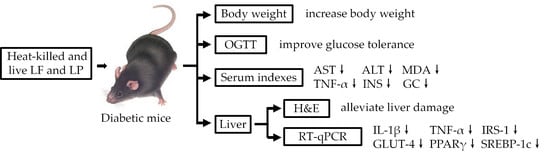
Effects of Lactobacillus on Mice with Diabetes Induced by High-Fat Diet with Streptozotocin (STZ)
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains
2.2. Mice
2.3. Experimental Model and Groups
2.4. Oral Glucose Tolerance Test (OGTT)
2.5. Colleciton of Blood Serum and Tissue Sample
2.6. Histological Observation
2.7. Measurement of Serum Indexes
2.8. Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
2.9. Statistical Analysis
3. Results
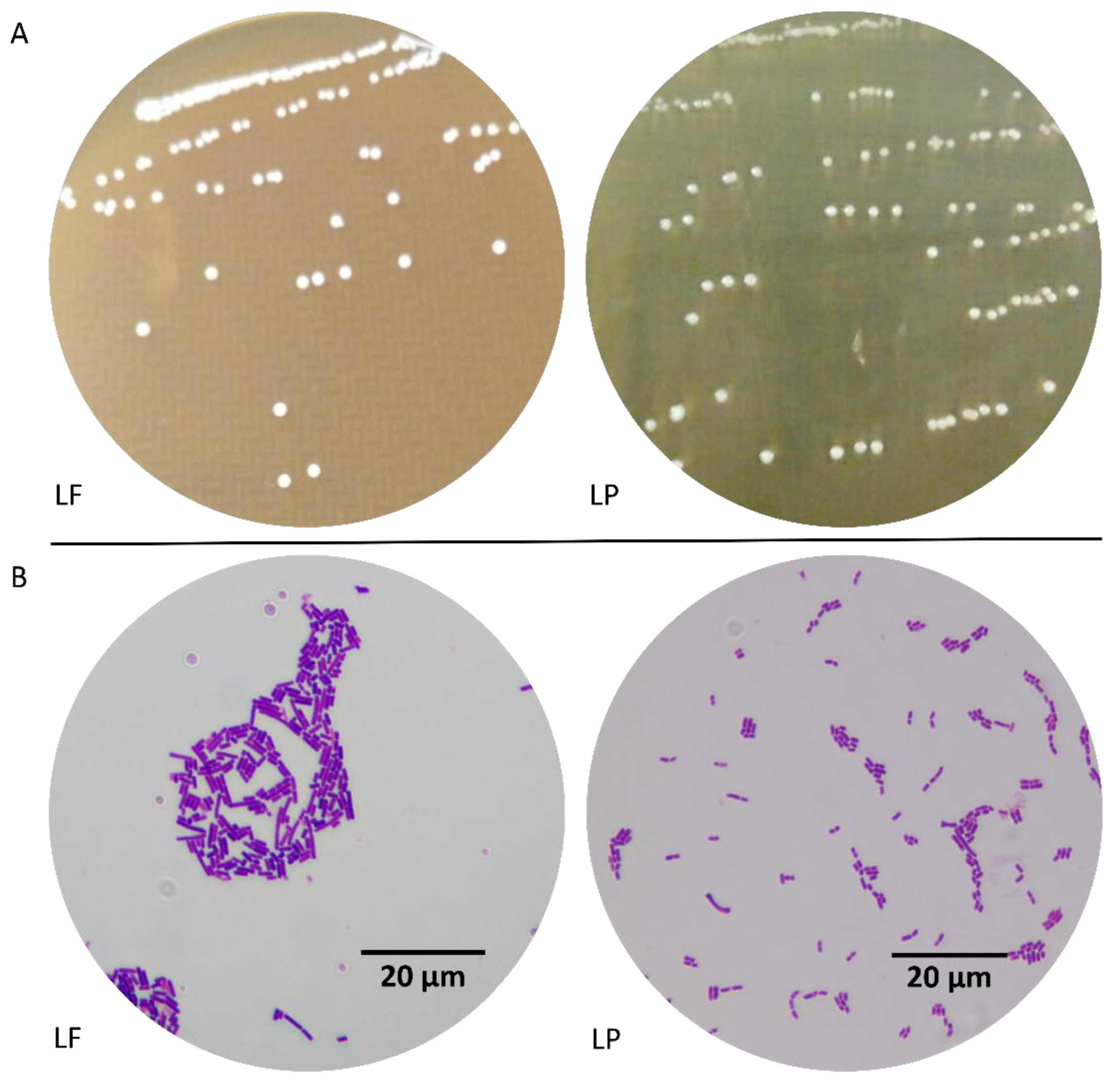
3.1. Morphological Characteristics
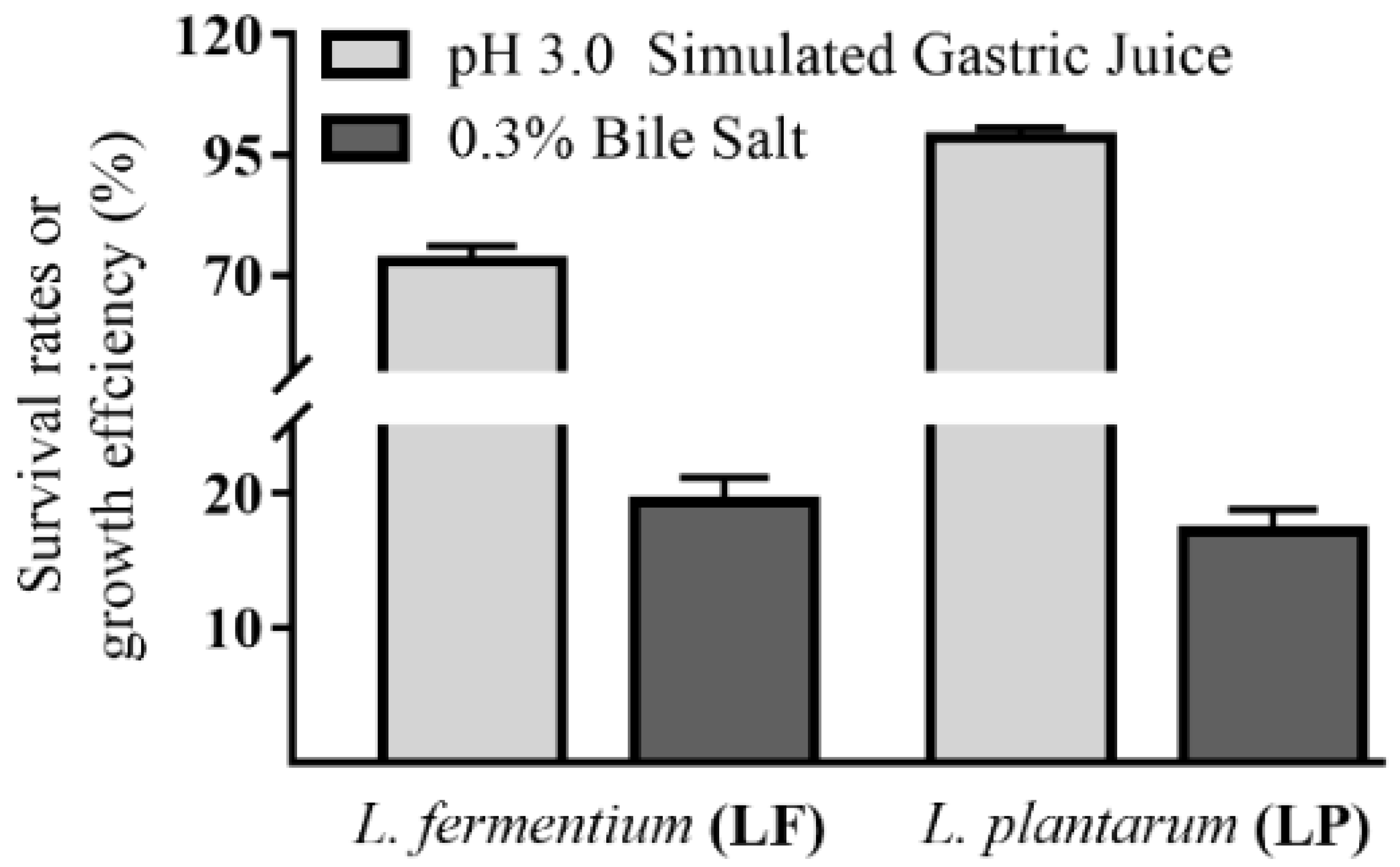
3.2. In Vitro Tolerance to Stimulated Gastric Juice and Bile Salt
3.3. Body Weight
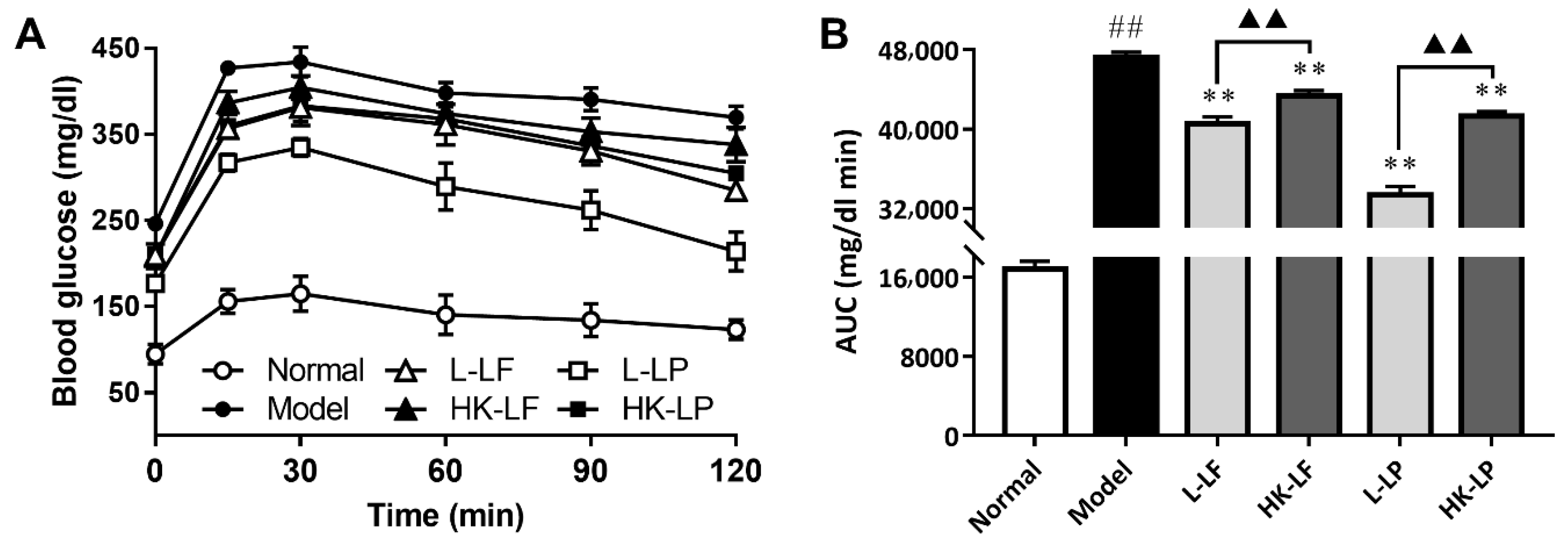
3.4. OGTT
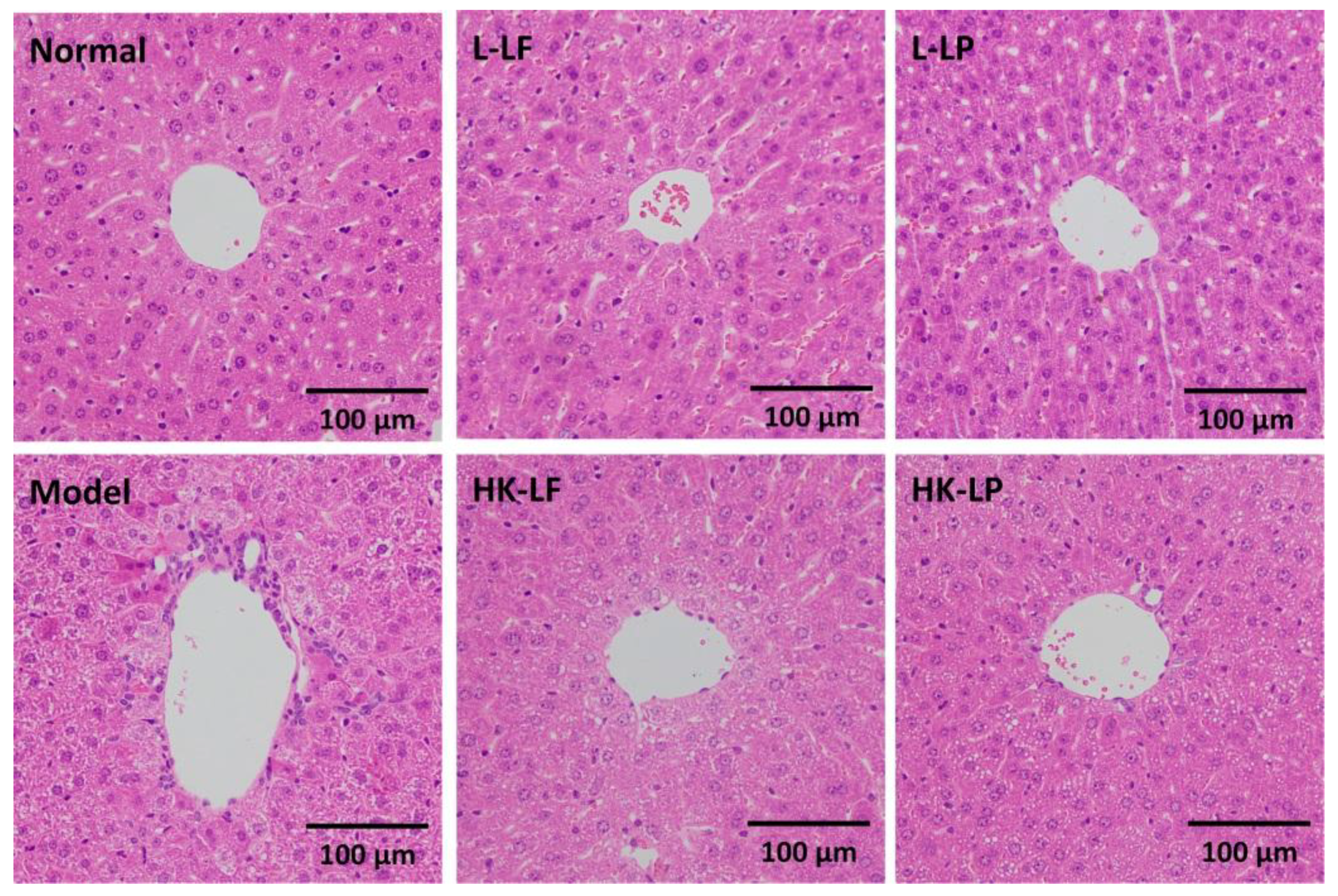
3.5. Pathological Observation
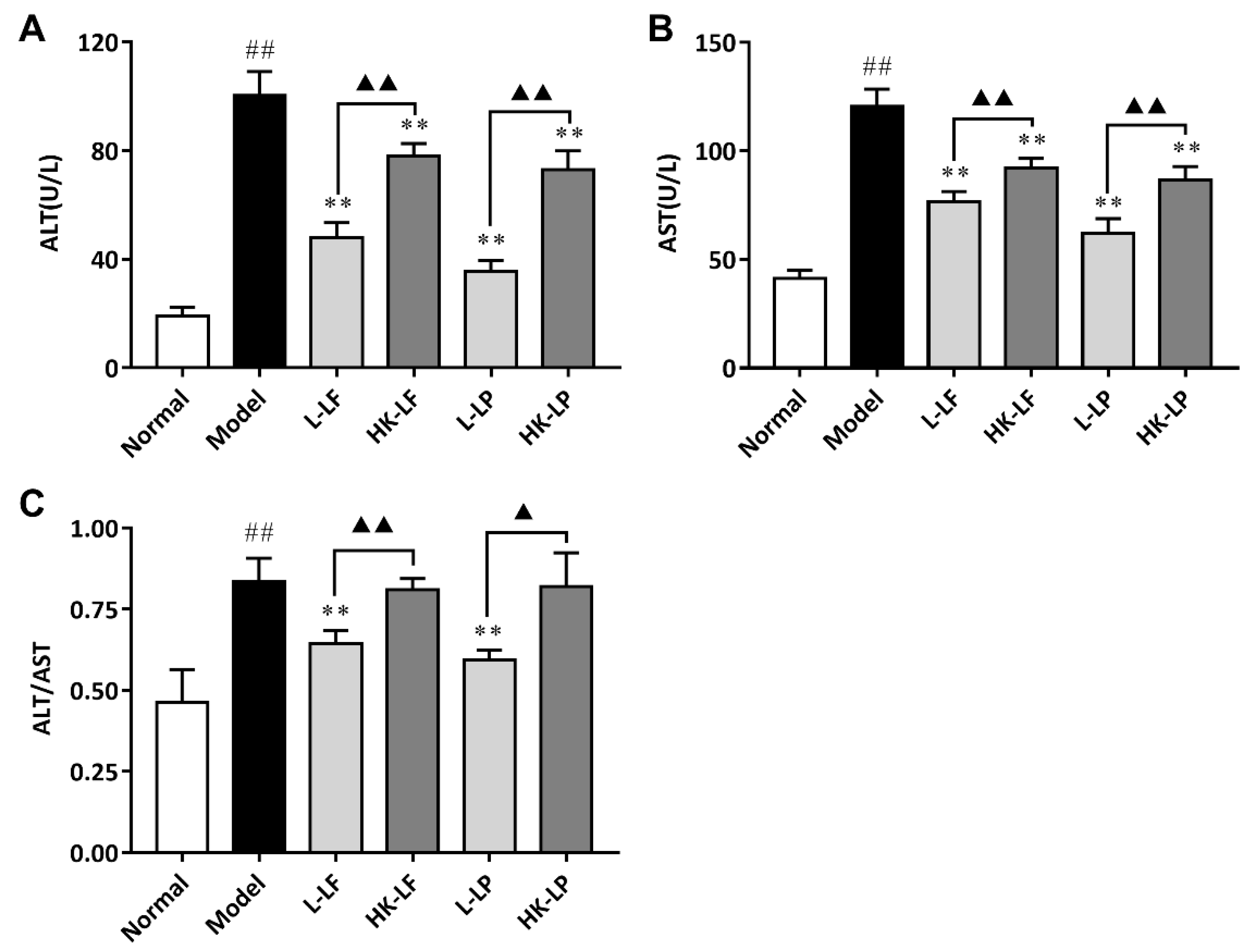
3.6. Measurement of ALT and AST in Serum
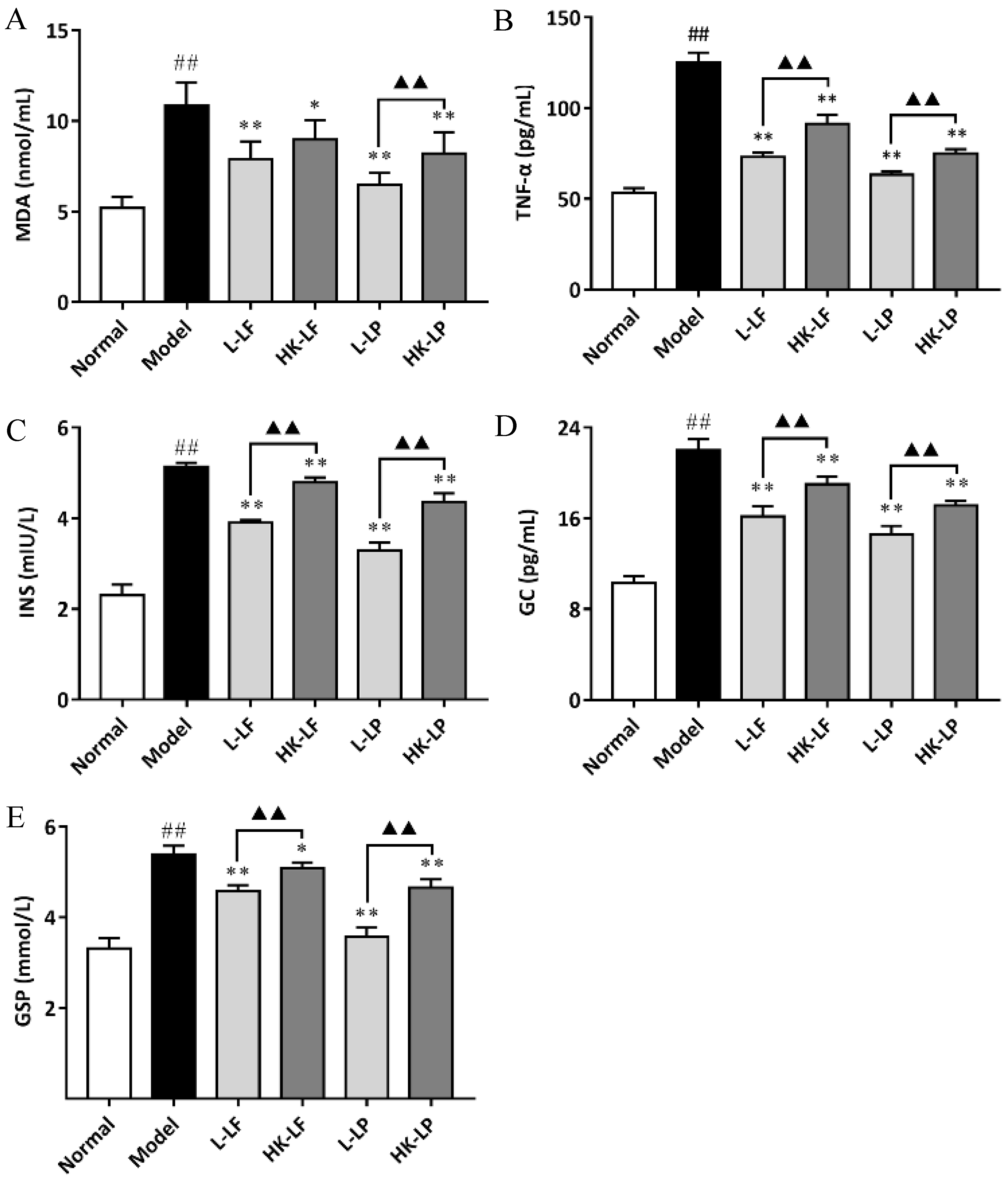
3.7. Measurement of MDA, TNF-α, GSP, INS, and GC in Serum
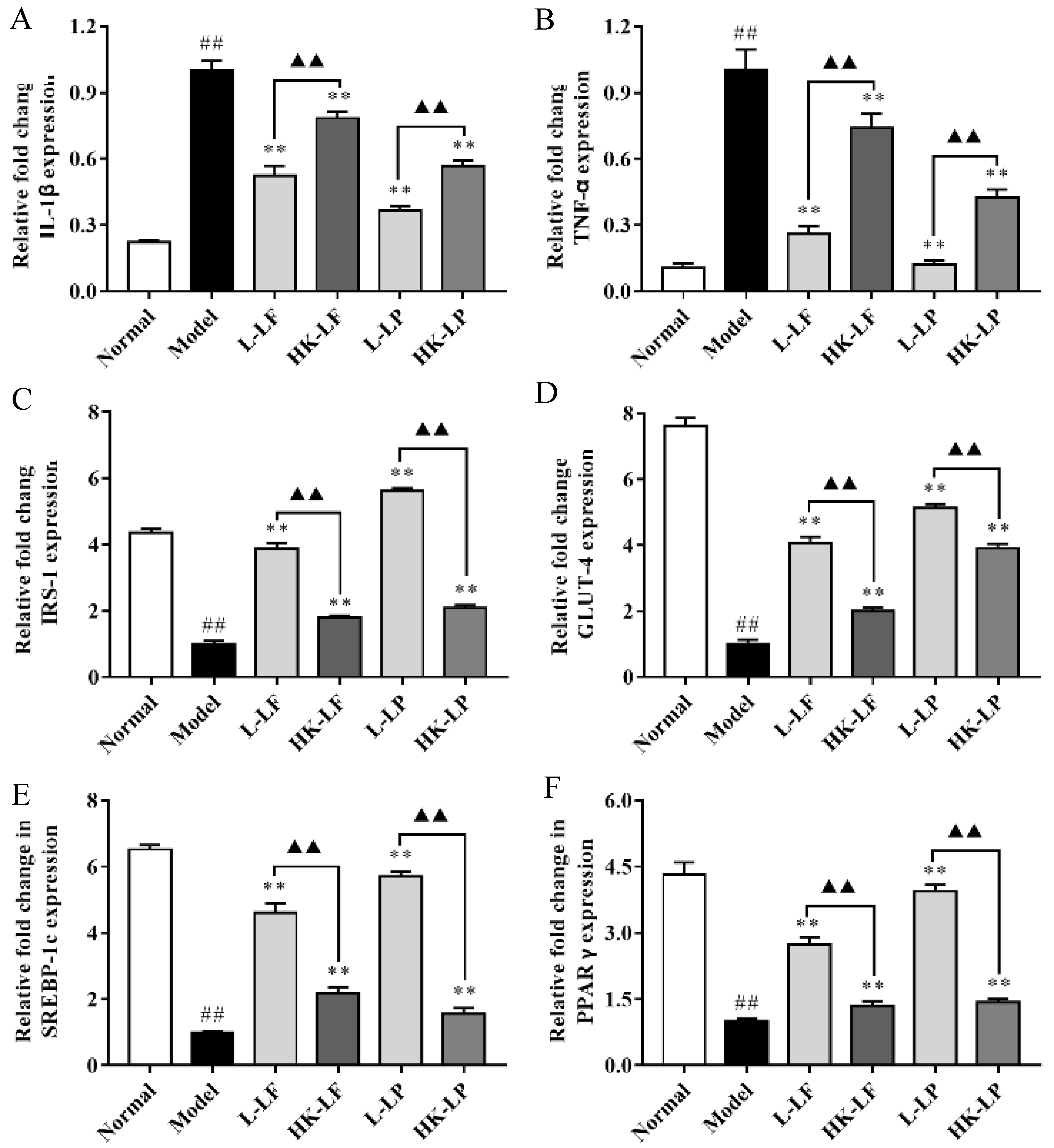
3.8. RT-qPCR
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rolfe, R.D. The role of probiotic cultures in the control of gastrointestinal health. J. Nutr. 2000, 130, 396S–402S. [Google Scholar] [CrossRef] [PubMed]
