Acridine-Triphenylamine Based Hole-Transporting and Hole-Injecting Material for Highly Efficient Phosphorescent-Based Organic Light Emitting Diodes
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
2.3. Synthesis of 4-(9,9-Diphenylacridin-10(9H)-yl)-N-(4-(9,9-diphenylacridin-10(9H)-yl)phenyl)-N-phenylaniline (TPA-1A)
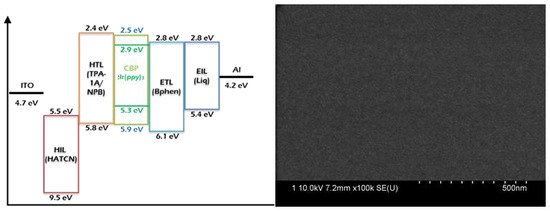
2.4. OLED Fabrication and Characterization
3. Results and Discussions
3.1. Thermal and Morphological Properties
3.2. Electrochemical and Photophysical Properties
3.3. Device Characteristics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kochapradist, P.; Prachumrak, N.; Tarsang, R.; Keawin, T.; Jungsuttiwong, S.; Sudyoadsuk, T.; Promarak, V. Multi-triphenylamine-substituted carbazoles: Synthesis, characterization, properties, and applications as hole-transporting materials. Tetrahedron Lett. 2013, 54, 3683–3687. [Google Scholar] [CrossRef]
- Wolak, M.A.; Delcamp, J.; Landis, C.A.; Lane, P.A.; Anthony, J.; Kafafi, Z. High-Performance Organic Light-Emitting Diodes Based on Dioxolane-Substituted Pentacene Derivatives. Adv. Funct. Mater. 2006, 16, 1943–1949. [Google Scholar] [CrossRef]
- Huh, D.H.; Kim, G.W.; Kim, G.H.; Kulshreshtha, C.; Kwon, J.H. High hole mobility hole transport material for organic light-emitting devices. Synth. Met. 2013, 180, 79–84. [Google Scholar] [CrossRef]
- Usluer, O.; Demic, S.; Egbe, D.A.; Birckner, E.; Tozlu, C.; Pivrikas, A.; Sariciftci, N.S. Fluorene-Carbazole Dendrimers: Synthesis, Thermal, Photophysical and Electroluminescent Device Properties. Adv. Funct. Mater. 2010, 20, 4152–4161. [Google Scholar] [CrossRef]
- Takizawa, S.Y.; Montes, V.A.; Anzenbacher, J.P. Phenylbenzimidazole-based new bipolar host materials for efficient phosphorescent organic light-emitting diodes. Chem. Mater. 2009, 21, 2452–2458. [Google Scholar] [CrossRef]
- Kuwabara, Y.; Ogawa, H.; Inada, H.; Noma, N.; Shirota, Y. Thermally stable multilared organic electroluminescent devices using novel starburst molecules, 4,4′,4″-Tri (N-carbazolyl) triphenylamine (TCTA) and 4,4′,4″-Tris (3-methylphenylphenylamino) triphenylamine (m-MTDATA), as hole-transport materials. Adv. Mater. 1994, 6, 677–679. [Google Scholar] [CrossRef]
- Long, L.; Zhang, M.; Xu, S.; Zhou, X.; Gao, X.; Shang, Y.; Wei, B. Cyclic arylamines functioning as advanced hole-transporting and emitting materials. Synth. Met. 2012, 162, 448–452. [Google Scholar] [CrossRef]
- Braveenth, R.; Bae, H.W.; Ko, I.J.; Qiong, W.; Nguyen, Q.P.B.; Jayashantha, P.G.S.; Kwon, J.H.; Chai, K.Y. Thermally stable efficient hole-transporting materials based on carbazole and triphenylamine core for red phosphorescent OLEDs. Org Electron. 2017, 51, 463–470. [Google Scholar] [CrossRef]
- Okumoto, K.; Shirota, Y. Development of new hole-transporting amorphous molecular materials for organic electroluminescent devices and their charge-transport properties. Mater. Sci. Eng. B 2001, 85, 135–139. [Google Scholar] [CrossRef]
