1. Introduction
A review of the previous research regarding the dryout of bottom-heated saturated porous media shows, by the large number of published papers, the remarkable interest of researchers in this phenomenon over recent decades. However, there is a lack of publications related to the dryout/replenishment phenomena in porous media with an overlying liquid layer limited by a free surface.
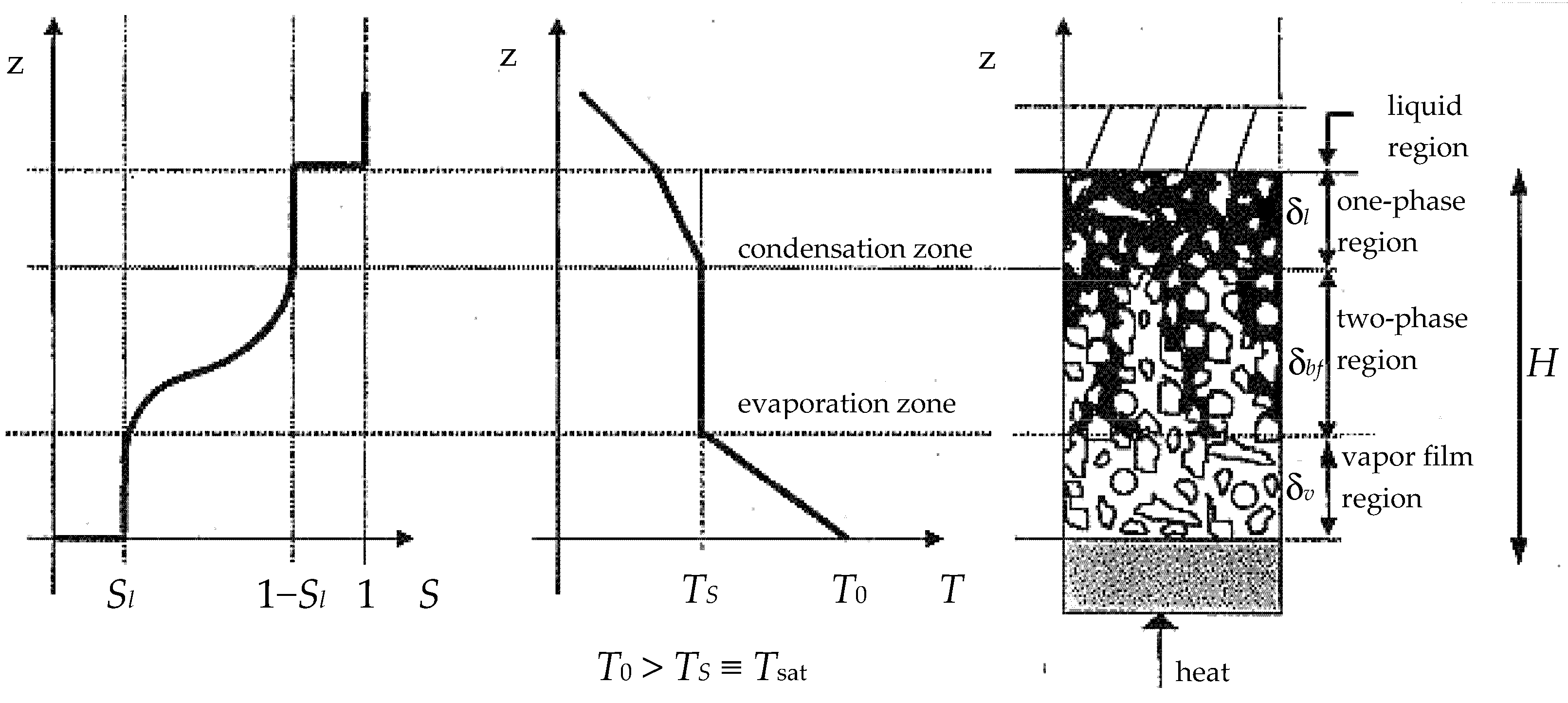
Dryout following boiling in porous media occurs in many engineering applications, such as geothermal systems, heat pipes, and post-accident analyses of liquid-cooled nuclear reactors. Since uncertainties exist about many aspects of the boiling process, experimental studies intend to elucidate the heat transfer process from the heating surface to the porous medium. A thin liquid layer in contact with the heating surface, a vapor layer, and a layer with both regions—vapor and liquid—can exist during this process. Experimental studies report the formation of an isothermal two-phase zone above the bottom whenever the temperature there exceeds saturation.
