Oxygen Carrier Aided Combustion (OCAC) of Wood Chips in a 12 MWth Circulating Fluidized Bed Boiler Using Steel Converter Slag as Bed Material
Abstract
:1. Introduction
1.1. Background
1.2. Chemical Looping Combustion and Oxygen Carriers
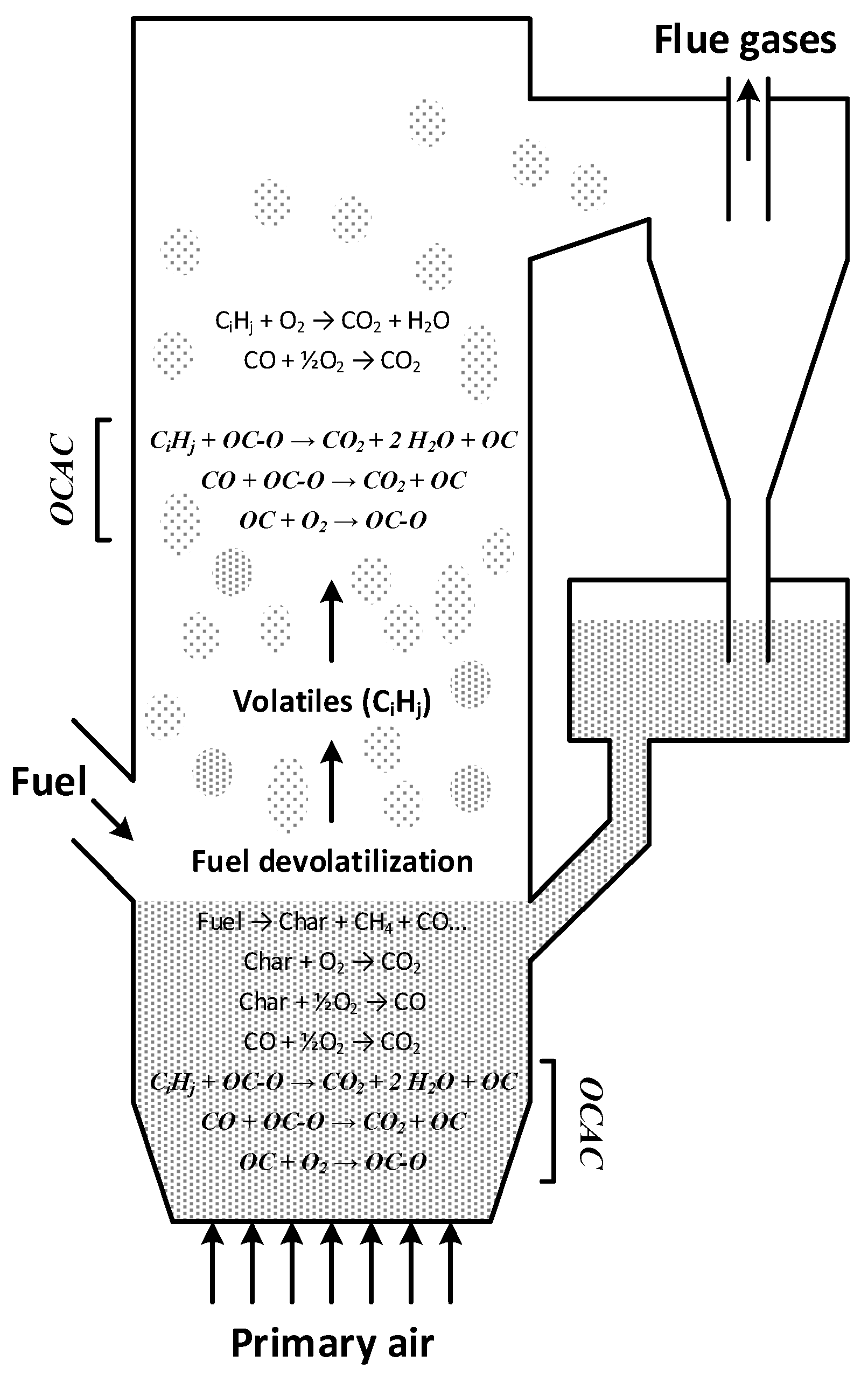
1.3. Oxygen Carrier Aided Combustion
- Gas phase fuel components can be oxidized not only by homogenous reactions with oxygen, but also by heterogeneous reactions with the oxygen carrier, as described in reaction (1).