- Braveenth, R.; Bae, I.J.; Han, J.H.; Qiong, W.; Seon, G.; Raagulan, K.; Yang, K.; Park, Y.H.; Kim, M.; Chai, K.Y. Utilizing a Spiro Core with Acridine- and Phenothiazine-Based New Hole-transporting materials for Highly Efficient Green Phosphorescent Organic Light-Emitting Diodes. Molecules 2018, 23, 713. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Jang, J.; Yook, K.S.; Lee, J.Y. Stable efficiency roll-off in phosphorescent organic light-emitting diodes. Appl. Phys. Lett. 2008, 92, 023513. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Liu, D.; Li, Y.; Lee, C.S.; Kwong, H.L.; Lee, S. A high Tg carbazole-based hole-transporting material for organic light-emitting devices. Chem. Mater. 2005, 17, 1208–1212. [Google Scholar] [CrossRef]
- Quinton, C.; Thiery, S.; Jeannin, O.; Tondelier, D.; Geffroy, B.; Jacques, E.; Berthelot, J.R.; Poriel, C. Electron-rich 4-substituted spirobifluorenes: Toward a new family of high triplet energy host materials for high-efficiency green and sky blue phosphorescent OLEDs. ACS Appl. Mater. Interfaces 2017, 9, 6194–6206. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.; Wang, D.; Zhang, C.; Wang, F.; Wu, H.; Lv, Z.; Zou, D. Synthesis of spiro [fluorene-9,9′-xanthene] derivatives and their application as hole-transporting materials for organic light-emitting devices. Synth. Met. 2012, 162, 614–620. [Google Scholar] [CrossRef]
- Feng, G.L.; Lai, W.Y.; Ji, S.J.; Huang, W. Synthesis of novel star-shaped carbazole-functionalized triazatruxenes. Tetrahedron Lett. 2006, 47, 7089–7092. [Google Scholar] [CrossRef]
- Hou, X.Y.; Li, T.C.; Yin, C.R.; Xu, H.; Lin, J.; Hua, Y.R.; Huang, W. Stable hole-transporting molecular glasses based on complicated 9, 9-diarylfluorenes (CDAFs). Synth. Met. 2009, 159, 1055–1060. [Google Scholar] [CrossRef]
- Tsutsui, T. Progress in electroluminescent devices using molecular thin films. Mrs Bull. 1997, 22, 39–45. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, J.M.; Lee, H.; Ko, K.Y.; Yook, K.S.; Lee, J.Y.; Baek, Y.G. Thermally stable triphenylene-based hole-transporting materials for organic light-emitting devices. Thin Solid Films 2011, 519, 5917–5923. [Google Scholar] [CrossRef]
- Tong, Q.X.; Lai, S.L.; Chan, M.Y.; Lai, K.H.; Tang, J.X.; Kwong, H.L.; Lee, S.T. High Tg triphenylamine-based starburst hole-transporting material for organic light-emitting devices. Chem. Mater. 2007, 19, 5851–5855. [Google Scholar] [CrossRef]
- Adachi, C.; Nagai, K.; Tamoto, N. Molecular design of hole transport materials for obtaining high durability in organic electroluminescent diodes. Appl. Phys. Lett. 1995, 66, 2679–2681. [Google Scholar] [CrossRef]
- Karthikeyan, C.S.; Wietasch, H.; Thelakkat, M. Highly efficient solid-state dye-sensitized TiO2 solar cells using donor-antenna dyes capable of multistep charge-transfer cascades. Adv. Mat. 2007, 19, 1091–1095. [Google Scholar] [CrossRef]
- Tang, C.W.; VanSlyke, S.A.; Chen, C.H. Electroluminescence of doped organic thin films. J. Appl. Phys. 1989, 65, 3610–3616. [Google Scholar] [CrossRef]
- Cias, P.; Slugovc, C.; Gescheidt, G. Hole transport in triphenylamine based OLED devices: From theoretical modeling to properties prediction. J. Phys. Chem. A 2011, 115, 14519–14525. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, L.; Liang, Y.; Yang, S.; Liang, J.; Cheng, F.; Chen, J. A thermally and electrochemically stable organic hole-transporting material with an adamantane central core and triarylamine moieties. Synth. Met. 2012, 162, 490–496. [Google Scholar] [CrossRef]
