Mixed Metal Oxide Systems Applied to Thermochemical Storage of Solar Energy: Benefits of Secondary Metal Addition in Co and Mn Oxides and Contribution of Thermodynamics
Abstract
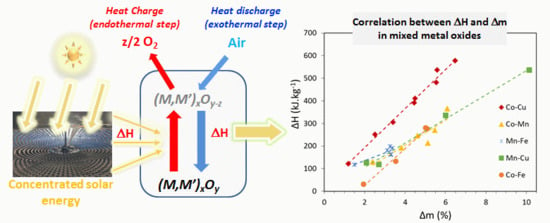
:1. Introduction
2. Materials and Methods
3. Experimental Assessment of Mixed Metal Oxide Systems for TCES
3.1. Co-Fe-O Mixed Oxide System
3.2. Co-Cu-O Mixed Oxide System
3.3. Mn-Fe-O Mixed Oxide System
3.4. Mn-Cu-O Mixed Oxide System
3.5. Mn-Co-O Mixed Oxide System
4. Contribution of Thermodynamic Calculations
- Existence of an accurate phase diagram for the pseudo-binary system,
- Existence of thermochemical data such as enthalpy of mixing between phases or heat capacity functions (cp(T)) for all phases of the system,
- Existence of a model established with the Calphad method, allowing equilibrium computations with a dedicated software.
4.1. Hausmannite Phase Boundaries
4.2. Phase Transitions in Mixed Oxide Systems
4.3. Calculations in the Cu-Fe-O System
5. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pardo, P.; Deydier, A.; Anxionnaz-Minvielle, Z.; Rougé, S.; Cabassud, M.; Cognet, P. A review on high temperature thermochemical heat energy storage. Renew. Sustain. Energy Rev. 2014, 32, 591–610. [Google Scholar] [CrossRef] [Green Version]
- Yan, T.; Wang, R.Z.; Li, T.X.; Wang, L.W.; Fred, I.T. A review of promising candidate reactions for chemical heat storage. Renew. Sustain. Energy Rev. 2015, 43, 13–31. [Google Scholar] [CrossRef]
- Abedin, A.H. A Critical Review of Thermochemical Energy Storage Systems. Open Renew. Energy J. 2011, 4, 42–46. [Google Scholar] [CrossRef]
- Pelay, U.; Luo, L.; Fan, Y.; Stitou, D.; Rood, M. Thermal energy storage systems for concentrated solar power plants. Renew. Sustain. Energy Rev. 2017, 79, 82–100. [Google Scholar] [CrossRef]
- Ströhle, S.; Haselbacher, A.; Jovanovic, Z.R.; Steinfeld, A. The effect of the gas-solid contacting pattern in a high-temperature thermochemical energy storage on the performance of a concentrated solar power plant. Energy Environ. Sci. 2016, 9, 1375–1389. [Google Scholar] [CrossRef]
- Bayon, A.; Bader, R.; Jafarian, M.; Fedunik-Hofman, L.; Sun, Y.; Hinkley, J.; Miller, S.; Lipiński, W. Techno-economic assessment of solid-gas thermochemical energy storage systems for solar thermal power applications. Energy 2018, 149, 473–484. [Google Scholar] [CrossRef]
- André, L.; Abanades, S.; Flamant, G. Screening of thermochemical systems based on solid-gas reversible reactions for high temperature solar thermal energy storage. Renew. Sustain. Energy Rev. 2016, 64, 703–715. [Google Scholar] [CrossRef]
- Fujii, I.; Tsuchiya, K.; Higano, M.; Yamada, J. Studies of an energy storage system by use of the reversible chemical reaction: CaO + H2O ⇌ Ca(OH)2. Sol. Energy 1985, 34, 367–377. [Google Scholar] [CrossRef]
- Pardo, P.; Anxionnaz-Minvielle, Z.; Rougé, S.; Cognet, P.; Cabassud, M. Ca(OH)2/CaO reversible reaction in a fluidized bed reactor for thermochemical heat storage. Sol. Energy 2014, 107, 605–616. [Google Scholar] [CrossRef]
