Study of the Effect of Pyrolysis Temperature on the Cd2+ Adsorption Characteristics of Biochar
Abstract
:Featured Application
Abstract
1. Materials and Methods
1.1. Preparation of Biochar
1.2. Biochar Characterisation
1.2.1. pH
1.2.2. Element Analysis
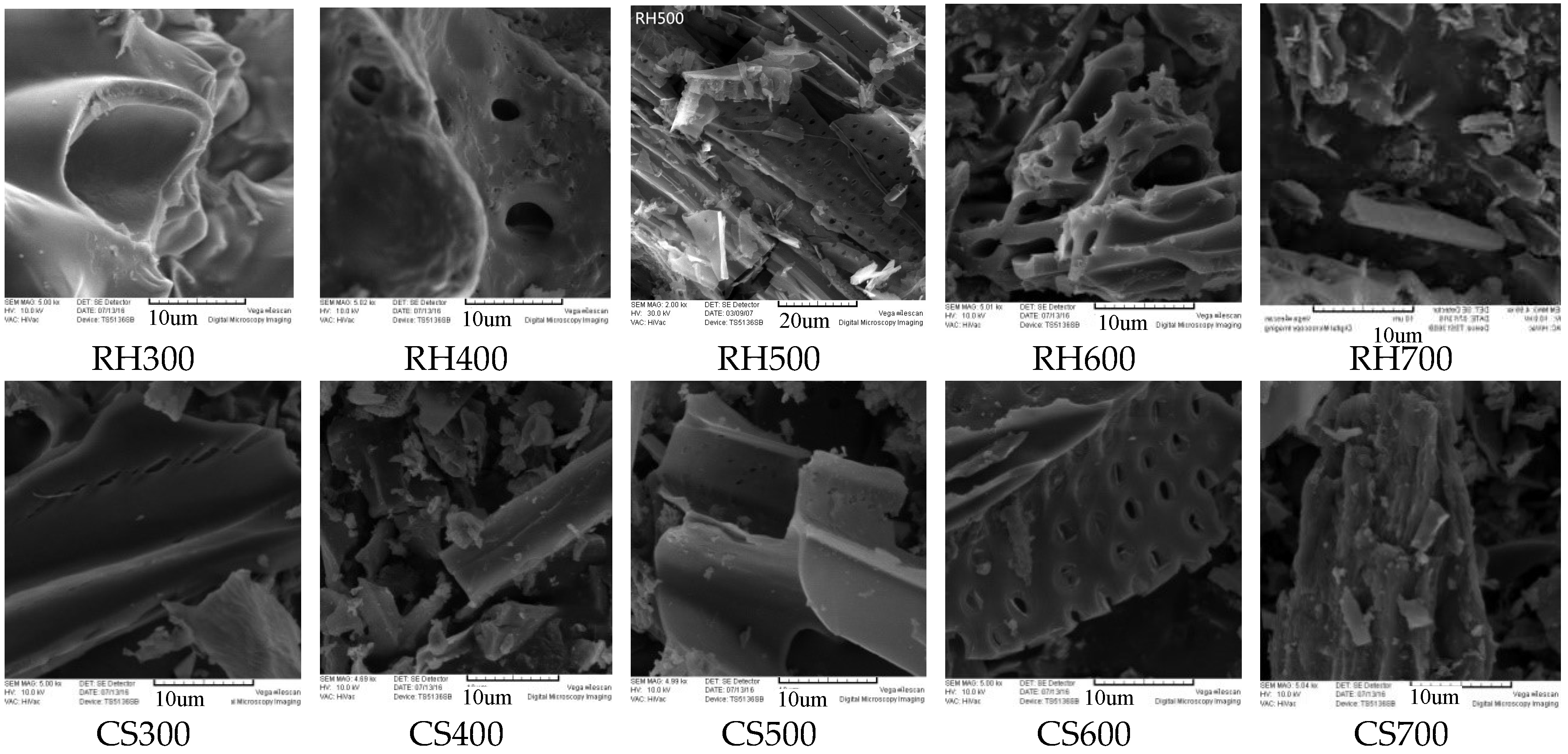
1.2.3. Surface Morphology Analysis
1.2.4. Specific Surface Area and Pore Grade
1.2.5. Surface Functional Groups
1.3. Adsorption Experiments
1.4. Data Processing
2. Results and Discussion
2.1. Biochar Yield and Ash Content at Various Pyrolysis Temperatures
2.2. Physiochemical Properties of Biochar
2.2.1. Biochar pH and Elemental Composition
2.2.2. Electron Microscopy Analysis of Biochar
2.2.3. Infrared Spectra of Biochar
2.3. Cd2+ Adsorption with Biochar
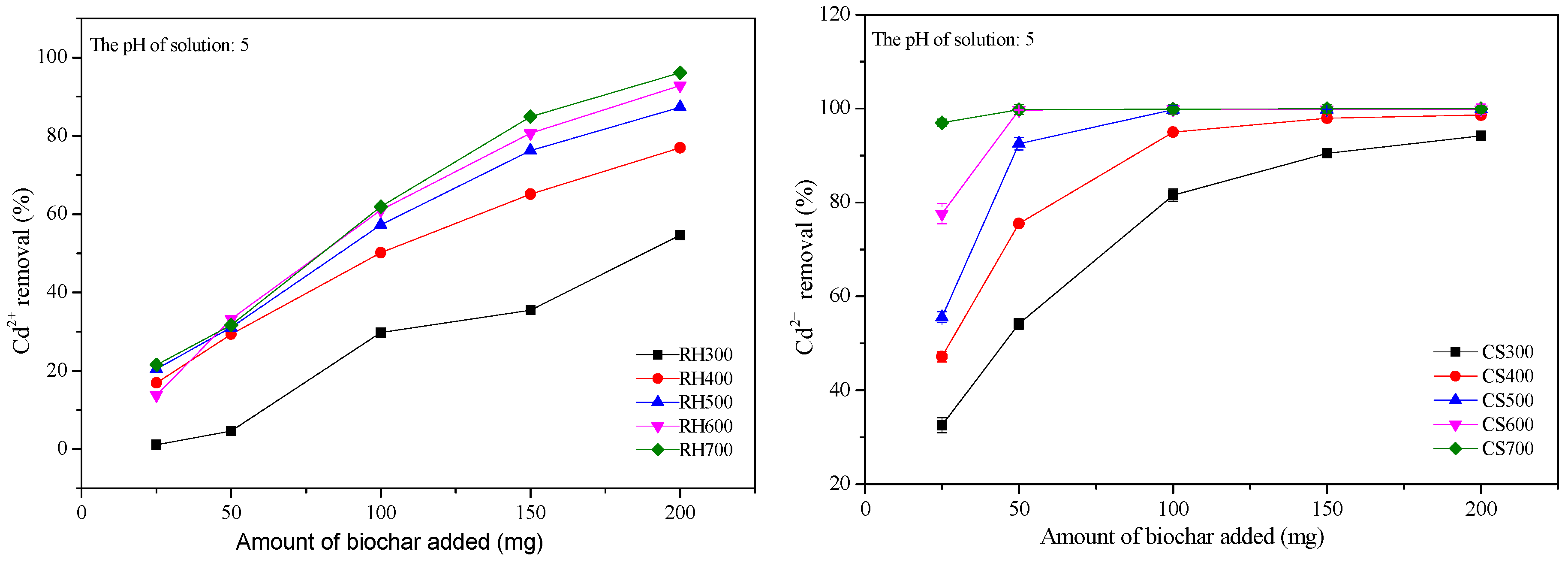