- Burroughes, J.H.; Bradley, D.D.C.; Brown, A.R.; Marks, R.N.; Mackay, K.; Friend, R.H.; Holmes, A.B. Light-emitting diodes based on conjugated polymers. Nature 1990, 347, 539–541. [Google Scholar] [CrossRef]
- Choi, H.; Ko, H.M.; Ko, J. Stable and efficient star-shaped hole-transporting materials with EDOT moiety as side arm for perovskite solar cells. Dyes Pigm. 2016, 126, 179–185. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, Q.; Yang, C.; Zhong, C.; Qin, J.; Ma, D. Multifunctional Triphenylamine/Oxadiazole Hybrid as Host and Exciton-Blocking Material: High Efficiency Green Phosphorescent OLEDs Using Easily Available and Common Materials. Adv. Funct. Mater. 2010, 20, 2923–2929. [Google Scholar] [CrossRef]
- Agarwala, P.; Kabra, D. A review on triphenylamine (TPA) based organic hole transport materials (HTMs) for dye sensitized solar cells (DSSCs) and perovskite solar cells (PSCs): Evolution and molecular engineering. J. Mater. Chem. A 2017, 5, 1348–1373. [Google Scholar] [CrossRef]
- Thelakkat, M. Star-Shaped, Dendrimeric and Polymeric Triarylamines as Photoconductors and Hole Transport Materials for Electro-Optical Applications. Macromol. Mater. Eng. 2002, 287, 442–461. [Google Scholar] [CrossRef]
- Torres, A.; Rego, L.G. Surface effects and adsorption of methoxy anchors on hybrid lead iodide perovskites: Insights for Spiro-MeOTAD attachment. J. Phys. Chem. C 2014, 118, 26947–26954. [Google Scholar] [CrossRef]
- Ohkuma, H.; Nakagawa, T.; Shizu, K.; Yasuda, T.; Adachi, C. Thermally activated delayed fluorescence from a spiro-diazafluorene derivative. Chem. Lett. 2014, 43, 1017–1019. [Google Scholar] [CrossRef]
- Reddy, S.S.; Sree, V.G.; Gunasekar, K.; Cho, W.; Gal, Y.S.; Song, M.; Jin, S.H. Highly Efficient Bipolar Deep-Blue Fluorescent Emitters for Solution-Processed Non-Doped Organic Light-Emitting Diodes Based on 9, 9-Dimethyl-9,10-dihydroacridine/Phenanthroimadazole Derivatives. Adv. Opt. Mater. 2016, 4, 1236–1246. [Google Scholar] [CrossRef]
- Méhes, G.; Nomura, H.; Zhang, Q.; Nakagawa, T.; Adachi, C. Enhanced Electroluminescence Efficiency in a Spiro-Acridine Derivative through Thermally Activated Delayed Fluorescence. Angew. Chem. 2012, 51, 11311–11315. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.N.; Chakravarthi, N.; Kranthiraja, K.; Reddy, S.S.; Kim, H.S.; Jin, S.H.; Park, N.G. Acridine-based novel hole-transporting material for high efficiency perovskite solar cells. J. Mater. Chem. A 2017, 5, 7603–7611. [Google Scholar] [CrossRef]
- Romain, M.; Tondelier, D.; Jeannin, O.; Geffroy, B.; Rault-Berthelot, J.; Poriel, C. Properties modulation of organic semi-conductors based on a donor-spiro-acceptor (D-spiro-A) molecular design: New host materials for efficient sky-blue PhOLEDs. J. Mater. Chem. C 2015, 3, 9701–9714. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.K.; Sun, Q.; Wu, S.F.; Yuan, Y.; Li, Q.; Jiang, Z.Q.; Liao, L.S. Thermally Activated Delayed Fluorescence Material as Host with Novel Spiro-Based Skeleton for High Power Efficiency and Low Roll-Off Blue and White Phosphorescent Devices. Adv. Funct. Mater. 2016, 26, 7929–7936. [Google Scholar] [CrossRef]
