Development of a Combined Trifluoroacetic Acid Hydrolysis and HPLC-ELSD Method to Identify and Quantify Inulin Recovered from Jerusalem artichoke Assisted by Ultrasound Extraction
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Ultrasound Assisted Inulin Extraction
2.2. Inulin Analysis
2.2.1. Inulin Hydrolysis
2.2.2. Removal of TFA
2.2.3. HPLC-ELSD Analysis
3. Results and Discussion
3.1. Effect of Hydrolysis Conditions
3.2. Linearity
3.3. Precision
3.4. Recovery
3.5. Stability of the Hydrolysate
3.6. Application of the New Developed Method for Quantification of Inulin from Jerusalem artichoke Root
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Waterhouse, A.L.; Chatterton, N.J. Glossary of fructan terms. In Science and Technology of Fructans; CRC Press: Boca Raton, FL, USA, 1993; pp. 1–7. [Google Scholar]
- López-Molina, D.; Navarro-Martínez, M.D.; Rojas-Melgarejo, F.; Hiner, A.N.P.; Chazarra, S.; Rodríguez-López, J.N. Molecular properties and prebiotic effect of inulin obtained from artichoke (Cynara scolymus L.). Phytochemistry 2005, 66, 1476–1484. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; He, J.; Liu, G.; Barba, F.J.; Koubaa, M.; Ding, L.; Bals, O.; Grimi, N.; Vorobiev, E. Recent insights for the green recovery of inulin from plant food materials using non-conventional extraction technologies: A review. Innov. Food Sci. Emerg. 2016, 33, 1–9. [Google Scholar] [CrossRef]
- Wei, L.; Wang, J.; Zheng, X.; Teng, D.; Yang, Y.; Cai, C.; Feng, T.; Zhang, F. Studies on the extracting technical conditions of inulin from Jerusalem artichoke tubers. J. Food Eng. 2007, 79, 1087–1093. [Google Scholar]
- Arcia, P.; Costell, E.; Tárrega, A. Inulin blend as prebiotic and fat replacer in dairy desserts: Optimization by response surface methodology. J. Dairy Sci. 2011, 94, 2192–2200. [Google Scholar] [CrossRef] [PubMed]
- Bayarri, S.; Chuliá, I.; Costell, E. Comparing λ-carrageenan and an inulin blend as fat replacers in carboxymethyl cellulose dairy desserts. Rheological and sensory aspects. Food Hydrocoll. 2010, 24, 578–587. [Google Scholar] [CrossRef] [Green Version]