2.3.1. Effect of the Addition of Various Amounts of Biochar on the Cd2+ Removal
2.3.2. Effect of Various Initial Biochar pH Values on Cd2+ Removal
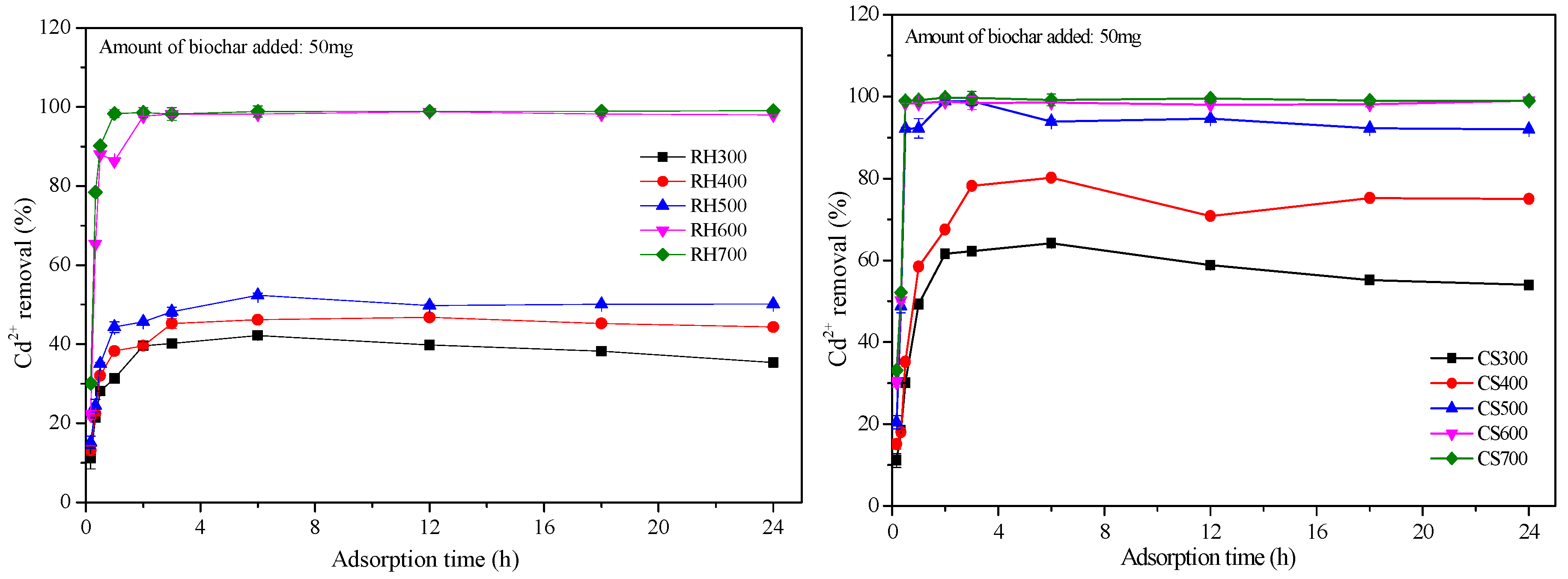
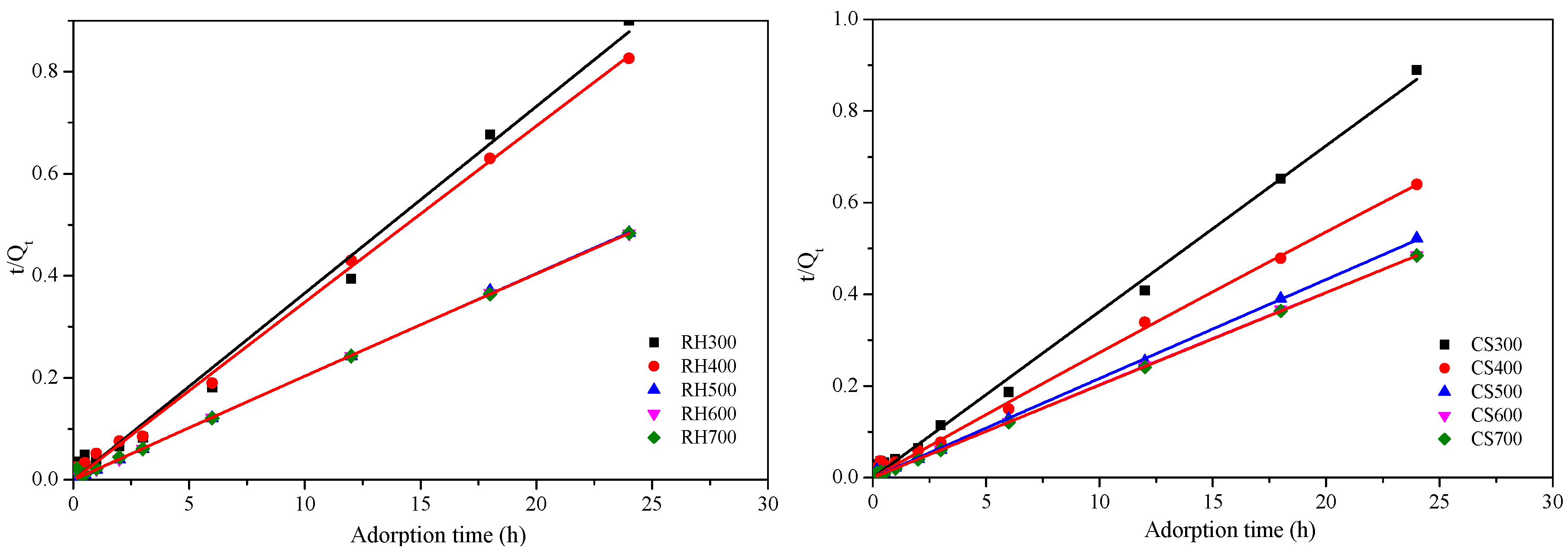
2.3.3. Cd2+ Adsorption Kinetics for Both Types of Biochar Samples
3. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Coen, N.; Mothersill, C.; Kadhim, M.; Wright, E.G. Heavy metals of relevance to human health induce genomic instability. J. Pathol. 2001, 195, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.J.; Ma, Y.; Zhu, Y.G.; Tang, Z.; McGrath, S.P. Soil contamination in China: Current status and mitigation strategies. Environ. Sci. Technol. 