Gelatin-Enabled Microsensor for Pancreatic Trypsin Sensing
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. IDE Sensor Fabrication and Film Deposition
2.2. Enzyme Solution Preparation
2.3. Impedance Sensing
2.4. QCM Sensing
2.5. Image/Data Analysis
3. Results
3.1. IDE Sensor and Film Structure and Morphology
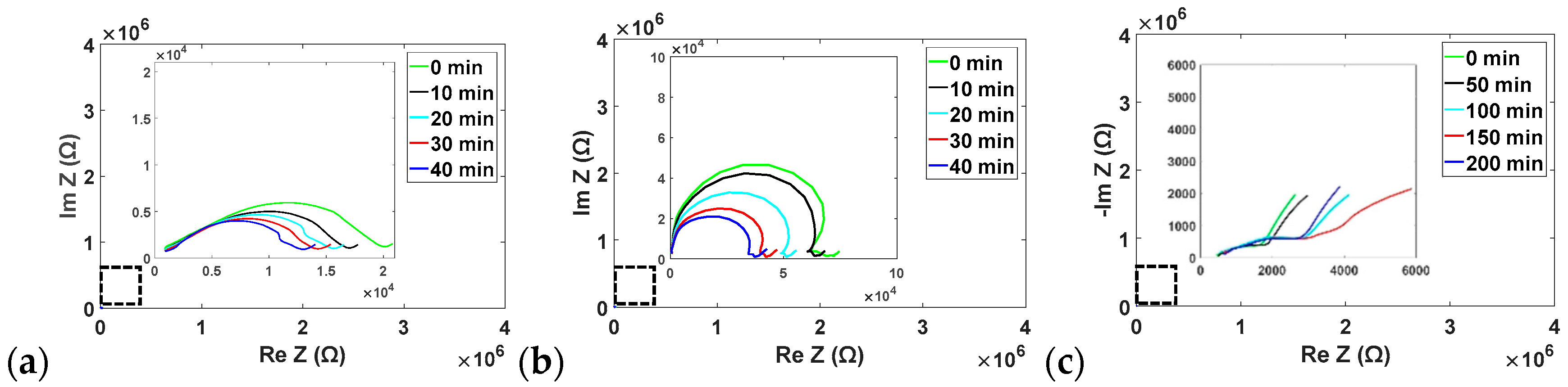
3.2. Trypsin Impedance Results
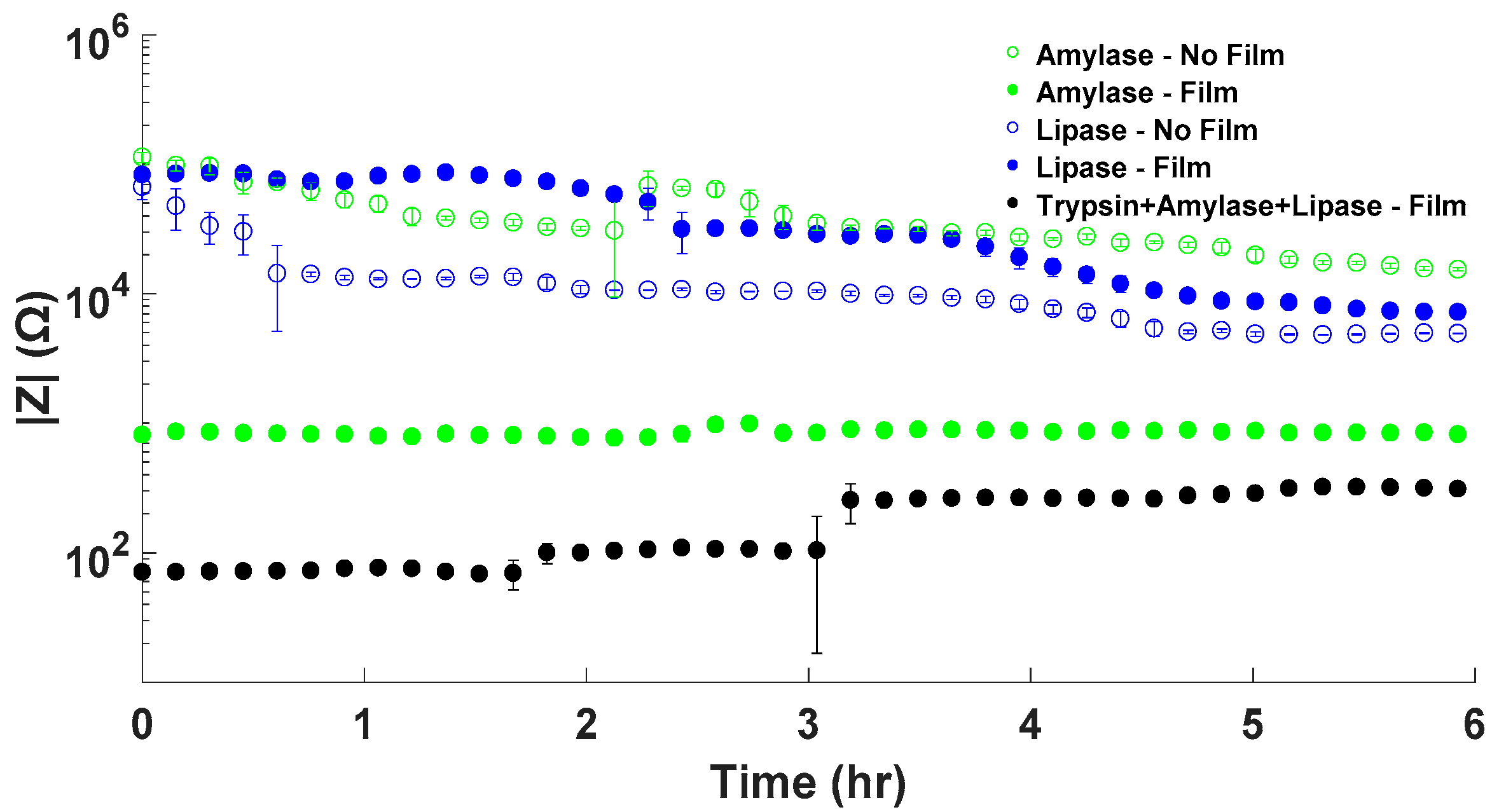
3.3. Enzyme Mixture Impedance Results
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pandol, S.J. The Exocrine Pancreas. Morgan Claypool Life Sci. 2010. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, M.; Imoto, M.; Dimagno, E.P. Rapid endoscopic secretin stimulation test and discrimination of chronic pancreatitis and pancreatic cancer from disease controls. Clin. Gastroenterol. Hepatol. 2003, 1, 397–403. [Google Scholar] [CrossRef]
- Goldberg, D.M.; Wormsley, K.G. The interrelationships of pancreatic enzymes in human duodenal aspirate. Gut 1970, 11, 859–866. [Google Scholar] [CrossRef] [PubMed]
