Adsorption Transformation of Heat: The Applicability in Various Climatic Zones of the Russian Federation
Abstract
:1. Introduction
2. Methodology of the Analysis
3. Results and Discussion
3.1. Analysis of the AHT Cycles Demanded in the RF and Appropriate Working Pairs
3.2. Refrigeration/Air Conditioning
3.3. Heating
3.4. Heat Storage
3.5. Heat Amplification Cycle “Heat from Сold” (HeCol)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aristov, Y.I. Novel materials for adsorptive heat pumping and storage: Screening and nanotailoring of sorption properties. J. Chem. Eng. Jpn. 2007, 40, 1242–1251. [Google Scholar] [CrossRef]
- Meunier, F. Adsorptive cooling: A clean technology. Clean. Prod. Proc. 2001, 3, 8–20. [Google Scholar] [CrossRef]
- Deng, J.; Wang, R.Z.; Han, G.Y. A review of thermally activated cooling technologies for combined cooling, heating and power systems. Prog. Energy Combust. Sci. 2011, 37, 172–203. [Google Scholar] [CrossRef]
- Wang, R.; Wang, L.; Wu, J. Adsorption Refrigeration Technology: Theory and Application; John Wiley & Sons: Singapore, 2014. [Google Scholar]
- Advances in Adsorption Technologies; Saha, B.; Ng, K.S. (Eds.) Nova Science Publishers: New York, NY, USA, 2010; ISBN 978-1-60876-833-2. [Google Scholar]
- Aristov, Y.I. Concept of adsorbent optimal for adsorptive cooling/heating. Appl. Therm. Eng. 2014, 72, 166–175. [Google Scholar] [CrossRef]
- Henninger, S.K.; Ernst, S.-J.; Gordeeva, L.; Bendix, P.; Fröhlich, D.; Grekova, A.; Bonaccorsi, L.; Aristov, Y.; Jaenchen, J. New materials for adsorption heat transformation and storage. Renew. Energy 2017, 110, 59–68. [Google Scholar] [CrossRef]
- Gordeeva, L.G.; Aristov, Y.I. Adsorptive heat storage and amplification: New cycles and adsorbents. Energy 2019, 167, 440–453. [Google Scholar] [CrossRef]
- Shmroukh, A.N.; Ali, A.H.H.; Ookawara, S. Adsorption working pairs for adsorption cooling chillers: A review based on adsorption capacity and environmental impact. Renew. Sustain. Energy Rev. 2015, 50, 445–456. [Google Scholar] [CrossRef]
- Scapino, L.; Zondag, H.A.; Bael, J.V.; Diriken, J.; Rindt, C.C.M. Sorption heat storage for long-term low-temperature applications: A review on the advancements at material and prototype scale. Appl. Energy 2017, 190, 920–948. [Google Scholar] [CrossRef]
- Askalany, A.A.; Salem, M.; Ismael, I.M.; Ali, A.H.H.; Morsy, M.G.; Saha, B.B. An overview on adsorption pairs for cooling. Renew. Sustain. Energy Rev. 2013, 19, 565–572. [Google Scholar] [CrossRef]
- Alahmer, A.; Wang, X.; Al-Rbaihat, R.; Alam, K.C.A.; Saha, B.B. Performance evaluation of a solar adsorption chiller under different climatic conditions. Appl. Energy 2016, 175, 293–304. [Google Scholar] [CrossRef]