- New mechanisms for oxygen transport in the boiler will be introduced, thus minimizing the presence of reducing zones and potentially reducing the emissions of CO, H2 and unburnt hydrocarbons.
- The bed of oxygen carrier material will act as an oxygen buffer, possibly thwarting negative effects of uneven fuel feeding and load changes.
- Enhanced fuel conversion in the dense bottom bed. In ordinary fluidized bed boilers the more stable fuel components (such as for example CH4) do not burn rapidly in the bottom bed since the moderate temperature (≈800–850 °C) and thermal inertia of the bed inhibits formation of hot flames. However, it has been shown in CLC studies [3] that CH4 is readily oxidized by oxygen carrying solids. The apparent reason would be that the heterogeneous reaction between CH4 and oxygen carrier is not hampered by temperature to the same extent as the homogeneous reaction. Consequently, in OCAC the conversion of CH4 should proceed more rapidly also inside the dense bottom bed, a phenomena that has also been demonstrated experimentally [8].
- OCAC may offer opportunities to reduce traditional problems in biomass combustion. This includes sintering, agglomeration, fouling and corrosion issues connected to combustion of biomass in fluidized beds [9].
- OCAC may allow for the use of less excess air than what is needed in conventional boilers.
1.4. LD-Slag as Oxygen Carrier
1.5. The Aims of This Study
2. Materials and Methods
2.1. Bed Material
2.2. Fuel
2.3. Experimental Facility
2.4. Methodology
- Temperature at different key locations. Can potentially indicate whether LD-slag facilitates fuel conversion in the dense bed and if less combustion takes place in the cyclone, compared to when sand is used.
- Pressure drop over key components. Indicates where the bed material is located, whether the bed fluidizes, the materials tendencies to agglomerate and if there is excessive attrition.
- Amount of elutriated ash and solids captured in the secondary cyclone and the subsequent textile filter.
- Bed, ash and fuel samples were also taken each day for various analyses, some of which will be referred to below.
- The baseline air flow was 2.15 kg/s.
- The baseline fuel flow was set to 1800 kg/h, which corresponds to roughly 5 MWth. The flow was adjusted depending on moisture content in order to achieve an outlet oxygen concentration of 3.5 vol% on dry basis.
- The set point for the oxygen concentration was step-wise decreased, which results in the regulator system increasing the fuel flow.
- The outlet oxygen concentration was decreased in 0.5 vol% steps down to 1.5 vol%. Each operating point was kept until the oxygen concentration had stabilized, and remained stable for at least 10 min.
- The target temperature was 840–870 °C in the dense bottom bed. Temperature could be regulated by exchanging a fraction of the fuel for wood pellets with lower moisture content than wood chips. Flue gas recirculation could also be altered for cooling the bed, but this was done only if necessary, since it can be expected to influence the fluid dynamics of the bed.
- = dry air volume added to the combustion chamber (mn3/mn3,fuel)
- = dry air volume needed for stoichiometric combustion of the fuel mix (mn3/mn3,fuel)
- = dry flue gas volume for stoichiometric combustion of the fuel mix (mn3/mn3,fuel)
- = CO2 fraction in dry flue gas for stoichiometric combustion of the fuel mix (-)
- = Measured CO2 fraction in dry flue gas (-)
- = Measured O2 fraction in dry flue gas (-)
- TA1 (first tendency towards agglomeration): This is the lowest temperature when a disturbance in the pressure drop over the bed can be verified. The point was chosen where a continuous reduction in pressure drop of about 1 Pa/min could be seen.
