A Review of the Applications of OCT for Analysing Pharmaceutical Film Coatings
Abstract
:1. Introduction
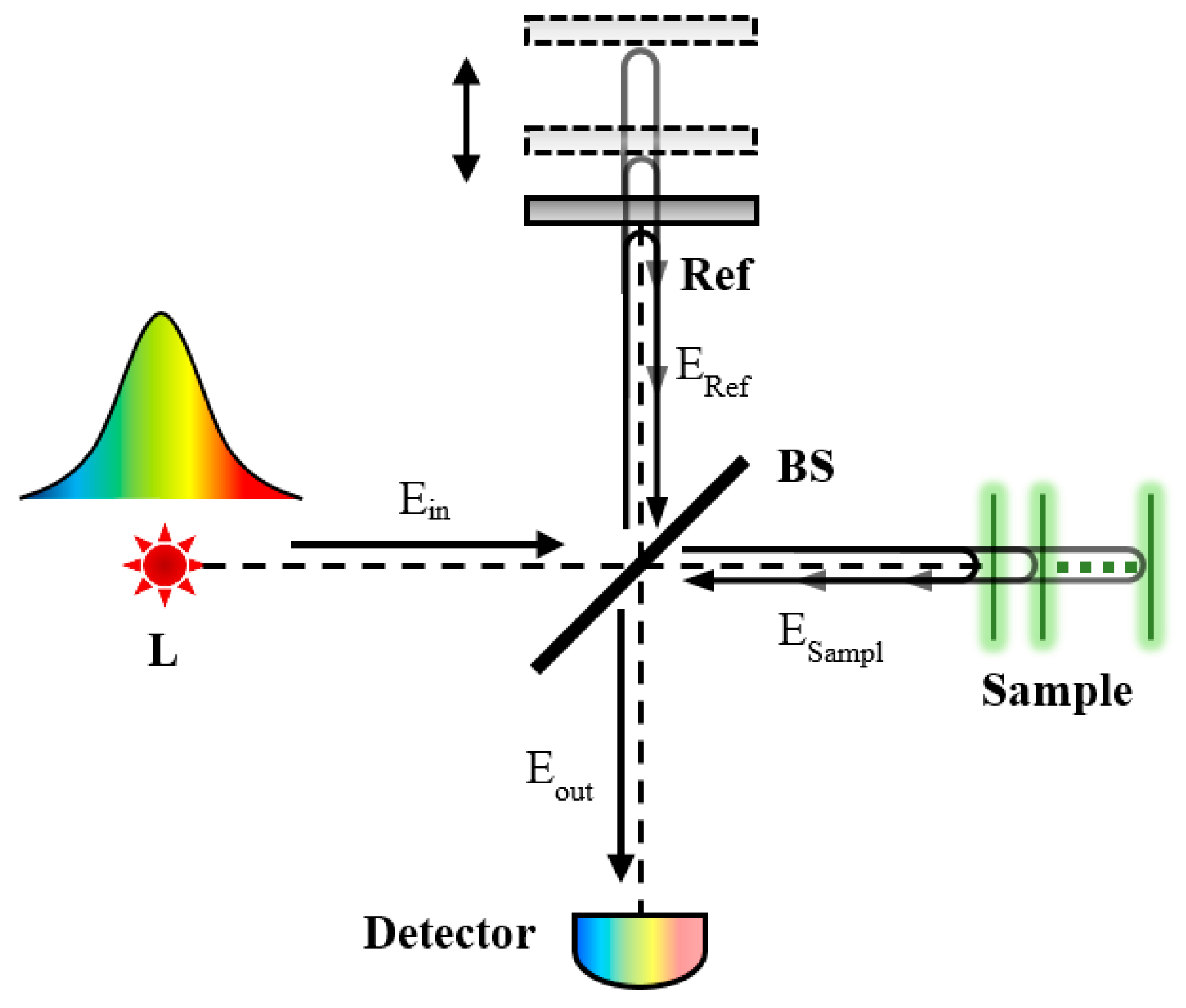
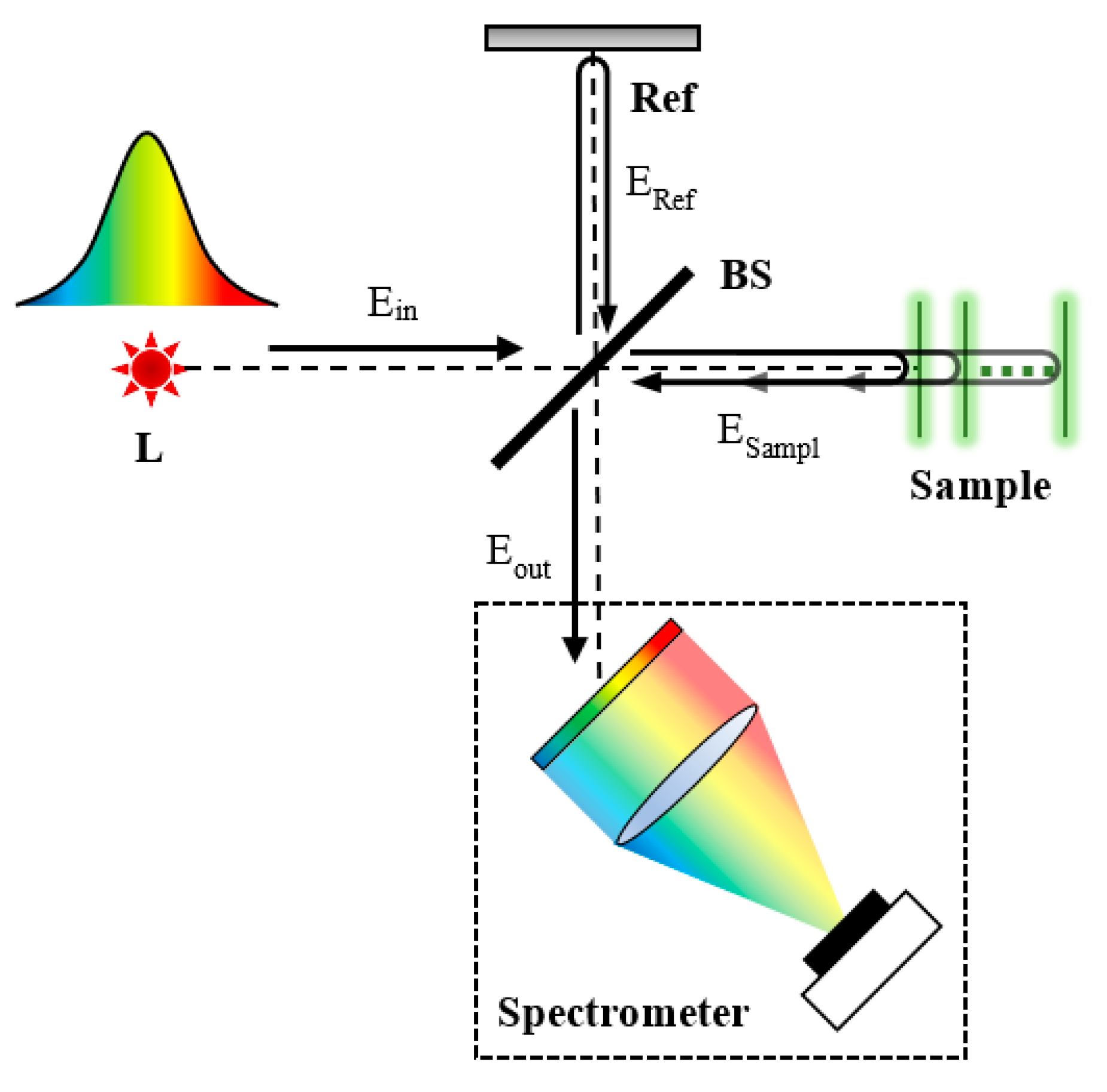
2. Overview of OCT Techniques
2.1. Time-Domain OCT
2.2. Fourier-Domain OCT
3. Pharmaceutical Film Coatings
3.1. Measurement Principle
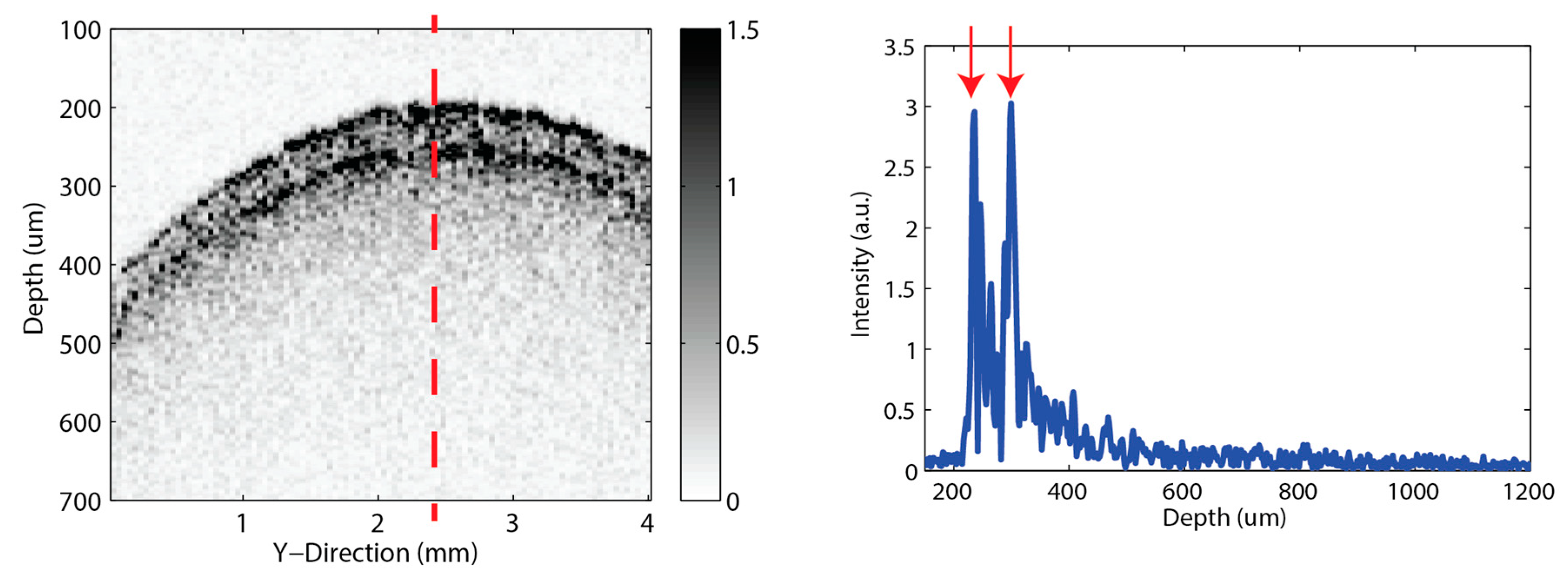
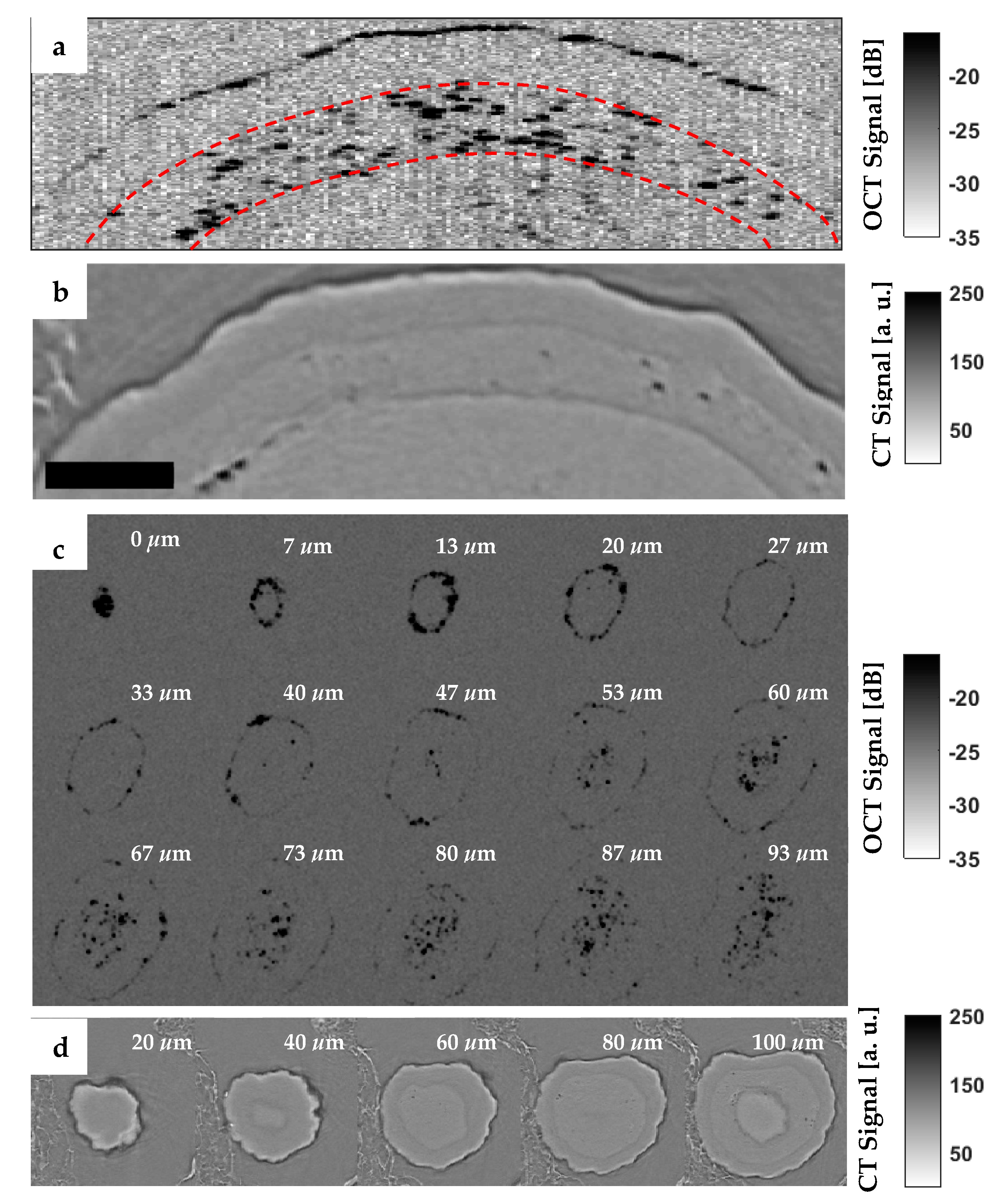
3.2. Off-Line Measurement
3.3. In-Line Measurement
4. Conclusions and Outlook
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Felton, L.A.; Porter, S.C. An update on pharmaceutical film coating for drug delivery. Expert Opin. Drug Deliv. 2013, 10, 421–435. [Google Scholar] [CrossRef] [PubMed]
- McGinity, J.W.; Felton, L.A. Aqueous Polymeric Coatings for Pharmaceutical Dosage Forms, 3rd ed.; Informa Healthcare: London, UK, 2008. [Google Scholar]
- Pandey, P.; Bharadwaj, R. Predictive Modeling of Pharmaceutical Unit Operations; Woodhead Publishing: Duxford, UK, 2017. [Google Scholar]
- Toschkoff, G.; Just, S.; Funke, A.; Djuric, D.; Knop, K.; Kleinebudde, P.; Scharrer, G.; Khinast, J.G. Spray models for discrete element simulations of particle coating processes. Chem. Eng. Sci. 2013, 101, 603–614. [Google Scholar] [CrossRef]
- Li, L.; Remmelgas, J.; Wachem, BG.; Corswant, C.; Johansson, M.; Folestad, S.; Rasmuson, A. Residence time distributions of different size particles in the spray zone of a Wurster fluid bed studied using DEM-CFD. Powder Technol. 2015, 280, 124–134. [Google Scholar] [CrossRef]
- Knop, K.; Kleinebudde, P. PAT-tools for process control in pharmaceutical film coating applications. Int. J. Pharm. 2013, 457, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Korasa, K.; Vrečer, F. Overview of PAT process analysers applicable in monitoring of film coating unit operations for manufacturing of solid oral dosage forms. Eur. J. Pharm. Sci. 2018, 111, 278–292. [Google Scholar] [CrossRef] [PubMed]
