Interleukin 6 Plays a Role in the Migration of Magnetically Levitated Mesenchymal Stem Cells Spheroids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Expansion Cell Culture
2.2. μ-Slide Chemotaxis 2D
2.3. Preparation of Array Membrane Samples
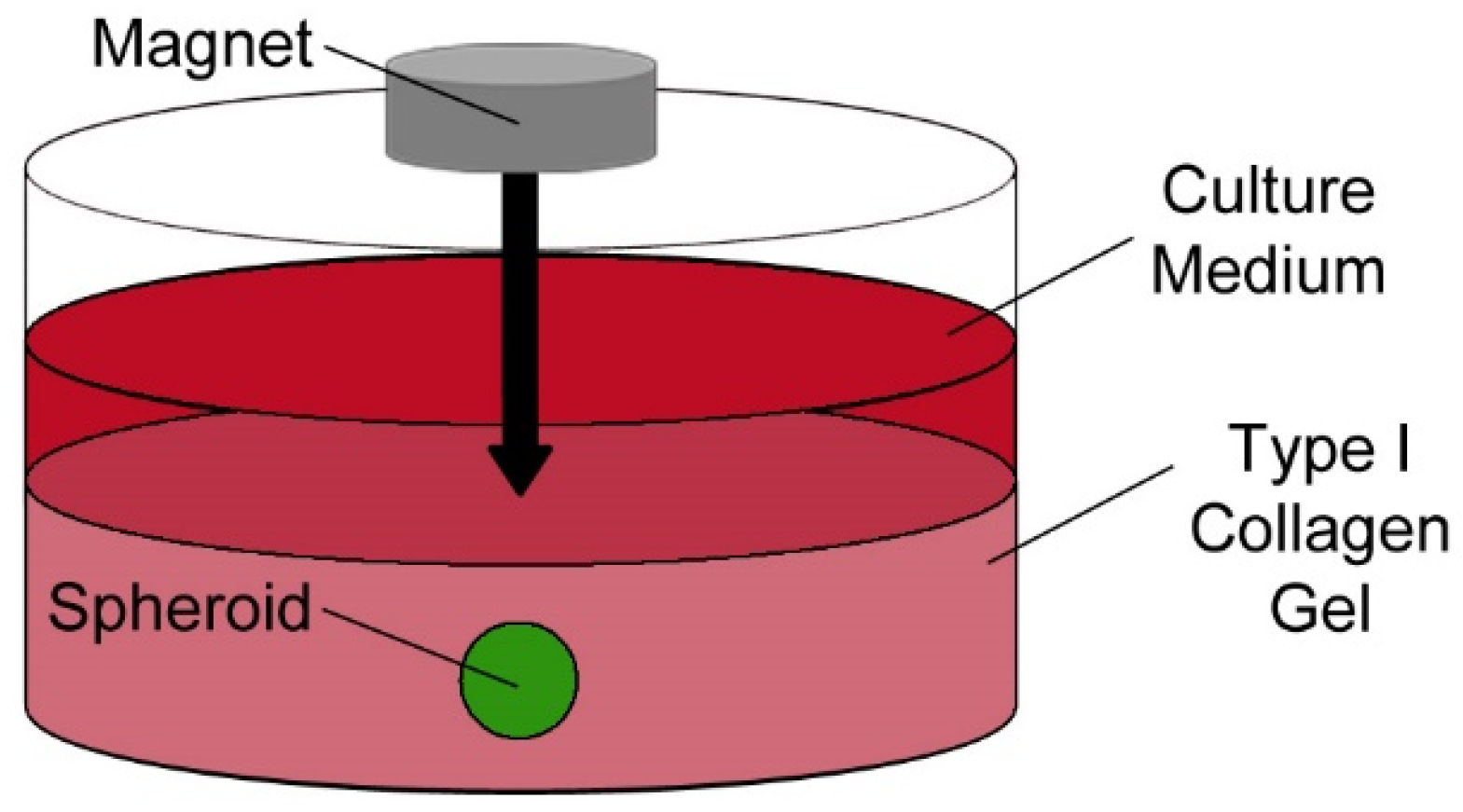
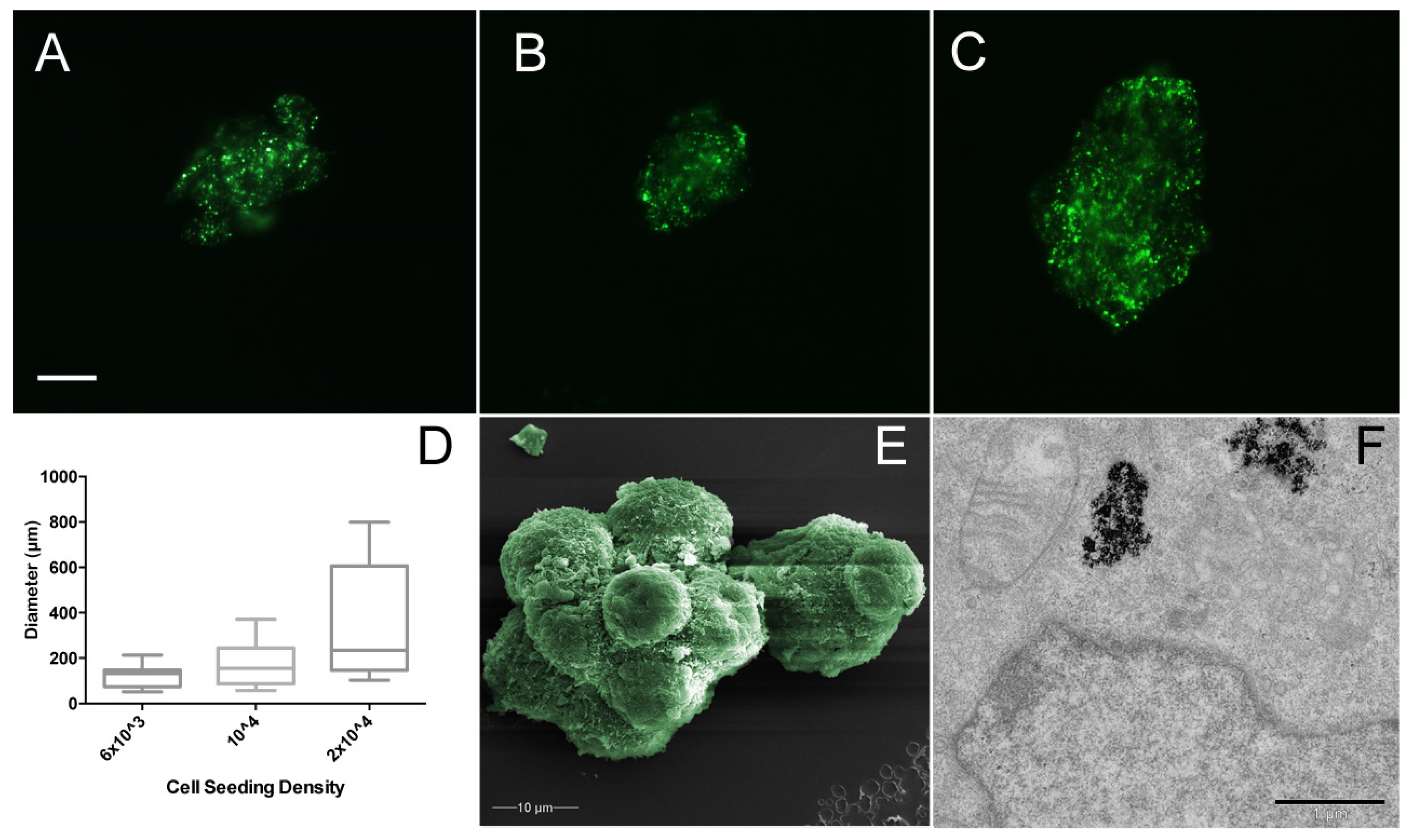
2.4. Generation of Three-Dimensional Spheroid Model System
2.5. Collagen Gel Preparation
2.6. Electron Microscopy Preparation
2.7. Inducing Migration and Preparation of Samples
2.8. Abcam Human MMP Antibody Array Membranes
3. Results and Discussion
3.1. MMP-1 and MMP-3 Facilitate MSC IL-6-Induced Migration in Monolayer
3.2. Magnetic Nanoparticles Enable MSC Spheroid Culture
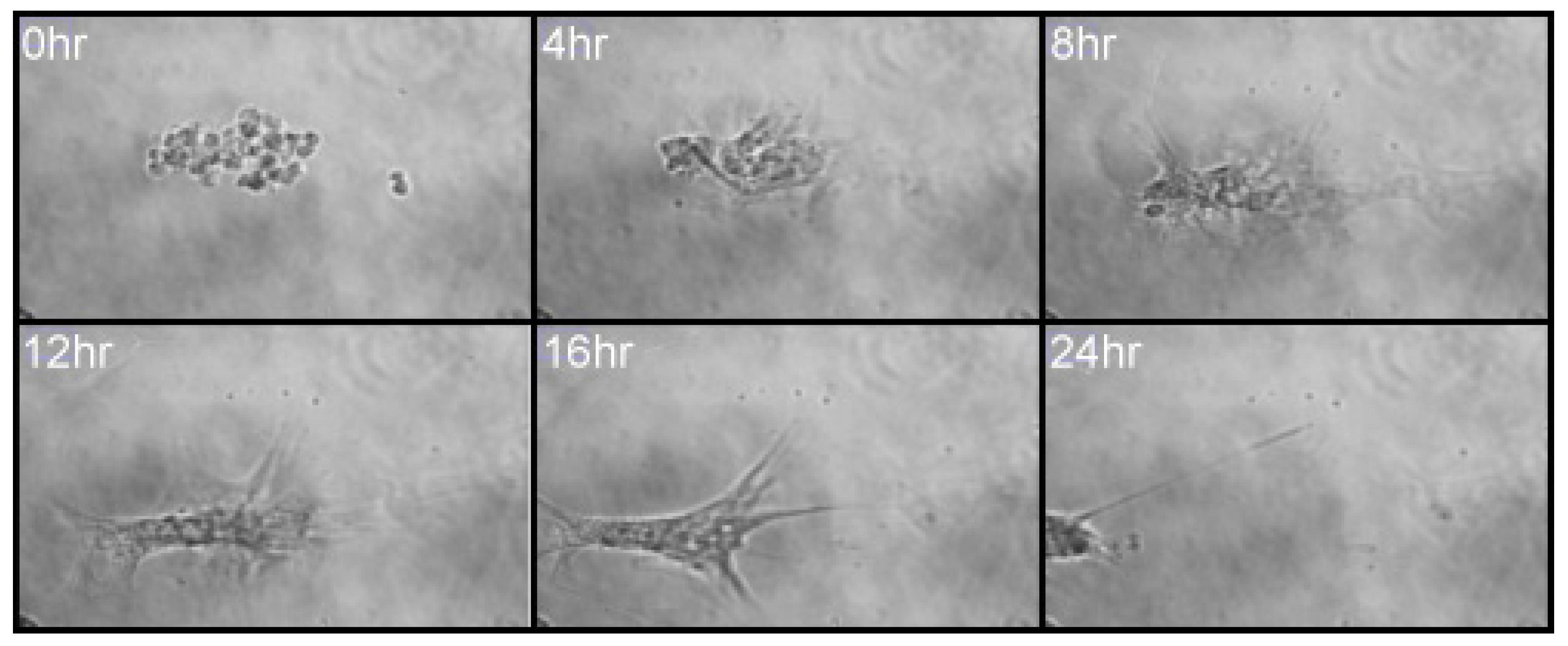
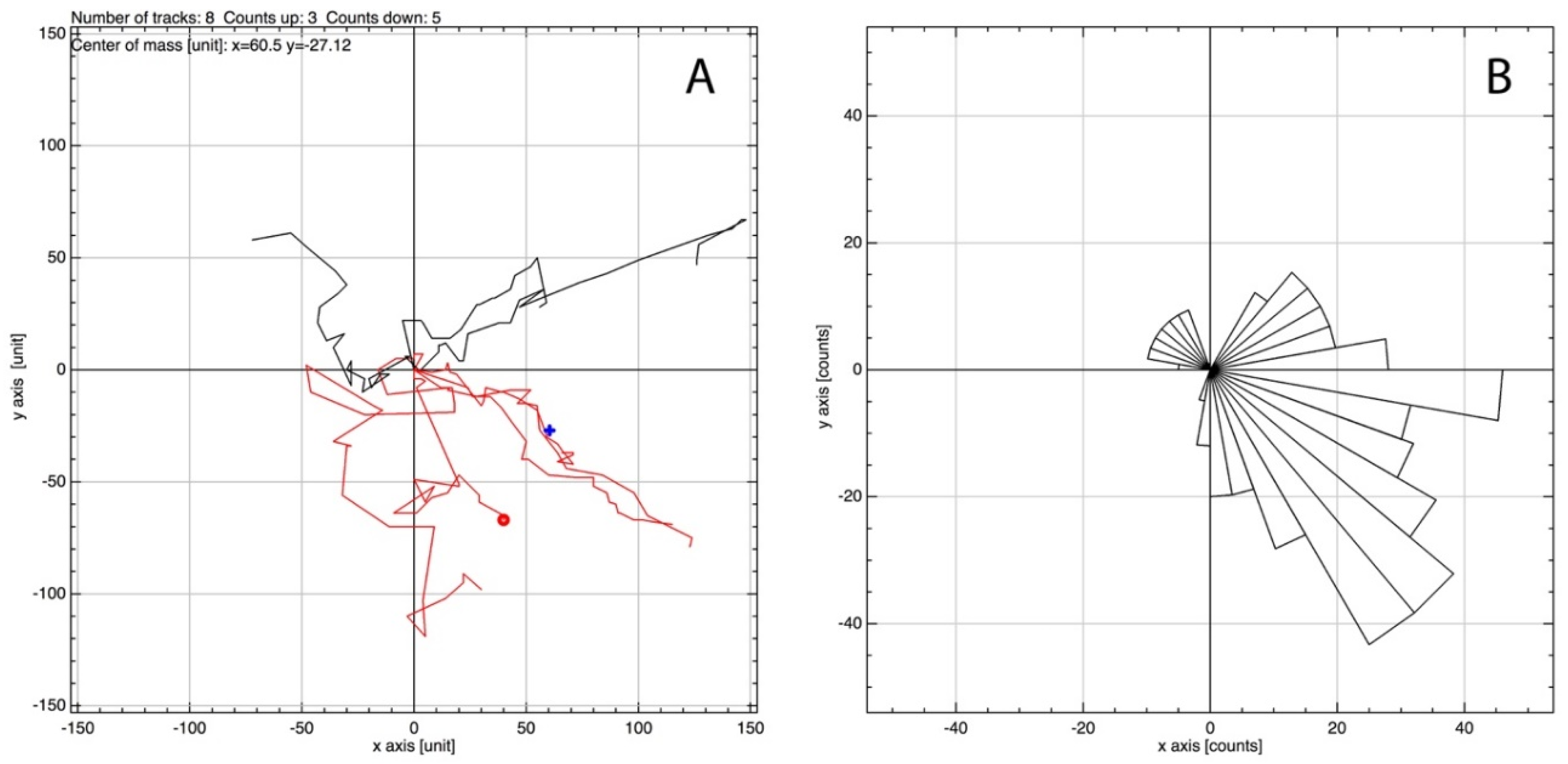
3.3. MSCs Migrate in Response to IL-6 in Three-Dimensional Spheroid Culture
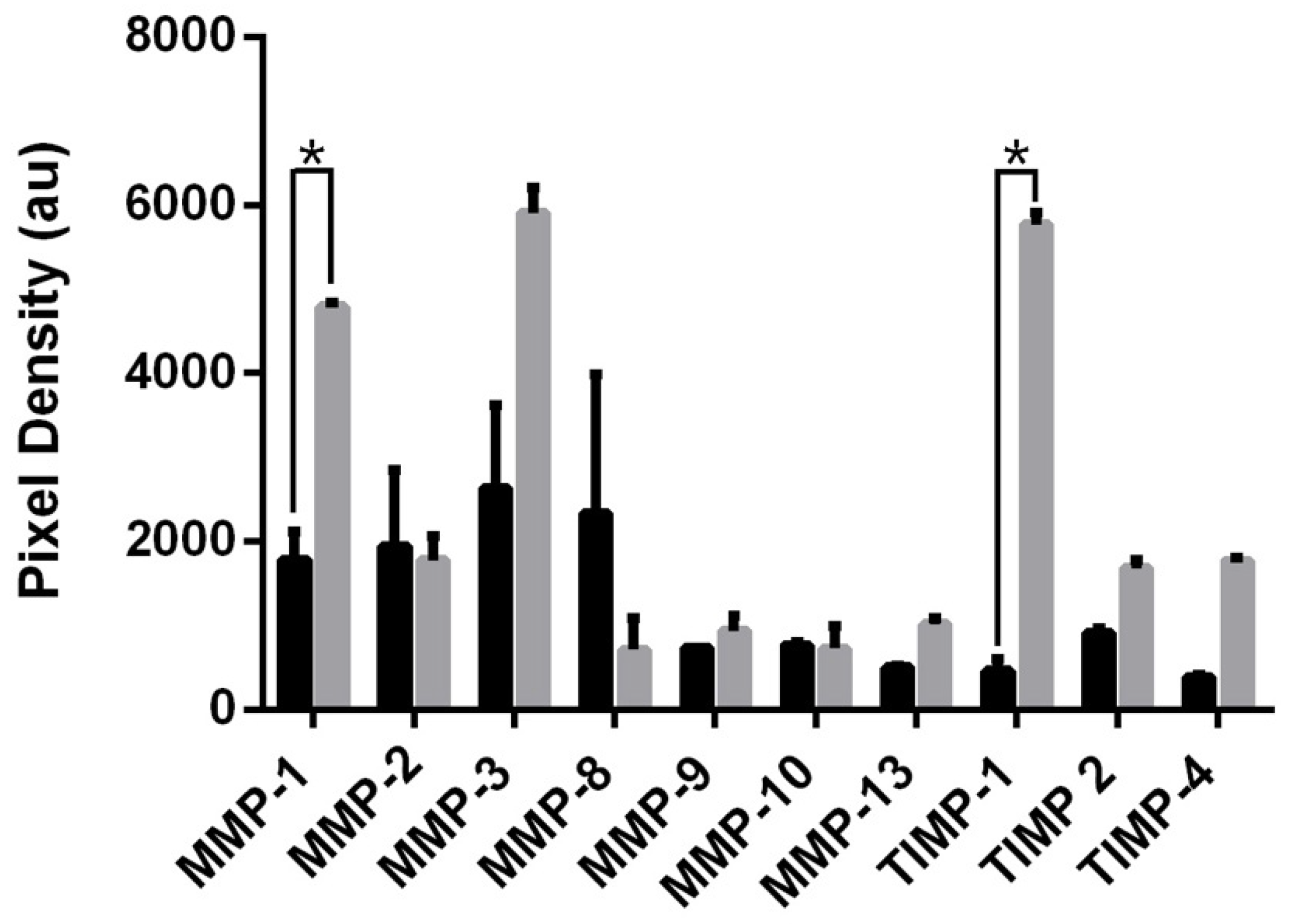
3.4. MMP-2 and MMP-8 Facilitate MSC IL-6-Induced Migration in 3D Spheroid Culture
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nadig, R.R. Stem cell therapy—Hype or hope? A review. J. Conserv. Dent. JCD 2009, 12, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I.; Bruder, S.P. Mesenchymal stem cells: Building blocks for molecular medicine in the 21st century. Trends Mol. Med. 2001, 7, 259–264. [Google Scholar] [CrossRef]
- Sohni, A.; Verfaillie, C.M. Mesenchymal stem cells migration homing and tracking. Stem Cells Int. 2013, 2013, 130763. [Google Scholar] [CrossRef] [PubMed]
- Baksh, D.; Song, L.; Tuan, R.S. Adult mesenchymal stem cells: Characterization, differentiation, and application in cell and gene therapy. J. Cell. Mol. Med. 2004, 8, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Bühring, H.J.; Battula, V.L.; Treml, S.; Schewe, B.; Kanz, L.; Vogel, W. Novel markers for the prospective isolation of human MSC. Ann. N. Y. Acad. Sci. 2007, 1106, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.L.B.K.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Bonab, M.M.; Alimoghaddam, K.; Talebian, F.; Ghaffari, S.H.; Ghavamzadeh, A.; Nikbin, B. Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 2006, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Meirelles, L.; Caplan, A.I.; Nardi, N.B. In search of the in vivo identity of mesenchymal stem cells. Stem Cells 2008, 26, 2287–2299. [Google Scholar] [CrossRef] [PubMed]
- Frenette, P.S.; Pinho, S.; Lucas, D.; Scheiermann, C. Mesenchymal stem cell: Keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annu. Rev. Immunol. 2013, 31, 285–316. [Google Scholar] [CrossRef] [PubMed]
- Bara, J.J.; Richards, R.G.; Alini, M.; Stoddart, M.J. Concise review: Bone marrow-derived mesenchymal stem cells change phenotype following in vitro culture: Implications for basic research and the clinic. Stem Cells 2014, 32, 1713–1723. [Google Scholar] [CrossRef] [PubMed]
- Ehninger, A.; Trumpp, A. The bone marrow stem cell niche grows up: Mesenchymal stem cells and macrophages move in. J. Exp. Med. 2011, 208, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.E.L.; Wheadon, H.; Lewis, N.; Yang, J.; Mullin, M.; Hursthouse, A.; Stirling, D.; Dalby, M.J.; Berry, C.C. A quiescent, regeneration-responsive tissue engineered mesenchymal stem cell bone marrow niche model via magnetic levitation. ACS Nano 2016, 10, 8346–8354. [Google Scholar] [CrossRef] [PubMed]
- Hannoush, E.J.; Sifri, Z.C.; Elhassan, I.O.; Mohr, A.M.; Alzate, W.D.; Offin, M.; Livingston, D.H. Impact of enhanced mobilization of bone marrow derived cells to site of injury. J. Trauma Acute Care Surg. 2011, 71, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Yong, X.; Li, C.; Lü, M.; Liu, D.; Chen, L.; Hu, J.; Teng, M.; Zhang, D.; Fan, Y.; et al. CXCL12/CXCR4 axis promotes mesenchymal stem cell mobilization to burn wounds and contributes to wound repair. J. Surg. Res. 2013, 183, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Bhadriraju, K.; Chen, C.S. Engineering cellular microenvironments to improve cell-based drug testing. Drug Discov. Today 2002, 7, 612–620. [Google Scholar] [CrossRef]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Antoni, D.; Burckel, H.; Josset, E.; Noel, G. Three-dimensional cell culture: A breakthrough in vivo. Int. J. Mol. Sci. 2015, 16, 5517–5527. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.E.; Child, H.W.; Hursthouse, A.; Stirling, D.; McCully, M.; Paterson, D.; Mullin, M.; Berry, C.C. The influence of particle size and static magnetic fields on the uptake of magnetic nanoparticles into three-dimensional cell-seeded collagen gel cultures. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Berry, C.C.; Curtis, A.S. Functionalisation of magnetic nanoparticles for applications in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R198. [Google Scholar] [CrossRef]
- Khmara, I.; Koneracka, M.; Kubovcikova, M.; Zavisova, V.; Antal, I.; Csach, K.; Kopcansky, P.; Vidlickova, I.; Csaderova, L.; Pastorekova, S.; et al. Preparation of poly-l-lysine functionalized magnetic nanoparticles and their influence on viability of cancer cells. J. Magn. Magn. Mater. 2017, 427, 114–121. [Google Scholar] [CrossRef]
- Yagi, H.; Soto-Gutierrez, A.; Kitagawa, Y.; Yarmush, M.L. Mesenchymal Stem Cell Therapy: Immunomodulation and Homing Mechanisms. In Stem Cells and Cancer Stem Cells; Springer: Dordrecht, The Netherlands, 2012; Volume 8, pp. 91–104. [Google Scholar]
- Maxson, S.; Lopez, E.A.; Yoo, D.; Danilkovitch-Miagkova, A.; LeRoux, M.A. Concise review: Role of mesenchymal stem cells in wound repair. Stem Cells Transl. Med. 2012, 1, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Roux, S.; Orcel, P. Bone loss: Factors that regulate osteoclast differentiation-an update. Arthritis Res. Ther. 2000, 2, 451–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshitake, F.; Itoh, S.; Narita, H.; Ishihara, K.; Ebisu, S. Interleukin-6 directly inhibits osteoclast differentiation by suppressing receptor activator of NF-κB signaling pathways. J. Boil. Chem. 2008, 283, 11535–11540. [Google Scholar] [CrossRef] [PubMed]
- Marriott, I.; Gray, D.L.; Tranguch, S.L.; Fowler, V.G.; Stryjewski, M.; Levin, L.S.; Hudson, M.C.; Bost, K.L. Osteoblasts express the inflammatory cytokine interleukin-6 in a murine model of Staphylococcus aureus osteomyelitis and infected human bone tissue. Am. J. Pathol. 2004, 164, 1399–1406. [Google Scholar] [CrossRef]
- Grellner, W.; Georg, T.; Wilske, J. Quantitative analysis of proinflammatory cytokines (IL-1β, IL-6, TNF-α) in human skin wounds. Forensic Sci. Int. 2000, 113, 251–264. [Google Scholar] [CrossRef]
- Birkedal-Hansen, H.; Moore, W.G.I.; Bodden, M.K.; Windsor, L.J.; Birkedal-Hansen, B.; DeCarlo, A.; Engler, J.A. Matrix metalloproteinases: A review. Crit. Rev. Oral Biol. Med. 1993, 4, 197–250. [Google Scholar] [CrossRef] [PubMed]
- Page-McCaw, A.; Ewald, A.J.; Werb, Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Boil. 2007, 8, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Almalki, S.G.; Agrawal, D.K. Effects of matrix metalloproteinases on the fate of mesenchymal stem cells. Stem Cell Res. Ther. 2016, 7, 129. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Mattila, P.K.; Lappalainen, P. Filopodia: Molecular architecture and cellular functions. Nat. Rev. Mol. Cell Biol. 2008, 9, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Arihiro, K.; Oda, H.; Kaneko, M.; Inai, K. Cytokines facilitate chemotactic motility of breast carcinoma cells. Breast Cancer 2000, 7, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Zhang, L.; Zhao, X.; Xu, G.; Zhang, Y.; Roberts, A.I.; Zhao, R.C.; Shi, Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2008, 2, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Sozzani, S.; Luini, W.; Molino, M.; Jílek, P.; Bottazzi, B.; Cerletti, C.; Matsushima, K.; Mantovani, A. The signal transduction pathway involved in the migration induced by a monocyte chemotactic cytokine. J. Immunol. 1991, 147, 2215–2221. [Google Scholar] [PubMed]
- Brew, K.; Dinakarpandian, D.; Nagase, H. Tissue inhibitors of metalloproteinases: Evolution, structure and function. Biochim. Biophys. Acta 2000, 1477, 267–283. [Google Scholar] [CrossRef]
- Yang, E.V.; Bane, C.M.; MacCallum, R.C.; Kiecolt-Glaser, J.K.; Malarkey, W.B.; Glaser, R. Stress-related modulation of matrix metalloproteinase expression. J. Neuroimmunol. 2002, 133, 144–150. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Chen, X.; Chen, J.; Gautam, S.C.; Xu, Y.; Chopp, M. MCP-1, MIP-1, IL-8 and ischemic cerebral tissue enhance human bone marrow stromal cell migration in interface culture. Hematology 2002, 7, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Lewis, N.S.; Lewis, E.E.; Mullin, M.; Wheadon, H.; Dalby, M.J.; Berry, C.C. Magnetically levitated mesenchymal stem cell spheroids cultured with a collagen gel maintain phenotype and quiescence. J. Tissue Eng. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, P.C.; Behrmann, I.; Haan, S.; Hermanns, H.M.; Müller-Newen, G.; Schaper, F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 2003, 374, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Radomski, A.; Jurasz, P.; Sanders, E.J.; Overall, C.M.; Bigg, H.F.; Edwards, D.R.; Radomski, M.W. Identification, regulation and role of tissue inhibitor of metalloproteinases-4 (TIMP-4) in human platelets. Br. J. Pharmacol. 2002, 137, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Rattigan, Y.; Hsu, J.M.; Mishra, P.J.; Glod, J.; Banerjee, D. Interleukin 6 mediated recruitment of mesenchymal stem cells to the hypoxic tumor milieu. Exp. Cell Res. 2010, 316, 3417–3424. [Google Scholar] [CrossRef] [PubMed]
- Menon, L.G.; Picinich, S.; Koneru, R.; Gao, H.; Lin, S.Y.; Koneru, M.; Mayer-Kuckuk, P.; Glod, J.; Banerjee, D. Differential gene expression associated with migration of mesenchymal stem cells to conditioned medium from tumor cells or bone marrow cells. Stem Cells 2007, 25, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.J.; Mishra, P.J.; Humeniuk, R.; Medina, D.J.; Alexe, G.; Mesirov, J.P.; Ganesan, S.; Glod, J.W.; Banerjee, D. Carcinoma-associated fibroblast–like differentiation of human mesenchymal stem cells. Cancer Res. 2008, 68, 4331–4339. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casson, J.; O’Kane, S.; Smith, C.-A.; Dalby, M.J.; Berry, C.C. Interleukin 6 Plays a Role in the Migration of Magnetically Levitated Mesenchymal Stem Cells Spheroids. Appl. Sci. 2018, 8, 412. https://doi.org/10.3390/app8030412
Casson J, O’Kane S, Smith C-A, Dalby MJ, Berry CC. Interleukin 6 Plays a Role in the Migration of Magnetically Levitated Mesenchymal Stem Cells Spheroids. Applied Sciences. 2018; 8(3):412. https://doi.org/10.3390/app8030412
Chicago/Turabian StyleCasson, Jake, Sam O’Kane, Carol-Anne Smith, Matthew John Dalby, and Catherine Cecilia Berry. 2018. "Interleukin 6 Plays a Role in the Migration of Magnetically Levitated Mesenchymal Stem Cells Spheroids" Applied Sciences 8, no. 3: 412. https://doi.org/10.3390/app8030412
APA StyleCasson, J., O’Kane, S., Smith, C.-A., Dalby, M. J., & Berry, C. C. (2018). Interleukin 6 Plays a Role in the Migration of Magnetically Levitated Mesenchymal Stem Cells Spheroids. Applied Sciences, 8(3), 412. https://doi.org/10.3390/app8030412