- Toral, M.; Gómez-Guzmán, M.; Jiménez, R.; Romero, M.; Sánchez, M.; Utrilla, M.P.; Garrido-Mesa, N.; Rodríguez-Cabezas, M.E.; Olivares, M.; Gálvez, J.; et al. The probiotic Lactobacillus coryniformis CECT5711 reduces the vascular pro-oxidant and pro-inflammatory status in obese mice. Clin. Sci. 2014, 127, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Persichetti, E.; De Michele, A.; Codini, M.; Traina, G. Antioxidative capacity of Lactobacillus fermentum LF31 evaluated in vitro by oxygen radical absorbance capacity assay. Nutrition 2014, 30, 936–938. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Nie, S.; Yu, Q.; Yin, J.; Xiong, T.; Gong, D.; Xie, M. Lactobacillus plantarum NCU116 attenuates cyclophosphamide-induced immunosuppression and regulates Th17/Treg cell immune responses in mice. J. Agric. Food Chem. 2016, 64, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Tabuchi, M.; Ozaki, M.; Tamura, A.; Yamada, N.; Ishida, T.; Hosoda, M.; Hosono, A. Antidiabetic effect of Lactobacillus GG in streptozotocin-induced diabetic rats. Biosci. Biotechnol. Biochem. 2003, 67, 1421–1424. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Li, Q.; Zhang, Z.; Sun, M.; Zhao, C.; Zhang, T. Effect of a novel potential probiotic Lactobacillus paracasei Jlus66 isolated from fermented milk on nonalcoholic fatty liver in rats. Food Funct. 2017, 8, 4539–4546. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, D.; Falony, G.; Vieira-Silva, S.; Wang, J.; Sailer, M.; Theis, S.; Verbeke, K.; Raes, J. Prebiotic inulin-type fructans induce specific changes in the human gut microbiota. Gut 2017, 66, 1968–1974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saavedra, J.M.; Bauman, N.A.; Oung, I.; Perman, J.A.; Yolken, R.H. Feeding of Bifidobacterium bifidum and Streptococcus thermophilus to infants in hospital for prevention of diarrhoea and shedding of rotavirus. Lancet 1994, 344, 1046–1049. [Google Scholar] [CrossRef]
- Adams, C.A. The probiotic paradox: Live and dead cells are biological response modifiers. Nutr. Res. Rev. 2010, 23, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Pantalone, K.M.; Hobbs, T.M.; Wells, B.J.; Kong, S.X.; Kattan, M.W.; Bouchard, J.; Yu, C.; Sakurada, B.; Milinovich, A.; Weng, W.; et al. Clinical characteristics, complications, comorbidities and treatment patterns among patients with type 2 diabetes mellitus in a large integrated health system. BMJ Open Diabetes Res. Care 2015, 3, e93. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Wang, X.; Jiang, X.; Kong, F.; Wang, S.; Yan, C. Antidiabetic effects of Morus alba fruit polysaccharides on high-fat diet- and streptozotocin-induced type 2 diabetes in rats. J. Ethnopharmacol. 2017, 199, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, Y.; Yan, H.; Lin, P.; Gu, W.; Yu, J. Antidiabetic effect of total saponins from Polygonatum kingianum in streptozotocin-induced daibetic rats. J. Ethnopharmacol. 