2015, 49, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liao, Q.; Chillrud, S.N.; Yang, Q.; Huang, L.; Bi, J.; Yan, B. Environmental Exposure to Cadmium: Health Risk Assessment and its Associations with Hypertension and Impaired Kidney Function. Sci Rep. 2016, 6, 29989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, G.; Liu, Y.; Tang, L.; Yang, G.; Pang, Y.; Zhang, Y.; Zhou, Y.; Li, Z.; Li, M.; Lai, M.; et al. Enhancement of Cd(II) adsorption by polyacrylic acid modified magnetic mesoporous carbon. Chem. Eng. J. 2015, 259, 153–160. [Google Scholar] [CrossRef]
- Chen, T.; Zhou, Z.; Han, R.; Meng, R.; Wang, H.; Lu, W. Adsorption of cadmium by biochar derived from municipal sewage sludge: Impact factors and adsorption mechanism. Chemosphere 2015, 134, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhou, Z.; Han, R.; Meng, R.; Wang, H.; Lu, W. Adsorption and desorption of phenanthrene on carbon nanotubes in simulated gastrointestinal fluids. Environ. Sci. Technol. 2011, 45, 6018–6024. [Google Scholar]
- Koptsik, G.N. Modern approaches to remediation of heavy metal polluted soils: A review. Eurasian Soil Sci. 2014, 47, 707–722. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science and Technology; Earthscan: London, UK, 2009; pp. 33–52. [Google Scholar]
- Liu, Z.; Zhang, F.S. Removal of lead from water using biochars prepared from hydrothermal liquefaction of biomass. J. Hazard. Mater. 2009, 167, 933–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ISO9277–1995 Determination of the Specific Surface Area of Solids by Gas Adsorption Using the BET Method; Powder Metallurgy Industry; International Organization for Standardization: Geneva, Switzerland, 1996.
