to the type of vegetation. Species native to meadow habitats showed greater efficiency in reducing the flow of rainwater: Silene uniflora Roth and Anthoxanthum odoratum L. displayed the highest levels of rainwater runoff reduction with respect to the Sedum genus. Other studies investigated the relationship between roots and the water quantity in substrate [21,30,31,32,33,34,35] and not all of them agree. Scott MacIvor and Lundholm [33] observed that some species can hold large quantities of water in relation to the density of their fibrous roots, reducing the soil’s average porosity and its water retention capacity. Dunnet et al. [21], on the other hand, in their study on the retention of water after rainfall, observed that plant height and root biomass are important factors in enabling a higher degree of rainwater retention. On the other hand, Schroll et al. [32] concluded that rainwater retention in winter was not affected by vegetation, and recommended planting a higher percentage of mosses and plants from humid climates, which remain active for most of the autumn, winter, and spring, even if it is very difficult to establish mosses that generally come naturally with time.
Recently, some studies carried out in the Mediterranean area—characterized by sharp seasonal and daily temperature variations—have evaluated the benefits of green roofs [4,19,23,30,35,36,37,38,39]. These studies considered the thermal and hydrological effects related to prolonged drought and irregular rainfall as well as looking at the capacity for survival of different species. Indeed, the selection of wild species typical of the Mediterranean area is a focal point of these studies. The wildflowers are adapted to natural thermal and hydrological stress due the climate of this area so their application in a green roof favors the ecological sustainability of this green infrastructure but also the conservation of urban biodiversity.
Any attempt to predict the seasonal water retention capacity of a green roof is further complicated by the effects of climate change in the Mediterranean area, with more irregular rainfall and the increased intensity of single meteorological events [40]. In this context, it would also be worthwhile to understand how green roofs can reduce the effects of the extreme meteorological events which are becoming more and more common in this area.
Our study aims to evaluate the way that three plant species: Frankenia laevis L., Dymondia margaretae Compton and Iris lutescens Lam., with different structures and growth forms influence the thermal and hydrological properties of the substrate in a Mediterranean green roof applying two minimal irrigation treatments and one treatment without artificial irrigation.
2. Materials and Methods
2.1. Climatic Characterization
We carried out the study between June 2009 and May 2010 in Caldes de Montbui (41°63′ N 2°16′ E), 205 meters above sea level and 30 Km (18.6 miles) north of Barcelona (Spain), along the Catalonian coastal mountain range. We used climatic data furnished by Caldes de Montbui weather station [41], placed 150 m from the green roof simulation.
This area present typical Mediterranean climate. The annual mean of temperature and rainfall, during 1991–2010, are 14.43 °C and 599.3 mm with January as the coldest month with 6.7. The maximum rainfall means results for September (89.1 mm), whereas the minimum mean value of evapotranspiration (ET0) correspond to December (16.5 mm). July was the hottest month with 23.5°C, and the driest month with 24.7 mm of rainfall, and with the maximum mean ET0 values (130.5 mm).
In particular, over the trial period, the mean rainfall and ET0 correspond respectively to 640 mm and 925 mm. Temperature monthly average varied between 6.1 °C and 25.1 °C, and rainfall monthly average varied between 5.2 mm and 125.2 mm, with ET0 levels between 20.4 mm and 131.0 mm. January and March presented minimum temperatures to −8 °C. During the summer months, ET0 values were high and precipitations low, with 135 mm of Et0 and 10.7 mm of precipitation in June. By contrast, August presented similar ET0 value (135.5 mm) but 27.4 mm rainfall value, concentrated in one single downpour.
2.2. Green Roof System
We created the green roof simulation using the Zinco® standardized system reproducing characteristic conditions found in a typical green roof. We structured a container of one square meter with substrate depth of 11 ± 1 cm, and the whole height of structure approximately 15 cm. We lined this with an SSM45 polypropylene synthetic fibre protective water retention membrane with a water retention capacity of approximately 5 L/m2. We covered the container with ZinCo Products [42]. The substrate had an apparent density of 0.85 (g/cm3), and a total porosity of 66%. PH values varied between 7.95 and 8.08, and electrical conductivity was between 158.4 and 194.2 μS/cm (microSiemens/cm).
2.3. Plant Material
Taking into account our previous study [43], we selected three different species: Dymondia margaretae Compton, Frankenia laevis L., and Iris lutescens Lam. (Table 1). We planted three species in different containers with a density of nine plants per square meter. We replicated each plot three times, once for every irrigation treatment, obtaining nine plots (Figure 1).

Table 1.
The selected species, their common English names, native habitats, and growth forms.
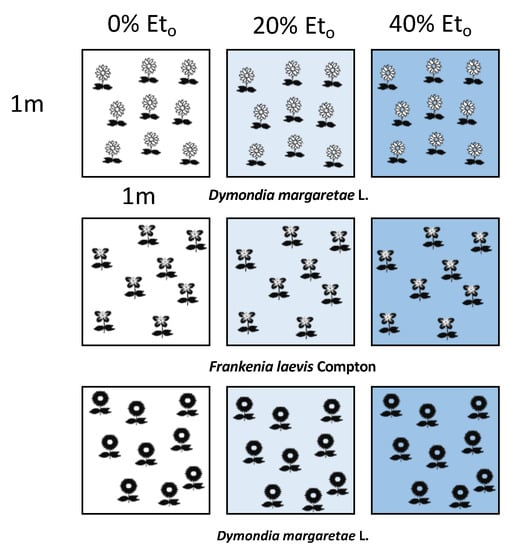
Figure 1.
The experimental design: 9 plots of 1 m2, each one with 9 plant of each species and for each irrigation treatment.
2.4. Irrigation System
In order to simulate the Mediterranean conditions, it has been applied two minimal irrigation treatments replacing 20%, and 40% of potential ET0, and one treatment receiving only natural rainwater (0% ET0). The ET0 levels used to calculate irrigation were obtained from the Caldes de Montbui meteorological station. Irrigation doses were recalculated each week by subtracting total rainfall from the average ET0 of the previous seven days (ET0–rainfall), of which 20% and 40% was used for the two treatments. The doses were distributed at regular intervals throughout the week.
Before laying the plants, we calibrated the entire structure over a period of several days measuring the distribution capacity of randomly chosen drips, obtaining an irrigation uniformity coefficient of 0.9. The 40% and 20% ET0 plots received, respectively, a total of 216 mm and 108 mm of water over the study period, 73% of which was distributed during the summer. Total rainfall throughout the summer was 281 mm, with an accumulated ET0 value of 332 mm. Irrigation was not provided during the winter months due to frequent rainfall. Moreover, we placed and calibrated a capacitive sensor (ECO 5 Decagon, Pullman, WA, USA) and a temperature sensor (EC-T5, Decagon, Pullman, WA, USA) in the substrate of each plot. We located all sensors horizontally at a depth of 7/8 cm, and we connected them to a central apparatus (Campbell CR1000, Campbell Sci, Shepshed, Leicestershire, UK) which recorded incoming data every 20 min. Finally, we calculated the water holding capacity according to standardized methodology: The substrate was water saturated by immersion and after drainage, water holding capacity was obtained measuring the water in tank, the difference between original value and this remaining water; it can be assumed as maximum water capacity storage in the substrate. In parallel water capacity was measured by means of soil water content sensors.