- James, O. The Lundh test. Gut 1973, 14, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.-H.; Lin, Y.-C.; Chen, W.-L.; Chen, Y.-H.; Chen, Y.-X.; Chen, C.-M.; Shiu, H.W.; Chang, L.-Y.; Chen, C.-H.; Chen, C.-H. Detecting trypsin at liquid crystal/aqueous interface by using surface-immobilized bovine serum albumin. Biosens. Bioelectron. 2016, 78, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Qin, H.; Cui, W.; Zhou, Y.; Du, J. Label–free, turn–on fluorescent sensor for trypsin activity assay and inhibitor screening. Talanta 2016, 161, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Neff, P.A.; Serr, A.; Wunderlich, B.K.; Bausch, A.R. Label-Free Electrical Determination of Trypsin Activity by a Silicon-on-Insulator Based Thin Film Resistor. ChemPhysChem 2007, 8, 2133–2137. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Alibakhshi, M.A.; Kim, D.-K.; Brown, C.M.; Craik, C.S.; Majumdar, A. Label-Free Electrical Detection of Enzymatic Reactions in Nanochannels. ACS Nano 2016, 10, 7476–7484. [Google Scholar] [CrossRef] [PubMed]
- Stoytcheva, M.; Zlatev, R.; Cosnier, S.; Arredondo, M.; Valdez, B. High sensitive trypsin activity evaluation applying a nanostructured QCM-sensor. Biosens. Bioelectron. 2013, 41, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Eastoe, J.E. The amino acid composition of mammalian collagen and gelatin. Biochem. J. 1955, 61, 589. [Google Scholar] [CrossRef] [PubMed]
- Li, M. Thin Films of Stimuli-Responsive Hydrogels. Ph.D. Thesis, Université Pierre et Marie Curie-Paris VI, Paris, France, 18 June 2015. [Google Scholar]
- Yeo, Y.; Geng, W.; Ito, T.; Kohane, D.S.; Burdick, J.A.; Radisic, M. Photocrosslinkable hydrogel for myocyte cell culture and injection. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 81, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Schyrr, B.; Boder-Pasche, S.; Ischer, R.; Smajda, R.; Voirin, G. Fiber-optic protease sensor based on the degradation of thin gelatin films. Sens. Biosens. Res. 2015, 3, 65–73. [Google Scholar] [CrossRef]
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Rubini, K.; Roveri, N. Mechanical and thermal properties of gelatin films at different degrees of glutaraldehyde crosslinking. Biomaterials 2001, 22, 763–768. [Google Scholar] [CrossRef]
