Processing and Prebiotics Characteristics of β-Glucan Extract from Highland Barley
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of β-Glucan Extract (GE) from Highland Barley
2.3. Determination of β-Glucan
2.4. Processing Characteristics of GE
2.4.1. Stability Assay
2.4.2. Solubility Assay
2.4.3. Foam Assay
2.5. Prebiotics Characteristics of GE
2.5.1. Anti-Digestibility Analysis
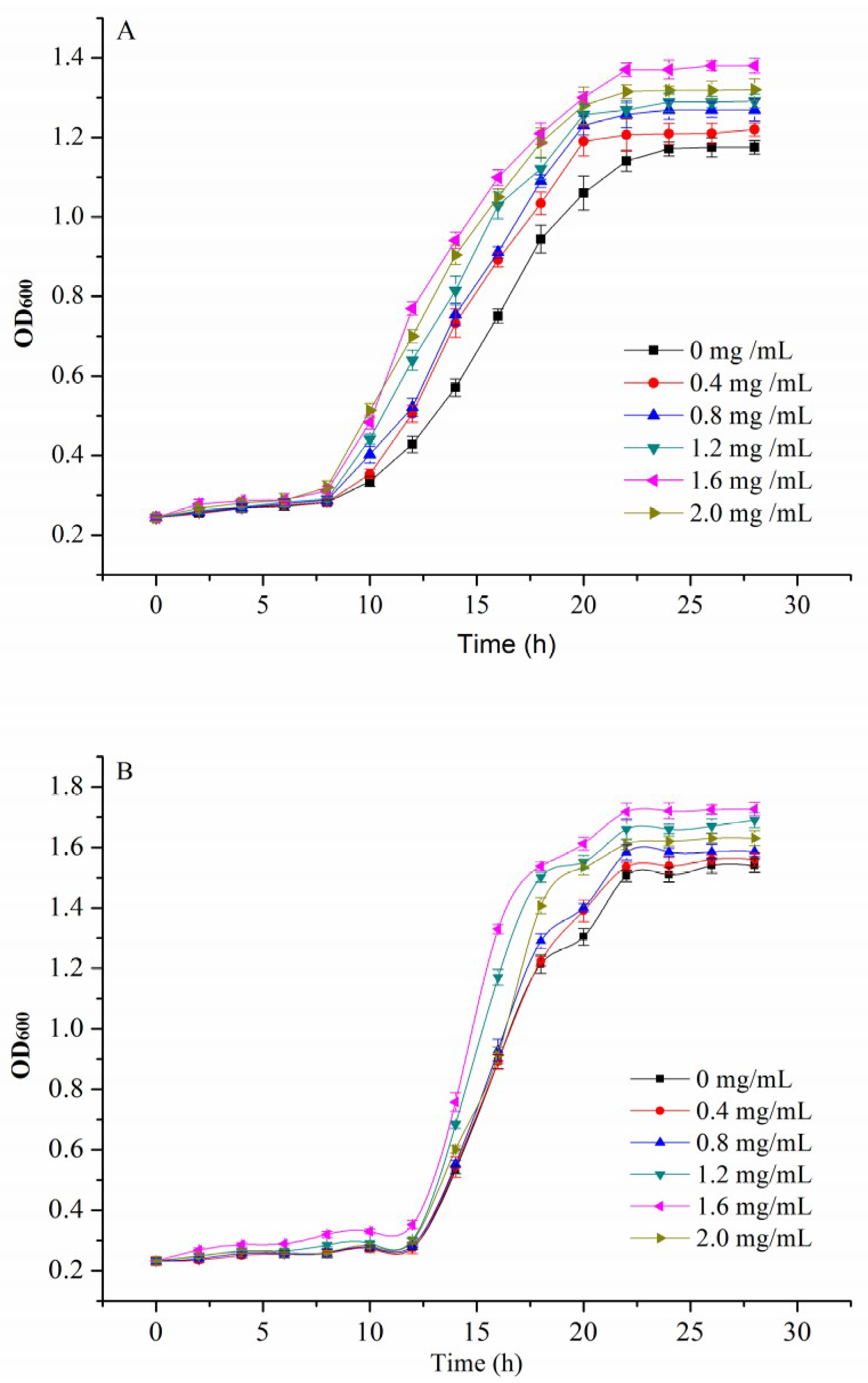
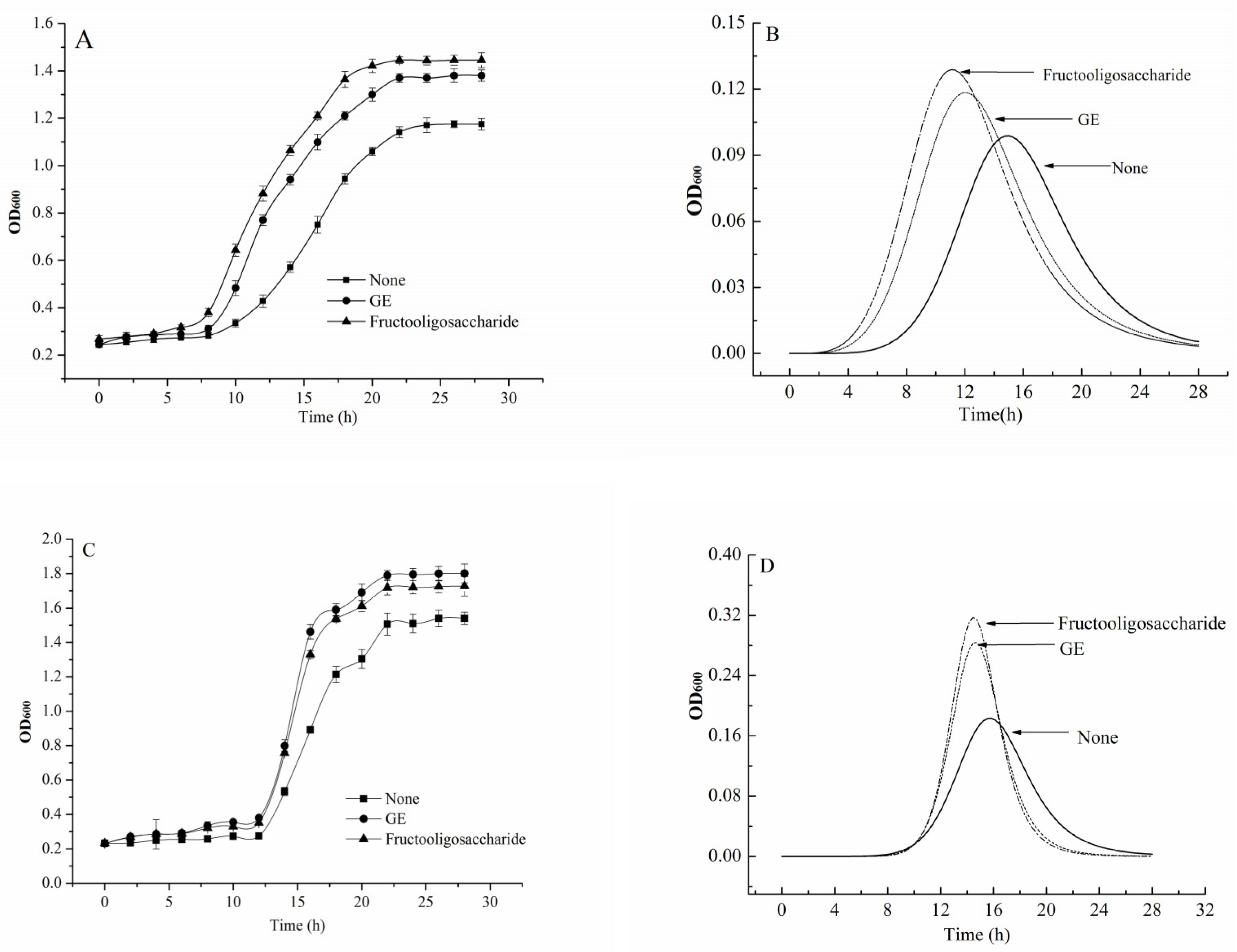
2.5.2. Effect of GE on Probiotics Growth
2.6. Statistical Analysis
3. Results and Discussion
3.1. Heat Stability and pH Stability of GE
3.2. Solubility of GE
3.3. Foaming Ability and Foam Stability of GE
3.4. Anti-Digestibility of GE
3.5. Improving Growth of Probiotics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, Y.; Christensen, D.A.; McKinnon, J.J.; Beattie, A.D.; Yu, P. Effect of altered carbohydrate traits in hulless barley (Hordeum vulgare L.) on nutrient profiles and availability and nitrogen to energy synchronization. J. Cereal Sci. 2013, 58, 182–190. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Xu, B. Superfine grinding improves functional properties and antioxidant capacities of bran dietary fibre from Qingke (hull-less barley) grown in Qinghai-Tibet Plateau, China. J. Cereal Sci. 2015, 65, 43–47. [Google Scholar] [CrossRef]
- Aman, P. Cholesterol-lowering effects of barley dietary fiber in humans: Scientific support for a generic health claim. Scand. J. Nutr. 2006, 50, 173–176. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, B.C.; Yan, F.; Tang, L.; Chen, F. Studies on “Water Method” Isolation and Characterization of Tibetan Hulless Barley β-glucan. J. Sichuan Univ. (Nat. Sci. Ed.) 2003, 40, 382–385. [Google Scholar]
- Drozdowski, L.A.; Reimer, R.A.; Temelli, F.; Bell, R.C.; Vasanthan, T.; Thomson, A.B. Beta-glucan extracts inhibit the in vitro intestinal uptake of long-chain fatty acids and cholesterol and down-regulate genes involved in lipogenesis and lipid transport in rats. J. Nutr. Biochem. 