- Singh, B.; Fang, Y.; Johnston, C.T. A Fourier-Transform Infrared Study of Biochar Aging in Soils. Soil Sci. Soc. Am. J. 2016, 80, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Nassar, N.N. Rapid removal and recovery of Pb(II) from wastewater by magnetic nanoadsorbents. J. Hazard. Mater. 2010, 184, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yip, K.; Kong, Z.; Li, C.Z.; Liu, D.; Yu, Y.; Gao, X. Removal and Recycling of Inherent Inorganic Nutrient Species in Mallee Biomass and Derived Biochars by Water Leaching. Ind. Eng. Chem. Res. 2011, 50, 12143–12151. [Google Scholar] [CrossRef]
- An, Z.L. Biochar Preparation and Its Pb2+ Adsorption Characteristics. Master’s Thesis, Huaqiao University, Xiamen, China, 2011. (In Chinese). [Google Scholar]
- Liou, T.H. Preparation and characterization of nano-structured silica from rice husk. Mater. Sci. Eng. A 2004, 364, 313–323. [Google Scholar] [CrossRef]
- Phuong, H.T.; Uddin, M.A.; Kato, Y. Characterization of Biochar from Pyrolysis of Rice Husk and Rice Straw. J. Biobased Mater. Bioenergy 2015, 9, 439–446. [Google Scholar] [CrossRef]
- Lehmann, J. A handful of carbon. Nature 2007, 443, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y. Characteristics of Biochar Derived from Arundo Donax and Its Inhibition of Soil Nitroous Oxide Discharge. Master’s Thesis, Ocean University of China, Qingdao, China, 2012. (In Chinese). [Google Scholar]
- Sinha, R.; Kumar, S.; Singh, R.K. Production of biofuel and biochar by thermal pyrolysis of linseed seed. Biomass Convers. Biorefin. 2013, 3, 327–335. [Google Scholar] [CrossRef]
- Tsai, W.T.; Huang, C.N.; Chen, H.R.; Cheng, H.Y. Pyrolytic Conversion of Horse Manure into Biochar and Its Thermochemical and Physical Properties. Waste Biomass Valoriz. 2015, 6, 975–981. [Google Scholar] [CrossRef]
- Nelson, N.O.; Agudelo, S.C.; Yuan, W.; Gan, J. Nitrogen and phosphorus availability in biochar-amended soils. Soil Sci. 2011, 176, 218–226. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, Y.; Wu, Y.; Shao, H.B.; Qin, S. Effects of Biochar Application on Nitrogen and Phosphorus Availability in Soils: A Review. Sci. Sin. Vitae 2016, 46, 1085–1090. (In Chinese) [Google Scholar]
- Hardie, M.; Clothier, B.; Bound, S.; Oliver, G.; Close, D. Does biochar influence soil physical properties and soil water availability? Plant Soil 2014, 376, 347–361. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Q.; Yan, D.; Fang, W.; Mao, L.; Wang, D.; Li, Y.; Ouyang, C.; Guo, M.; Cao, A. Effects of Biochar Amendment on Chloropicrin Adsorption and Degradation in Soil. Energies 2016, 9, 869. [Google Scholar] [CrossRef]