- Leitgeb, R.; Hitzenberger, C.; Fercher, A.F. Performance of fourier domain vs. Time domain optical coherence tomography. Opt. Express 2003, 11, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Fercher, A.F.; Drexler, W.; Hitzenberger, C.K.; Lasser, T. Optical coherence tomography-principles and applications. Rep. Prog. Phys. 2003, 66, 239. [Google Scholar] [CrossRef]
- Walther, J.; Gaertner, M.; Cimalla, P.; Burkhardt, A.; Kirsten, L.; Meissner, S.; Koch, E. Optical coherence tomography in biomedical research. Anal. Bioanal. Chem. 2011, 400, 2721–2743. [Google Scholar] [CrossRef]
- Tomlins, P.H.; Wang, R. Theory, developments and applications of optical coherence tomography. J. Phys. D Appl. Phys. 2005, 38, 2519. [Google Scholar] [CrossRef]
- Park, H.; Chodorow, M.; Kompfner, R. High resolution optical ranging system. Appl. Opt. 1981, 20, 2389–2394. [Google Scholar] [CrossRef]
- Fujimoto, J.G.; De Silvestri, S.; Ippen, E.P.; Puliafito, E.P.; Margolis, R.; Oseroff, A. Femtosecond optical ranging in biological systems. Opt. Lett. 1986, 11, 150–152. [Google Scholar] [CrossRef]
- Huang, D.; Wang, J.; Lin, C.P.; Puliafito, C.A.; Fujimoto, J.G. Micron-resolution ranging of cornea anterior chamber by optical reflectometry. Lasers Surg. Med. 1991, 11, 419–425. [Google Scholar] [CrossRef]
- Fercher, A.F.; Hitzenberger, C.K.; Kamp, G.; Elzaiat, S.Y. Measurement of intraocular distances by backscattering spectral interferometry. Opt. Commun. 1995, 117, 43–48. [Google Scholar] [CrossRef]
- Hausler, G.; Lindner, M.W. “Coherence radar” and “spectral radar”-new tools for dermatological diagnosis. J. Biomed. Opt. 1998, 3, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, T. Dynamic range of optical reflectometry with spectral interferometry. Jpn. J. Appl. Phys. 1999, 38, 6133. [Google Scholar] [CrossRef]