- Romain, M.; Tondelier, D.; Geffroy, B.; Shirinskaya, A.; Jeannin, O.; Berthelot, J.R.; Poriel, C. Spiro-configured phenyl acridine thioxanthene dioxide as a host for efficient PhOLEDs. Chem. Commun. 2015, 51, 1313–1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jäger, L.; Schmidt, T.D.; Brütting, W. Manipulation and control of the interfacial polarization in organic light-emitting diodes by dipolar doping. AIP Adv. 2016, 6, 095220. [Google Scholar] [CrossRef] [Green Version]
- Hu, N.X.; Xie, S.; Popovic, Z.D.; Ong, B.; Hor, A.M. Novel high Tg hole-transport molecules based on indolo [3,2-b] carbazoles for organic light-emitting devices. Synth. Met. 2000, 111, 421–424. [Google Scholar] [CrossRef]
- Poriel, C.; Berthelot, J.R. Structure-property relationship of 4-substituted-spirobifluorenes as hosts for phosphorescent organic light emitting diodes: An overview. J. Mater. C 2017, 5, 3869–3897. [Google Scholar] [CrossRef]
- Cho, Y.J.; Kim, O.Y.; Lee, J.Y. Synthesis of an aromatic amine derivative with novel double spirobifluorene core and its application as a hole transport material. Organ. Electron. 2012, 13, 351–355. [Google Scholar] [CrossRef]







| HTM | Tg a (°C) | Td b (°C) | UV-Vis c (nm) | PL max d (nm) | HOMO e (eV) | LUMO f (eV) | Eg g (eV) | ET h (eV) |
|---|---|---|---|---|---|---|---|---|
| TPA-1A | 176 | 474 | 319 | 425 | −5.78 | −2.45 | 3.33 | 2.58 |
| Characteristic | TPA-1A (HTL) | NPB (Reference) | TPA-1A (HIL) |
|---|---|---|---|
| Current (mA) | 0.006 a | 0.006 a | 0.01 a |
| 0.10 b | 0.18 b | 0.18 b | |
| Current efficiency (cd/A) | 44.29 a | 33.38 a | 37.09 a |
| 49.13 b | 27.21 b | 30.57 b | |
| Power efficiency (lm/W) | 31.62 a | 30.85 a | 32.37 a |
| 27.56 b | 19.88 b | 19.21 b | |
| Luminescence (cd/m2) | 62.81 a | 48.4 a | 146.2 a |
| 1282 b | 1197 b | 1341 b | |
| External quantum efficiency (EQE %) | 15.50 a | 13.39 a | 14.59 a |
| 13.74 b | 7.64 b | 8.53 b | |
| International Commission on Illumination CIE (x,y) b | 0.33, 0.62 | 0.33, 0.63 | 0.34, 0.61 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braveenth, R.; Bae, I.-J.; Wang, Y.; Kim, S.H.; Kim, M.; Chai, K.Y. Acridine-Triphenylamine Based Hole-Transporting and Hole-Injecting Material for Highly Efficient Phosphorescent-Based Organic Light Emitting Diodes. Appl. Sci. 2018, 8, 1168. https://doi.org/10.3390/app8071168
Braveenth R, Bae I-J, Wang Y, Kim SH, Kim M, Chai KY. Acridine-Triphenylamine Based Hole-Transporting and Hole-Injecting Material for Highly Efficient Phosphorescent-Based Organic Light Emitting Diodes. Applied Sciences. 2018; 8(7):1168. https://doi.org/10.3390/app8071168
Chicago/Turabian StyleBraveenth, Ramanaskanda, Il-Ji Bae, Yan Wang, Soo Hyeon Kim, Miyoung Kim, and Kyu Yun Chai. 2018. "Acridine-Triphenylamine Based Hole-Transporting and Hole-Injecting Material for Highly Efficient Phosphorescent-Based Organic Light Emitting Diodes" Applied Sciences 8, no. 7: 1168. https://doi.org/10.3390/app8071168
APA StyleBraveenth, R., Bae, I.-J., Wang, Y., Kim, S. H., Kim, M., & Chai, K. Y. (2018). Acridine-Triphenylamine Based Hole-Transporting and Hole-Injecting Material for Highly Efficient Phosphorescent-Based Organic Light Emitting Diodes. Applied Sciences, 8(7), 1168. https://doi.org/10.3390/app8071168