The dryout condition requires accurate knowledge of how the vapor condenses on the capillary surface of the two-phase region in the porous media, and how the interface of the two-phase plain liquid saturated regions moves upward. In this regard, the works of the researchers Leverett [
1], Philip and DeVries [
2], Ecker and Faghri [
3], Su [
4], Stemmelen et al. [
5], Shahraeeni [
6,
7] and many others have contributed to better knowledge of the aforementioned phenomena.
The transport of heat from the liquid–vapor countercurrent in a two-phase liquid layer region was studied by Sondergeld and Turcotte [
8]. When boiling occurs, an almost isothermal two-phase zone forms near the bottom. Bau and Torrance [
9] reported experimental observations of boiling in a vertical circular cylinder heated from below and cooled from above. A simple one-dimensional model is used to predict the high of this zone, the necessary condition for its formation, and the dryout heat flux.
The effects of gravity, capillarity, multiphase flow, and phase change in a fluid-filled porous media were studied by Udell [
10,
11] by means of one-dimensional analysis considering steady-state conditions. If a critical heat flux is exceeded, a vapor zone will occur at the heated surface. In this study, Udell established the criteria for determining the heat flux at the dryout.
The dryout heat flux in particulate beds heated through the base has also been studied. Jones et al. [
12], in a laboratory study, discussed the role of capillary forces in bed dryout. Then, Ramesh and Torrance [
13] reviewed the interaction between boiling and convection phenomena in a fluid-saturated porous layer heated from below and cooled from above. They used a stability diagram to provide a qualitative understanding of the experimental results.
With regards to model boiling and dryout in particle beds, and concurrent with the experimental research, significant efforts have been made to develop understanding in this area. The aim of most of these models is to predict the incipient dryout power, namely the necessary power level to cause the appearance of a dry area in the bed. Dhir and Catton [
14] presented a model based on their experiments. A step forward was made by Hardee and Nilson [
15] when they considered the combined resistances of liquid and vapor flow and developed their dryout criterion based on the conservation laws for mass, momentum, and energy. Shires and Stevens [
16] extended the Hardee-Nilson model to include the effect of capillary forces and Lipinski [
17,
18] went further in the same direction, assuming a capillary force and using the relative permeability to express two-phase friction. For the dryout in channeled porous beds, the modeling efforts for determining the heat flux refer only to shallow beds. Most of the related studies, such as those by Jones et al. [
12], Dhir and Catton [
14], and Dhir and Barleon [
19], involved bottom-heated cases.
On the other hand, the studies of heat transfer, boiling, and dryout in porous media, with and without channels and surfactant addition to the saturating liquid phase, are limited. Tzan and Yang [
20], Carbonell [
21] and Cheng et al. [
22] have studied the effects of surfactant solutions on critical heat flux under boiling conditions.
The work presented in this paper is also intended to study the replenishment phenomenon of the porous media occurring immediately after dryout. The discharge of liquid from the overlaying layer to the porous medium should be interpreted as a result of the gravitational instability of a layer of water on steam. This interface instability has attracted much attention in the scientific community because of its wide applicability in the fields of geothermal reservoirs. The papers published by Schubert and Straus [
23], Eastwood and Spanos [
24], Pestov [
25], Hager and Whitaker [
26], and Tsypkin and Il’icher [
27] are examples.
Finally, the thermal instability in a porous medium of a non-Newtonian fluid is also attracting much attention. As a recent example, Celli et al. [
28] presented a two-dimensional analysis of thermal convective instability of a power-law fluid flowing in a horizontal porous layer. They compare and contrast the theoretical results with experimental work.