2010, 21, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Cai, F.; Shen, R.; Liu, Y. Hypoglycaemic effects and inhibitory effect on intestinal disaccharidases of oat beta-glucan in streptozotocin-induced diabetic mice. Food Chem. 2011, 129, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Schulze, C.; Wetzel, M.; Reinhardt, J.; Schmidt, M.; Felten, L.; Mundt, S. Screening of microalgae for primary metabolites including β-glucans and the influence of nitrate starvation and irradiance on β-glucan production. J. Appl. Phycol. 2016, 28, 2719–2725. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, T.; Fu, X.; Brennan, M.; Abbasi, A.M.; Zheng, B.S.; Liu, R.H. The use of an enzymatic extraction procedure for the enhancement of highland barley (Hordeum vulgare L.) phenolic and antioxidant compounds. Int. J. Food Sci. Technol. 2016, 51, 1916–1924. [Google Scholar] [CrossRef]
- Du, B.; Zhu, F.M.; Xu, B.J. β-Glucan extraction from bran of hull-less barley by accelerated solvent extraction combined with response surface methodology. J. Cereal Sci. 2014, 59, 95–100. [Google Scholar] [CrossRef]
- Burkus, Z.; Temelli, F. Stabilization of emulsions and foams using barley β-glucan. Food Res. Int. 2000, 33, 27–33. [Google Scholar] [CrossRef]
- Shen, R.L.; Zhang, W.J.; Dong, J.L. Preparation, structural characteristics and digestibility of resistant starches from highland barley, oats and buckwheat starches. J. Food Nutr. Res. 2016, 55, 303–312. [Google Scholar]
- Xi, X.G.; Wei, X.L.; Wang, Y.F.; Chu, Q.J.; Xiao, J.B. Determination of tea polysaccharides in Camellia sinensis by a modified phenol-sulfuric acid method. Arch. Biol. Sci. 2010, 62, 669–676. [Google Scholar] [CrossRef]
- Hansford, G.S.; Bailey, A.D. The logistic equation for modelling bacterial oxidation kinetics. Miner. Eng. 1992, 5, 1355–1364. [Google Scholar] [CrossRef]
- Temelli, F. Extraction and functional properties of barley beta-glucan as affected by temperature and pH. J. Food Sci. 1997, 62, 1194–1201. [Google Scholar] [CrossRef]
- Gamel, T.H.; Abdel-Aal, E.M.; Tosh, S.M. Effect of yeast-fermented and sour-dough making processes on physicochemical characteristics of β-glucan in whole wheat/oat bread. LWT-Food Sci. Technol. 2015, 60, 78–85. [Google Scholar] [CrossRef]