- Nartey, O.D.; Zhao, B. Biochar Preparation, Characterization, and Adsorptive Capacity and Its Effect on Bioavailability of Contaminants: An Overview. Adv. Mater. Sci. Eng. 2014, 2014, 715398. [Google Scholar] [CrossRef]
- Xu, N.N.; Lin, D.S.; Xu, Y.M.; Xie, Z.L.; Liang, X.F.; Guo, W.J. Adsorption of Aquatic Cd2+ by Biochar Obtained from Corn Stover. J. Agro-Environ. Sci. 2014, 33, 958–964. (In Chinese) [Google Scholar]
- Chia, C.H.; Gong, B.; Joseph, S.D.; Marjo, C.E.; Munroe, P.; Rich, A.M. Imaging of mineral-enriched biochar by FTIR, Raman and SEM–EDX. Vib. Spectrosc. 2012, 62, 248–257. [Google Scholar] [CrossRef]
- Xu, X.; Cao, X.; Zhao, L.; Wang, H.; Yu, H.; Gao, B. Removal of Cu, Zn, and Cd from aqueous solutions by the dairy manure-derived biochar. Environ. Sci. Pollut. Res. 2013, 20, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.D.; Harris, W. Properties of dairy-manure-derived biochar pertinent to its potential use in remediation. Bioresour. Technol. 2010, 101, 5222–5228. [Google Scholar] [CrossRef] [PubMed]
- Hai, T.N.; You, S.J.; Chao, H.P. Adsorption Capacity and Mechanism of Cadmium on Orange Peel- Derived Biochar at Different Pyrolysis Temperatures and Times. J. Solid Waste Technol. Manag. 2016, 42, 709–720. [Google Scholar]
- Guo, W.J.; Liang, X.F.; Lin, D.S.; Xu, Y.M.; Wang, L.; Sun, Y.B.; Qin, X. Adsorption of Cd2+ on biochar from aqueous solution. Environ. Sci. 2013, 34, 3716–3721. [Google Scholar]
- Wang, X.P.; Xue, Y.W.; Cheng, X.R.; Liu, Y. An Overview of Heavy Metal Removal Using Biochar. China Rural Water Hydropower 2013, 12, 51–56. [Google Scholar]
- Zheng, H.; Wang, Z.; Zhao, J.; Herbert, S.; Xing, B. Sorption of antibiotic sulfamethoxazole varies with biochars produced at different temperatures. Environ. Pollut. 2013, 181, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Qin, H.Z.; Li, L.Q.; Pan, G.X.; Zhang, X.H.; Zheng, J.W.; Han, X.J.; Yu, X.Y. Adsorption of Cd2+ and Pb2+ in aqueous solution by biochars produced from the pyrolysis of different crop feedstock. Ecol. Environ. Sci. 2012, 21, 146–152. (In Chinese) [Google Scholar]
- Zhang, H.M.; Xu, M.G.; Lv, J.L.; Li, J.M.; Liu, H.X. Study on the effects of pH on cadmium adsorption and analysis by soil machine components. J. Agro-Environ. Sci. 2005, 24, 320–324. (In Chinese) [Google Scholar]
- Kołodyńska, D.; Wnętrzak, R.; Leahy, J.J.; Hayes, M.H.; Kwapiński, W.; Hubicki, Z. Kinetic and adsorptive characterization of biochar in metal ions removal. Chem. Eng. J. 2012, 197, 295–305. [Google Scholar] [CrossRef]
- Chen, X.; Chen, G.; Chen, L.; Chen, Y.; Lehmann, J.; McBride, M.B.; Hay, A.G. Adsorption of copper and zinc by biochars produced from pyrolysis of hardwood and corn straw in aqueous solution. Bioresour. Technol. 2011, 102, 8877–8884. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.F.; Zhao, B.W.; Diao, J.R. Adsorptive characteristics of cadmium onto biochar produced from pyrolysis of wheat straw in aqueous solution. China Environ. Sci. 2017, 37, 551–559. (In Chinese) [Google Scholar]