- Linder, M.; Roßkopf, C.; Schmidt, M.; Wörner, A. Thermochemical energy storage in kW-scale based on CaO/Ca(OH)2. Energy Procedia 2013, 49, 888–897. [Google Scholar] [CrossRef]
- Dai, L.; Long, X.F.; Lou, B.; Wu, J. Thermal cycling stability of thermochemical energy storage system Ca(OH)2/CaO. Appl. Therm. Eng. 2018, 133, 261–268. [Google Scholar] [CrossRef]
- Criado, Y.A.; Huille, A.; Rougé, S.; Abanades, J.C. Experimental investigation and model validation of a CaO/Ca(OH)2 fluidized bed reactor for thermochemical energy storage applications. Chem. Eng. J. 2017, 313, 1194–1205. [Google Scholar] [CrossRef]
- Yan, J.; Zhao, C.Y. First-principle study of CaO/Ca(OH)2 thermochemical energy storage system by Li or Mg cation doping. Chem. Eng. Sci. 2014, 117, 293–300. [Google Scholar] [CrossRef]
- Criado, Y.A.; Alonso, M.; Abanades, J.C. Enhancement of a CaO/Ca(OH)2 based material for thermochemical energy storage. Sol. Energy 2016, 135, 800–809. [Google Scholar] [CrossRef]
- Ortiz, C.; Romano, M.C.; Valverde, J.M.; Binotti, M.; Chacartegui, R. Process integration of Calcium-Looping thermochemical energy storage system in concentrating solar power plants. Energy 2018, 155, 535–551. [Google Scholar] [CrossRef]
- Chen, X.; Jin, X.; Liu, Z.; Ling, X.; Wang, Y. Experimental investigation on the CaO/CaCO3 thermochemical energy storage with SiO2 doping. Energy 2018, 155, 128–138. [Google Scholar] [CrossRef]
- Benitez-Guerrero, M.; Valverde, J.M.; Sanchez-Jimenez, P.E.; Perejon, A.; Perez-Maqueda, L.A. Multicycle activity of natural CaCO3 minerals for thermochemical energy storage in Concentrated Solar Power plants. Sol. Energy 2017, 153, 188–199. [Google Scholar] [CrossRef]
- André, L.; Abanades, S. Evaluation and performances comparison of calcium, strontium and barium carbonates during calcination/carbonation reactions for solar thermochemical energy storage. J. Energy Storage 2017, 13, 193–205. [Google Scholar] [CrossRef]
- Wu, S.; Zhou, C.; Doroodchi, E.; Nellore, R.; Moghtaderi, B. A review on high-temperature thermochemical energy storage based on metal oxides redox cycle. Energy Convers. Manag. 2018, 168, 421–453. [Google Scholar] [CrossRef]
- Bulfin, B.; Vieten, J.; Agrafiotis, C.; Roeb, M.; Sattler, C. Applications and limitations of two step metal oxide thermochemical redox cycles; A review. J. Mater. Chem. A 2017, 5, 18951–18966. [Google Scholar] [CrossRef]
- Alonso, E.; Pérez-Rábago, C.; Licurgo, J.; Fuentealba, E.; Estrada, C.A. First experimental studies of solar redox reactions of copper oxides for thermochemical energy storage. Sol. Energy 2015, 115, 297–305. [Google Scholar] [CrossRef]
- Haseli, P.; Jafarian, M.; Nathan, G.J. High temperature solar thermochemical process for production of stored energy and oxygen based on CuO/Cu2O redox reactions. Sol. Energy 2017, 153, 1–10. [Google Scholar] [CrossRef]
- Hänchen, M.; Stiel, A.; Jovanovic, Z.R.; Steinfeld, A. Thermally driven copper oxide redox cycle for the separation of oxygen from gases. Ind. Eng. Chem. Res. 2012, 51, 7013–7021. [Google Scholar] [CrossRef]