- Akhlaghinia, M.; Torabi, F.; Chan, C.W. Experimental investigation of temperature effect on three-phase relative permeability isoperms in heavy oil systems. Fuel 2014, 118, 281–290. [Google Scholar] [CrossRef]
- Lusk, L.T.; Duncombe, G.R.; Kay, S.B.; Navarro, A.; Ryder, D. Barley beta-glucan and beer foam stability. J. Am. Soc. Brew. Chem. 2001, 59, 183–186. [Google Scholar]
- Steiner, E.; Auer, A.; Becker, T.; Gastl, M. Comparison of beer quality attributes between beers brewed with 100% barley malt and 100% barley raw material. J. Sci. Food Agric. 2012, 92, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, P.P.; Chen, Z.; Cai, S.N.; Jing, T.; Fan, H.H.; Mo, F.; Zhang, J.; Lin, R. Preparation, characterization, and evaluation of amphotericin B-loaded MPEG-PCL-g-PEI micelles for local treatment of oral Candida albicans. Int. J. Nanomed. 2017, 12, 4269–4283. [Google Scholar] [CrossRef] [PubMed]
- Richardson, C.T.; Walsh, J.H.; Hicks, M.I.; Fordtran, J.S. Studies on mecheanisms of food-stimulated gastric-acid secretion in normal human subjects. J. Clin. Invest. 1976, 58, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.Y.; Cheung, P.C.K. Fermentation of β-glucans derived from different sources by Bifidobacteria: evaluation of their Bifidogenic effect. J. Agric. Food Chem. 2011, 59, 5986–5992. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Martínez, I.; Walter, J.; Keshavarzian, A.; Rose, D.J. In vitro characterization of the impact of selected dietary fibers on fecal microbiota composition and short chain fatty acid production. Anaerobe 2013, 23, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Rosburg, V.; Boylston, T.; White, P. Viability of Bifidobacteria strains in yogurt with added oat beta-glucan and corn starch during cold storage. J. Food Sci. 2010, 75, C439–C444. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wang, J.; Yao, H. Feruloyl oligosaccharides stimulate the growth of Bifidobacterium bifidum. Anaerobe 2005, 11, 225–229. [Google Scholar] [CrossRef] [PubMed]