2.5. Measurements’ Design
For each species we recorded the following parameters: volumetric water content (VWC; θ m3/m−3), as the maximum amount of water absorbable by the substrate; relative extractable water (REW), as the amount of substrate water available to the plants; rainwater retention capacity; maximum–minimum average substrate temperatures; green cover; final root and aerial biomasses.
To evaluate the maximum water holding capacity of the substrate we subtract the field capacity (VWC field capacity) by the lowest water content registered during the whole trial period (minimum VWC). Later, we used this result to obtain the amount of REW according to the equation defined by Granier [44] and Fernández et al. [45]:
REW = (actual VWC − minimum VWC)/(maximum VWC − minimum VWC) × 100
In calculating the rainwater holding capacity of the substrate we looked at five different types of rainfall as classified by AEMET. We selected as ‘very heavy’ the rainfall event of 14 September 2009; as ‘heavy’ the rainfall events of 9 August 2009 and 20 September 2009; as ‘moderate’ the rainfall events of 8 February 2010 and 7 March 2010. For each of these rainfall types we looked at the VWC variation over a period of 24 h before the rainfall and immediately afterwards, when the substrate was saturated. We also calculated water content variation in the saturated substrate over a period of 60 h following rainfall. Holding capacity values were expressed as L/m2 by multiplying the water volume (cm3/cm−3) by the substrate depth of 110 mm.
EcH2O probes estimate the substrate water content by indirectly measuring the dielectric substrate permittivity. A relationship between substrate water content and dielectric permittivity was estimated in the laboratory following Nemali et al. [46]. To obtain a range of water contents from dry to near saturation, different volumes of deionized water were added to the substrate and mixed thoroughly to obtain uniformity. The weight of the container with substrate and different water contents was taken and a sensor was inserted, and dielectric permittivity was measured according to Topp [47]. Finally, the daily changes of substrate water content were calculated from data recorded each 10 min.
To calculate the insulation capacity of the substrate and plants in high temperature conditions, we looked at the experimental hottest period, since July to September, and compared average maximum substrate temperatures for each species and each irrigation treatment plot (0%, 20%, 40%) with average maximum air temperatures. Later, to evaluate insulation capacity in conditions of low temperatures we compared daily minimum substrate and air temperatures during the coldest period, from November to March. Finally, we compared the average minimum temperature values for each species in all irrigation treatment plots with air temperatures to evaluate the differences.
We measured the plant cover photographically with a Nikon EOS 500 also. Later, we processed the images with Greenpix digital analysis software according to Casadesús et al. [48,49]. Finally, to calculate final biomass, we removed all plants from the soil and we separated the upper sections (leaves and stems) from the roots, before washing them carefully. In this way, we located all resulting material in an oven at 65 °C until it was at a constant weight.
2.6. Statistical Analysis
We based our experiment on a factorial design with following factors: irrigation, species, block and data collection times only in the vegetation cover analysis. Irrigation factor had 3 levels (40%, 20% and 0%), species factor had 3 levels (Dimondia, Frankenia and Iris) and block factor (3). We analysed the data by analysis of variance (ANOVA) and mean separation using the Proc Mixed procedures in SAS version 9.2 (SAS Institute Inc. Cary, NC, USA) with irrigation and species as a fixed factor and block and collection time as a random factor. We performed a post hoc test, by the Tukey Kramer method, to determine significant differences between irrigation treatments and collection dates. We carried out all analyses with the SAS 9.2 software. Finally, we compared the average values of VWC, REW, and temperature, using the Tukey–Kramer HSD test and JMP 10 software.
3. Results
3.1. Volumetric Water Content (VWC), Relative Extractable Water (REW) and Rainwater Holding Capacity
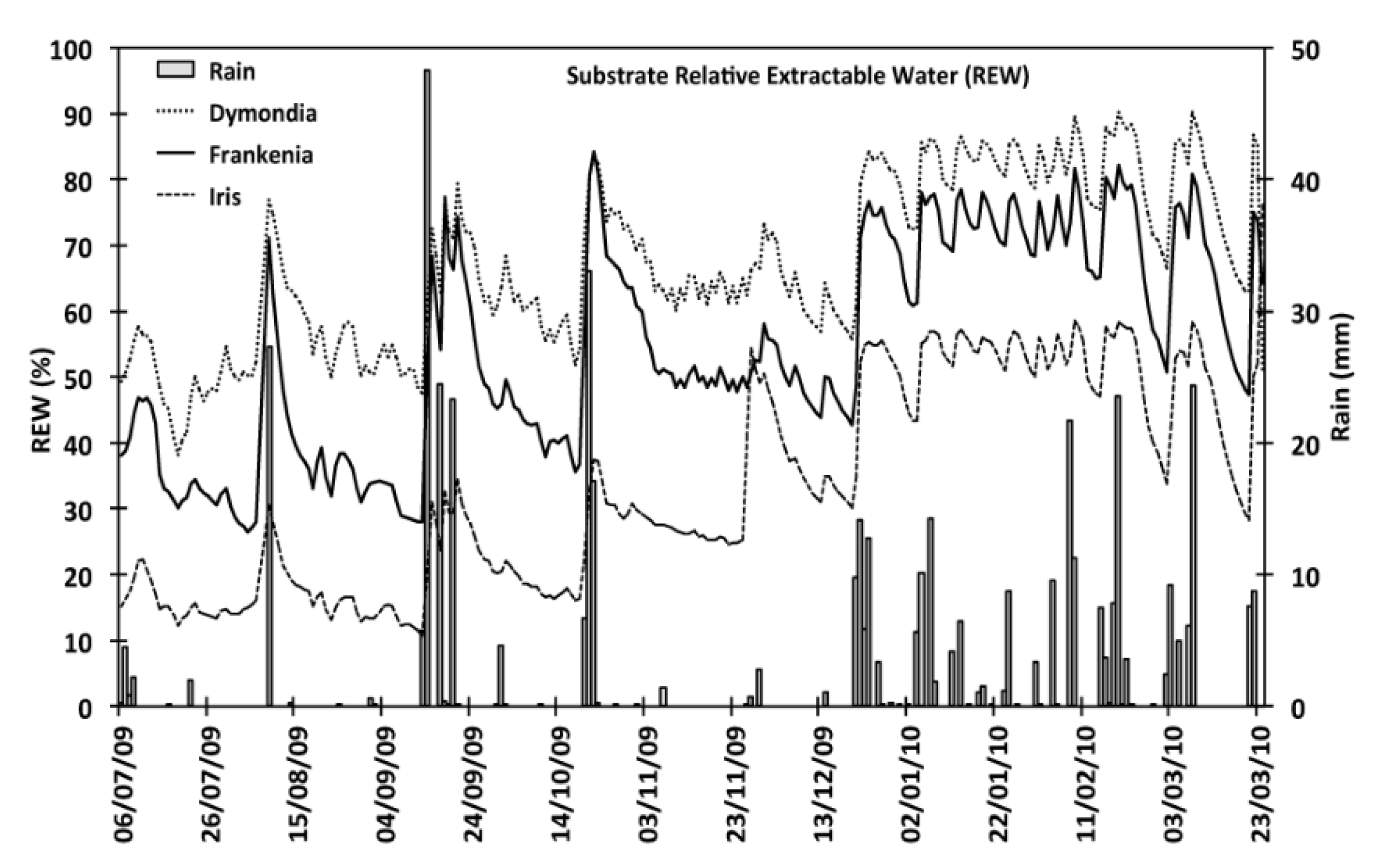
The differences of the water content in three treatments (VWC) and for the three species resulted statistically siginificant with (p < 0.05). The water content (VWC) values of D. margaretae substrate (15.5%) was significantly higher than F. laevis (12.5%) and I. lutescens (7.8%) substrates. In this way, the seasonal evaluation confirms these results indeed the summer VWC values were 13.5% for D. margaretae, 10.1% for F. laevis and 4.6% for Iris lutescens; from November to March they were 17%, 14.5% and 10.3% (Figure 2).