- TA2 (apparent agglomeration): The lowest temperature when agglomeration clearly is taking place. The bed is considered to be agglomerating when the pressure drop is reduced continuously with 5 Pa/min or more.
- TA3 (complete agglomeration): The temperature when the pressure drop is stabilized at its minimum value.
- The amounts of material removed and added were always attuned to achieve the desired bed composition and a fixed pressure drop over the whole bed of 5.5 kPa.
- It was assumed that the composition of the bed material removed was the same as it would be as if the whole bed was perfectly mixed.
- It was assumed that there was no accumulation of ash or fuel in the bed, that is, only LD-slag and sand counts.
3. Results
3.1. Operation with 100% LD-Slag
- At startup with completely fresh LD-slag (day 2), the CO concentration was initially low. However, soon an increasing trend could be seen and after 7 h of operation the CO concentration was 100 ppm and still increasing. This is a considerably higher concentration than what would be expected for silica after a similar time of operation.
- In the next morning (day 3), the trend accelerated and the CO concentration soon exceeded 1200 ppm, which is outside the preferred measuring range of the instruments used. During operation with silica sand and wood fuel, such an increase in emissions would be expected to take place only after several days of operation without regeneration of bed material.
3.2. Addition of Sulphur Granulates to the Boiler
3.3. Addition of Ammonium Sulphate to the Vortex Finder
3.4. Mixing of LD-Slag with Sand
3.5. Effect on Boiler Temperature Profile during Operation
- There is always a temperature drop over the cyclone, which is to be expected since it is water-cooled. The temperature drop is reduced by the fact that final combustion is also taking place here. It is clear that the temperature drop is much more pronounced for experiments involving LD-slag compared to when only sand is used. This suggests that, with LD-slag, significantly less combustibles enter the cyclone and burn there. Considering that the concentration of incombustibles (CO, H2, CH4, THC) in the stack are on ppm level, this suggests that more combustion must take place inside the actual boiler body when LD-slag is used, in comparison to the sand case.
- Also, from the temperature difference between the bottom of the boiler and the top of the dense bed it can be seen that more heat is generated in the dense bed when LD-slag is present for the 10% and 30% mixtures, than when only sand is used. This corroborates with the observation above.
- For the case with 100% LD-slag with addition of ammonium sulphate, the picture is somewhat unclear and can be interpreted in different ways. There are reaction enthalpies for dissolution of ammonium sulphate, oxidation of ammonia and sulphation of K, which can be expected to affect the temperature in the cyclone outlet. One should also consider the unit on the y-axis and the temperature in the bottom bed, which was significantly higher than in other experiments at 880 °C. So as of this time insufficient information is available to explain precisely what is taking place.