| Stability | Solubility | Foaming Ability | ||||
|---|---|---|---|---|---|---|
| Treatment | β-Glucan Retain Ratio (%) | Treatment | Solubility (g/100 g) | Treatment | Foam Capacity (%) | Foam Stability (%) |
| Heat (°C) | - | Temperature (°C) | Temperature (°C) | - | ||
| Boiling | - | 20 | 0.28 ± 0.04a | 20 | 45.65 ± 1.24b | 55.55 ± 1.23e |
| 100 | 100 ± 0.14a | 40 | 0.39 ± 0.02b | 40 | 45.54 ± 1.41b | 50.15 ± 0.35d |
| Dry heat (°C) | - | 60 | 0.66 ± 0.02c | 60 | 44.12 ± 1.83b | 25.14 ± 1.02c |
| 140 | 100 ± 0.21a | 80 | 0.79 ± 0.02d | 80 | 41.67 ± 2.42ab | 13.98 ± 1.32b |
| 160 | 99.96 ± 0.55a | 100 | 0.91 ± 0.06e | 100 | 38.21 ± 2.68a | 7.21 ± 0.80a |
| 180 | 99.36 ± 1.01a | pH | - | pH | - | - |
| 200 | 99.01 ± 0.88a | 3 | 0.41 ± 0.03a | 3 | 20.35 ± 1.24a | 25.87 ± 2.12a |
| 220 | 98.21 ± 1.83a | 5 | 0.66 ± 0.03b | 5 | 31.08 ± 1.92b | 32.26 ± 1.82b |
| - | - | 7 | 0.91 ± 0.05c | 7 | 45.76 ± 1.23d | 55.55 ± 1.23e |
| - | - | 9 | 0.87 ± 0.02c | 9 | 39.55 ± 2.06c | 51.28 ± 1.37d |
| - | - | 11 | 0.86 ± 0.05c | 11 | 32.67 ± 1.34b | 46.87 ± 1.95c |
| pH | - | NaCl concentration (%, w/v) | NaCl concentration (%, w/v) | |||
| 3 | 70.45 ± 3.78a | 0 | 0.91 ± 0.01c | 0 | 45.58 ± 1.34a | 55.61 ± 1.08a |
| 5 | 92.96 ± 2.83b | 1 | 0.87 ± 0.02b | 1 | 48.21 ± 1.02b | 56.25 ± 1.84a |
| 7 | 100 ± 0.85c | 2 | 0.84 ± 0.02b | 2 | 54.67 ± 2.25c | 64.81 ± 1.23b |
| 9 | 100 ± 1.04c | 3 | 0.81 ± 0.02ab | 3 | 61.27 ± 1.32d | 68.85 ± 2.18c |
| 11 | 99.27 ± 0.57c | 4 | 0.78 ± 0.01a | 4 | 65.73 ± 1.86e | 69.23 ± 1.56c |
| pH | DH of GE Acted with α-Amylase (%) | pH | DH of GE Acted with SGA (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| t = 0 h | t = 2 h | t = 4 h | t = 6 h | t = 0 h | t = 2 h | t = 4 h | t = 6 h | ||
| 4 | 0 | 0 | 0 | 0 | 1 | 0 | 4.05 ± 0.24 | 4.95 ± 0.26 | 4.95 ± 0.34 |
| 5 | 0 | 0.12 ± 0.02 | 0.12 ± 0.03 | 0.12 ± 0.01 | 2 | 0 | 2.21 ± 0.17 | 2.72 ± 0.07 | 2.72 ± 0.15 |
| 6 | 0 | 0.74 ± 0.04 | 0.82 ± 0.06 | 0.82 ± 0.07 | 3 | 0 | 0.74 ± 0.05 | 0.82 ± 0.05 | 0.82 ± 0.09 |
| 7 | 0 | 2.21 ± 0.12 | 2.72 ± 0.12 | 2.72 ± 0.14 | 4 | 0 | 0.31 ± 0.02 | 0.31 ± 0.03 | 0.31 ± 0.04 |