- Clayton, C.K.; Whitty, K.J. Measurement and modeling of decomposition kinetics for copper oxide-based chemical looping with oxygen uncoupling. Appl. Energy 2014, 116, 416–423. [Google Scholar] [CrossRef]
- Alonso, E.; Pérez-Rábago, C.; Licurgo, J.; Gallo, A.; Fuentealba, E.; Estrada, C.A. Experimental aspects of CuO reduction in solar-driven reactors: Comparative performance of a rotary kiln and a packed-bed. Renew. Energy 2017, 105, 665–673. [Google Scholar] [CrossRef]
- Schrader, A.J.; Muroyama, A.P.; Loutzenhiser, P.G. Solar electricity via an Air Brayton cycle with an integrated two-step thermochemical cycle for heat storage based on Co3O4/CoO redox reactions: Thermodynamic analysis. Sol. Energy 2015, 118, 485–495. [Google Scholar] [CrossRef]
- Block, T.; Knoblauch, N.; Schmücker, M. The cobalt-oxide/iron-oxide binary system for use as high temperature thermochemical energy storage material. Thermochim. Acta 2014, 577, 25–32. [Google Scholar] [CrossRef]
- Agrafiotis, C.; Roeb, M.; Schmücker, M.; Sattler, C. Exploitation of thermochemical cycles based on solid oxide redox systems for thermochemical storage of solar heat. Part 2: Redox oxide-coated porous ceramic structures as integrated thermochemical reactors/heat exchangers. Sol. Energy 2015, 114, 440–458. [Google Scholar] [CrossRef]
- Neises, M.; Tescari, S.; de Oliveira, L.; Roeb, M.; Sattler, C.; Wong, B. Solar-heated rotary kiln for thermochemical energy storage. Sol. Energy 2012, 86, 3040–3048. [Google Scholar] [CrossRef]
- Tescari, S.; Agrafiotis, C.; Breuer, S.; De Oliveira, L.; Neises-Von Puttkamer, M.; Roeb, M.; Sattler, C. Thermochemical solar energy storage via redox oxides: Materials and reactor/heat exchanger concepts. Energy Procedia 2013, 49, 1034–1043. [Google Scholar] [CrossRef]
- Singh, A.; Tescari, S.; Lantin, G.; Agrafiotis, C.; Roeb, M.; Sattler, C. Solar thermochemical heat storage via the Co3O4/CoO looping cycle: Storage reactor modelling and experimental validation. Sol. Energy 2017, 144, 453–465. [Google Scholar] [CrossRef]
- Karagiannakis, G.; Pagkoura, C.; Halevas, E.; Baltzopoulou, P.; Konstandopoulos, A.G. Cobalt/cobaltous oxide based honeycombs for thermochemical heat storage in future concentrated solar power installations: Multi-cyclic assessment and semi-quantitative heat effects estimations. Sol. Energy 2016, 133, 394–407. [Google Scholar] [CrossRef]
- Pagkoura, C.; Karagiannakis, G.; Zygogianni, A.; Lorentzou, S.; Kostoglou, M.; Konstandopoulos, A.G.; Rattenburry, M.; Woodhead, J.W. Cobalt oxide based structured bodies as redox thermochemical heat storage medium for future CSP plants. Sol. Energy 2014, 108, 146–163. [Google Scholar] [CrossRef]
- André, L.; Abanades, S. Investigation of metal oxides, mixed oxides, perovskites and alkaline earth carbonates/hydroxides as suitable candidate materials for high-temperature thermochemical energy storage using reversible solid-gas reactions. Mater. Today Energy 2018, 10, 48–61. [Google Scholar] [CrossRef]