- Trapani, F.; Polyzoidis, A.; Loebbecke, S.; Piscopo, C.G. On the general water harvesting capability of metal-organic frameworks under well-defined climatic conditions. Microporous Mesoporous Mater. 2016, 230, 20–24. [Google Scholar] [CrossRef]
- Habib, K.; Saha, B.B.; Chakraborty, A.; TaekOh, S.; Koyama, S. Study on solar driven combined adsorption refrigeration cycles in tropical climate. Appl. Therm. Eng. 2013, 50, 1582–1589. [Google Scholar] [CrossRef]
- Allouhi, A.; Kousksou, T.; Jamil, A.; Agrouaz, Y.; Bouhal, T.; Saidur, R.; Benbassou, A. Performance evaluation of solar adsorption cooling systems for vaccine preservation in Sub-Saharan Africa. Appl. Energy 2016, 170, 232–241. [Google Scholar] [CrossRef]
- Alam, K.C.A.; Saha, B.B.; Akisawa, A. Adsorption cooling driven by solar collector: A case study for Tokyo solar data. Appl. Therm. Eng. 2013, 50, 1603–1609. [Google Scholar] [CrossRef]
- Rouf, R.A.; Alam, K.C.A.; Khan, M.A.H. Solar Adsorption Cooling and Hot Water Supply for Climatic Condition of Dhaka. Procedia Eng. 2015, 105, 705–712. [Google Scholar] [CrossRef]
- Polanyi, M. Theories of the adsorption of gas. Trans. Faraday Soc. 1932, 28, 16. [Google Scholar]
- Dubinin, M.M. Theory of physical adsorption of gases and vapour and adsorption properties of adsorbents of various natures and porous structures. Bull. Div. Chem. Soc. 1960, 9, 1072–1078. [Google Scholar] [CrossRef]
- Aristov, Y.I.; Sharonov, V.E.; Tokarev, M.M. Universal relation between the boundary temperatures of a basic cycle of sorption heat machines. Chem. Eng. Sci. 2008, 63, 2907–2912. [Google Scholar] [CrossRef]
- Grekova, A.; Gordeeva, L.; Aristov, Y. Composite sorbents “Li/Ca halogenides inside Multi-wall Carbon Nano-tubes” for Thermal Energy Storage. Sol. Energy Mater. Sol. Cells 2016, 155, 176–183. [Google Scholar] [CrossRef]
- Wilson, S.T.; Lok, B.M.; Messina, C.A.; Cannan, T.R.; Flanigen, E.M. Aluminophosphate molecular sieves: A new class of microporous crystalline inorganic solids. J. Am. Chem. Soc. 1982, 104, 1146–1147. [Google Scholar] [CrossRef]
- Martens, J.A.; Jacobs, P. Crystalline microporous phosphates: A family of versatile catalysts and adsorbents, in advanced zeolite science and application. Stud. Surf. Sci. Catal. 1994, 85, 653–685. [Google Scholar] [CrossRef]
- Ng, E.P.; Mintova, S. Nanoporous materials with enhanced hydrophilicity and high water sorption capacity. Microporous Mesoporous Mater. 2008, 114, 1–26. [Google Scholar] [CrossRef]
- Kitagawa, S.; Kitaura, R.; Noro, S. Functional porous coordination polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef] [PubMed]