The aim of this paper is to elucidate the influence of the physical properties of both phases—solid matrix and saturating liquid—of bottom-heated porous media with an overlying plain water layer. Then, the main contribution of this work is in theoretical and experimental studies that lead us to a better understanding of the particle diameter and surface tension roles; in particular, the roles that they play in the thermal state evolution on the dryout and stability of the system water layer-vapor region of porous medium heated from below with an overlying water layer limited by a free surface.
4. Discussion
4.1. Heating Process to Dryout-Replenishment
Figure 4 shows that the temperature distribution in the bronze powder porous media is very different from the temperature distribution in sand porous media. Moreover, the influence of the nature of the saturating liquid in sand porous media is very important.
To clarify the causes of such differences, the value of the Rayleigh number and the Bond number were calculated for the temperature difference between the ends of the porous medium when the heating plate reaches the temperature of 100 °C. (
Table 4): Δ
T = 70 °C for B+DS and B+W, and Δ
T = 35 °C in the S+DS and S+W). The uncertainty values of Rayleigh and Bond numbers shown in
Table 4, as in the rest of tables, were obtained by propagation of error analysis according to Coleman and Steele [
30].
The values obtained from the Rayleigh number for the porous media B+W and B+DS, are much lower than the limit for instability to appear, set at 27.10 according to Nield and Bejan [
31]. Nevertheless, the values for the sand-based porous media significantly exceed the limit of instability; consequently, the process of heat transfer in this medium is conduction driven by convection/advection, while in the former it is practically by conduction (Fourier). On the other hand, the value of the Bond number is less than 1 in all porous media; therefore, the flow is dominated by capillarity.
A more detailed analysis allows us to elucidate that the process of heating the porous media consists of several stages with notable differences between them. For this purpose, the temperature ratios of the thermocouples T5/T6 and T4/T6 are depicted in
Figure 6.
By observing
Figure 6, the process of heating to dryout-replenishment is characterized by several thermic stages. The peculiarities of each stage are as follows:
Stage A–B. Heating of the porous medium essentially by non-stationary conduction (B+DS), or by conduction/convection/non-stationary advection (S+DS) until the surface temperature of the heating plate reaches 100 °C.
Stage B–C. Progressive increase of the temperature in the porous medium until boiling occurs at the plate-porous medium interface, keeping the plate temperature constant at approximately 102 °C.
Stage C–D. Boiling extends throughout the porous medium, which is maintained at a constant temperature slightly higher than 100 °C until the beginning of the dryout. Subsequently, continuous rising of the temperature of the plate above 100 °C occurs while the porous medium keeps its temperature slightly higher than 100 °C.
Stage D–E–F. For the sand-based porous media only, the relative evolution of the temperature porous medium-plate when the dryout-replenishment occurs is seen. From the beginning of the process to the end of the CD stage, displacement of liquid from the porous medium to the tray takes place. Liquid displacement results in the formation of a two-phase zone in a porous medium.
4.2. Heat Transfer from the Heating Plate to the Porous Medium
The magnitude of the temperature difference in the heating plate interface (T6) and the porous medium (T5) informs us about the process of heat transfer. In the boiling stage the temperature difference is 2 °C in the S+DS, and approximately 3 °C in the B+DS. At the end of this stage, the dryout starts in the S+DS and finishes with a temperature difference in the interface of 14 °C. A pronounced temperature rise occurs at the plate, then the B+DS is kept boiling. This is possibly due to the formation of a vapor layer with very low thermal conductivity at the interface.
The calculation of the heat transfer between the heating plate and the porous medium has been carried out by the energy balance method, according to the following equation:
where
is the power transferred by the heating plate to the porous medium,
is the electrical power supplied to the heating plate and
is the power dissipated for the heating of the plate. This power is calculated by the equation:
where
A is the area of the surface of the plate in contact with the porous medium,
e is its thickness,
ρ and
cp are the density and the specific heat of the aluminum, and
is the temperature variation per unit of time at the point
z of the plate and instant
t of the heating process.
Figure 7 shows the results obtained for all the porous media studied throughout the experimentation.