2016, 179, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Duarte, S.; Baber, J.; Fujii, T.; Coito, A.J. Matrix metalloproteinases in liver injury, repair and fibrosis. Matrix Biol. 2015, 44–46, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Li, Q.; Zhong, W.; Dong, J.; Wang, Z.; Wang, C. l-carnitine ameliorated fatty liver in high-calorie diet/STZ-induced type 2 diabetic mice by improving mitochondrial function. Diabetol. Metab. Syndr. 2011, 3, 31. [Google Scholar] [CrossRef] [PubMed]
- Bloomgarden, Z.T. Second World Congress on the Insulin Resistance Syndrome: Hypertension, cardiovascular disease, and treatment approaches. Diabetes Care 2005, 28, 2073–2080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.J.; Kim, H.K. Anti-diabetic effects of electrolyzed reduced water in streptozotocin-induced and genetic diabetic mice. Life Sci. 2006, 79, 2288–2292. [Google Scholar] [CrossRef] [PubMed]
- Bolzán, A.D.; Bianchi, M.S. Genotoxicity of Streptozotocin. Mutat. Res. 2002, 512, 121–134. [Google Scholar] [CrossRef]
- Lenzen, S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia 2008, 51, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Malekifard, F.; Delirezh, N.; Hobbenaghi, R.; Malekinejad, H. Immunotherapeutic effects of pentoxifylline in type 1 diabetic mice and its role in the response of T-helper lymphocytes. Iran J. Basic Med. Sci. 2015, 18, 247–252. [Google Scholar] [PubMed]