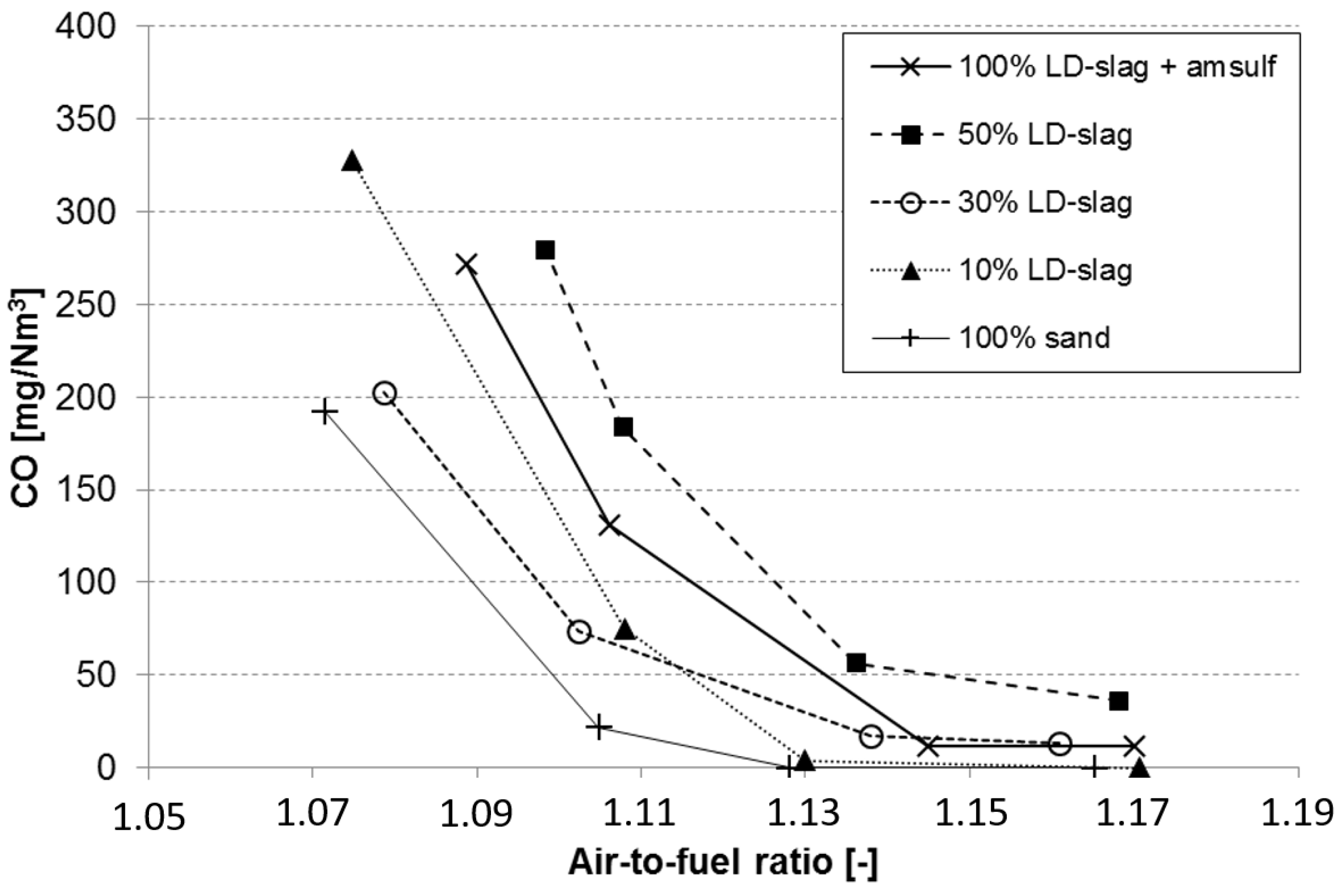
3.6. Effect on CO Emissions during Reduced Air-to-Fuel Ratio
- The reference experiments with silica sand provided the lowest emissions. Here it should be pointed out that the data for the reference case refers to experiments performed at slightly higher temperature (870 °C) and in a clean boiler directly at start-up of the firing season. Both factors are likely to contribute to slightly better than expected performance with respect to emissions.
- The results suggest that increased substitution of LD-slag increases CO emissions. The mix with 50% LD-slag clearly has higher emissions than the mix with 10% LD-slag. This is the opposite effect of what could be expected.
- For experiments with 100% LD-slag, the emissions were far above what is shown in the graph. However, as can be seen, addition of ammonium sulphate in the cyclone leg reduced the CO emissions to acceptable levels, as has been explained above.
3.7. Effect on NO Emissions
- An increase in air-to-fuel ratio results in higher NO emissions. This is a well-known phenomenon within combustion chemistry.
- The sand reference has the lowest NO emissions, meaning that no improvements could be verified by using LD-slag as bed material.
- Of the experiments that involved LD-slag, those with lowest CO emissions also had the lowest NO emissions.