- Chinn, S.R.; Swanson, E.A.; Fujimoto, J.G. Optical coherence tomography using a frequency-tunable optical source. Opt. Lett. 1997, 22, 340–342. [Google Scholar] [CrossRef]
- Yun, S.H.; Tearney, G.J.; Bouma, B.E.; Park, B.H.; de Boer, J.F. High-speed spectral-domain optical coherence tomography at 1.3 µm wavelength. Opt. Express 2003, 11, 3598–3604. [Google Scholar] [CrossRef] [PubMed]
- Choma, M.A.; Sarunic, M.V.; Yang, C.H.; Izatt, J.A. Sensitivity advantage of swept source and Fourier domain optical coherence tomography. Opt. Express 2003, 11, 2183. [Google Scholar] [CrossRef]
- Schmitt, J.M. Optical coherence tomography (OCT): A review. IEEE J. Sel. Top. Quantum Electron. 1999, 5, 1205–1215. [Google Scholar] [CrossRef]
- Stifter, D. Beyond biomedicine: A review of alternative applications and developments for optical coherence tomography. Appl. Phys. B Lasers Opt. 2007, 88, 337–357. [Google Scholar] [CrossRef]
- Lin, H.; Dong, Y.; Shen, Y.C.; Zeitler, J.A. Quantifying pharmaceutical film coating with optical coherence tomography and terahertz pulsed imaging: An evaluation. J. Pharm. Sci. 2015, 104, 3377–3385. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Dong, Y.; Markl, D.; Williams, B.M.; Zheng, Y.; Shen, Y.C.; Zeitler, J.A. Measurement of the inter-tablet coating uniformity of a pharmaceutical pan coating process with combined terahertz and optical coherence tomography in-line sensing. J. Pharm. Sci. 2017, 106, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Mauritz, J.M.; Morrisby, R.S.; Hutton, R.S.; Legge, C.H.; Kaminski, C.F. Imaging pharmaceutical tablets with optical coherence tomography. J. Pharm. Sci. 2010, 99, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Shen, H.; Shen, Y.