- Gordeeva, L.G.; Aristov, Y.I. Composites “salt inside porous matrix” for adsorption heat transformation: A current state of the art and new trends”. Int. J. Low Carb. Tech. 2012, 7, 288–302. [Google Scholar] [CrossRef]
- Henninger, S.K.; Jeremias, F.; Kummer, H.; Schlossig, P.; Henning, H.-M. Novel sorption materials for solar heating and cooling. Energy Procedia 2012, 30, 279–288. [Google Scholar] [CrossRef]
- Aristov, Y.I.; Tokarev, M.M.; Freni, A.; Glaznev, I.S.; Restuccia, G. Kinetics of water adsorption on silica Fuji Davison RD. Microporous Mesoporous Mater. 2006, 96, 65–71. [Google Scholar] [CrossRef]
- Thua, K.; Chakrabortyc, A.; Sahad, B.B.; Ng, K.C. Thermo-physical properties of silica gel for adsorption desalination cycle. Appl. Therm. Eng. 2013, 50, 1596–1602. [Google Scholar] [CrossRef]
- El-Sharkawy, I.I.; Hassan, M.; Saha, B.B.; Koyama, S.; Nasr, M.M. Study on adsorption of methanol onto carbon based adsorbents. Int. J. Refrig. 2009, 32, 1579–1586. [Google Scholar] [CrossRef]
- Henninger, S.K.; Schicktanz, M.; HuЁgenell, P.P.C.; Sievers, H.; Henning, H.-M. Evaluation of methanol adsorption on activated carbons for thermally driven chillers part I: Thermophysical characterization. Int. J. Refrig. 2012, 35, 543–553. [Google Scholar] [CrossRef]
- Brancato, V.; Frazzica, A. Characterisation and comparative analysis of zeotype water dsorbents for heat transformation applications. Sol. Energy Mater. Sol. Cells 2018, 15, 91–102. [Google Scholar] [CrossRef]
- Kohler, T.; Hinze, M.; Miller, K.; Schwieger, W. Temperature independent description of water adsorption on zeotypes showing a type V adsorption isotherm. Energy 2017, 135, 227–236. [Google Scholar] [CrossRef]
- Kim, S.-N.; Kim, J.; Kim, H.-Y.; Cho, H.-Y.; Ahn, W.-S. Adsorption/catalytic properties of MIL-125 and NH2-MIL-125. Catal. Today 2013, 204, 85–93. [Google Scholar] [CrossRef]
- Jasuja, H.; Huang, Y.; Walton, K.S. Adjusting the Stability of Metal−Organic Frameworks under Humid Conditions by Ligand Functionalization. Langmuir 2012, 28, 16874–16880. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, G.; Matsuda, R.; Sato, H.; Hori, A.; Takata, M.; Kitagawa, S. Effect of functional groups in MIL-101 on water sorption behavior. Microporous Mesoporous Mater. 2012, 157, 89–93. [Google Scholar] [CrossRef]
- Küsgens, P.; Rose, M.; Senkovska, I.; Fröde, H.; Henschel, A.; Siegle, S.; Kaskel, S. Characterization of metal-organic frameworks by water adsorption. Microporous Mesoporous Mater. 2009, 120, 325–330. [Google Scholar] [CrossRef]
- Grekova, A.D.; Gordeeva, L.G.; Aristov, Y.I. Composite “LiCl/vermiculite” as advanced water sorbent for thermal energy storage. Appl. Therm. Eng. 2017, 124, 1401–1408. [Google Scholar] [CrossRef]