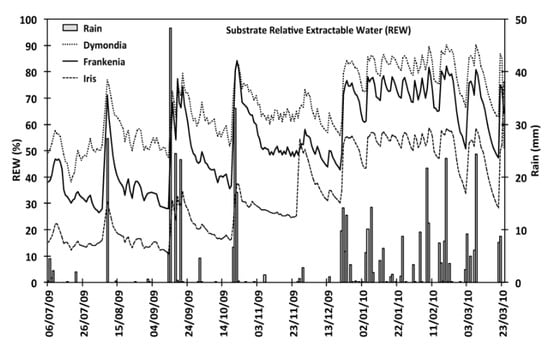
Figure 2.
Relative extractable water (REW) of the substrate from July to March for all irrigation treatments in each species.
Overall average values for relative extractable water (REW) highlighted in Figure 1 showed that D. margareta’s substrate contained more water, 38% in summer and 63% in winter. The REW value of F. laevis were 26% in summer and 52% in winter. Finally, those of I. lutescens’s substrate varied between 10% in summer and 28% in winter.
Our data shows (Table 2) variations in substrate volumetric water content for each species before and after rainfall events, when gravitational water (as water contained in the macropores) had drained away, 60 h after the rain had ended. These results indicate a greater degree of variation after the August and September rains than after rainfall in winter. F. laevis was the species with the widest variations.

Table 2.
Variation of substrate water content after different rainfall events (volumetric water content (VWC) before–VWC after) and between field capacity and 60 h after the rainfall events in each species.
3.2. Soil Temperature
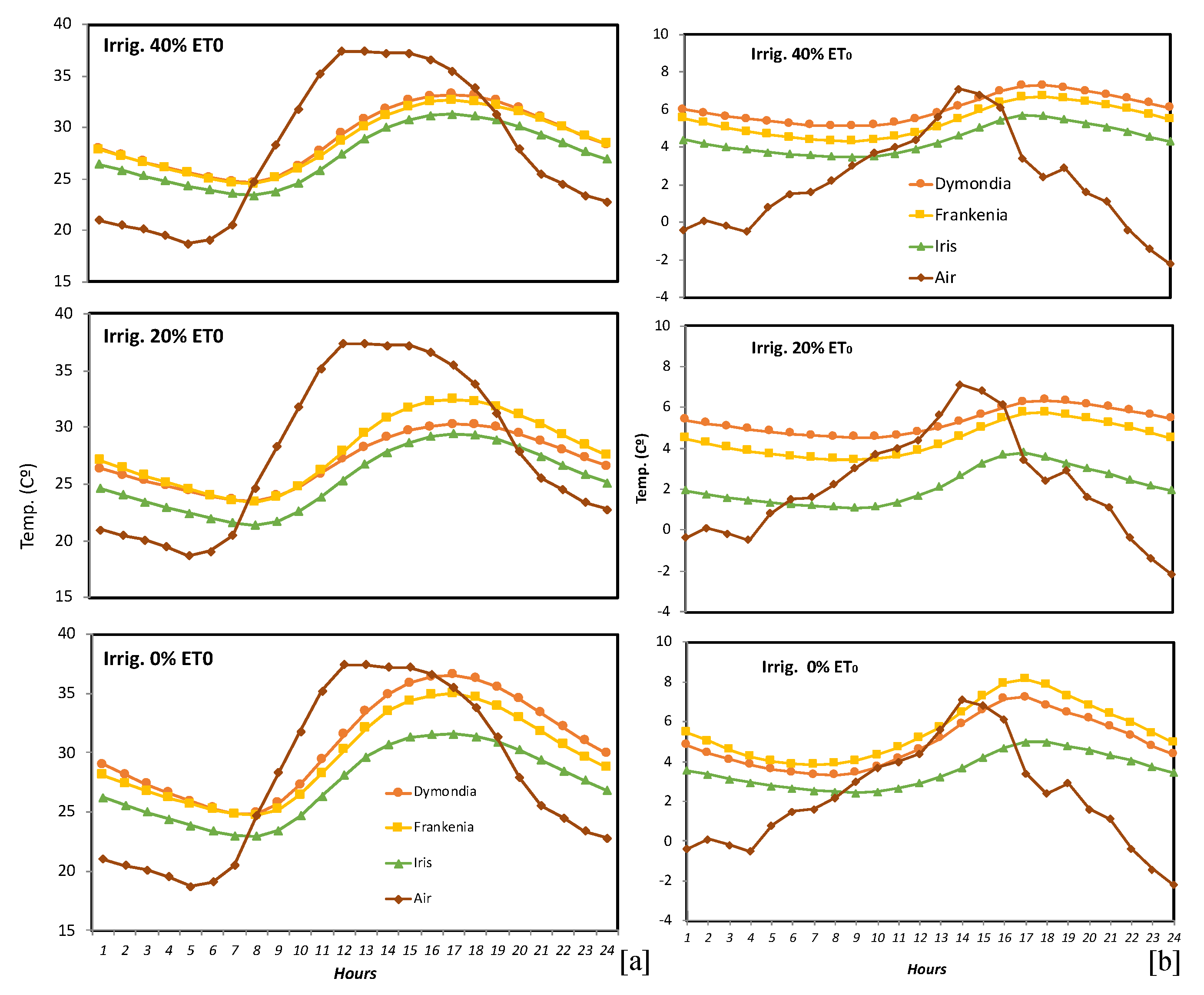
The average hourly air and substrate temperature variations in the three irrigation treatments of each of the three species during a typical day (17 August 2009; Figure 3a). Air temperatures underwent an oscillation of 18.7 °C over the course of one day; in the irrigated substrates temperatures oscillated between 6.8 and 9 °C, and in the non-irrigated substrate oscillation was between 8.6 and 11.7 °C. Maximum substrate temperature usually occurred 4/5 h after maximum air temperature. The hourly minimum temperatures throughout a typical winter’s day (17 December 2009, Figure 3b), in the 40% ET0 treatment varied between 5 and 10 °C, in the 20% ET0 treatment they varied between 2.5 and 9 °C, and in the non-irrigated treatment they were between 4.2 and 10.8 °C. On the same day, air temperatures varied between 1.7 and 13 °C.