- Wada, T.; Sugatani, J.; Terada, E.; Ohguchi, M.; Miwa, M. Physicochemical characterization and biological effects of inulin enzymatically synthesized from sucrose. J. Agric. Food Chem. 2005, 53, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.; Bayarri, S.; Tárrega, A.; Costell, E. Inulin as texture modifier in dairy products. Food Hydrocoll. 2011, 25, 1881–1890. [Google Scholar] [CrossRef]
- Chi, Z.-M.; Zhang, T.; Cao, T.-S.; Liu, X.-Y.; Cui, W.; Zhao, C.-H. Biotechnological potential of inulin for bioprocesses. Bioresour. Technol. 2011, 102, 4295–4303. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Guan, Q.; Koubaa, M.; Barba, F.J.; Roohinejad, S.; Cravotto, G.; Yang, X.; Li, S.; He, J. HPLC-DAD-ESI-MS2 analytical profile of extracts obtained from purple sweet potato after green ultrasound-assisted extraction. Food Chem. 2017, 215, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Wu, Q.; Di, X.; Li, S.; Barba, F.J.; Koubaa, M.; Roohinejad, S.; Xiong, X.; He, J. Multistage recovery process of seaweed pigments: Investigation of ultrasound assisted extraction and ultra-filtration performances. Food Bioprod. Process. 2017, 104, 40–47. [Google Scholar] [CrossRef]
- Chen, G.; Kan, J. Ultrasound-assisted extraction, characterization, and antioxidant activity in vitro and in vivo of polysaccharides from chestnut rose (Rosa roxburghii tratt) fruit. J. Food Sci. Technol. 2018, 55, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Bals, O.; Grimi, N.; Ding, L.; Vorobiev, E. Better damage of chicory tissue by combined electroporation and ohmic heating for solute extraction. Food Bioprod. Process. 2015, 94, 248–254. [Google Scholar] [CrossRef]
- Zhu, Z.; Bals, O.; Grimi, N.; Vorobiev, E. Pilot scale inulin extraction from chicory roots assisted by pulsed electric fields. Int. J. Food Sci. Technol. 2012, 47, 1361–1368. [Google Scholar] [CrossRef]
- Khuenpet, K.; Fukuoka, M.; Jittanit, W.; Sirisansaneeyakul, S. Spray drying of inulin component extracted from Jerusalem artichoke tuber powder using conventional and ohmic-ultrasonic heating for extraction process. J. Food Eng. 2017, 194, 67–78. [Google Scholar] [CrossRef]
- Zhu, Z.; Bals, O.; Grimi, N.; Ding, L.; Vorobiev, E. Qualitative characteristics and dead-end ultrafiltration of chicory juice obtained from pulsed electric field treated chicories. Ind. Crop. Prod. 2013, 46, 8–14. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Glibowski, P.; Wasko, A. Effect of thermochemical treatment on the structure of inulin and its gelling properties. Int. J. Food Sci. Technol. 2008, 43, 2075–2082. [Google Scholar] [CrossRef]
- Li, W.; Zhang, J.; Yu, C.; Li, Q.; Dong, F.; Wang, G.; Gu, G.; Guo, Z. Extraction, degree of polymerization determination and prebiotic effect evaluation of inulin from Jerusalem artichoke. Carbohydr. Polym. 2015, 121, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Lopes, S.M.; Krausová, G.; Rada, V.; Gonçalves, J.E.; Gonçalves, R.A.; de Oliveira, A.J. Isolation and characterization of inulin with a high degree of polymerization from roots of Stevia rebaudiana (bert.) Bertoni. Carbohydr. Res. 2015, 411, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Sun, Z.; Chen, C.; Zhang, L.; Zhu, S. Simultaneous separation and determination of fructose, sorbitol, glucose and sucrose in fruits by HPLC–ELSD. Food Chem. 2014, 145, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Shanmugavelan, P.; Kim, S.Y.; Kim, J.B.; Kim, H.W.; Cho, S.M.; Kim, S.N.; Kim, S.Y.; Cho, Y.S.; Kim, H.R. Evaluation of sugar content and composition in commonly consumed korean vegetables, fruits, cereals, seed plants, and leaves by HPLC-ELSD. Carbohydr. Res. 2013, 380, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X. Study on hydrolysis technology of chicory inulin. J. Anhui Agric. Sci. 2008, 36, 8874–8875. [Google Scholar]
- Fan, Y.; Xing, S. Kinetics of acid-hydrolysis of inulin under atmospheric pressure. Guangzhou Chem. 1999, 4. Available online: http://en.cnki.com.cn/Article_en/CJFDTOTAL-GZHX199904005.htm (accessed on 26 March 2018). (In Chinese).




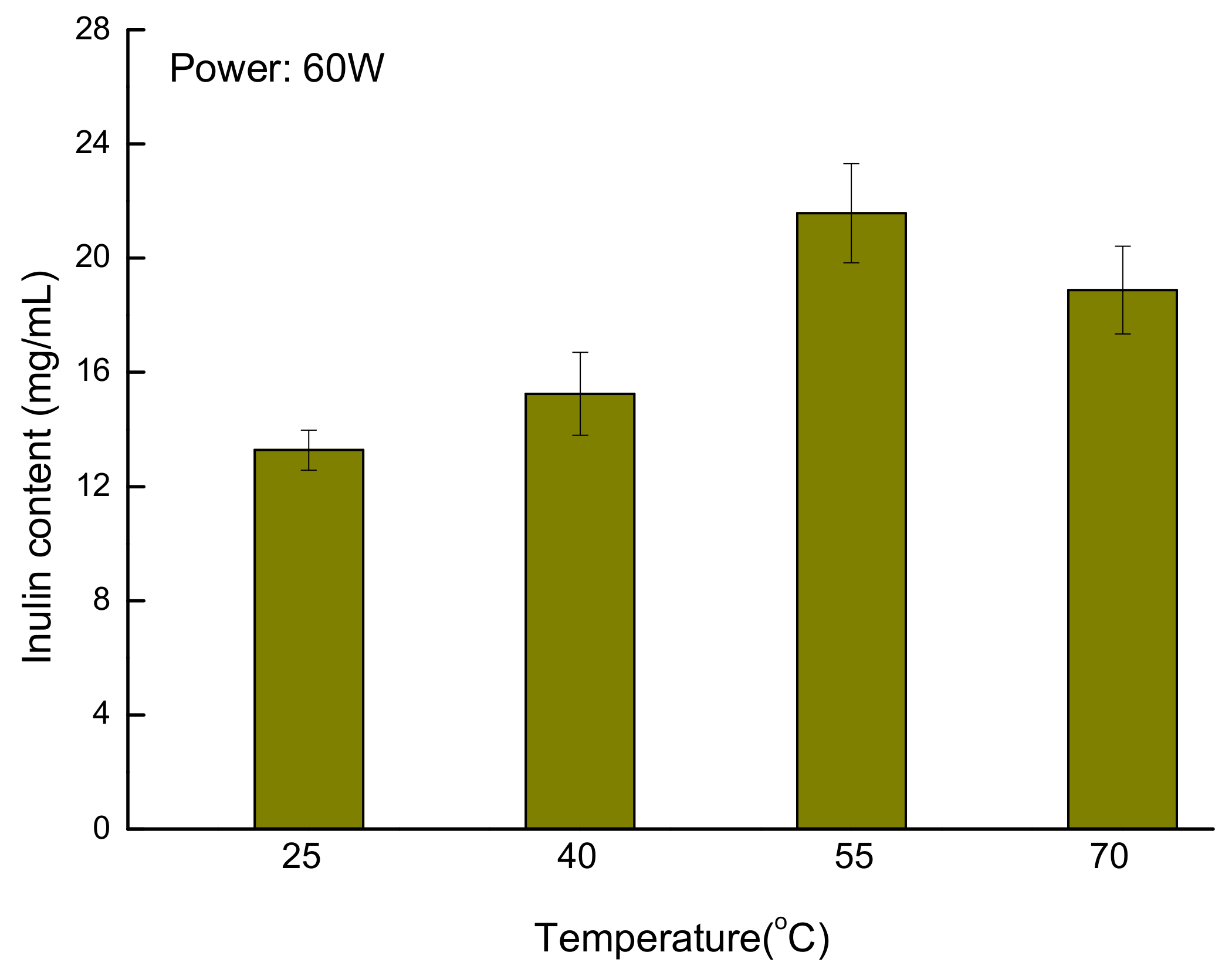
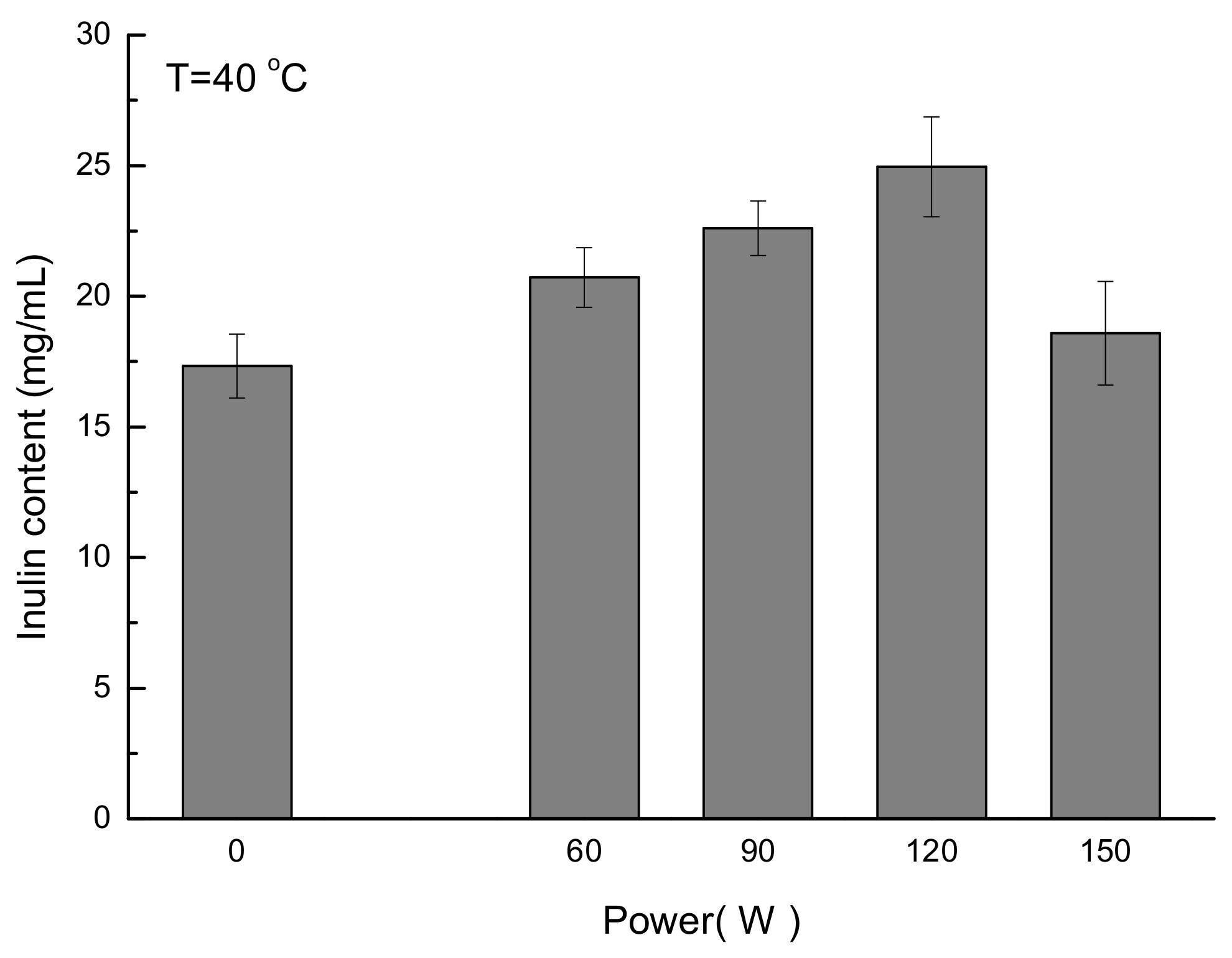
| Analysis | Concentration of Fructose (mg/mL) | Mean Concentration (mg/mL) | RSD (%) |
|---|---|---|---|
| 1 | 0.4011 | 0.3973 | 1.11 |
| 2 | 0.4032 | ||
| 3 | 0.3980 | ||
| 4 | 0.3927 | ||
| 5 | 0.3971 | ||
| 6 | 0.3921 |
| Fructose Level (mL) | Recovery (%) | Mean Recovery (%) | RSD (%) |
|---|---|---|---|
| 1.0 | 101.53 | 100.27 | 1.11 |
| 2.0 | 99.44 | ||
| 3.0 | 99.81 |
| Time (h) | 0 | 1 | 2 | 3 | 4 |
| Concentration of Fructose (mg/mL) | 0.4011 | 0.3980 | 0.3950 | 0.4005 | 0.4031 |
| RSD (%) | 0.78 | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Wu, Q.; Yin, F.; Zhu, Z.; He, J.; Barba, F.J. Development of a Combined Trifluoroacetic Acid Hydrolysis and HPLC-ELSD Method to Identify and Quantify Inulin Recovered from Jerusalem artichoke Assisted by Ultrasound Extraction. Appl. Sci. 2018, 8, 710. https://doi.org/10.3390/app8050710
Li S, Wu Q, Yin F, Zhu Z, He J, Barba FJ. Development of a Combined Trifluoroacetic Acid Hydrolysis and HPLC-ELSD Method to Identify and Quantify Inulin Recovered from Jerusalem artichoke Assisted by Ultrasound Extraction. Applied Sciences. 2018; 8(5):710. https://doi.org/10.3390/app8050710
Chicago/Turabian StyleLi, Shuyi, Qian Wu, Fangfang Yin, Zhenzhou Zhu, Jingren He, and Francisco J. Barba. 2018. "Development of a Combined Trifluoroacetic Acid Hydrolysis and HPLC-ELSD Method to Identify and Quantify Inulin Recovered from Jerusalem artichoke Assisted by Ultrasound Extraction" Applied Sciences 8, no. 5: 710. https://doi.org/10.3390/app8050710
APA StyleLi, S., Wu, Q., Yin, F., Zhu, Z., He, J., & Barba, F. J. (2018). Development of a Combined Trifluoroacetic Acid Hydrolysis and HPLC-ELSD Method to Identify and Quantify Inulin Recovered from Jerusalem artichoke Assisted by Ultrasound Extraction. Applied Sciences, 8(5), 710. https://doi.org/10.3390/app8050710