- Gordeeva, L.G.; Freni, A.; Krieger, T.A.; Restuccia, G.; Aristov, Y.I. Composites “lithium halides in silica gel pores”: Methanol sorption equilibrium. Microporous Mesoporous Mater. 2008, 112, 264–271. [Google Scholar] [CrossRef]
- Simonova, I.A.; Freni, A.; Restuccia, G.; Aristov, Y.I. Water sorption on composite “silica modified by calcium nitrate”. Microporous Mesoporous Mater. 2009, 122, 223–228. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, H.; Yang, F.; Zhang, N.; Cao, X. Inorganic composite sorbents for water vapor sorption: A research progress. Renew. Sustain. Energy Rev. 2016, 54, 761–776. [Google Scholar] [CrossRef]
- Meteonorm. Available online: http://www.meteonorm.com/ (accessed on 20 December 2018).
- Aristov, Y.I. Adsorptive transformation of ambient heat: A new cycle. Appl. Therm. Eng. 2017, 124, 521–524. [Google Scholar] [CrossRef]
- The Map Climatic zones of the Russian Federation. Available online: http://meteoinfo.ru/climate (accessed on 20 December 2018).
- Aristov, Y.I. A new adsorptive cycle “HeCol” for upgrading ambient heat: The current state of the art. Int. J. Refrig. 2018. submitted. [Google Scholar] [CrossRef]
 ), P2 = 127.5 (
), P2 = 127.5 (  ), P3 = 217.6 (
), P3 = 217.6 (  ) mbar (b).
) mbar (b).
 ), P2 = 127.5 (
), P2 = 127.5 (  ), P3 = 217.6 (
), P3 = 217.6 (  ) mbar (b).
) mbar (b).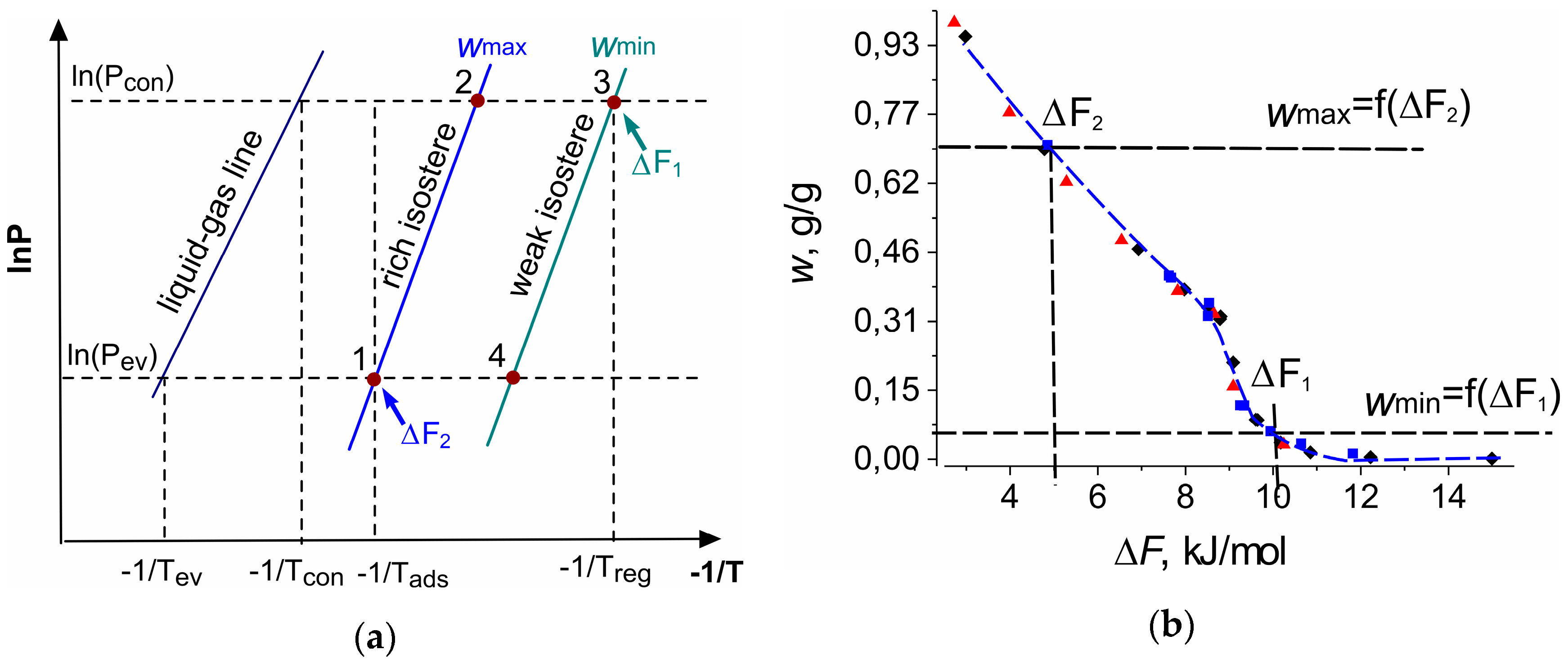







| City | Treg, °C | ΔF, kJ/mol | Adsorbent | Δw, g/g | |
|---|---|---|---|---|---|
| ΔF2 | ΔF1 | ||||
| Air conditioning | |||||
| Astrakhan | 85 | 4.0 | 6.9 | LiCl/MWCNT | 0.6 |
| Refrigeration | |||||
| Astrakhan | 85 | 5.2 | 6.9 | LiCl/vermiculite LiCl/MWCNT | 0.3 0.4 |
| Omsk | 75 | 4.8 | 5.9 | LiCl/vermiculite ALPO—18 | 0.5 0.2 |
| Yakutsk | 65 | 4.5 | 4.8 | CAU10 | 0.2 |
| Moscow | 65 | 4.2 | 5.0 | CAU10 | 0.3 |
| Oimyakon | 65 | 4.2 | 4.8 | CAU10 | 0.3 |
| Vladivostok | 60 | 3.9 | 4.7 | MIL125NH2 | 0.3 |
| Arkhangelsk | 60 | 3.8 | 5.1 | MIL125NH2 | 0.3 |
| City | Treg, °C | ΔF2, kJ/mol | ΔF1, kJ/mol | Δw, g/g | Period | |
|---|---|---|---|---|---|---|
| Astrakhan | 90 | 2.5–3.8 | 6.2 | LiCl/Ver | 0.6–0.9 | May–Sept |
| LiCl/MWCNT | 0.3–0.5 | |||||
| 85 | 4.8 | MIL125NH2 | 0.3–0.4 | |||
| 75 | 2.5–2.9 | 4.1 | FAMZ01 | 0.2 | Jun–Aug | |
| LiCl/Ver | 0.1–0.3 | |||||
| Moscow | 90 | 3.7–5.0 | 6.2 | LiCl/Ver | 0.5–0.6 | May–Sept |
| 80 | 3.7–4.0 | 4.8 | CAU10 | 0.3 | Jun–Aug | |
| MIL125NH2 | 0.2–0.4 | |||||
| Vladivostok | 90 | 3.5–5.4 | 6.2 | LiCl/Ver | 0.4–0.6 | May–Oct |
| Omsk | 90 | 3.4–5.0 | 6.2 | LiCl/Ver | 0.4–0.6 | May–Sept |
| 80 | 3.4–4.0 | 4.8 | MIL125NH2 | 0.2–0.4 | Jun–Aug | |
| Arkhangelsk | 90 | 4.1–4.7 | 6.2 | LiCl/Ver | 0.4–0.6 | Jun–Aug |