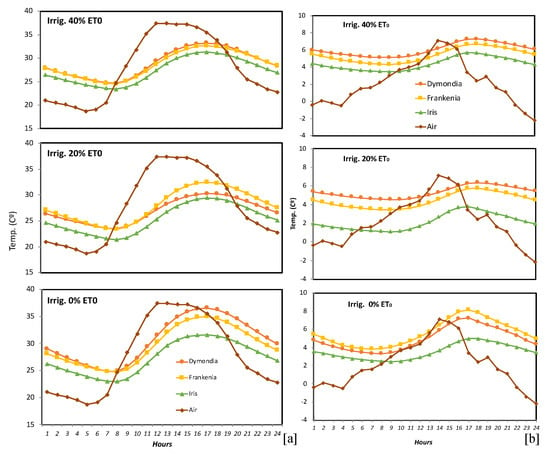
Figure 3.
Hourly air and substrate temperatures for all three irrigation treatments of D. margaretae, F. laevis, and I. lutescens: (a) on a typical summer’s day (17 August 2009); (b) on a typical winter’s day (17 December 2009).
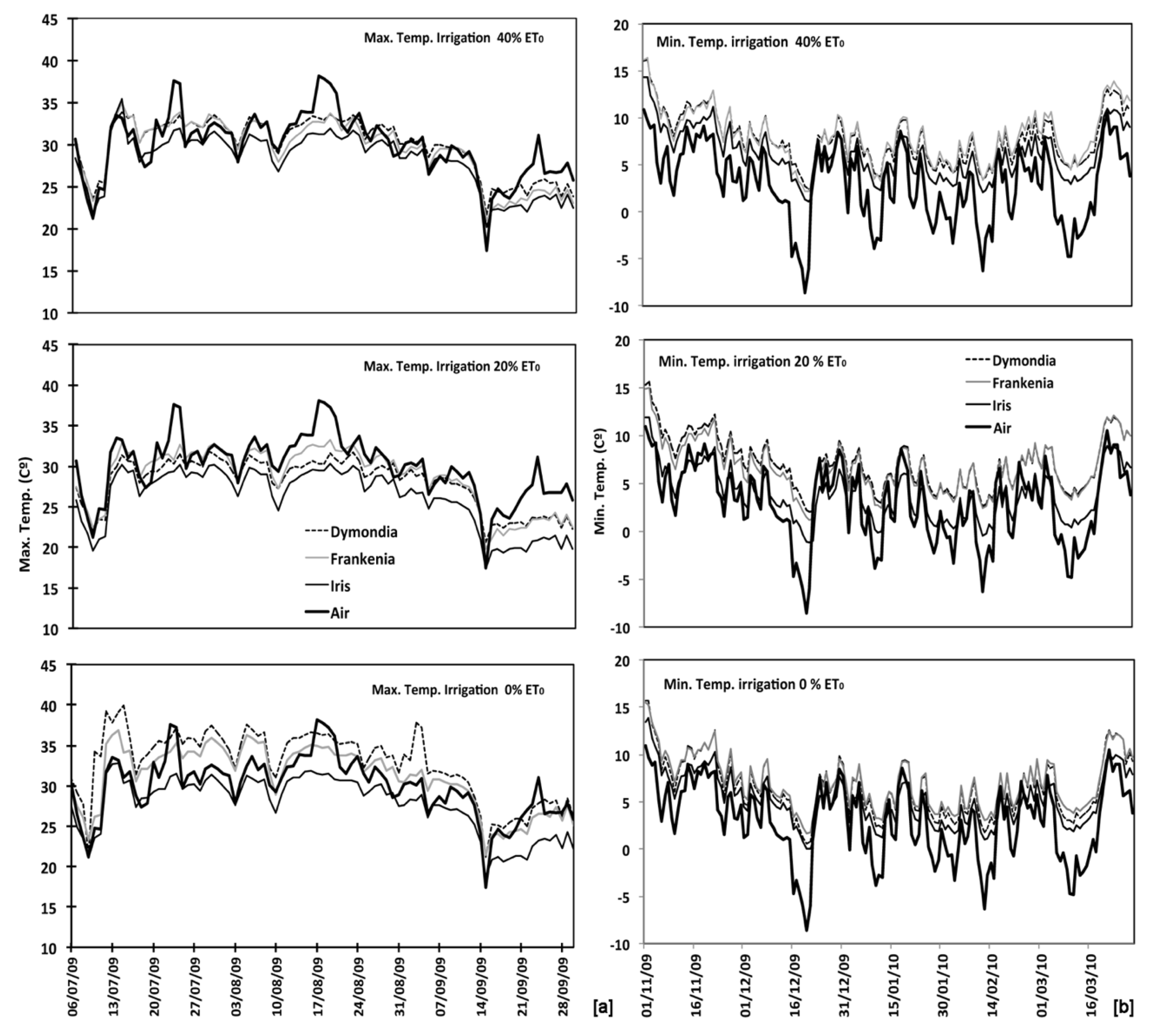
The average daily maximum temperature during the summer season resulted not significantly different from average air temperature for all species and irrigation treatments. In particular, the maximum substrate temperature, in the summer season (Figure 4a, Table A1), of the non-irrigated plot of D. margaretae and F. laevis resulted significantly higher than irrigated substrates, and they were also higher than the air temperature respectively of 2.8 °C and 1.1 °C. By contrast, the substrate temperature of 20% ET0 plots for both species were significantly lower than air temperature respectively of 1.9 °C and 1.2 °C. The two species displayed similar behaviour in the 40% ET0 plot didn’t presenting significant differences between air and substrate temperatures. The average maximum daily substrates’ temperature of Iris lutescens plots for all irrigation treatments was significantly lower than air temperature of 3.8 °C in 20% ET0 plot; 1.7 °C in the 40% ET0 plot, and 1.2 °C in the non-irrigated plot. The relationship between maximum air temperatures and substrate temperatures for all three species and each irrigation treatment resulted statistically significant (r > 0.2540 por n > 100 α = 0.01).
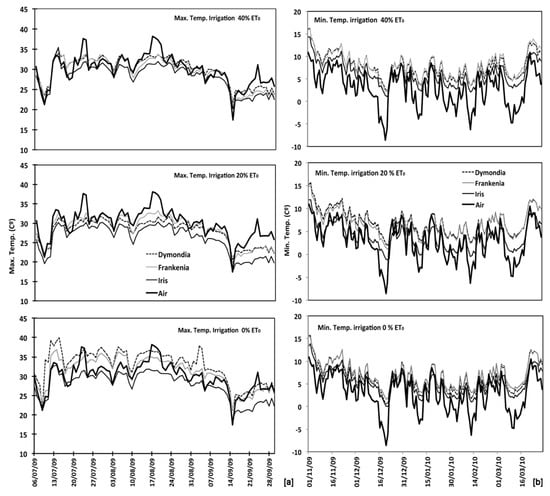
Figure 4.
Temperature soil recorded for each species and irrigation treatments (0–20–40% ET0) in relation to air temperature: (a) maximum temperatures since July to September; (b) minimum temperatures since November to March.