- Zang, F.; Gerasopoulos, K.; Fan, X.Z.; Brown, A.D.; Culver, J.N.; Ghodssi, R. Real-time monitoring of macromolecular biosensing probe self-assembly and on-chip ELISA using impedimetric microsensors. Biosens. Bioelectron. 2016, 81, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.W.; Meyer, M.T.; Berkovich, A.; Subramanian, S.; Iliadis, A.A.; Bentley, W.E.; Ghodssi, R. A surface acoustic wave biofilm sensor integrated with a treatment method based on the bioelectric effect. Sens. Actuators Phys. 2016, 238, 140–149. [Google Scholar] [CrossRef]
- Kim, Y.W.; Sardari, S.E.; Meyer, M.T.; Iliadis, A.A.; Wu, H.C.; Bentley, W.E.; Ghodssi, R. An ALD aluminum oxide passivated Surface Acoustic Wave sensor for early biofilm detection. Sens. Actuators B Chem. 2012, 163, 136–145. [Google Scholar] [CrossRef]
- Jaiswal, N.; Prakash, O.; Talat, M.; Hasan, S.H.; Pandey, R.K. α-Amylase immobilization on gelatin: Optimization of process variables. J. Genet. Eng. Biotechnol. 2012, 10, 161–167. [Google Scholar] [CrossRef]