- Cani, P.D.; Delzenne, N.M.; Amar, J.; Burcelin, R. Role of gut microflora in the development of obesity and insulin resistance following high-fat diet feeding. Pathol. Biol. 2008, 56, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, X.; Wang, H.; Yang, Z.; Li, J.; Suo, H. Prevent effects of Lactobacillus Fermentum HY01 on dextran sulfate sodium-induced colitis in mice. Nutrients 2017, 9, 545. [Google Scholar] [CrossRef] [PubMed]
- Bensalah, F.; Delorme, C.; Renault, P. Characterisation of thermotolerant cocci from indigenous flora of ‘leben’ in algerian arid area and DNA identification of atypical Lactococcus lactis strains. Curr. Microbiol. 2009, 59, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, F.C.; Lee, C.L.; Chai, C.Y.; Chen, W.T.; Lu, Y.C.; Wu, C.S. Oral administration of Lactobacillus reuteri GMNL-263 improves insulin resistance and ameliorates hepatic steatosis in high fructose-fed rats. Nutr. Metab. 2013, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, F.; Lan, C.E.; Huang, T.; Chen, K.; Chai, C.; Chen, W.; Fang, A.; Chen, Y.; Wu, C. Heat-killed and live Lactobacillus reuteri GMNL-263 exhibit similar effects on improving metabolic functions in high-fat diet-induced obese rats. Food Funct. 2016, 7, 2374–2388. [Google Scholar] [CrossRef] [PubMed]