- Scheidegger, A.M.; Sparks, D.L. Kinetics of the formation and the dissolution of nickel surface precipitates on pyrophyllite. Chem. Geol. 1996, 132, 157–164. [Google Scholar] [CrossRef]
- Li, R.Y.; Chen, D.; Li, L.Q.; Pan, G.X.; Chen, J.Q.; Guo, H. Adsorption of Pb2+ and Cd2+ in Aqueous Solution by Biochars Derived from Different Crop Residues. J. Agro-Environ. Sci. 2015, 34, 1001–1008. (In Chinese) [Google Scholar]
- Yang, Y.T.; Zhang, Y.P. The Characteristics of Cu2+ And Pb2+ Electrostatic and Specific Adsorptions of Constant Charge Soil Colloids. Acta Pedol. Sin. 2003, 40, 102–109. (In Chinese) [Google Scholar]
- Cho, H.H.; Wepasnick, K.; Smith, B.A.; Bangash, F.K.; Fairbrother, D.H.; Ball, W.P. Sorption of aqueous Zn(II) and Cd(II) by multiwall carbon nanotubes: The relative roles of oxygen-containing functional groups and carbon. Langmuir 2009, 26, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.C.; Dougherty, D.A. The cation-π interaction. Chem. Rev. 1997, 97, 1303–1324. [Google Scholar] [CrossRef] [PubMed]
- Inyang, M.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.R.; Pullammanappallil, P.; Cao, X. Removal of heavy metals from aqueous solution by biochars derived from anaerobically digested biomass. Bioresour. Technol. 2012, 110, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Liu, Y.S. Adsorption of Pb2+ and Cd2+ onto Biochars Derived from Pyrolysis of Four Kinds of Biomasses. Acta Sci. Nat. Univ. Pekin. 2013, 49, 1075–1082. (In Chinese) [Google Scholar]



| Biochar | RH300 | RH400 | RH500 | RH600 | RH700 | CS300 | CS400 | CS500 | CS600 | CS700 |
|---|---|---|---|---|---|---|---|---|---|---|
| Yield | 37.52 | 28.74 | 27.6 | 24.85 | 23.32 | 51.64 | 33.61 | 22.24 | 20.91 | 19.92 |
| (%) | ±0.01 | ±0.01 | ±0.01 | ±0.00 | ±0.00 | ±0.03 | ±0.02 | ±0.02 | ±0.00 | ±0.01 |
| Ash content | 64.19 | 66.06 | 66.56 | 75.35 | 76.2 | 5.54 | 8.52 | 11.45 | 12.29 | 12.15 |
| (%) | ±0.01 | ±0.00 | ±0.01 | ±0.01 | ±0.01 | ±0.00 | ±0.00 | ±0.02 | ±0.01 | ±0.02 |
| Biochar | pH | Element Content (%) | Atomic Ratio | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | C | H | O | S | H/C | O/C | (O + N)/C | C/N | ||
| RH300 | 5.31 | 0.67 | 45.99 | 2.48 | 26.55 | 0.01 | 0.05 | 0.58 | 0.59 | 68.64 |
| RH400 | 6.49 | 0.57 | 44.23 | 2.42 | 20.46 | 0.01 | 0.05 | 0.46 | 0.48 | 77.60 |
| RH500 | 8.15 | 0.54 | 42.63 | 2.00 | 14.87 | 0.10 | 0.05 | 0.35 | 0.36 | 78.94 |
| RH600 | 9.20 | 0.39 | 38.84 | 1.40 | 10.03 | 0.03 | 0.04 | 0.26 | 0.27 | 99.59 |
| RH700 | 9.42 | 0.31 | 28.44 | 1.00 | 7.62 | 0.10 | 0.04 | 0.27 | 0.28 | 91.74 |