4.3. Liquid Evaporation from the Tray
For the calculation of the evaporation rate of the liquid phase in the free surface of the tray, the equation proposed by Shah [
32] was adopted:
where
is the evaporation rate,
ρw and
Ww are the density and specific humidity of the air at the saturated water temperature, and
ρr and
Wr are the density and specific humidity of the air at ambient temperature and humidity.
The calculation of
involves knowing the water temperature at the free surface. To carry out this calculation, the method developed by Chu and Goldstein [
33] has been applied. The method derives a relationship between the temperature distributions in dimensionless form
and a dimensionless distance
, where
z is the distance to the bottom of the liquid tray at temperature
Tz. Moreover,
δ is the thickness of the boundary layer as
, while
hc is the water layer thickness and the Nusselt number has the following expression in function of the Rayleigh number:
The value of ΔT corresponding to the thickness of the liquid layer in the tray (2, 3, 3, 4 and 5 cm) has been calculated by a successive approximation procedure from the value of the Rayleigh number Ra < 1708, which ensures a stable regime, and from the value of the temperature of the liquid at the bottom of the tray, measured by thermocouple 1 (T1). The results obtained that the entire liquid layer is practically at the same temperature as the one measured by thermocouple 1, corresponding to the bottom of the tray.
Once the value of this temperature is known, the evaporation rate can be calculated by means of Equation (3). From these values, the evaporative volumetric rate
is calculated and, afterwards, the volume of liquid evaporated
Vev versus time by the integral:
4.4. Total Volume of Liquid Displaced from Porous Medium to the Tray
The real total volume (referred to as the initial state) of liquid displaced from each porous medium during the heating process,
Vdt, is the sum of the accumulated volume in the tray
Vac (calculated from the limnimeter measurements) and the evaporated volume,
Vev, subtracting the volume due to thermal expansion,
Vet, using (
Figure 9):
4.5. Power Required for the Evaporation of the Total Volume of Liquid
Assuming that the total volume of liquid displaced from the porous medium per unit of time is previously evaporated at the interface of the plate and porous medium, the required thermal power is expressed as:
At all times, the values of
t,
, and thus
and
are known. The value of
is calculated from the function
. Having neglected the term
because it is much smaller than
, the use of Equation (7) in relation to the porous media B+DS and S+DS gives the results shown in
Figure 10.
In
Figure 10, the curves
of
Figure 6 have also been drawn to identify the intervals of
t in which the thermal power transferred to the porous medium by the heating plate,
, is lower than that required for the evaporation of the total volume of liquid displaced from the porous medium per unit of time.
The thermal power required to evaporate the total volume of liquid displaced per unit of time is much higher than the power available during practically all the heating time of the porous medium. This means that the displacement of liquid is almost entirely due to a piston effect created by capillary action.
The vapor generated by the thermal power that the plate transfers at each instant to the porous medium at the interface produces an increase in the pressure in the two-phase region. This pressure promotes the displacement of liquid from the saturated region until, by expansion, it reaches a new state of equilibrium. This displacement occurs as soon as the following condition is satisfied:
where
pc is the capillary pressure,
ecs is the thickness of the saturated layer,
h is the height of the liquid layer in the tray, and Δ
pR is the resistance to flow in the capillary defined by Rumpf-Gupte’s law.
4.6. Power Required for the Evaporation of the Liquid in the Tray
The power required at each instant to evaporate liquid from the tray is calculated by the equation:
where
Acb is the area of the free surface of the liquid in the tray, and ė is defined by Equation (3).
Figure 11 shows the results.
4.7. Heating the Porous Media
The heat accumulated by the porous medium at time t, once the temperature field is known, is very difficult to determine due to the following reasons: it is a non-stationary process, specific heats of both phases are a function of temperature, and the ignorance of the value of the interstitial heat transfer coefficient.
However, even if the existence of these factors is accepted, one can ask whether, given the slowness with which the temperature varies, the application of the Fourier law or the methods developed for the non-stationary regime, such as those by Price [
34] and Nozad et al. [
35], can be accepted as a good approximation assuming the local thermal equilibrium condition.