-C.; Zeitler, J.A.; Ho, L.; Evans, M.; Taday, P.F.; Pepper, M.; Rades, T.; Gordon, K.C.; et al. Noninvasive 3D Characterization using Terahertz Pulsed Imaging and Infrared Optical Coherence Tomography. In Proceedings of the 34th International Conference on Infrared, Millimeter, and Terahertz Waves (IRMMW-THz 2009), Busan, Korea, 21–25 September 2009. [Google Scholar]
- Zhong, S.; Shen, Y.-C.; Ho, L.; May, R.K.; Zeitler, J.A.; Evans, M.; Taday, P.F.; Pepper, M.; Rades, T.; Gordon, K.C.; et al. Non-destructive quantification of pharmaceutical tablet coatings using terahertz pulsed imaging and optical coherence tomography. Opt. Lasers Eng. 2011, 49, 361–365. [Google Scholar] [CrossRef] [Green Version]
- Koller, D.M.; Hannesschlager, G.; Leitner, M.; Khinast, J.G. Nondestructive analysis of tablet coatings with optical coherence tomography. Eur. J. Pharm. Sci. 2011, 44, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Markl, D.; Sacher, S.; Leitner, M.; Khinast, J.G.; Buchsbaum, A. Automated pharmaceutical tablet coating layer evaluation of optical coherence tomography images. Meas. Sci. Technol. 2015, 26, 035701. [Google Scholar] [CrossRef]
- Markl, D.; Hannesschläger, G.; Sacher, S.; Leitner, M.; Buchsbaum, A.; Pescod, R.; Baele, T.; Khinast, J.G. In-line monitoring of a pharmaceutical pan coating process by optical coherence tomography. J. Pharm. Sci. 2015, 104, 2531–2540. [Google Scholar] [CrossRef]
- Dong, Y.; Lin, H.; Abolghasemi, V.; Gan, L.; Zeitler, J.A.; Shen, Y.-C. Investigating Intra-Tablet Coating Uniformity With Spectral-Domain Optical Coherence Tomography. J. Pharm. Sci. 2017, 106, 546–553. [Google Scholar] [CrossRef]
- Juuti, M.; Tuononen, H.; Prykäri, T.; Kontturi, V.; Kuosmanen, M.; Alarousu, E.; Ketolainen, J.; Myllylä, R.; Peiponen, K.-E. Optical and terahertz measurement techniques for flat-faced pharmaceutical tablets: A case study of gloss, surface roughness and bulk properties of starch acetate tablets. Meas. Sci. Technol. 2009, 20, 015301. [Google Scholar] [CrossRef]
- Markl, D.; Wahl, P.; Pichler, H.; Sacher, S.; Khinast, J.G. Characterization of the coating and tablet core roughness by means of 3D optical coherence tomography. Int. J. Pharm. 2018, 536, 459–466. [Google Scholar] [CrossRef]
- Lin, H.; Dong, Y.; Markl, D.; Zhang, Z.; Shen, Y.C.; Zeitler, J.A. Pharmaceutical Film Coating Catalog for Spectral Domain Optical Coherence Tomography. J. Pharm. Sci. 2017, 106, 3171–3176. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zeitler, J.A.; Dong, Y.; Shen, Y.C. Non-destructive evaluation of polymer coating structures on pharmaceutical pellets using full-field optical coherence tomography. J. Pharm. Sci. 2014, 103, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Dubois, A.; Vabre, L.; Boccara, A.C.; Beaurepaire, E. High-resolution full-field optical coherence tomography with a linnik microscope. Appl. Opt. 2002, 41, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Dubois, A.; Grieve, K.; Moneron, G.; Lecaque, R.; Vabre, L.; Boccara, C. Ultrahigh-resolution full-field optical coherence tomography. Appl. Opt. 2004, 43, 2874–2883. [Google Scholar] [CrossRef] [PubMed]
- Markl, D.; Hannesschlager, G.; Sacher, S.; Leitner, M.; Khinast, J.G. Optical coherence tomography as a novel tool for in-line monitoring of a pharmaceutical film-coating process. Eur. J. Pharm. Sci. 2014, 55, 58–67. [Google Scholar] [CrossRef] [PubMed]
- May, R.K.; Evans, M.J.; Zhong, S.; Warr, I.; Gladden, L.F.; Shen, Y.C.; Zeitler, J.A. Terahertz in- line sensor for direct coating thickness measurement of individual tablets during film coating in real-time. J. Pharm. Sci. 2011, 100, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Markl, D.; Zettl, M.; Hannesschlager, G.; Sacher, S.; Leitner, M.; Buchsbaum, A.; Khinast, J.G. Calibration-free in-line monitoring of pellet coating processes via optical coherence tomography. Chem. Eng. Sci. 2015, 125, 200–208. [Google Scholar] [CrossRef]