- Sepodes, B.; Maio, R.; Pinto, R.; Marques, C.; Mendes-do-Vale, J.; McDonald, M.C.; Thiemermann, C.; Mota-Filipe, H. Tempol, an intracelullar free radical scavenger, reduces liver injury in hepatic ischemia-reperfusion in the rat. Transpl. Proc. 2004, 36, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Eliza, J.; Daisy, P.; Ignacimuthu, S.; Duraipandiyan, V. Antidiabetic and antilipidemic effect of eremanthin from Costus speciosus (Koen.)Sm., in STZ-induced diabetic rats. Chem. Biol. Interact. 2009, 182, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Chou, L.S.; Weimer, B. Isolation and characterization of acid- and bile-tolerant isolates from strains of Lactobacillus acidophilus. J. Dairy Sci. 1999, 82, 23–31. [Google Scholar] [CrossRef]
- Jadoon, S.; Malik, A. A review article on the formation, mechanism and biochemistry of MDA and MDA as a biomarker of oxidative stress. Int. J. Adv. Res. 2017, 5, 811–818. [Google Scholar] [CrossRef]
- Zingoni, A.; Sornasse, T.; Cocks, B.G.; Tanaka, Y.; Santoni, A.; Lanier, L.L. Cross-talk between activated human NK cells and CD4+ T cells via OX40-OX40 ligand interactions. J. Immunol. 2004, 173, 3716–3724. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Guo, L.; Tian, S.; Wang, T. Effects of curcumin on antioxidation in diabetic rats. Pak. J. Pharm. Sci. 2015, 28, 371–373. [Google Scholar] [PubMed]