- Alteriis, E.D.; Parascandola, P.; Salvadore, S.; Scardi, V. Enzyme immobilisation within insolubilised gelatin. J. Chem. Technol. Biotechnol. Biotechnol. 1985, 35, 60–64. [Google Scholar] [CrossRef]
- Fadnavis, N.W.; Sheelu, G.; Kumar, B.M.; Bhalerao, M.U.; Deshpande, A.A. Gelatin Blends with Alginate: Gels for Lipase Immobilization and Purification. Biotechnol. Prog. 2003, 19, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, N.; Prakash, O. Immobilization of Soybean α-amylase on Gelatin and its Application as a Detergent Additive. Asian J. Biochem. 2011, 6, 337–346. [Google Scholar] [CrossRef]
- Caldwell, M.L.; Dickey, E.S.; Hanrahan, V.M.; Kung, H.C.; Kung, J.T.; Misko, M. Amino Acid Composition of Crystalline Pancreatic Amylase from Swine1. J. Am. Chem. Soc. 1954, 76, 143–147. [Google Scholar] [CrossRef]
- Bianchetta, J.D.; Bidaud, J.; Guidoni, A.A.; Bonicel, J.J.; Rovery, M. Porcine Pancreatic Lipase. Eur. J. Biochem. 1979, 97, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Thimann, K.V. The Effect of Salts on the Ionisation of Gelatin. J. Gen. Physiol. 