- Pei, C.; Lin, H.; Markl, D.; Shen, Y.C.; Zeitler, J.A.; Elliott, J.A. A quantitative comparison of in-line coating thickness distributions obtained from a pharmaceutical tablet mixing process using discrete element method and terahertz pulsed imaging. Chem. Eng. Sci. 2018, 192, 34–45. [Google Scholar] [CrossRef]
- Lin, H.; Pei, C.; Markl, D.; Shen, Y.C.; Elliott, J.A.; Zeitler, J.A. Steps towards numerical verification of the terahertz in-line measurement of tablet mixing by means of discrete element modelling. IET Mcrow. Antenna Propag. 2018, 12, 1775–1779. [Google Scholar] [CrossRef]
- Wang, S.; Singh, M.; Lopez, A.L.; Wu, C.; Raghunathan, R.; Schill, A.; Li, J.; Larin, K.V.; Larina, I.V. Direct four-dimensional structural and functional imaging of cardiovascular dynamics in mouse embryos with 15 MHz optical coherence tomography. Opt. Lett. 2015, 40, 4791–4794. [Google Scholar] [CrossRef]

| Reference | Measurement Type | Light Source | Central Wavelength/FWHM Bandwidth (nm) | Axial Resolution (um) | Lateral Resolution (um) | Acquisition Rate |
|---|---|---|---|---|---|---|
| Juuti et al. (2009) [32] | Off-line | / | / | / | 5 | / |
| Mauritz et al. (2009) [25] | Off-line | Spectral radar OCT OCP930SR (Thorlabs) | 930/100 | 6.5 | 9 | 15 KHz |
| Swept source OCT Microscope OCM1300SS (Thorlabs) | 1325/130 | 12 | 15 | 16 KHz | ||
| Zhong et al. (2009, 2010) [26,27] | Off-line | Tungsten Halogen Lamp | 700/236 | 0.9 | / | / |
| Koller et al. (2011) [28] | Off-line | Supercontinuum Lasers (NKT Photonics) | 820/150 | <4 | 4.3 | 20 KHz |
| Li et al. (2013) [35] | Off-line (FF-OCT) | LED | 850/90 | 3.6 | 11 | 120 FPS |
| Markl et al. (2014) [38] | Off-line | TELESTOTM OCT (Thorlabs) | 1325/150 | 7.5 | / | / |
| SLD (Superlum Diode) | 830/62 | 4.9 | 9.51 | 27.8 KHz | ||
| Markl et al. (2015) [29] | Off-line | Supercontinuum Lasers (NKT Photonics) | 820/150 | 1.98 | 13.05 | 20 KHz |
| Lin et al. (2015) [23] | Off-line | SLD (EXALOS) | 840/55 | Theo: 5.7 | 16 | / |
| Dong et al. (2017) [31] | Off-line | SLD (EXALOS) | 844/131 | Theo: 2.4 | / | 45 KHz |
| Lin et al. (2017a) [34] | Off-line | SLD (EXALOS) | 840/55 | Theo: 5.7 | 20 | / |
| Markl et al. (2018) [33] | Off-line | SLD (Superlum Diode) | 832/75 | Theo: 4.1 | 10 | 59.2 KHz |
| Markl et al. (2015) [30] | In-line | SLD (Superlum Diode) | 832/75 | Theo: 4.1 | 14 | 59.2 KHz |
| Markl et al. (2015) [40] | In-line | SLD broadlighter (Superlum Diode Ltd.) | 830/62 | 4.9 | 9.51 | 27.8 KHz |
| Lin et al. (2017) [24] | In-line | SLD (EXALOS) | 840/55 | 7.5 | 20 | 14.3 KHz |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.; Zhang, Z.; Markl, D.; Zeitler, J.A.; Shen, Y. A Review of the Applications of OCT for Analysing Pharmaceutical Film Coatings. Appl. Sci. 2018, 8, 2700. https://doi.org/10.3390/app8122700
Lin H, Zhang Z, Markl D, Zeitler JA, Shen Y. A Review of the Applications of OCT for Analysing Pharmaceutical Film Coatings. Applied Sciences. 2018; 8(12):2700. https://doi.org/10.3390/app8122700
Chicago/Turabian StyleLin, Hungyen, Zijian Zhang, Daniel Markl, J. Axel Zeitler, and Yaochun Shen. 2018. "A Review of the Applications of OCT for Analysing Pharmaceutical Film Coatings" Applied Sciences 8, no. 12: 2700. https://doi.org/10.3390/app8122700
APA StyleLin, H., Zhang, Z., Markl, D., Zeitler, J. A., & Shen, Y. (2018). A Review of the Applications of OCT for Analysing Pharmaceutical Film Coatings. Applied Sciences, 8(12), 2700. https://doi.org/10.3390/app8122700