- Sonksen, P.; Sonksen, J. Insulin: Understanding its action in health and disease. Br. J. Anaesth. 2000, 85, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, S.A.; Rios Candelore, M.; Xie, D.; Yang, X.; Tota, L.M.; Ding, V.D.; Li, Z.; Bansal, A.; Miller, C.; Cohen, S.M.; et al. A novel glucagon receptor antagonist inhibits glucagon-mediated biological effects. Diabetes 2004, 53, 3267–3273. [Google Scholar] [CrossRef] [PubMed]
- Rotter, V.; Nagaev, I.; Smith, U. Interleukin-6 (il-6) reduces gene and protein expression of IRS-1 and GLUT 4 and is overexpressed in human fat cells from insulin-resistant subjects. Diabetes 2002, 51, A303. [Google Scholar]
- Dussault, I.; Forman, B.M. Prostaglandins and fatty acids regulate transcriptional signaling via the peroxisome proliferator activated receptor nuclear receptors. Prostaglandins Other Lipid Mediat. 2000, 62, 1–13. [Google Scholar] [CrossRef]
- Ferre, P.; Foufelle, F. Hepatic steatosis: A role for de novo lipogenesis and the transcription factor SREBP-1c. Diabetes Obes. Metab. 2010, 12 (Suppl. 2), 83–92. [Google Scholar] [CrossRef] [PubMed]


| Gene | Sequence (5′-3′) |
|---|---|
| IRS-1 | Forward: CCGCGTTCAAGGAGGTCTG |
| Reverse: GCGGTAGATGCCAATCAGGT | |
| GLUT-4 | Forward: ACACTGGTCCTAGCTGTATTCT |
| Reverse: CCAGCCACGTTGCATTGTA | |