- André, L.; Abanades, S.; Cassayre, L. High-temperature thermochemical energy storage based on redox reactions using Co-Fe and Mn-Fe mixed metal oxides. J. Solid State Chem. 2017, 253, 6–14. [Google Scholar] [CrossRef]
- André, L.; Abanades, S.; Cassayre, L. Experimental Investigation of Co–Cu, Mn–Co, and Mn–Cu Redox Materials Applied to Solar Thermochemical Energy Storage. ACS Appl. Energy Mater. 2018, 1, 3385–3395. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Serrano, D.P.; Pizarro, P.; Coronado, J.M. Improving the Thermochemical Energy Storage Performance of the Mn2O3/Mn3O4 Redox Couple by the Incorporation of Iron. ChemSusChem 2015, 8, 1947–1954. [Google Scholar] [CrossRef] [PubMed]
- Karagiannakis, G.; Pagkoura, C.; Zygogianni, A.; Lorentzou, S.; Konstandopoulos, A.G. Monolithic ceramic redox materials for thermochemical heat storage applications in CSP plants. Energy Procedia 2013, 49, 820–829. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Serrano, D.P.; Pizarro, P.; Coronado, J.M. Thermochemical heat storage based on the Mn2O3/Mn3O4 redox couple: Influence of the initial particle size on the morphological evolution. J. Mater. Chem. A 2014, 2, 19435–19443. [Google Scholar] [CrossRef]
- Gillot, B.; El Guendouzi, M.; Laarj, M. Particle size effects on the oxidation-reduction behavior of Mn3O4 hausmannite. Mater. Chem. Phys. 2001, 70, 54–60. [Google Scholar] [CrossRef]
- Block, T.; Schmücker, M. Metal oxides for thermochemical energy storage: A comparison of several metal oxide systems. Sol. Energy 2016, 126, 195–207. [Google Scholar] [CrossRef]
- Monazam, E.R.; Breault, R.W.; Siriwardane, R. Kinetics of magnetite (Fe3O4) oxidation to hematite (Fe2O3) in air for chemical looping combustion. Ind. Eng. Chem. Res. 2014, 53, 13320–13328. [Google Scholar] [CrossRef]
- Kozuka, H. Handbook of Sol-Gel Science and Technology; Volume 1: Sol-gel Processing; Sakka, S., Ed.; Springer Science & Business Media: Berlin, Germany, 2005; 680 p. [Google Scholar]
- Carrillo, A.J.; Serrano, D.P.; Pizarro, P.; Coronado, J.M. Understanding Redox Kinetics of Iron-Doped Manganese Oxides for High Temperature Thermochemical Energy Storage. J. Phys. Chem. C 2016, 120, 27800–27812. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Moya, J.; Bayón, A.; Jana, P.; De La Peña O’Shea, V.A.; Romero, M.; Gonzalez-Aguilar, J.; Serrano, D.P.; Pizarro, P.; Coronado, J.M. Thermochemical energy storage at high temperature via redox cycles of Mn and Co oxides: Pure oxides versus mixed ones. Sol. Energy Mater. Sol. Cells 2014, 123, 47–57. [Google Scholar] [CrossRef]
- Motuzas, J.; Diniz Da Costa, J.C. Copper aided exchange in high performance oxygen production by CuCo binary oxides for clean energy delivery. J. Mater. Chem. A 2015, 3, 17344–17350. [Google Scholar] [CrossRef]
- Kang, Y.B.; Jung, I.H. Thermodynamic modeling of oxide phases in the Fe–Mn–O system. J. Phys. Chem. Solids 2016, 98, 237–246. [Google Scholar] [CrossRef]
- Adánez-Rubio, I.; Izquierdo, M.T.; Abad, A.; Gayán, P.; de Diego, L.F.; Adánez, J. Spray granulated Cu-Mn oxygen carrier for chemical looping with oxygen uncoupling (CLOU) process. Int. J. Greenh. Gas Control 2017, 65, 76–85. [Google Scholar] [CrossRef]
- Golikov, Y.V.; Tubin, S.Y.; Barkhatov, V.P.; Balakirev, V.F. Phase Diagrams of the Co-Mn-O System in Air. J. Phys. Chem. Solids 1985, 46, 539–544. [Google Scholar] [CrossRef]
- Driessens, F.C.M.; Rieck, G.D. Phase Equilibria in the System Cu-Mn-O. Z. Anorg. Allg. Chem. 1967, 351, 48–62. [Google Scholar] [CrossRef]
- Wei, P.; Bieringer, M.; Cranswck, L.M.D.; Petric, A. In-situ High Temperature X-ray and Neutron Diffraction of Cu-Mn Oxide Phases. J. Mater. Sci. 2010, 45, 1056–1064. [Google Scholar] [CrossRef]
- Shishin, D.; Hidayat, T.; Jak, E.; Decterov, S.A. Critical assessment and thermodynamic modeling of the Cu–Fe–O system. Calphad 2013, 41, 160–179. [Google Scholar] [CrossRef]
- Jacob, K.T.; Fitzner, K.; Alcock, C.B. Activities in the spinel solid solution, phase equilibria and thermodynamic properties of ternary phases in the system Cu-Fe-O. Met. Trans. B 1977, 8, 451–460. [Google Scholar] [CrossRef]
- Zhang, W.-W.; Chen, M. Thermodynamic Modeling of the Co-Fe-O System. Calphad 2013, 41, 76–88. [Google Scholar] [CrossRef]
- Jung, I.-H.; Decterov, S.A.; Pelton, A.D.; Kim, H.-M.; Kang, Y.-B. Thermodynamic Evaluation and Modeling of the Fe-Co-O System. Acta Mater. 2004, 52, 507–519. [Google Scholar] [CrossRef]
- Zabdyr, L.A.; Fabrichnaya, O.B. Phase Equilibria in the Cobalt Oxide-Copper Oxide System. J. Phase Equilib. 2002, 23, 149–155. [Google Scholar] [CrossRef]
- Crum, J.V.; Riley, B.J.; Vienna, J.D. Binary Phase Diagram of the Manganese Oxide-Iron Oxide System. J. Am. Ceram. Soc. 2009, 92, 2378–2384. [Google Scholar] [CrossRef]
- Kjellqvist, L.; Selleby, M. Thermodynamic assessment of the Cr-Mn-O system. J. Alloys Coump. 2010, 507, 84–92. [Google Scholar] [CrossRef]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Gheribi, A.E.; Hack, K.; Jung, I.H.; Kang, Y.B.; Melançon, J.; et al. FactSage Thermochemical Software and Databases, 2010–2016. Calphad 2016, 54, 35–53. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Serrano, D.P.; Pizarro, P.; Coronado, J.M. Manganese oxide-based thermochemical energy storage: Modulating temperatures of redox cycles by Fe-Cu co-doping. J. Energy Storage 2016, 5, 169–176. [Google Scholar] [CrossRef]
- Pashkova, E.V.; Novosadvoa, E.B.; Chalyi, V.P.; Antishko, A.N. Phase Diagram of the Manganese, Iron, and Cobalt oxides. Ukr. Chem. J. 1987, 53, 26–29. [Google Scholar]
- Lalanne, M.; Barnabé, A.; Mathieu, F.; Tailhades, P. Synthesis and Thermostructural Studies of a CuFe1−xCrxO2 Delafossite Solid Solution with 0 ≤ x ≤ 1. Inorg. Chem. 2009, 48, 6065–6071. [Google Scholar] [CrossRef] [PubMed]











| TCES System | Onset Temperature (°C) | Temperature Gap (°C) | Conversion (%) | Exp. Reaction Enthalpy (kJ/kg) | Phase Transition & Redox Couple | |||
|---|---|---|---|---|---|---|---|---|
| Reduction | Oxidation | |||||||
| Co3O4/CoO | 909 | 843 | 66 | 95.6 | 597 | |||
| Mn2O3/Mn3O4 | 944 | 772 | 172 | 19.2 | 148 | |||
| CuO/Cu2O | 1028 | 1002 | 26 | 87.1 | 536 | |||
| Fe2O3/Fe3O4 | 1391 | 1350 | 41 | 86 | 183 | |||
| Co-Cu-O | x(Cu) | 0.03 | 896 | 860 | 36 | 94.3 | 574 | |
| 0.1 | 867 | 845 | 22 | 97.4 | 570 | |||
| 0.2 | 864 | 824 | 40 | 97.3 | 520 | |||
| 0.25 | 863 | 820 | 43 | 99.7 | 503 | |||
| 0.3 | 864 | 813 | 51 | 97.5 | 436 | |||
| 0.4 | 864 | 816 | 48 | 93 | 327 | |||
| 0.6 | 861 | 818 | 43 | 98.8 | 351 | |||
| 0.8 | 867 | 813 | 54 | 93.4 | 212 | |||
| Co-Fe-O | x(Fe) | 0.05 | 921 | 848 | 73 | 84.4 | 454 | |
| 0.1 | 931 | 896 | 35 | 83.9 | 365 | |||
| 0.25 | 933 | 914 | 19 | 82.4 | 224 | |||
| 0.4 | 941 | 945 | 4 | 82.5 | 51 | |||
| Mn-Co-O | x(Co) | 0.5 | 989 (Ar) | - | - | 64.2 | 133 | |
| 0.6 | 942 (Ar) | - | - | 84.7 | 200 | |||
| 0.7 | 930 | 877 | 53 | 96.7 | 306 | |||
| 0.8 | 933 | 873 | 60 | 96.6 | 296 | |||
| 0.9 | 920 | 871 | 49 | 97.9 | 427 | |||
| 0.95 | 937 | 991 | 54 | 94.9 | 319 | |||
| Mn-Cu-O | x(Cu) | 0.05 | 900 | no re-ox. | - | 21.2 | 126 | |
| 0.1 | 877 | no re-ox. | - | 10.7 | - | |||
| 0.2 | 811 | no re-ox. | - | 1.6 | - | |||
| 0.3 | 976 | 1040 | 64 | 90.8 | - | - | ||
| 0.4 | 960 | 969 | 9 | 96.9 | - | |||
| 0.5 | 962 | 886 | 76 | 88.2 | - | |||
| 0.8 | 954 | 1000 | 46 | 89.7 | 354 | |||
| Mn-Fe-O | x(Fe) | 0.1 | 990 | 774 | 216 | 31 | 168 | |
| 0.15 | 981 | 870 | 111 | 99.1 | 171 | |||
| 0.2 | 980 | 860 | 120 | 98.2 | 194 | |||
| 0.3 | 984 | 866 | 118 | 94.1 | 195 | |||
| 0.4 | 998 | 903 | 95 | 95.3 | 189 | |||
| 0.5 | 1014 | 937 | 77 | 94.7 | 189 | |||
| Oxide System | Phase Diagram | Model |
|---|---|---|
| Cu-Fe-O | Jacob, 1977 [53] | Shishin, 2013 [52] |
| Co-Fe-O | Zhang, 2013 [54] | Jung, 2004 [55] Zhang, 2013 [54] |
| Co-Cu-O | Zabdyr, 2002 [56] | Zabdyr, 2002 [56] |
| Mn-Fe-O | Crum, 2009 [57] | Kjellqvist, 2010 [58] Kang, 2016 [47] |
| Mn-Co-O | Golikov, 1985 [49] | None |
| Mn-Cu-O | Driessens, 1967 [50] Wei, 2009 [51] | None |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
André, L.; Abanades, S.; Cassayre, L. Mixed Metal Oxide Systems Applied to Thermochemical Storage of Solar Energy: Benefits of Secondary Metal Addition in Co and Mn Oxides and Contribution of Thermodynamics. Appl. Sci. 2018, 8, 2618. https://doi.org/10.3390/app8122618
André L, Abanades S, Cassayre L. Mixed Metal Oxide Systems Applied to Thermochemical Storage of Solar Energy: Benefits of Secondary Metal Addition in Co and Mn Oxides and Contribution of Thermodynamics. Applied Sciences. 2018; 8(12):2618. https://doi.org/10.3390/app8122618
Chicago/Turabian StyleAndré, Laurie, Stéphane Abanades, and Laurent Cassayre. 2018. "Mixed Metal Oxide Systems Applied to Thermochemical Storage of Solar Energy: Benefits of Secondary Metal Addition in Co and Mn Oxides and Contribution of Thermodynamics" Applied Sciences 8, no. 12: 2618. https://doi.org/10.3390/app8122618
APA StyleAndré, L., Abanades, S., & Cassayre, L. (2018). Mixed Metal Oxide Systems Applied to Thermochemical Storage of Solar Energy: Benefits of Secondary Metal Addition in Co and Mn Oxides and Contribution of Thermodynamics. Applied Sciences, 8(12), 2618. https://doi.org/10.3390/app8122618