The average daily minimum temperature during the winter season for all three species and all irrigation treatments (Figure 4b) resulted significantly different from average air temperature. For D. margaretae, the substrate’s temperature resulted higher than air temperature for all plots: 4.8 °C for 40% ET0; 4.2 °C for 20% ET0 plot, and 3.6 °C in the non-irrigated substrate. Moreover, there were significant differences between the 40% ET0 treatment and the non-irrigated treatment. In this way, also F. laevis the substrate in 40% ET0 plot was warmer of 5 °C, in the 20% ET0 plot of 4 °C, and the non-irrigated substrate of 3.7 °C. There were no significant differences between the 20% ET0 substrate and the non-irrigated substrate, but they both showed significant differences with respect to the 40% ET0 substrate. At last, temperatures in substrates planted with I. lutescens that were irrigated with 40% ET0 and with 0% ET0 were significantly higher than air temperatures, by 3.2 °C and 2.2 °C respectively. The temperature of the substrate irrigated with 20% ET0 was 0.8 °C higher than air temperature, constituting a not significant difference. In the same way, the comparison between minimum air temperatures and minimum substrate temperatures during the same period. In all cases the difference was significant (r > 0.2540 por n > 100 α = 0.01).
3.3. Plant Development: Vegetation Cover and Biomass
Total biomass was greater for the irrigated than for the not-irrigated plots. Upper biomass measurements for two species displayed significant differences between the two irrigation treatment, while only one species showed significant differences between irrigation treatments for total (aerial + root) biomass (Table 3).

Table 3.
Means of dry weight (g) × m2 of the tested species in the three irrigation protocols.
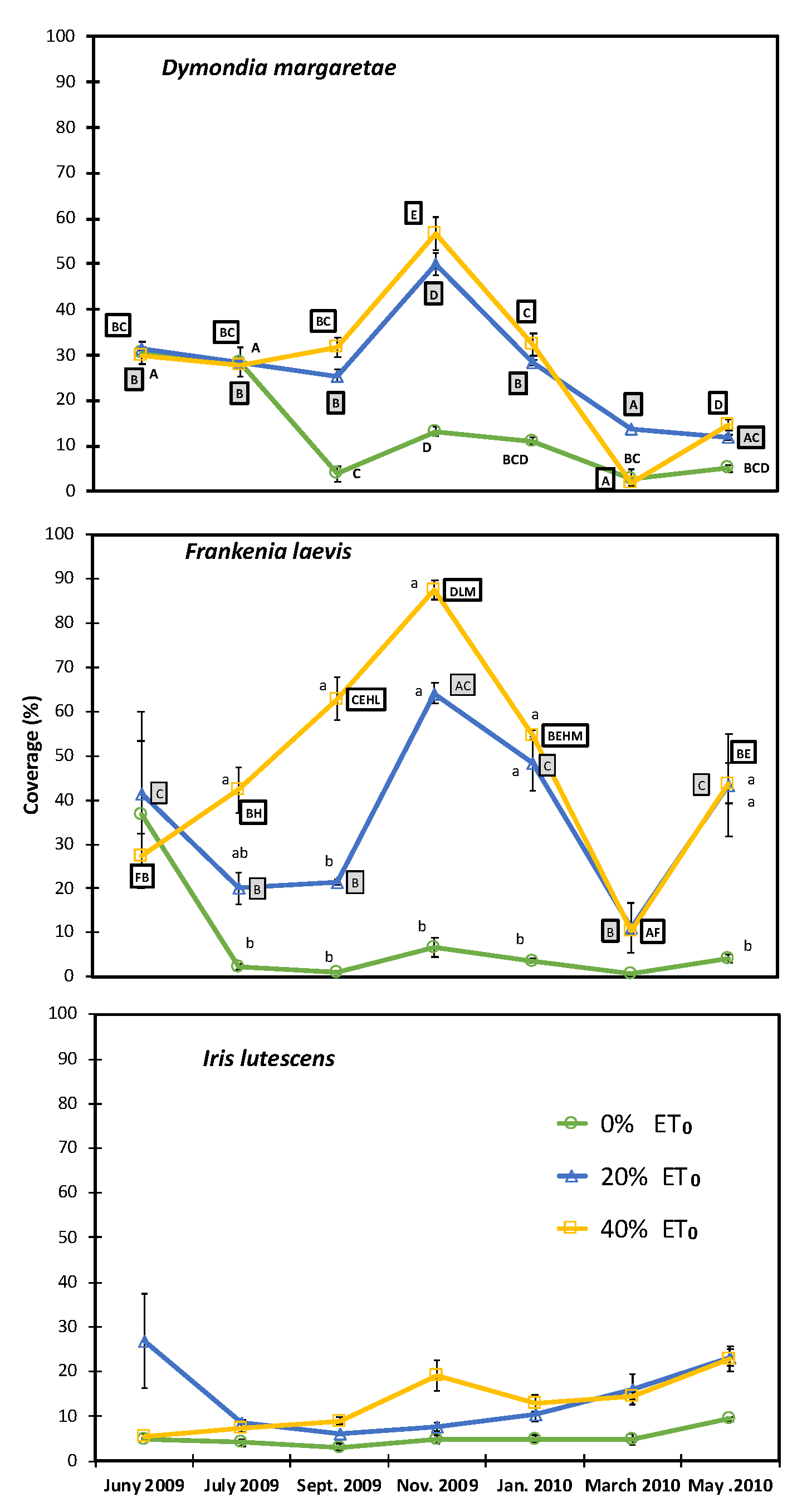
The irrigated plots containing D. margaretae and F. laevis reached their highest level of cover in November and the lowest in March, due to the late arrival of cold snaps in the same month (Figure 5). Both species saw a rise in cover as the irrigation result. The plants that were not irrigated showed the lowest levels of surface cover (>10%). I. lutescens tended to develop slowly and constantly without the seasonal variations that characterised the other two species (Figure 3), and without significant differences between treatments and between different time periods.
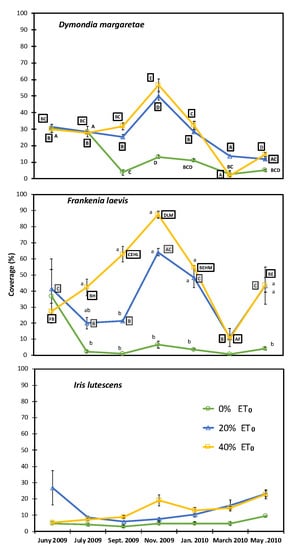
Figure 5.
Annual vegetation cover for each species and irrigation treatments (0–20–40% ET0). Capital letters indicate significant differences throughout the trial period for the same treatment. Lowercase letters indicate significant differences between irrigation treatments in the same month.
4. Discussion
Our results demonstrate that the substrate contained different levels of volumetric water content (VCW) according to species, suggesting that the plants’ seasonal development and biomass structures were the primary cause of these differences [21,33]. We also found that biomass alone is not sufficient to explain the behavior of various species, although it is important to understand the biomass distribution. The water content in substrate of D. margaretae was higher than other species in both season due to the compact nature of its roots and leaves, limiting also the evaporation process of the substrate. This species has a compact low-growing structure, developing leaves and roots on the substrate, and it is the only species whose root biomass was greater than its upper parts. F. laevis, a semi-shrub species, has very thin roots occupying all available substrate. Its more complex rooting stem structure allowed the substrate to store less water than D. margaretae, suggesting that it consumed a larger amount of water. Therefore, the biomass of I. lutescens was mainly concentrated in its large rhizomes, developing principally in the spring and summer. The substrate of this species stored the least water amount. We suggest that root biomass, improving the soil structure, helps in the rainwater conservation and using different plant combinations makes it possible to achieve specific results according to the context in which a green roof is built. In a Mediterranean climate, D. margaretae’s ability to keep humidity in the substrate above minimum levels could be a positive sign with regard to the survival of vegetation on green roofs.