3.8. Observations with Respect to General Operability
4. Discussion
4.1. CO Emissions
4.2. Commercial Outlook for LD-Slag as Bed Material
4.3. Implications for the Viability of Chemical Looping Combustion
5. Conclusions
- From an operational point of view, LD-slag worked well as bed material in a CFB boiler. The material was easy to handle, to fill into and remove. No problems were encountered related to agglomeration.
- The production of fly ash was three times as high as for similar operation with silica sand, but this did not result in any operational problems. The high production of fly ash is likely at least partly a result of the 10–15 wt% fine particles below 90 μm present in the starting material.
- The temperature profile of the boiler suggests that the presence of LD-slag facilitates fuel conversion in the boiler, with the clearest sign being a much greater temperature drop in the cyclone in comparison to when only silica sand is used.
- The use of 100% LD-slag resulted in much higher emissions of CO than the sand reference. It is believed that this is connected to poor absorption of ash components, in particular potassium.
- During operation with 100% LD-slag, CO emissions could successfully be suppressed by addition of small amounts of ammonium sulphate to vortex finder of the primary cyclone.
- Operation with 10%, 30% and 50% LD-slag were feasible from a practical point of view and resulted in reasonably low CO emissions.
- LD-slag did not show potential for reduction of NO emissions. For a given air-to-fuel ratio, NO emissions were always higher when LD-slag was included in the bed compared to the sand reference. This behavior was similar to previous experiments with ilmenite [10] and manganese ore [14] in the same boiler.
- In order to constitute a commercially attractive substitute for silica sand in fluidized bed combustion of biomass, the issue with CO emissions will need to be further examined and better understood. Also, the potential cost of LD-slag in the suitable size range and impact of changed ash properties needs to be carefully assessed.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fan, L.S. Chemical Looping Systems for Fossil Energy Conversions; Wiley: New York, NY, USA, 2010. [Google Scholar]
- Fennel, P.; Anthony, B. Calcium and Chemical Looping Technology for Power Generation and Carbon Dioxide (CO2) Capture; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Adánez, J.; Abad, A.; Garcia-Labiano, F.; Gayán, P.; de Diege, L.F. Progress in Chemical-Looping Combustion and Reforming technologies. Prog. Energy Combust. Sci. 2011, 38, 215–282. [Google Scholar] [CrossRef]
- Adánez, J.; Abad, A.; Mendiara, T.; Gayán, P.; de Diege, L.F.; Garcia-Labiano, F. Chemical looping combustion of solid fuels. Prog. Energy Combust. Sci. 2018, 65, 6–66. [Google Scholar] [CrossRef]
- Lyngfelt, A.; Leckner, B. A 1000 MWth boiler for chemical-looping combustion of solid fuels—Discussion of design and costs. Appl. Energy 2015, 157, 475–487. [Google Scholar] [CrossRef]
- Mattisson, T.; Lyngfelt, A.; Leion, H. Chemical-looping with oxygen uncoupling for combustion of solid fuels. Int. J. Greenh. Gas Control. 2009, 3, 11–19. [Google Scholar] [CrossRef]
- Lyngfelt, A.; Mattisson, T.; Rydén, M.; Linderholm, C. 10,000 h of Chemical-Looping Combustion operation—Where are we and where do we want to Go? In Proceedings of the 5th International Conference on Chemical Looping, Park City, UT, USA, 24–27 September 2018. [Google Scholar]
- Chadeesingh, D.R.; Hayhurst, A.N. The combustion of a fuel-rich mixture of methane and air in a bubbling fluidised bed of silica sand at 700 °C and also with particles of Fe2O3 or Fe present. Fuel 2014, 127, 169–177. [Google Scholar] [CrossRef]
- Khan, A.A.; de Jong, W.; Jansens, P.J.; Spliethoff, H. Biomass combustion in fluidized bed boilers: Potential problems and remedies. Fuel Process. Technol. 2009, 90, 21–50. [Google Scholar] [CrossRef]
- Thunman, H.; Lind, F.; Breitholtz, C.; Berguerand, N.; Seemann, M. Using an oxygen-carrier as bed material for combustion of biomass in a 12-MWth circulating fluidized-bed boiler. Fuel 2013, 113, 300–309. [Google Scholar] [CrossRef]
- Corcoran, A.; Marinkovic, J.; Lind, F.; Thunman, H.; Knutsson, P.; Seemann, M. Ash Properties of Ilmenite Used as Bed Material for Combustion of Biomass in a Circulating Fluidized Bed Boiler. Energy Fuels 2014, 28, 7672–7679. [Google Scholar] [CrossRef]
- Lind, F.; Corcoran, A.; Thunman, H. Validation of the oxygen buffering ability of bed materials used for OCAC in a large scale CFB boiler. Powder Technol. 2017, 316, 462–468. [Google Scholar] [CrossRef]
- Corcoran, A.; Knutsson, P.; Lind, F.; Thunman, H. Comparing the structural development of sand and rock ilmenite during long-term exposure in a biomass fired 12 MWth CFB-boiler. Fuel Process. Technol. 2017, 171, 39–44. [Google Scholar] [CrossRef]
- Rydén, M.; Hanning, M.; Corcoran, A.; Lind, F. Oxygen carrier aided combustion (OCAC) of wood chips in a semi-commercial circulating fluidized bed boiler using manganese ore as bed material. Appl. Sci. 2016, 6, 347. [Google Scholar] [CrossRef]
- Wang, P.; Leion, H.; Yang, H. Oxygen-Carrier-Aided Combustion in a bench-scale fluidized bed. Energy Fuels 2017, 31, 6463–6471. [Google Scholar] [CrossRef]
- Jerndal, E.; Mattisson, T.; Lyngfelt, A. Thermal analysis of chemical-looping combustion. Chem. Eng. Res. Des. 2006, 84, 795–806. [Google Scholar] [CrossRef]
- Rydén, M.; Leion, H.; Lyngfelt, A.; Mattisson, T. Combined oxides as oxygen carrier material for chemical-looping combustion with oxygen uncoupling. Appl. Energy 2014, 113, 1924–1932. [Google Scholar] [CrossRef]
- Azimi, G.; Rydén, M.; Leion, H.; Mattisson, T.; Lyngfelt, A. (MnzFe1−z)yOx combined oxides as oxygen carrier for chemical-looping with oxygen uncoupling. AICHE J. 2013, 59, 582–588. [Google Scholar] [CrossRef]
- Källén, M.; Rydén, M.; Lyngfelt, A.; Mattisson, T. Chemical-Looping Using Combined Iron/Manganese/Silica Oxygen Carriers. Appl. Energy 2015, 157, 330–337. [Google Scholar] [CrossRef]
- Kassman, H.; Bäfver, L.; Åmand, L.-E. The importance of SO2 and SO3 for sulphation of gaseous KCl—An experimental investigation in a biomass fired CFB boiler. Combust. Flame 2010, 157, 1649–1657. [Google Scholar] [CrossRef]
- Davisson, K.O.; Åmand, L.-E.; Steenari, B.-M.; Elled, A.-L.; Eskilsson, D.; Leckner, B. Countermeasures against alkali-related problems during combustion of biomass in a circulating fluidized bed boiler Biomass combustion in fluidized bed boilers: Potential problems and remedies. Chem. Eng. Sci. 2008, 63, 5314–5329. [Google Scholar] [CrossRef]
- Hindiyarti, L.; Frandsen, F.; Livbjerg, H.; Glarborg, P. Influence of potassium chloride on moist CO oxidation under reducing conditions: Experimental and kinetic modeling study. Fuel 2006, 85, 978–988. [Google Scholar] [CrossRef]
- Hildor, F.; Mattisson, T.; Leion, H.; Linderholm, C.; Hanning, M.; Lind, F.; Rydén, M. LD slag as an oxygen carrier for combustion processes. In Proceedings of the 5th International Conference on Chemical Looping, Park City, UT, USA, 24–27 September 2018. [Google Scholar]
- Hanning, M.; Corcoran, A.; Zhao, D.; Lind, F.; Rydén, M. Characterization of Oxygen Carriers during Combustion and CLC Conditions in a 12 MWth Circulating Fluidized Bed Biomass Boiler. Biomass Bioenergy 2018, 119, 179–190. [Google Scholar] [CrossRef]
- Marinkovic, J.; Thunman, H.; Knutsson, P.; Seemann, M. Characteristics of olivine as a bed material in an indirect biomass gasifier. Chem. Eng. J. 2015, 279, 555–566. [Google Scholar] [CrossRef]
- Lindau, L.; Skog, E. CO-Reduktion i FB-Panna via Dosering av Elementärt Svavel; Värmeforsk Rapport 812; Värmeforsk: Stockholm, Sweden, 2003. (In Swedish) [Google Scholar]
- Kassman, H.; Andersson, C.; Carlsson, J.; Björklund, U.; Strömberg, B. Minskade Utsläpp av CO och NOx Genom Dosering av Ammoniumsulfat i Förbränningsrummet; Värmeforsk Rapport 908; Värmeforsk: Stockholm, Sweden, 2005. (In Swedish) [Google Scholar]
- Moldenhauer, P.; Linderholm, C.; Rydén, M.; Lyngfelt, A. Experimental investigation of chemical-looping combustion and chemical-looping gasification of biomass-based fuels using steel converter slag as oxygen carrier. In Proceedings of the 1st International Conference on Negative CO2 Emissions, Göteborg, Sweden, 22–24 May 2018. [Google Scholar]





| Material | LD-Slag | Baskarp B28 |
|---|---|---|
| Composition (wt%) | 31.7% Ca, 17.1% Fe, 5.9% Mg, 5.6% Si, 2.6% Mn, 1.5% V, 0.8% Al, 0.25% P, 0.10% S, 0.05% K, balance O | 74% quartz, 22% feldspar, 2% biotite |
| Treatment | Crushing, drying, sieving | - |
| Size span | 100–400 μm (nominal) | 90–355 μm |
| Fraction below 90 μm | 10–15% | - |
| Bulk density (aerated) | ≈1600 kg/m3 | 1500 kg/m3 |
| Bulk density (tapped) | ≈1700 kg/m3 | - |
| Component | Week 1 | Week 2 |
|---|---|---|
| Carbon (wt%) | 50.4 | 50.1 |
| Hydrogen (wt%) | 6.0 | 6.0 |
| Nitrogen (wt%) | 0.1 | 0.1 |
| Sulphur (wt%) | <0.02 | <0.02 |
| Chlorine (wt%) | <0.01 | <0.01 |
| Aluminium (wt%) | 0.003 | 0.004 |
| Silicon (wt%) | 0.009 | 0.016 |
| Iron (wt%) | 0.003 | 0.003 |
| Titanium (wt%) | <0.001 | <0.001 |
| Manganese (wt%) | 0.008 | 0.008 |
| Magnesium (wt%) | 0.019 | 0.020 |
| Calcium (wt%) | 0.12 | 0.12 |
| Barium (wt%) | 0.001 | 0.001 |
| Sodium (wt%) | 0.004 | 0.005 |
| Potassium (wt%) | 0.061 | 0.071 |
| Phosphorus (wt%) | 0.007 | 0.009 |
| Higher heating value (MJ/kg) | 20.23 | 20.1 |
| Lower heating value (MJ/kg) | 18.93 | 18.78 |