| 8 | 0 | 1.51 ± 0.08 | 1.75 ± 0.14 | 1.75 ± 0.09 | 5 | 0 | 0 | 0 | 0 |
| β-Glucan (mg/mL) | Lactobacillus bulgaricus | |||
| N0 (OD600) | Nmax (OD600) | tmax (h) | μmax (h−1) | |
| 0 | 0.264 ± 0.011 | 1.196 ± 0.059 | 14.98 ± 0.247 | 0.098 ± 0.005 |
| 0.4 | 0.260 ± 0.014 | 1.235 ± 0.024 | 13.58 ± 0.356 | 0.111 ± 0.006 |
| 0.8 | 0.263 ± 0.013 | 1.295 ± 0.013 | 13.58 ± 0.236 | 0.111 ± 0.008 |
| 1.2 | 0.259 ± 0.008 | 1.310 ± 0.018 | 12.73 ± 0.121 | 0.112 ± 0.009 |
| 1.6 | 0.261 ± 0.006 | 1.391 ± 0.029 | 12.16 ± 0.147 | 0.118 ± 0.007 |
| 2.0 | 0.261 ± 0.015 | 1.340 ± 0.023 | 12.16 ± 0.054 | 0.112 ± 0.005 |
| β-Glucan (mg/mL) | Bifidobacterium adolescentis | |||
| N0 (OD600) | Nmax (OD600) | tmax (h) | μmax (h−1) | |
| 0 | 0.240 ± 0.008 | 1.537 ± 0.036 | 15.83 ± 0.214 | 0.183 ± 0.006 |
| 0.4 | 0.242 ± 0.006 | 1.566 ± 0.047 | 15.83 ± 0.175 | 0.195 ± 0.012 |
| 0.8 | 0.245 ± 0.011 | 1.594 ± 0.032 | 15.55 ± 0.236 | 0.206 ± 0.014 |
| 1.2 | 0.251 ± 0.010 | 1.666 ± 0.025 | 15.55 ± 0.147 | 0.234 ± 0.008 |
| 1.6 | 0.259 ± 0.008 | 1.710 ± 0.017 | 14.70 ± 0.089 | 0.283 ± 0.013 |
| 2.0 | 0.253 ± 0.014 | 1.640 ± 0.028 | 14.98 ± 0.072 | 0.263 ± 0.007 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.; Xie, H.; Liu, L.; Jia, D.; Yao, K.; Chi, Y. Processing and Prebiotics Characteristics of β-Glucan Extract from Highland Barley. Appl. Sci. 2018, 8, 1481. https://doi.org/10.3390/app8091481
Ren Y, Xie H, Liu L, Jia D, Yao K, Chi Y. Processing and Prebiotics Characteristics of β-Glucan Extract from Highland Barley. Applied Sciences. 2018; 8(9):1481. https://doi.org/10.3390/app8091481
Chicago/Turabian StyleRen, Yao, Haoyu Xie, Li Liu, Dongying Jia, Kai Yao, and Yuanlong Chi. 2018. "Processing and Prebiotics Characteristics of β-Glucan Extract from Highland Barley" Applied Sciences 8, no. 9: 1481. https://doi.org/10.3390/app8091481
APA StyleRen, Y., Xie, H., Liu, L., Jia, D., Yao, K., & Chi, Y. (2018). Processing and Prebiotics Characteristics of β-Glucan Extract from Highland Barley. Applied Sciences, 8(9), 1481. https://doi.org/10.3390/app8091481