1930, 14, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Barsoukov, E.; Macdonald, R. Impedance Spectroscopy: Theory, Experiment, and Applications, 2nd ed.; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Daniels, J.S.; Pourmand, N. Label-Free Impedance Biosensors: Opportunities and Challenges. Electroanalysis 2007, 19, 1239–1257. [Google Scholar] [CrossRef] [PubMed]
- Erwanto, Y.; Kawahara, S.; Katayama, K.; Takenoyama, S.; Fujino, H.; Yamauchi, K.; Morishita, T.; Kai, Y.; Watanabe, S.; Muguruma, M. Microbial Transglutaminase Modifies Gel Properties of Porcine Collagen. Asian-Australas. J. Anim. Sci. 2003, 16, 269–276. [Google Scholar] [CrossRef]
- Franson, K.; Rössner, S. Fat intake and food choices during weight reduction with diet, behavioural modification and a lipase inhibitor. J. Intern. Med. 2000, 247, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Konig, V.; Vertesy, L.; Schneider, T.R. Structure of the alpha-amylase inhibitor tendamistat at 0.93 A. Acta Crystallogr. 2003, 59, 1737–1743. [Google Scholar]
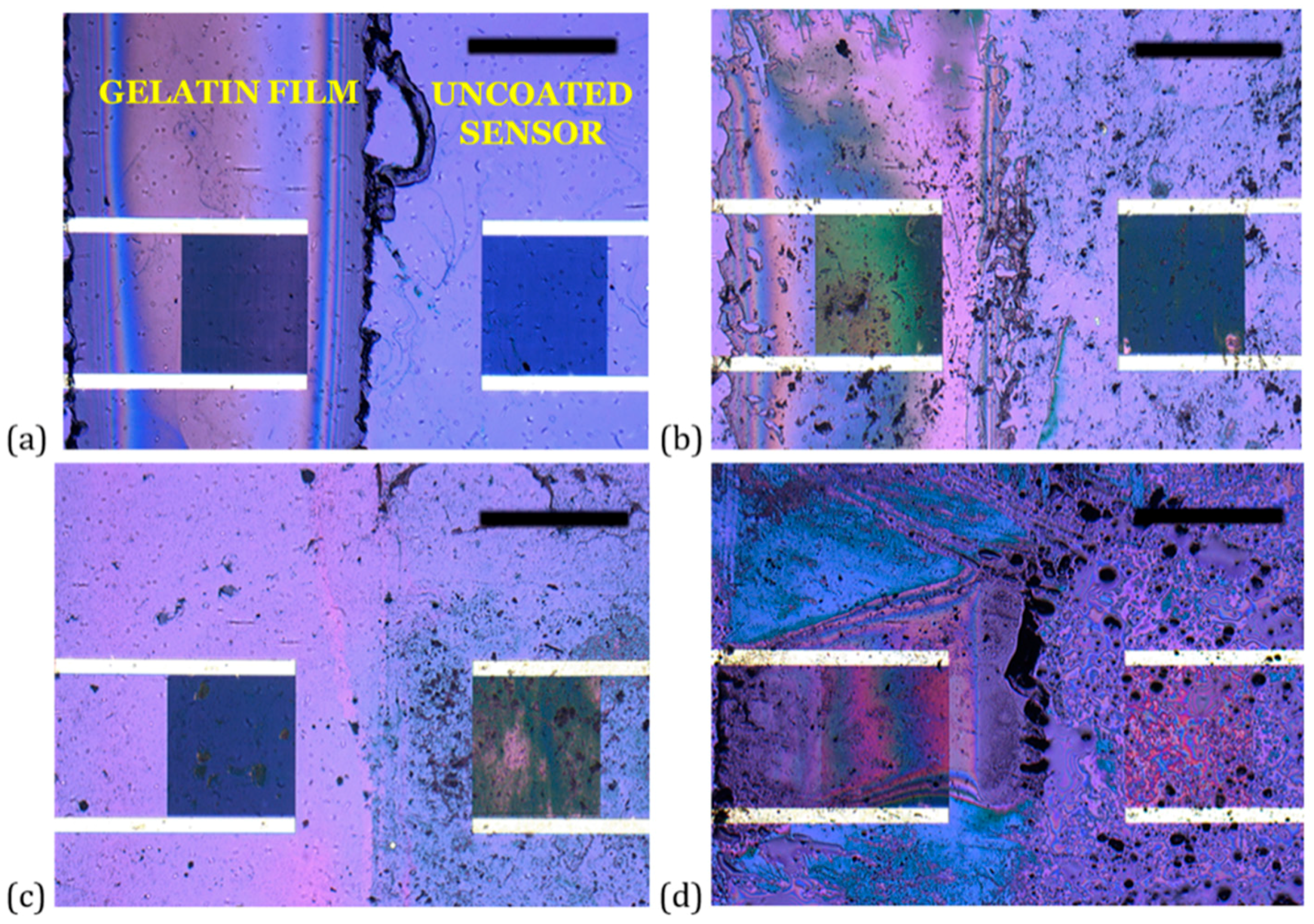
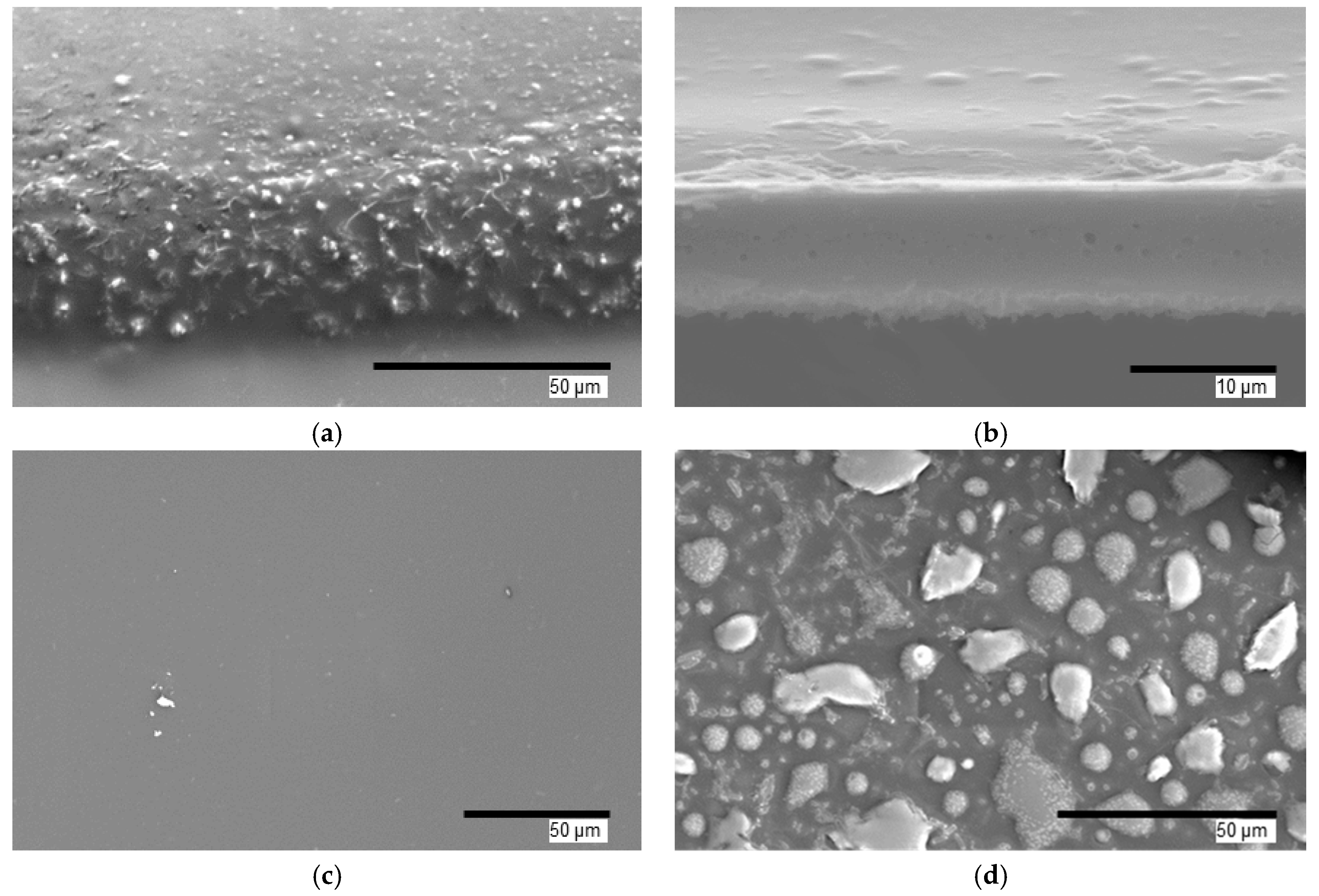
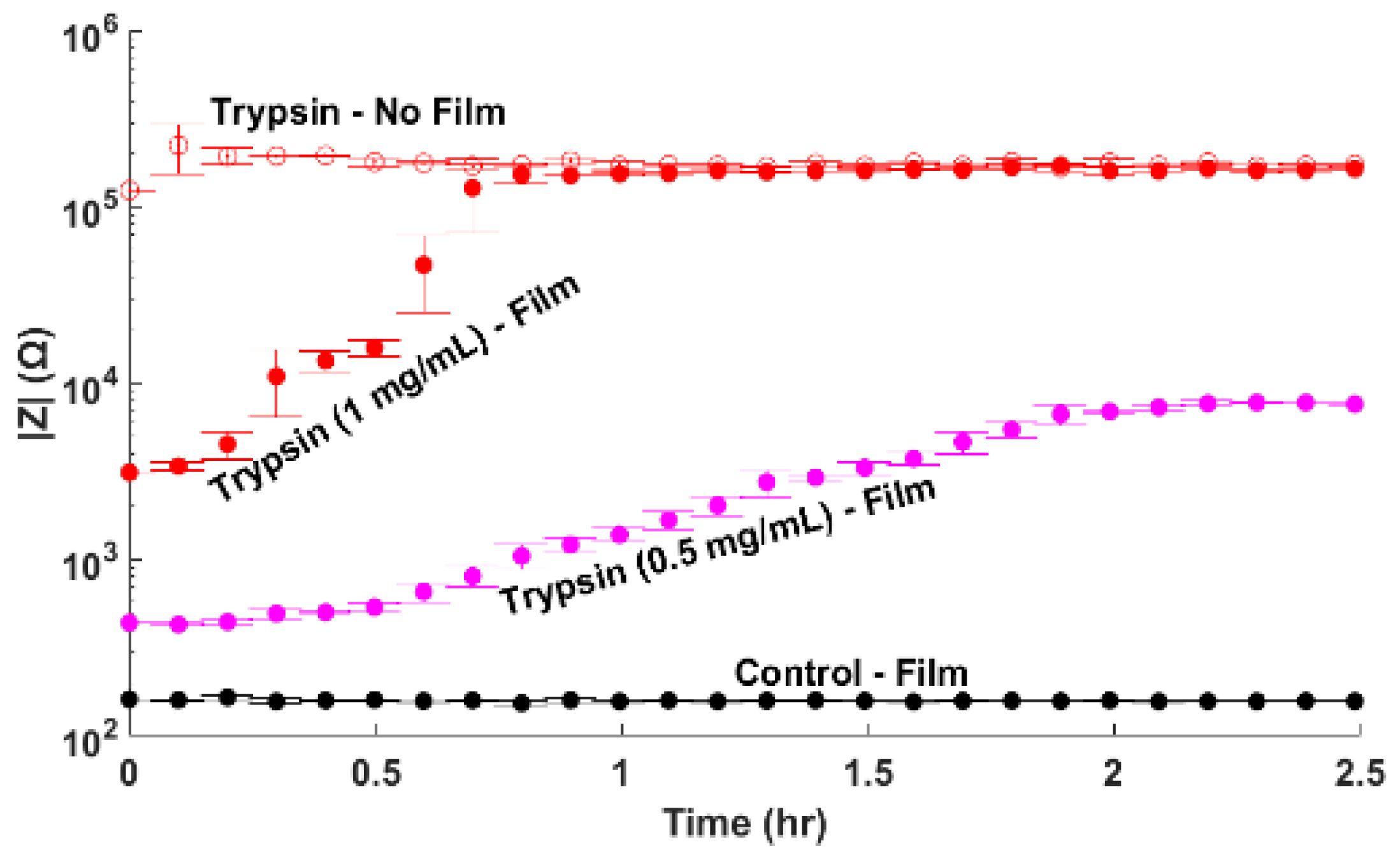
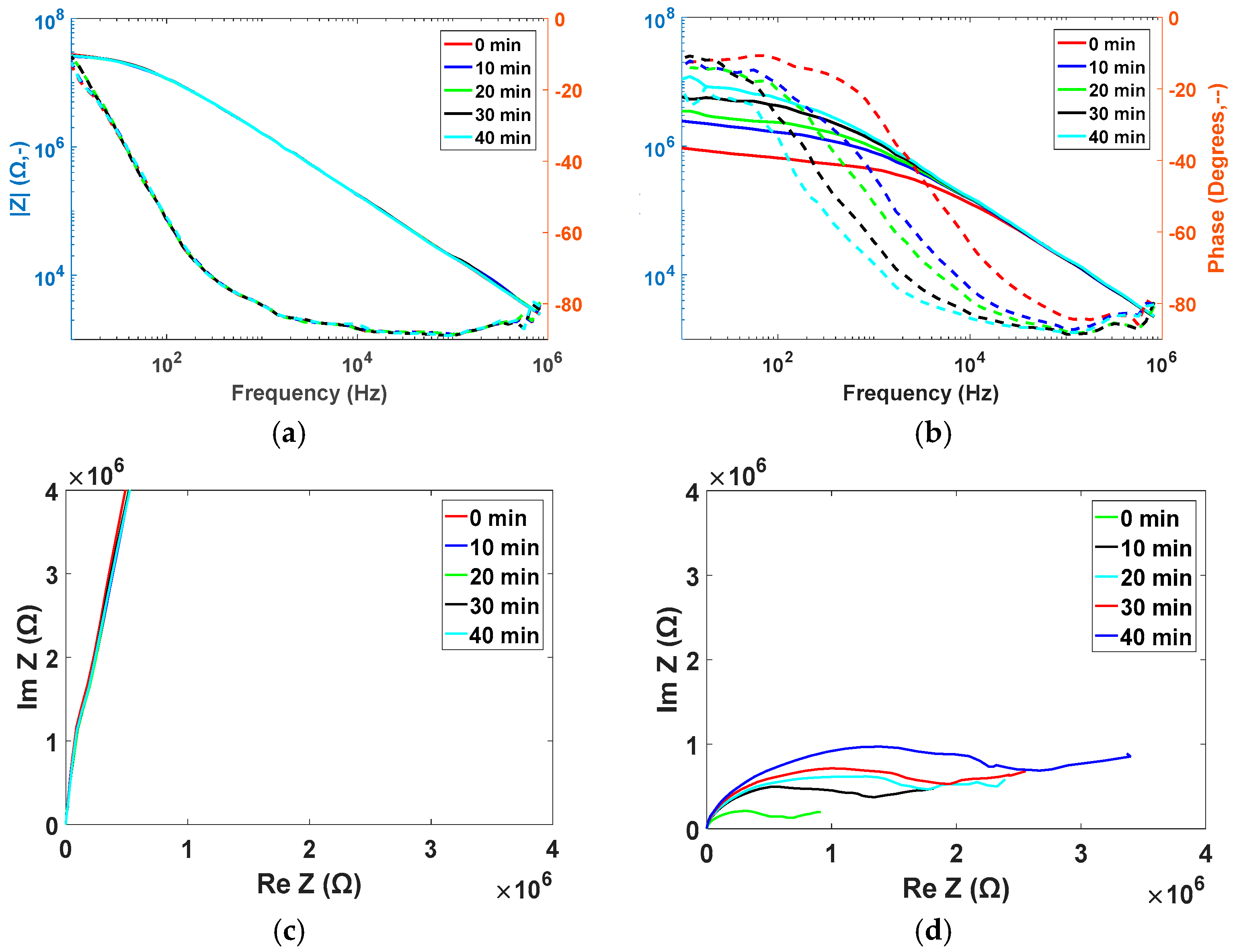
| Condition | Film Thickness Remaining (%) |
|---|---|
| PBS (Phosphate Buffered Saline) | 78.6 ± 0.05 |
| Amylase (1 mg/mL) | 24.3 ± 0.08 |
| Lipase (1 mg/mL) | 52.4 ± 0.30 |
| Trypsin (1 mg/mL) | 11.1 ± 0.03 |
| Trypsin (0.5 mg/mL) | 21.7 ± 0.10 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banis, G.; Beardslee, L.A.; Ghodssi, R. Gelatin-Enabled Microsensor for Pancreatic Trypsin Sensing. Appl. Sci. 2018, 8, 208. https://doi.org/10.3390/app8020208
Banis G, Beardslee LA, Ghodssi R. Gelatin-Enabled Microsensor for Pancreatic Trypsin Sensing. Applied Sciences. 2018; 8(2):208. https://doi.org/10.3390/app8020208
Chicago/Turabian StyleBanis, George, Luke A. Beardslee, and Reza Ghodssi. 2018. "Gelatin-Enabled Microsensor for Pancreatic Trypsin Sensing" Applied Sciences 8, no. 2: 208. https://doi.org/10.3390/app8020208
APA StyleBanis, G., Beardslee, L. A., & Ghodssi, R. (2018). Gelatin-Enabled Microsensor for Pancreatic Trypsin Sensing. Applied Sciences, 8(2), 208. https://doi.org/10.3390/app8020208