| CS300 | 6.61 | 1.23 | 64.04 | 3.81 | 29.36 | 0.06 | 0.06 | 0.46 | 0.48 | 52.07 |
| CS400 | 8.51 | 1.34 | 70.23 | 3.34 | 22.00 | 0.06 | 0.05 | 0.31 | 0.33 | 52.41 |
| CS500 | 9.72 | 1.01 | 74.24 | 3.18 | 17.99 | 0.30 | 0.04 | 0.24 | 0.26 | 73.50 |
| CS600 | 10.24 | 0.94 | 79.15 | 2.41 | 14.46 | 0.23 | 0.03 | 0.18 | 0.19 | 84.20 |
| CS700 | 10.42 | 1.03 | 77.13 | 2.13 | 16.39 | 0.57 | 0.03 | 0.21 | 0.23 | 74.88 |
| Biochar | Brunauer–Emmett–Teller (BET) Area (m2·g−1) | Mean Pore Size (nm) | Micropore Volume (cm3·g−1) |
|---|---|---|---|
| RH300 | 78.401 | 3.697 | 0.009 |
| RH400 | 101.295 | 3.670 | 0.008 |
| RH500 | 292.595 | 3.772 | 0.015 |
| RH600 | 377.717 | 3.815 | 0.032 |
| RH700 | 406.244 | 3.820 | 0.049 |
| CS300 | 35.258 | 3.417 | 0.010 |
| CS400 | 117.536 | 3.065 | 0.011 |
| CS500 | 300.103 | 4.096 | 0.013 |
| CS600 | 500.807 | 3.807 | 0.014 |
| CS700 | 553.709 | 3.785 | 0.052 |
| Sample | First-Order Kinetics | Second-Order Kinetics | ||||
|---|---|---|---|---|---|---|
| K1 (min−1) | qm (mg·g−1) | R2 | K2 (mg·g−1·min−1) | qm (mg·g−1) | R2 | |
| RH300 | 1.02 | 31.08 | 0.783 | 3.32 × 10−4 | 103.13 | 0.990 |
| RH400 | 1.43 | 29.90 | 0.880 | 4.57 × 10−4 | 76.54 | 0.997 |
| RH500 | 4.21 | 50.24 | 0.688 | 5.72 × 10−4 | 35.91 | 0.999 |
| RH600 | 3.06 | 49.69 | 0.908 | 9.09 × 10−4 | 22.62 | 0.999 |
| RH700 | 2.95 | 49.00 | 0.847 | 8.68 × 10−4 | 26.66 | 0.998 |
| CS300 | 1.50 | 29.15 | 0.939 | 4.71 × 10−4 | 168.97 | 0.995 |
| CS400 | 1.22 | 38.07 | 0.970 | 5.76 × 10−4 | 115.76 | 0.995 |
| CS500 | 2.74 | 51.11 | 0.872 | 6.13 × 10−4 | 191.24 | 0.998 |
| CS600 | 3.00 | 59.90 | 0.842 | 7.58 × 10−4 | 127.14 | 0.999 |
| CS700 | 3.09 | 62.30 | 0.859 | 6.31 × 10−4 | 110.29 | 0.999 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Y.; Shi, S.; Liu, J.; Su, S.; Liang, Q.; Zeng, X.; Li, T. Study of the Effect of Pyrolysis Temperature on the Cd2+ Adsorption Characteristics of Biochar. Appl. Sci. 2018, 8, 1019. https://doi.org/10.3390/app8071019
Jia Y, Shi S, Liu J, Su S, Liang Q, Zeng X, Li T. Study of the Effect of Pyrolysis Temperature on the Cd2+ Adsorption Characteristics of Biochar. Applied Sciences. 2018; 8(7):1019. https://doi.org/10.3390/app8071019
Chicago/Turabian StyleJia, Yuehui, Shengli Shi, Jie Liu, Shiming Su, Qiong Liang, Xibai Zeng, and Tingshu Li. 2018. "Study of the Effect of Pyrolysis Temperature on the Cd2+ Adsorption Characteristics of Biochar" Applied Sciences 8, no. 7: 1019. https://doi.org/10.3390/app8071019
APA StyleJia, Y., Shi, S., Liu, J., Su, S., Liang, Q., Zeng, X., & Li, T. (2018). Study of the Effect of Pyrolysis Temperature on the Cd2+ Adsorption Characteristics of Biochar. Applied Sciences, 8(7), 1019. https://doi.org/10.3390/app8071019