| PPARγ | Forward: GGAAGACCACTCGCATTCCTT |
| Reverse: GTAATCAGCAACCATTGGGTCA | |
| SREBP-1c | Forward: GCAGCCACCATCTAGCCTG |
| Reverse: CAGCAGTGAGTCTGCTTGAT | |
| TNF-α | Forward: CAGGCGGTGCCTATGTCTC |
| Reverse: CGATCACCCCGAAGTTCAGTAG | |
| IL-1β | Forward: GAAATGCCACCTTTTGACAGTG |
| Reverse: TGGATGCTCTCATCAGGACAG | |
| β-actin | Forward: GAGAAAATCTGGCACCACACCT |
| Reverse: GCACAGCCTGGATAGCAACGTA |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Tan, F.; Yi, R.; Mu, J.; Zhao, X.; Yang, Z. Effects of Lactobacillus on Mice with Diabetes Induced by High-Fat Diet with Streptozotocin (STZ). Appl. Sci. 2018, 8, 1249. https://doi.org/10.3390/app8081249
Chen X, Tan F, Yi R, Mu J, Zhao X, Yang Z. Effects of Lactobacillus on Mice with Diabetes Induced by High-Fat Diet with Streptozotocin (STZ). Applied Sciences. 2018; 8(8):1249. https://doi.org/10.3390/app8081249
Chicago/Turabian StyleChen, Xiaoyong, Fang Tan, Ruokun Yi, Jianfei Mu, Xin Zhao, and Zhennai Yang. 2018. "Effects of Lactobacillus on Mice with Diabetes Induced by High-Fat Diet with Streptozotocin (STZ)" Applied Sciences 8, no. 8: 1249. https://doi.org/10.3390/app8081249
APA StyleChen, X., Tan, F., Yi, R., Mu, J., Zhao, X., & Yang, Z. (2018). Effects of Lactobacillus on Mice with Diabetes Induced by High-Fat Diet with Streptozotocin (STZ). Applied Sciences, 8(8), 1249. https://doi.org/10.3390/app8081249