| Gas Component | Measuring Method |
|---|---|
| Methane (CH4) | NDIR, GC |
| Carbon monoxide (CO) | NDIR, GC |
| Carbon dioxide (CO2) | NDIR, GC |
| Oxygen (O2) | PMOD, GC |
| Hydrogen (H2) | GC |
| Nitrogen (N2) | GC |
| Nitric oxide (NO) | CL |
| Nitric dioxide (NO2) | CL |
| Dinitrogen oxide (N2O) | GC |
| Total hydrocarbons (THC) | FID |
| Day | Date | LD-Slag (wt%) | Si-Sand (wt%) | Comment |
|---|---|---|---|---|
| 1 | 2017-11-13 | ≈100 | Preparations for start-up. | |
| 2 | 2017-11-14 | ≈100 | Start-up and operation with 100% LD-slag. | |
| 3 | 2017-11-15 | ≈100 | 100% LD-slag, ammonium sulphate experiments. | |
| 4 | 2017-11-16 | ≈100 | 100% LD-slag, ammonium sulphate experiments. | |
| 5 | 2017-11-17 | ≈100 | 100% LD-slag, experiments with sulphur granulates. | |
| Boiler regeneration during weekend (days 6–7). This was done in order to replace all LD-slag with fresh silica sand. Boiler stop day 7 to get rid of as much remaining slag as possible. | ||||
| 8 | 2017-11-20 | ≈1 | ≈99 | Start-up and operation with mostly silica sand. |
| 9 | 2017-11-21 | ≈10 | ≈90 | Operation with ≈10% LD-slag. Lambda experiments. |
| 10 | 2017-11-22 | ≈30 | ≈70 | Operation with ≈30% LD-slag. Lambda experiments. |
| 11 | 2017-11-23 | ≈50 | ≈50 | Operation with ≈50% LD-slag. Lambda experiments. |
| 12 | 2017-11-24 | ≈50 | ≈50 | Conclusion, preparations for next campaign. |
| Sample | TA1 (°C) | TA2 (°C) | TA3 (°C) | Comments |
|---|---|---|---|---|
| LD-slag (fresh) | - | - | - | Successfully reached maximum temperature without problems. |
| 100% LD-slag (extracted 20171117) | - | - | - | Successfully reached maximum temperature without problems. Some micro-agglomerations noted during cleaning of reactor. |
| 30/70% mixture LD-slag/sand (extracted 20171122) | 1010 | 1060 | 1081 | Agglomerates and increased bulk density noted during cleaning of reactor. |
| Source of Ash | LD-Slag | Si-Sand |
|---|---|---|
| Secondary cyclone (kg/24 h) | 360 | 132 |
| Textile filter (kg/24 h) | 43 | 11 |
| Ash sweeping (kg/24 h) | 155 | 50 |
| Total (kg/24 h) | 558 | 193 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rydén, M.; Hanning, M.; Lind, F. Oxygen Carrier Aided Combustion (OCAC) of Wood Chips in a 12 MWth Circulating Fluidized Bed Boiler Using Steel Converter Slag as Bed Material. Appl. Sci. 2018, 8, 2657. https://doi.org/10.3390/app8122657
Rydén M, Hanning M, Lind F. Oxygen Carrier Aided Combustion (OCAC) of Wood Chips in a 12 MWth Circulating Fluidized Bed Boiler Using Steel Converter Slag as Bed Material. Applied Sciences. 2018; 8(12):2657. https://doi.org/10.3390/app8122657
Chicago/Turabian StyleRydén, Magnus, Malin Hanning, and Fredrik Lind. 2018. "Oxygen Carrier Aided Combustion (OCAC) of Wood Chips in a 12 MWth Circulating Fluidized Bed Boiler Using Steel Converter Slag as Bed Material" Applied Sciences 8, no. 12: 2657. https://doi.org/10.3390/app8122657
APA StyleRydén, M., Hanning, M., & Lind, F. (2018). Oxygen Carrier Aided Combustion (OCAC) of Wood Chips in a 12 MWth Circulating Fluidized Bed Boiler Using Steel Converter Slag as Bed Material. Applied Sciences, 8(12), 2657. https://doi.org/10.3390/app8122657