Substrate water content variations before and immediately after rainfall, could also be described as the soil’s recharge capacity. Our study confirms that, this recharge capacity varied seasonally between summer and winter rainfall events, and in relation to the species. Moreover, in our work, two rainfall events occurring in the hottest period, although different in intensity, resulted in similar substrate humidity variations, confirming that the soil’s storage capacity changed according to the season [50]. In accordance with Spolek [7], our study found that average water content was higher in the winter, leaving the substrate with a reduced capacity for storage during the rainiest period of the year.
Humidity variations in the substrate 60 h after a rainfall event can supply us with information about what happens to the water stored in the soil. Comparing the data, F. laevis (semi-shrub) > D. margaretae (groundcover) > I. lutescens (Rhizomatous-herbaceous). The highest variations occurred in F. laevis, suggesting that water consumption is linked to vegetation cover and structural complexity and that this species can be a good performing species in a well irrigated green roof having a high level of water consumption [51].
In all treatments, mainly in irrigated ones, interaction between air and water in the substrate seems to be the factor that most affected thermal isolation in our green roof simulations during summer. Our experiment confirmed the findings of Del Barrio [52] and Kannelloupoulou [53], in that we noted a significant relationship between maximum air temperature and maximum substrate temperature, with the lowest line equation slope values in the irrigated plots. The importance of water content is further demonstrated by the fact that temperatures in a non-irrigated plot were higher than the air temperatures of two irrigated plots. Although many studies have suggested that vegetation cover and leaf structure are important factors in reducing the temperatures of a green roof [39,52,53,54], our study highlighted that the behaviour of the species was not sufficiently linear. We did, however, observe that daily substrate temperature fluctuations were different for irrigated plants and not irrigated (which showed higher temperatures). Generally, the non-irrigated plant substrates displayed higher temperatures and lower insulation values. In a Mediterranean climate, the energy consumption of air-conditioning units in summer can be higher than the winter heating systems [7]. However, our study showed that the green roof offered more insulation from low temperatures than it did protection from heat according to the findings of Ekşi and Uzun [19] in Istanbul. All plots highlighted a reduction in temperature range, especially towards the lower end of the scale. An analysis of the number of days on which substrate temperature was lower than air temperature for all three species, taking into account the non-irrigation in winter, suggested that the plant structure and biomass influence thermal insulation. Indeed, the most efficient insulation was observed in the plants treated with 40% ET0, which had the greatest biomass. I. lutescens, in all three treatments, was the species which offered the least insulation from cold temperatures and was also the species with the most compact structure and which offered the least cover. In this way, D. margaretae, in the 0% ET0 treatment, displayed no measurable biomass and, therefore, achieved the worst results in terms of insulation.
5. Conclusions
D. margaretae achieved a higher water content level in the substrate than other species both in summer and winter and after all rainfall events. F. laevis displayed high water consumption and its ability to reduce water runoff in Mediterranean green roofs. Of the three species, I. lutescens came last with regard to hydrological performance, so it is recommendable to use it only in combination with other species.
A balanced interaction among substrate, water, and species is important in order to soften the effects of temperature extremes in the environment. The relationship between air and water in a substrate was a more influential factor during warmer periods. During the coldest period, on the other hand, the most influential factor was the interaction vegetation cover-substrate: the best results were achieved by the species with the highest biomass and cover values.
The results of our study suggest that finding one single species that offers an all-round optimal performance is not simple, and it is important, therefore, to use a flexible approach when planning a green roof, and combine species whose properties complement each other. In a Mediterranean climate, it may be necessary to choose between limiting the extreme effects of storm water and reducing energy consumption. Our findings suggest that the species in our study should only be used in a green roof when supplementary irrigation is available.
Author Contributions
A.V., R.S. and C.B. conceived and designed the experiments; A.V. performed the experiments; A.V., R.S. and C.B. analyzed the data; A.V. and F.B. wrote the paper.
Acknowledgments
This research was funded by the Spanish government for financial assistance received through the MINECO, IMPACTO, and CUMED projects. We are also grateful for technical support in the field and the laboratory provided by the IRTA’s Marc Ferrer, Laia Serra, Cristian Morales, Beatriz Grau, and Inma Funes. We would also like to thank Roberto Casalini and Justin Bradshaw for their precious advice and help in revising the text of this article.
Conflicts of Interest
We wish to draw the attention of the Editor to the following facts which may be considered as potential conflicts of interest and to significant financial contributions to this work. We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome. We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us. We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property. We understand that the Corresponding Author is the sole contact for the Editorial process (including Editorial Manager and direct communications with the office). He is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs. We confirm that we have provided a current, correct email address which is accessible by the Corresponding Author and which has been configured to accept email from flavia.bartoli@uniroma3.it.
Appendix A

Table A1.
The daily data of Maximum (From July to September) and the Minimum (From November to March) values of substrate’s Temperatures in relation to the air temperature.
Table A1.
The daily data of Maximum (From July to September) and the Minimum (From November to March) values of substrate’s Temperatures in relation to the air temperature.