This question can be answered by determining the values of the characteristic time of conduction and the value of the Biot number of the porous media being studied. The characteristic time of conduction is defined as
, and the Biot number as
, where
,
αf is the thermal diffusivity,
λf is the thermal conductivity and
hsf is the interstitial heat transfer coefficient, the value of which is calculated by applying the Wakao and Kaguei [
36] correlation for spherical particles, as follows:
where
and
. Once the values of
tc and
Bi are calculated,
Table 5 and
Table 6 show magnitude values and the results corresponding to the condition of maximum velocity of the liquid to the tray, together with the residence time
tr. The uncertainty in each of the fluid properties was estimated to be 1%.
From the results, it can be seen that the characteristic time is greater than the residence time in all the porous media studied. Then, at the interface of the solid and boundary layer of the liquid flow, the temperature variation of the solid shows a large delay with respect to the liquid. Therefore, the local thermal equilibrium is far from being reached.
Owing to the values of
tc/
tr and the Biot number, the calculation of the heating rate of the porous medium over time cannot be carried out with the two-temperature model. Instead, an approximate indirect procedure—the thermal power balance approach—is used:
In this equation, the terms
(increase of the sensible heat of the liquid of the tray and the evaporated liquid in the interface plate-porous medium) and
(losses due to insulation failure and increase in the temperature of the metallic cover of the porous medium) can be neglected, and then the equation results in:
By taking into account the previous Equations (1) and (9), the results are shown in
Figure 12.
4.8. Formation of the Two-Phase Region
4.8.1. Thickness and Hydrostatic Pressure
As a result, of the displacement of liquid from the porous medium to the tray, its saturation progressively decreases, causing the formation of a two-phase region in which thickness increases over time. This thickness,
zbf, is calculated as a function of time by means of the equation:
and Equation (6). The thickness of the saturated layer of the porous medium,
hsat, is calculated as:
where
hmp is the initial thickness.
Figure 13 shows the values of the thickness of the saturated layer of the porous media.
The hydrostatic pressure
Phs in this region can be evaluated as:
where
zpl is the distance to the plate of the interface between the two-phase region and the saturated region of the porous medium. By knowing the density values at the temperature of the liquid in the saturated region
and in the tray
, Equation (15) is applied to the porous media studied, giving the results shown in
Figure 14.
We proceed now to compare the vapor pressure corresponding to the surface temperature of the plate in contact with the porous medium,
Pv(
Tpl), with pressure at the temperature at the interface, (
Psat)
d, and the hydrostatic pressure,
Phs. The saturation vapor pressure in the thin layer of capillary condensation, assuming negligible solute effects, is calculated by the Kelvin equation (Carey [
37]):
where
Psat(
Tδ) is the saturated water vapor pressure at the vapor absolute temperature
Tδ;
σ is the surface tension of liquid water in contact with its own vapor,
ρl is the liquid density, and
rm is the mean meniscus radius.
Figure 15 shows the results of these magnitudes.
By observing
Figure 15, it is verified that the vapor pressure in the interface is higher than the hydrostatic pressure. The pressure difference, or capillary pressure, promotes its displacement and, consequently, the displacement of liquid to the tray, meanwhile the vapor expands to reach a new state of equilibrium.
4.8.2. Temporal Evolution of the Saturation in the Porous Media
The saturation value is calculated by the following this equation:
where
Vsat is the initial saturated liquid volume in the porous medium (1007.5 cm
3 for the sand-based medium and 954.4 cm
3 for those based on bronze powder), and
Vd,t(
t) is the total volume of liquid displaced from the porous medium at time
t.
Figure 16 shows the results obtained corresponding to the state of dryout prior to the replenishment of the porous medium.
4.8.3. Interface Displacement and Capillary Liquid Velocities
The interface displacement velocity between the two-phase and saturated zones has been approximately calculated by dividing the derivative of
Vdt(
t) by
Ae. The results are shown in
Figure 17.