| Yakutsk | 90 | 3.5–4.1 | 6.2 | LiCl/Ver | 0.6 | Jun–Aug |
| 80 | 4.8 | CAU10 | 0.3 | |||
| MIL125NH2 | 0.2–0.4 | |||||
| Oimyakon | 90 | 4.2–4.8 | 6.2 | LiCl/Ver | 0.5–0.6 | Jun–Aug |
| City | ΔF2, kJ/mol | ΔF1, kJ/mol | Adsorbent | Δw, g/g | Heating Period |
|---|---|---|---|---|---|
| Astrakhan | 3.8 | 8.0 | LiCl/MWCNT | 0.9 | Apr, May, Sept, Oct |
| Moscow | 4.3 | 9.1 | LiCl/SiO2 | 0.6 | Apr, May, Sept, Oct |
| Vladivostok | 4.6 | 9.0 | UiO67 | 0.4 | Apr, May, Sept, Oct |
| Omsk | 4.9 | 8.9 | MaxSorbIII | 0.3 | Apr, May, Sept, Oct |
| Oimyakon | 5.1 | 9.7 | LiBr/SiO2 | 0.4 | May, Sept |
| Oimyakon | 8.1 | 9.7 | LiBr/SiO2 | 0.1 | Apr, Oct |
| Arkhangelsk | 5.3 | 9.6 | LiBr/SiO2 | 0.3 | Apr, May, Sept, Oct |
| Yakutsk | 6.6 | 8.9 | LiBr/SiO2, MaxSorbIII | 0.1 | Apr, May, Sept, Oct |
| TM, °C | TH, °C | ΔF2, kJ/mol | ΔF1, kJ/mol | Adsorbent | Δw, g/g | Period |
|---|---|---|---|---|---|---|
| Yakutsk | ||||||
| 3 | 30 | 3.8 | 5.0–7.2 | LiCl/MWCNT | 0.97 | Nov–Feb |
| 35 | 4.5 | 5.0–7.2 | LiCl/SiO2 | 0.38 | Nov–Feb | |
| 40 | 5.1 | 6.4–7.2 | MaxSorbIII | 0.14 | Dec–Feb | |
| 10 | 30 | 2.7 | 4.8–8.4 | LiCl/MWCNT | 1.37 | Nov–March |
| 40 | 4.0 | 4.8–8.4 | LiCl/MWCNT, LiCl/SiO2 | 0.67 0.70 | Nov–March | |
| 50 | 5.4 | 7.4–8.4 | MaxSorbIII | 0.19 | Dec–Feb | |
| Oimyakon | ||||||
| 3 | 30 | 3.8 | 6.7–9.0 | LiCl/MWCNT | 0.97 | Nov–March |
| 35 | 4.5 | 6.7–9.0 | LiCl/SiO2 | 0.38 | Nov–March | |
| 40 | 5.1 | 8.4–9.0 | MaxSorbIII | 0.14 | Dec–Feb | |
| 10 | 30 | 2.7 | 7.9–10.2 | LiCl/MWCNT | 1.37 | Nov–March |
| 40 | 4.0 | 7.9–10.2 | LiCl/MWCNT, LiCl/SiO2 | 0.67 0.70 | Nov–March | |
| 50 | 5.4 | 9.6–10.2 | LiBr/SiO2 | 0.26 | Dec–Feb | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grekova, A.; Gordeeva, L.; Sapienza, A.; Aristov, Y. Adsorption Transformation of Heat: The Applicability in Various Climatic Zones of the Russian Federation. Appl. Sci. 2019, 9, 139. https://doi.org/10.3390/app9010139
Grekova A, Gordeeva L, Sapienza A, Aristov Y. Adsorption Transformation of Heat: The Applicability in Various Climatic Zones of the Russian Federation. Applied Sciences. 2019; 9(1):139. https://doi.org/10.3390/app9010139
Chicago/Turabian StyleGrekova, Alexandra, Larisa Gordeeva, Alessio Sapienza, and Yuri Aristov. 2019. "Adsorption Transformation of Heat: The Applicability in Various Climatic Zones of the Russian Federation" Applied Sciences 9, no. 1: 139. https://doi.org/10.3390/app9010139
APA StyleGrekova, A., Gordeeva, L., Sapienza, A., & Aristov, Y. (2019). Adsorption Transformation of Heat: The Applicability in Various Climatic Zones of the Russian Federation. Applied Sciences, 9(1), 139. https://doi.org/10.3390/app9010139