| Days | D. margaretae | F. laevis | I. lutescens | Air | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 40% ET0 | 20% ET0 | 0% ET0 | 40% ET0 | 20% ET0 | 0% ET0 | 40% ET0 | 20% ET0 | 0% ET0 | ||
| Maximum temperatures since July to September | ||||||||||
| 06/07/09 | 30 | 27 | 31 | 30 | 27 | 30 | 28 | 26 | 28 | 30,6 |
| 13/07/09 | 33 | 30 | 38 | 33 | 31 | 36 | 34 | 29 | 33 | 33,5 |
| 20/07/09 | 32 | 29 | 35 | 32 | 31 | 33 | 29 | 28 | 29 | 32,9 |
| 27/07/09 | 32 | 30 | 36 | 32 | 32 | 34 | 31 | 29 | 30 | 31,8 |
| 03/08/09 | 30 | 28 | 32 | 29 | 28 | 32 | 28 | 26 | 28 | 27,9 |
| 10/08/09 | 29 | 27 | 31 | 28 | 27 | 29 | 27 | 25 | 27 | 29,3 |
| 11/08/09 | 31 | 29 | 34 | 29 | 29 | 31 | 28 | 26 | 29 | 31 |
| 17/08/09 | 33 | 30 | 37 | 33 | 32 | 35 | 31 | 29 | 32 | 38,1 |
| 24/08/09 | 33 | 31 | 35 | 32 | 31 | 34 | 31 | 29 | 30 | 33,7 |
| 31/08/09 | 30 | 28 | 32 | 29 | 29 | 31 | 28 | 26 | 27 | 28,9 |
| 07/09/09 | 30 | 29 | 32 | 30 | 29 | 31 | 28 | 26 | 28 | 28,7 |
| 14/09/09 | 26 | 24 | 26 | 25 | 24 | 26 | 24 | 21 | 23 | 24,1 |
| 21/09/09 | 25 | 23 | 26 | 24 | 22 | 25 | 23 | 20 | 21 | 26,1 |
| 28/09/09 | 24 | 22 | 26 | 23 | 23 | 26 | 22 | 20 | 22 | 26,8 |
| Minimum temperatures since November to March | ||||||||||
| 01/11/09 | 16 | 15 | 16 | 16 | 15 | 15 | 14 | 12 | 13 | 11 |
| 16/11/09 | 11 | 10 | 10 | 10 | 9 | 10 | 9 | 6 | 8 | 6 |
| 01/12/09 | 7 | 7 | 5 | 7 | 6 | 6 | 5 | 3 | 4 | 1 |
| 16/12/09 | 5 | 4 | 2 | 4 | 3 | 3 | 3 | 1 | 2 | −5 |
| 31/12/09 | 9 | 9 | 9 | 10 | 9 | 9 | 8 | 6 | 7 | 8 |
| 15/01/10 | 7 | 6 | 6 | 7 | 6 | 6 | 5 | 2 | 4 | 2 |
| 30/01/10 | 6 | 5 | 4 | 6 | 5 | 5 | 4 | 2 | 3 | 2 |
| 31/01/10 | 5 | 4 | 3 | 5 | 4 | 4 | 3 | 1 | 2 | 0 |
| 14/02/10 | 5 | 4 | 3 | 5 | 4 | 4 | 3 | 1 | 2 | −2 |
| 01/03/10 | 8 | 7 | 7 | 9 | 7 | 7 | 6 | 4 | 5 | 3 |
| 16/03/10 | 6 | 5 | 4 | 7 | 5 | 5 | 4 | 2 | 3 | −1 |
References
- Brenneisen, S. Space for urban wildlife: Designing green roofs as habitats in Switzerland. Urban Habitats 2006, 4, 27–36. [Google Scholar]
- Carter, T.; Butler, C. Ecological impacts of replacing traditional roofs with green roofs in two urban areas. Cities Environ. 2008, 1, 1–17. [Google Scholar] [CrossRef]
- Lundholm, J.; Peck, S.W. Introduction: Frontiers of green roof ecology. Urban Ecosyst. 2008, 11, 335–337. [Google Scholar] [CrossRef]
- Benvenuti, S. Wildflower green roofs for urban landscaping, ecological sustainability and biodiversity. Landsc. Urban Plan. 2014, 124, 151–161. [Google Scholar] [CrossRef]
- Berardi, U.; GhaffarianHoseini, A.; GhaffarianHoseini, A. State-of-the-art analysis of the environmental benefits of green roofs. Appl. Energy 2014, 115, 411–428. [Google Scholar] [CrossRef]
- Vijayaraghavan, K. Green roofs: A critical review on the role of components, benefits, limitations and trends. Renew. Sustain. Energy Rev. 2016, 57, 740–752. [Google Scholar] [CrossRef]
- Spolek, G. Performance monitoring of three eco-roofs in Portland, Oregon. Urban Ecosyst. 2008, 11, 349–359. [Google Scholar] [CrossRef]
- Stovin, V. The potential of green roofs to manage. Urban Stormwater. Water Environ. J. 2010, 24, 192–199. [Google Scholar] [CrossRef]
- Berndtsson, J.C. Green roof performance towards management of runoff water quantity and quality: A review. Ecol. Eng. 2010, 36, 351–360. [Google Scholar] [CrossRef]
- Mentens, J.; Raes, D.; Hermy, M. Green roofs as a tool for solving the rainwater runoff problem in the urbanized 21st century? Landsc. Urban Plan. 2005, 77, 217–226. [Google Scholar] [CrossRef]
- Uhl, M.; Schiedt, L. Green roof storm water retention–monitoring results. In Proceedings of the 11th International Conference on Urban Drainage, Edinburgh, UK, 31 August–5 September 2008. [Google Scholar]
- Santamouris, M. Cooling the cities—A review of reflective and green roof mitigation technologies to fight heat island and improve comfort in urban environments. Sol. Energy 2014, 103, 682–703. [Google Scholar] [CrossRef]
- Liu, K.; Baskaran, B. Thermal Performance of Extensive Green Roofs in Cold Climates. In Proceedings of the 2005 World Sustainable Building Conference, Tokyo, Japan, 27–29 September 2005; Available online: http://www.nrc-cnrc.gc.ca/obj/irc/doc/pubs/nrcc48202/nrcc48202.pdf (accessed on 15 March 2010).
- Spala, A.; Bagiorgas, H.S.; Assimakopoulos, M.N.; Kalavrouziotis, J.; Matthopoulos, D.; Mihalakakou, G. On the green roof system. Selection, state of the art and energy potential investigation of a system installed in an office building in Athens, Greece. Renew. Energy 2008, 33, 173–177. [Google Scholar] [CrossRef]
- Desjarlais, A.O.; Zaltash, A.; Atchley, J.A. Thermal Performance of Vegetative Roofing Systems. In Proceedings of the 25th RCI International Convention, Orlando, FL, USA, 25–30 March 2010. [Google Scholar]
- Niachou, A.; Papakonstantinou, K.; Santamouris, M.; Tsangrassoulis, A.; Mihalakakou, G. Analysis of the green roof thermal properties and investigation of its energy performance. Energy Build. 2001, 33, 719–729. [Google Scholar] [CrossRef]
- Susca, T.; Gaffin, S.R.; Dell’Osso, G.R. Positive effects of vegetation: Urban heat island and green roofs. Environ. Pollut. 2011, 159, 2119–2126. [Google Scholar] [CrossRef] [PubMed]
- Blanusa, T.; Vaz Monteiro, M.M.; Fantozzi, F.; Vysini, E.; Li, Y.; Cameron, R.W.F. Alternatives to Sedum on greenroofs: Can broad leaf perennial plants offer better ‘cooling service’? Build. Environ. 2013, 59, 99–106. [Google Scholar] [CrossRef]
- Ekşi, M.; Uzun, A. Investigation of thermal benefits of an extensive green roof in Istanbul climate. Sci. Res. Essays 2013, 8, 623–632. [Google Scholar] [CrossRef]
- Stovin, V.; Poë, S.; De-Ville, S.; Berretta, C. The influence of substrate and vegetation configuration on green roof hydrological performance. Ecol. Eng. 2015, 85, 159–172. [Google Scholar] [CrossRef]
- Dunnett, N.; Nagase, A.; Booth, R.; Grime, P. Influence of vegetation composition on runoff in two simulated green roof experiments. Urban Ecosyst. 2008, 11, 385–398. [Google Scholar] [CrossRef]
- Nagase, A.; Dunnett, N. Amount of water runoff from different vegetation types on extensive green roofs: Effects of plant species, diversity and plant structure. Landsc. Urban Plan. 2011, 104, 356–363. [Google Scholar] [CrossRef]
- Van Mechelen, C.; Dutoit, T.; Hermy, M. Mediterranean open habitat vegetation offers great potential for extensive green roof design. Landsc. Urban Plan. 2014, 121, 81–91. [Google Scholar] [CrossRef]
- Sendo, T.; Kanechi, M.; Uno, Y.; Inagaki, N. Evaluation of growth and green coverage of ten ornamental species for planting as urban rooftop greening. J. Jpn. Soc. Hortic. Sci. 2010, 79, 69–76. [Google Scholar] [CrossRef]
- Caneva, G.; Kumbaric, A.; Savo, V.; Casalini, R. Ecological approach in selecting extensive green roof plants: A dataset of Mediterranean plants. Plant Biosyst. 2015, 149, 374–383. [Google Scholar] [CrossRef]
- Lundholm, J.; Williams, N.S.G. Effects of Vegetation on Green Roof Ecosystem Services. In Green Roof Ecosystems; Sutton, R.K., Ed.; Springer: Cham, Switzerland, 2015; Volume 9, pp. 211–232. [Google Scholar]
- Feng, C.; Meng, Q.; Zhang, Y. Theoretical and experimental analysis of the energy balance of extensive green roofs. Energy Build. 2010, 42, 959–965. [Google Scholar] [CrossRef]
- Jaffal, I.; Ouldboukhitine, S.E.; Belarbi, R. A comprehensive study of the impact of green roofs on building energy performance. Renew. Energy 2012, 43, 157–164. [Google Scholar] [CrossRef]
- Kumar, R.; Kaushik, S.C. Performance evaluation of green roof and shading for thermal protection of buildings. Build. Environ. 2005, 40, 1505–1511. [Google Scholar] [CrossRef]
- Casalini, R.; Bartoli, F.; Caneva, G. Investigation of seed germination of twelve Mediterranean wildflowers for evaluating their potential use on extensive green roofs. Acta Hortic. 2017, 1189, 263–266. [Google Scholar] [CrossRef]
- Wolf, D.; Lundholm, J.T. Water uptake in green roof microcosms: Effects of plant species and water availability. Ecol. Eng. 2008, 33, 179–186. [Google Scholar] [CrossRef]
- Schroll, E.; Lambrinos, J.G.; Sandrock, D. An Evaluation of Plant Selections and Irrigation Requirements for Extensive Green Roofs in the Pacific Northwestern United States. HortTechnology 2011, 21, 314–322. [Google Scholar]
- Scott MacIvor, J.; Lundholm, J. Performance evaluation of native plants suited to extensive Green roof conditions in a maritime climate. Ecol. Eng. 2011, 37, 407–417. [Google Scholar] [CrossRef]
- Nardini, A.; Andri, S.; Crasso, M. Influence of substrate depth and vegetation type on temperature and water runoff mitigation by extensive green roofs: Shrubs versus herbaceous plants. Urban Ecosyst. 2012, 15, 697–708. [Google Scholar] [CrossRef]
- Raimondo, F.; Trifilò, P.; Lo Gullo, M.A.; Andri, S.; Savi, T.; Nardini, A. Plant performance on Mediterranean green roofs: Interaction of species-specific hydraulic strategies and substrate water relations. AoB Plants 2015, 7, plv007. [Google Scholar] [CrossRef]
- Fioretti, R.; Palla, A.; Lanza, L.G.; Principi, P. Green roof energy and water related performance in Mediterranean climate. Build. Environ. 2010, 45, 1890–1904. [Google Scholar] [CrossRef]
- Pérez, G.; Coma, J.; Solé, C.; Castell, A.; Cabeza, L.F. Green roofs as passive system for energy savings when using rubber crumbs as drainage layer. Energy Procedia 2012, 30, 452–460. [Google Scholar] [CrossRef]
- Zinzi, M.; Agnoli, S. Cool and green roofs. An energy and comfort comparison between passive cooling and mitigation urban heat island techniques for residential buildings in the Mediterranean region. Energy Build. 2012, 55, 66–76. [Google Scholar] [CrossRef]
- Olivieri, F.; Di Perna, C.; D’Orazio, M.; Olivieri, L.; Neila, J. Experimental measurements and numerical model for the summer performance assessment of extensive green roofs in a Mediterranean coastal climate. Energy Build. 2013, 63, 1–14. [Google Scholar] [CrossRef]
- IPCC. Climate Change. Five Assessment Report. 2013. Available online: http://www.ipcc.ch/publications_and_data/publications_and_data_reports.shtml-1 (accessed on 10 January 2009).
- Available online: http://www.ruralcat.net/web/guest/agrometeo.estacions (accessed on 10 January 2009).
- ZinCo 2010. Retrieved January 22 2010. Available online: http://www.zincogreenroof.com/EN/downloads/pdfs/ZinCo_Product_List.pdf (accessed on 20 December 2008).
- Vestrella, A.; Savé, R.; Biel, C. An Experimental Study in Simulated Greenroof in Mediterranean Climate. J. Agric. Sci. 2015, 7, 95–111. [Google Scholar] [CrossRef]
- Granier, A. Evaluation of transpiration in a Douglas-fir stand by means of sap flow measurements. Tree Physiol. 1987, 3, 309–320. [Google Scholar] [CrossRef]
- Fernández, J.E.; Moreno, F.; Girón, I.F.; Blázquez, O.M. Stomatal control of water use in olive tree leaves. Plant Soil 1997, 190, 179–192. [Google Scholar] [CrossRef]
- Nemali, K.S.; Montesano, F.; Dove, S.K.; van Iersel, M.W. Calibration and performance of moisture sensors in soilless substrates: ECH2O and Theta probes. Sci. Hortic. 2007, 112, 227–234. [Google Scholar] [CrossRef]
- Topp, G.C. State of the art of measuring soil water content. Hydrol. Process. 2003, 17, 2993–2996. [Google Scholar] [CrossRef]
- Casadesús, J.; Biel, C.; Savé, R. Turf color measurement with conventional digital cameras. In Proceedings of the EFITA/WCCA Joint Congress on It in Agriculture, Vila Real, Portugal, 25–28 July 2005. [Google Scholar]
- Casadesús, J.; Kaya, Y.; Bort, J.; Nachit, M.M.; Araus, J.L.; Amor, S.; Ferrazzano, G.; Maalouf, F.; Maccaferri, M.; Martos, V.; et al. Water Use Efficiency. Using vegetation indices derived from conventional digital cameras as selection criteria for wheat breeding in water-limited environments. Ann. Appl. Biol. 2007, 150, 227–236. [Google Scholar] [CrossRef]
- Carson, T.B.; Marasco, D.E.; Culligan, P.J.; McGillis, W.R. Hydrological performance of extensive green roofs in New York City: Observations and multi-year modeling of three full-scale systems. Environ. Res. Lett. 2013, 8, 1–13. [Google Scholar] [CrossRef]
- Farrell, C.; Szota, C.; Williams, N.S.G.; Arndt, S.K. High water users can be drought tolerant: Using physiological traits for Greenroof plant selection. Plant Soil 2013, 372, 177. [Google Scholar] [CrossRef]
- Del Barrio, E.P. Analysis of the green roofs cooling potential in buildings. Energy Build. 1998, 27, 179–193. [Google Scholar] [CrossRef]
- Kanellopoulou, K. Cooling performance of green roofs. In Proceedings of the PLEA 2008–25th Conference on Passive and Low Energy Architecture, Dublin, Ireland, 22–24 October 2008. [Google Scholar]
- Wong, N.H.; Cheong, D.K.V.; Yan, H.; Soh, J.; Ong, C.L.; Sia, A. The effects of rooftop garden on energy consumption of a commercial building in Singapore. Energy Build. 2003, 35, 353–364. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).