This method of calculation is a rough approximation. The average velocity of liquid displacement by the capillaries in the saturated region of the porous medium must be calculated with the following equation:
where
pc is the capillary pressure in the interface between the two-phase and saturated zones, and
Tsi and
Tsc are the liquid temperatures in the interface and the upper end of the porous medium, respectively. The capillary pressure is calculated by using Equation (8) and its gradient as:
where
τ is the capillary tortuosity and
hhst is the distance to the interface from the free surface of the liquid in the tray. It is possible to relate the capillary pressure in the two-phase zone with the global saturation measured as propose by Stubos [
38,
39]. The form proposed by Lipinski [
18], to fit the data given by Scheidegger on sands, is:
By knowing the value of Sef for the porous media at any time t, the pressure gradient is calculated by means of Equation (19) and the average velocity of the liquid displaced to the tray by using Equation (18), but only if Darcy’s law and the Boussinesq approximation are accomplished.
As an example, the velocity of the liquid has been calculated through the saturated layer of the porous medium B+DS at time 5220 s, obtaining a velocity of 0.03 mm·s−1. The same order of magnitude obtained from the experimental results is 0.02 mm·s−1.
4.9. Dryout and Replenishment
The dryout occurs in all porous media studied but appears earlier and more frequently in sand-based media than in bronze media. Replenishment only occurs in sand media and is more pronounced in those saturated by a surfactant solution.
The dryout process is progressive: the drying of the porous medium in contact with the heating plate occurs gradually. From the experimental results, several conditions of the dryout process concur, such as cessation of the saturation liquid displacement of the porous media to the tray, minimum saturation value of the porous medium, progressive elevation of the surface temperature of the heating plate in contact with the porous medium, and the maximum value of the vapor pressure in the two-phase zone.
Table 7 includes the values of the magnitudes corresponding to the state of total dryout: the maximum volume of liquid displaced to the tray (
Vdt), maximum temperature reached by the surface of the plate in contact with the porous medium (
Tpl), and maximum vapor pressure in the two-phase zone (
Pvbf). The time corresponding to the state of total dryout (
tdr) and the degree of saturation (
S) have also been included.
Regarding the phenomenon of replenishment, it occurs when total dryout is reached.
Figure 18 shows the significant magnitudes in the phenomenon of dryout-replenishment corresponding to the porous media S+W in a time interval between the replenishment and the dryout.
With regards to the influence of the vapor pressure, it is observed that once boiling occurs throughout the porous medium, the absolute pressure in the two-phase zone is 1.023 ≤
pv ≤ 1.036 bar. These limits represent an overpressure of 0.009 ≤ Δ
pv ≤ 0.022 bar, where the maximum value is measured in the piezometer 4 (0.022 ± 0.001 bar), corresponding to a time prior to the dryout-replenishment in the S+DS porous medium.
Figure 19 and
Figure 20 depict the correlations between three of the magnitudes that identify the dryout-replenishment phenomena.
6. Conclusions
The dryout process studied here is different from the processes carried out by Dhir and Catton [
45], Barleon and Werle [
46], and Turland and Moore [
47], among others. The investigations of these authors concern the safety of fast nuclear reactors, and study how to extract the heat from beds of fuel particles after an accident. The work presented investigates how dryout occurs in bottom-heated saturated porous media, behavior during and after the dryout, evolution and temperature, the value of the heat flow, and the influence of the physical properties of the solid matrix and the saturating liquid phase.
6.1. Influence of the Nature and Physical Properties of the Solid Phase
There are five physical properties of the solid phase that influence the fluid-thermal response of the porous medium: size of the particles, specific surfaces of the particles, density, thermal capacity, and thermal conductivity of the material.
Comparing the experimental results obtained with porous media based on sand and based on bronze saturated with the same liquid, the following conclusions can be drawn:
A larger particle size ensures a higher value of the intrinsic permeability for the same value—practically, the volume of the pore. The value of the Bond, Reynolds, and Rayleigh numbers of the porous medium increases, causing a change in mass flow rate and heat transfer.
The greater particle size, the lower value of the specific surface, capacity, and thermal conductivity, producing a state of thermal non-equilibrium.
The heating intensity of the porous medium decreases with the particle size and with a greater difference of the thermal conductivity between the liquid phase and the solid matrix.
6.2. Influence of the Surfactant
The addition of surfactant to the liquid phase of a porous medium is characterized by: